Adsorption Properties and Mechanisms of Methylene Blue by Modified Sphagnum Moss Bio-Based Adsorbents
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of the Materials
2.3. Analytical Methods
2.4. Batch Adsorption Experiments
2.5. Adsorption Isotherms
2.6. Adsorption Kinetics
2.7. Thermodynamics
2.8. Adsorption Regeneration Experiment
3. Result and Discussion
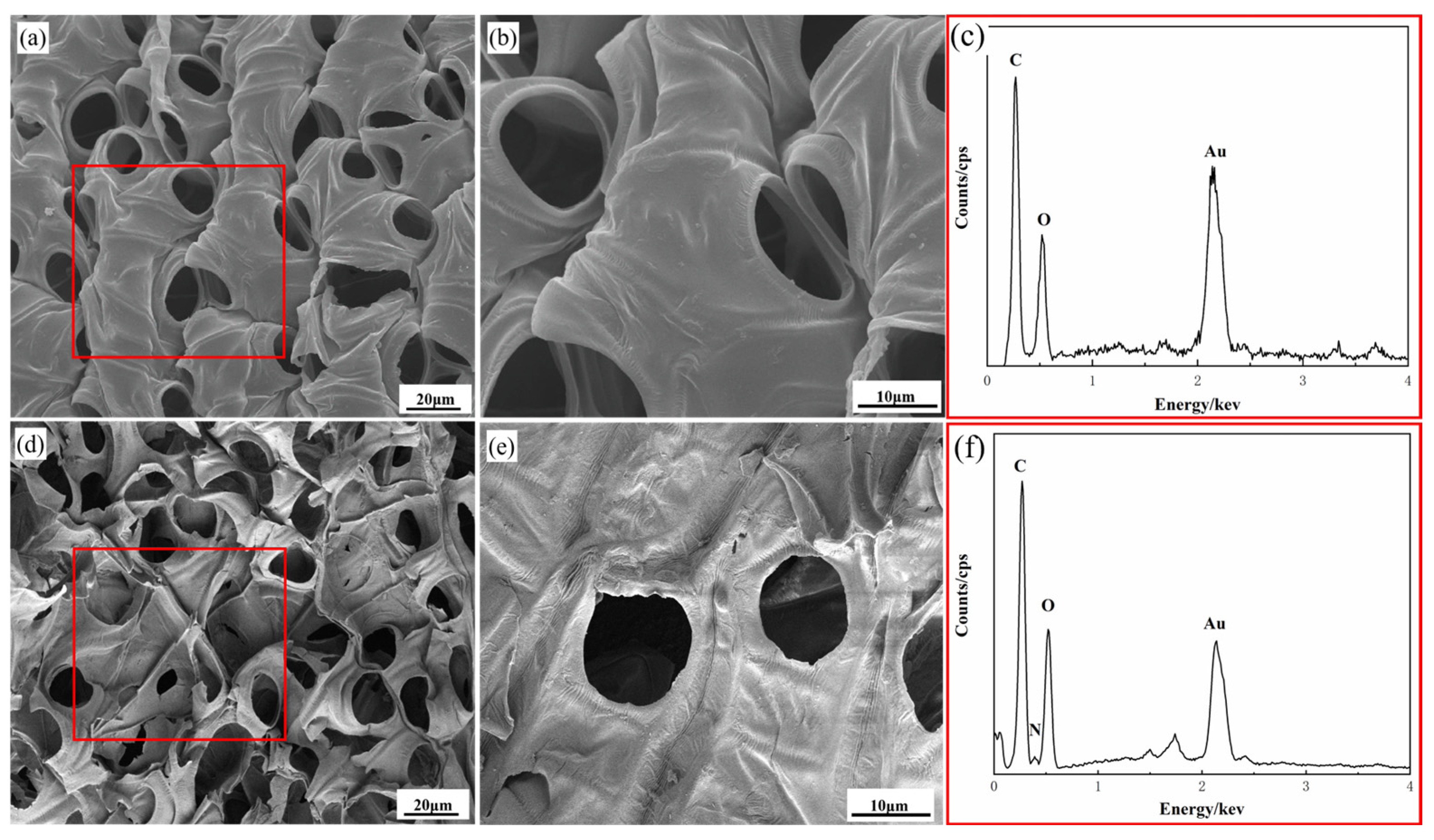
3.1. Analyses of Morphologies
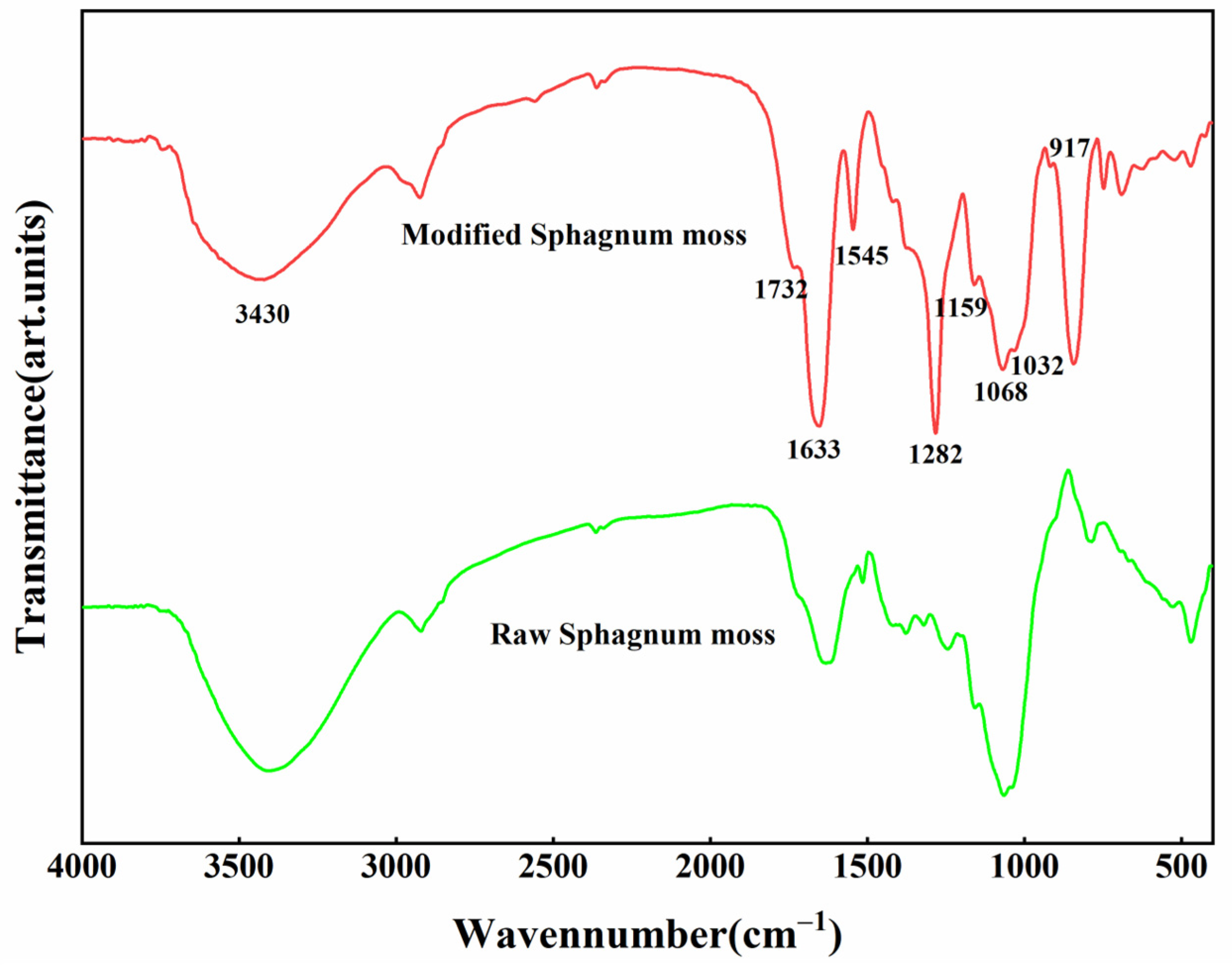
3.2. Infrared Spectroscopic Analysis
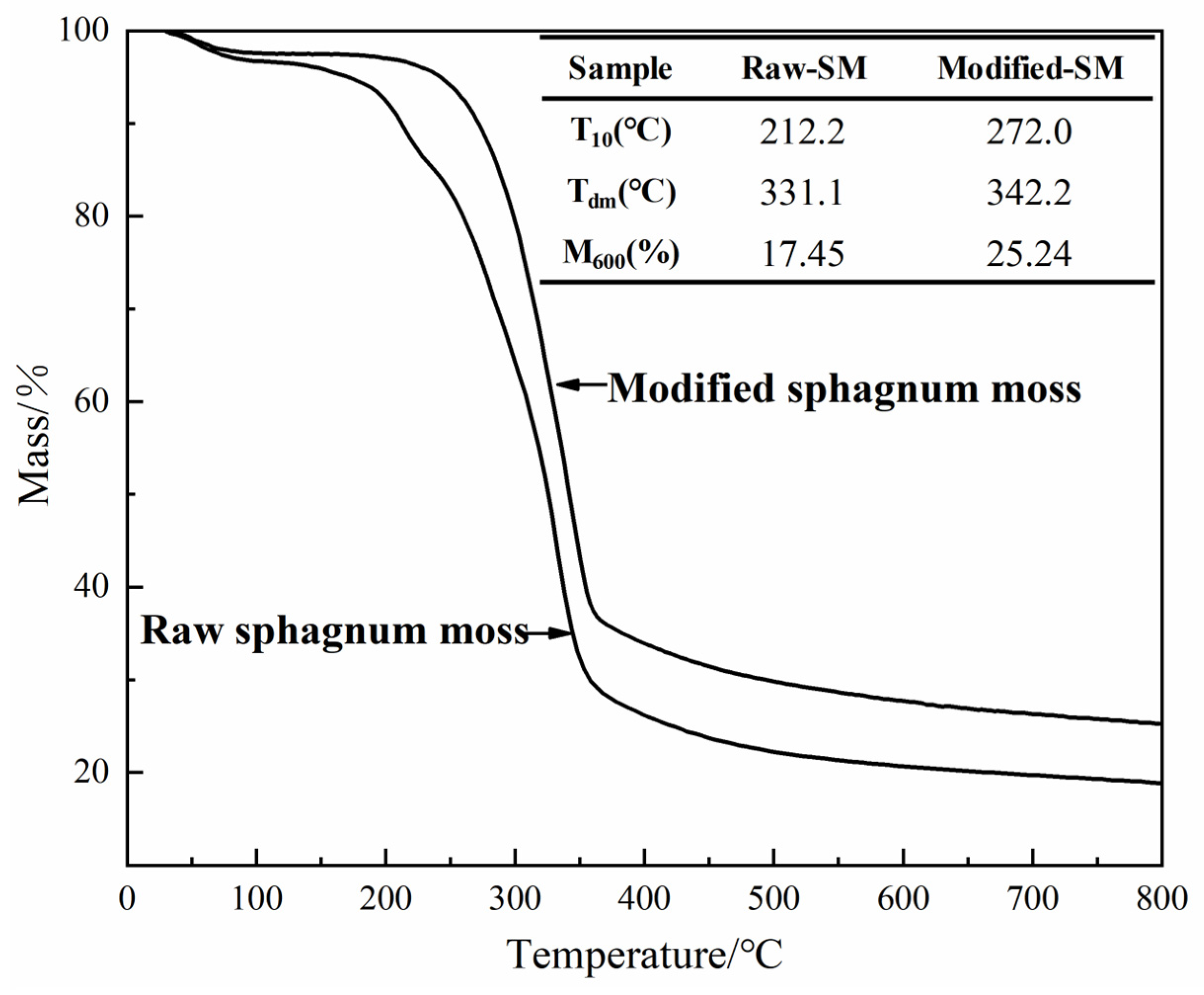
3.3. Thermogravimetric Analysis
3.4. Adsorption Experiments
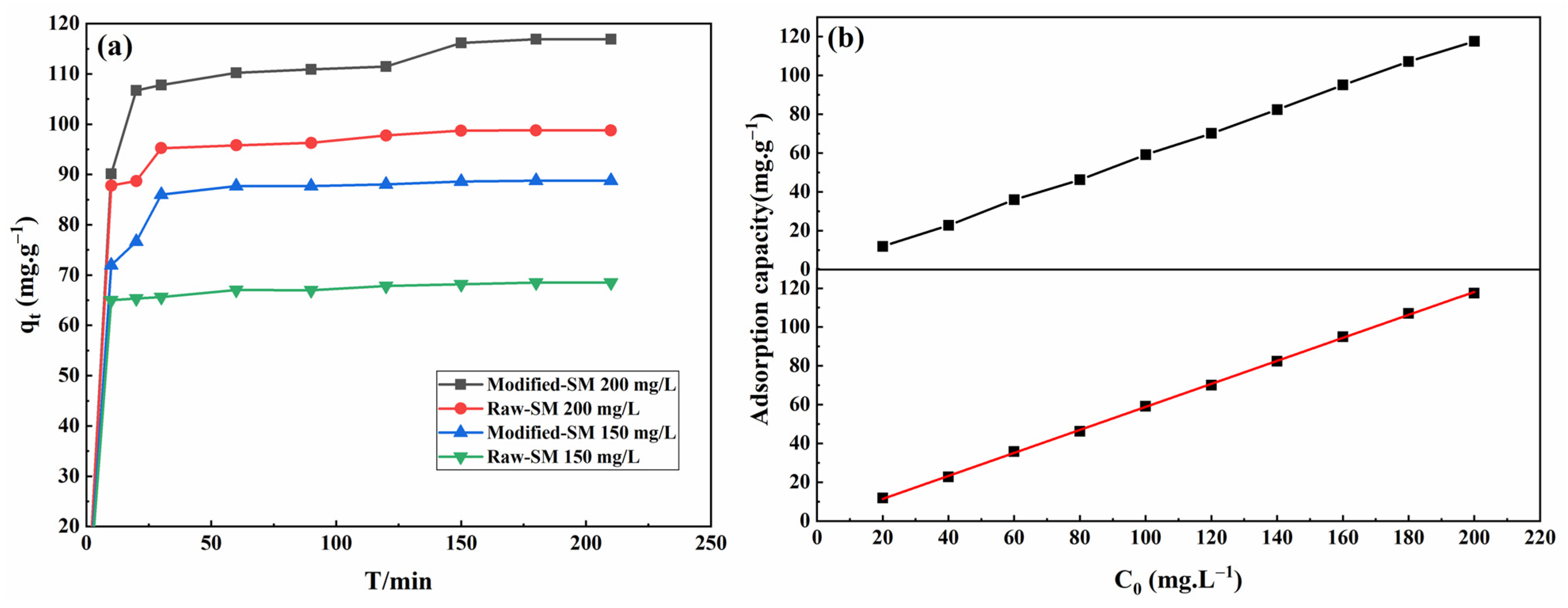
3.4.1. The Influence of Contact Time and Initial MB Concentration on Adsorption Effect
3.4.2. The Influence of the MB Solution pH on Adsorption Effect
3.4.3. The Influence of Adsorbent Dosage on Adsorption Effect
3.4.4. Adsorption Isotherms
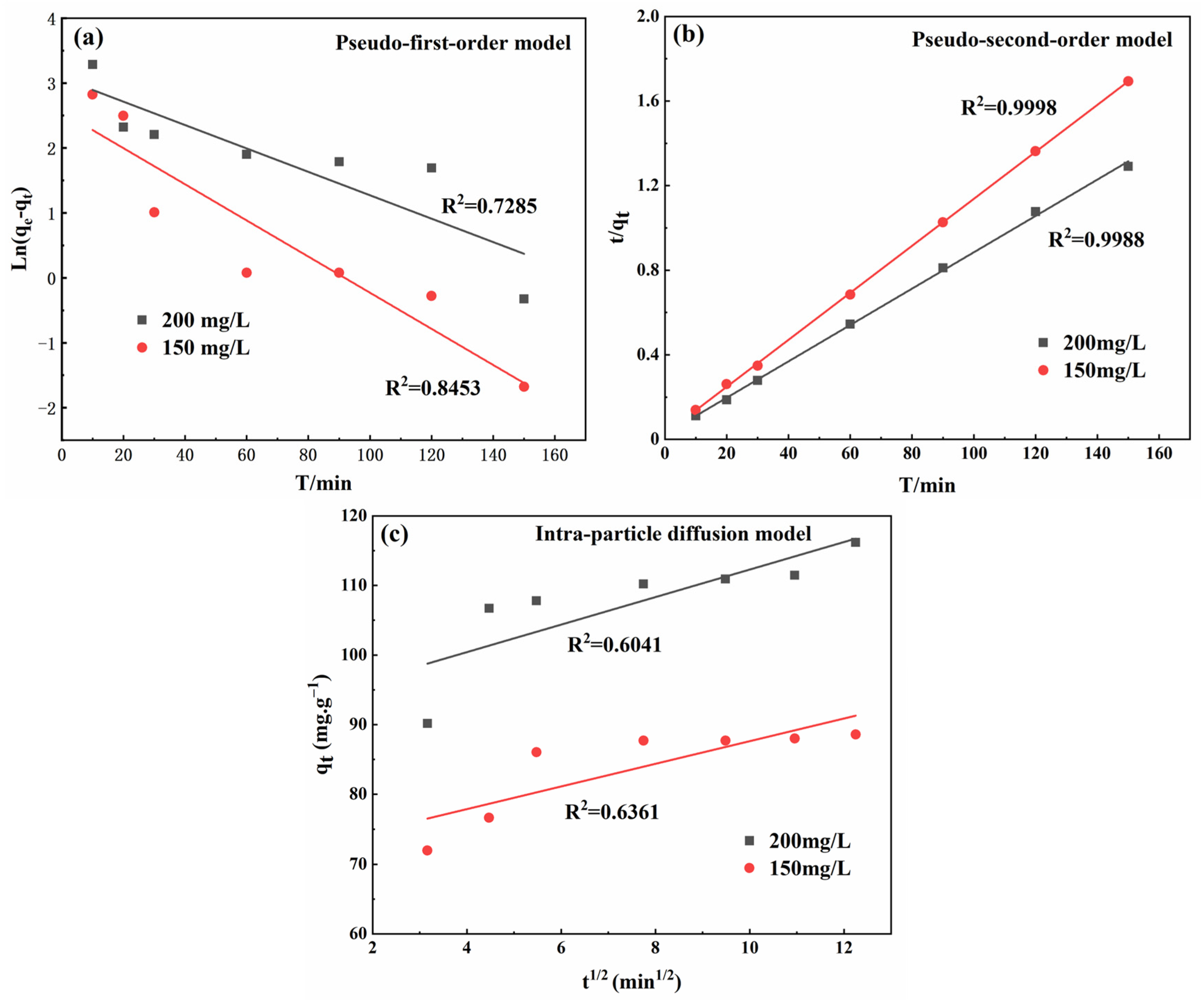
3.4.5. Adsorption Kinetics
3.4.6. Adsorption Thermodynamics
3.4.7. Adsorption–Desorption Cycle Experiments
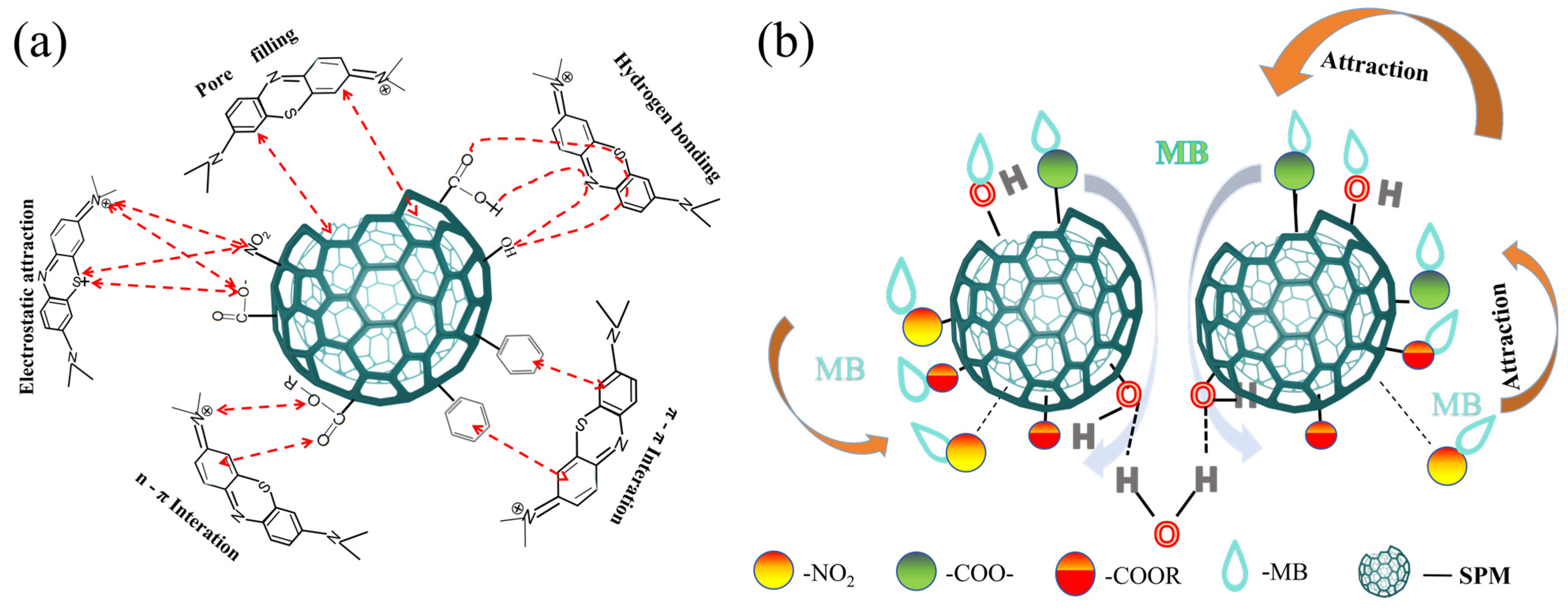
3.5. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Kammah, M.; Elkhatib, E.; Gouveia, S.; Cameselle, C.; Aboukila, E. Enhanced removal of indigo carmine dye from textile effluent using green cost-efficient nanomaterial: Adsorption, kinetics, thermodynamics and mechanisms. Sustain. Chem. Pharm. 2022, 29, 100753. [Google Scholar] [CrossRef]
- Zhang, L.; Zaoui, A.; Sekkal, W. Adsorption efficiency of highly methylene blue dye concentrations with multilayer chitosan-modified clays for a precise nanofiltration performance of polluted water. J. Water Process. Eng. 2024, 57, 104651. [Google Scholar] [CrossRef]
- Dilshad, S.; Muslim, M.; Ahmed, A.; Ali, A.; Firdaus, S.; Alam, M.J.; Ahmad, S.; Ahmad, M.; Ahmad, A. Design and synthesis of a new coordination polymer of zinc (ii): Crystal structure and evaluation for its adsorption studies in removing perilous organic dye pollutants in aqueous medium. J. Mol. Struct. 2024, 1301, 137350. [Google Scholar] [CrossRef]
- Guezzen, B.; Medjahed, B.; Benhelima, A.; Guendouzi, A.; Didi, M.A.; Zidelmal, S.; Abdelkrim Boudia, R.; Adjdir, M. Improved pollutant management by kinetic and box-behnken design analysis of HDTMA-modified bentonite’s adsorption of indigo carmine dye. J. Ind. Eng. Chem. 2023, 125, 242–258. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on methylene blue: Its properties, uses, toxicity and photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Gupta, S.V.; Kulkarni, V.V.; Ahmaruzzaman, M. Fabrication of a bio-adsorbent material by grafting CeO2 quantum dots (QDTs) over areca nut shell biochar using saccharum officinarum extract as a solvent/capping agent for adsorption of methylene blue dye: Synthesis, material analyses, adsorption kinetics and isotherms studies. Colloids Surf. A Physicochem. Eng. Asp. 2024, 680, 132611. [Google Scholar]
- Zavala-Flores, E.; Flores-López, L.Z.; Alonso-Nuñez, G.; Espinoza-Gómez, H. Removal and adsorption kinetics of methylene blue dye by pristine cotton husk bracts (Gossypium hirsutum L.) From agroindustrial waste. Ind. Crops Prod. 2024, 209, 117947. [Google Scholar] [CrossRef]
- Boughrara, L.; Zaoui, F.; Guezzoul, M.; Sebba, F.Z.; Bounaceur, B.; Kada, S.O. New alginic acid derivatives ester for methylene blue dye adsorption: Kinetic, isotherm, thermodynamic, and mechanism study. Int. J. Biol. Macromol. 2022, 205, 651–663. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene blue dye: Toxicity and potential elimination technology from wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Li, H.; Kong, J.; Zhang, H.; Gao, J.; Fang, Y.; Shi, J.; Ge, T.; Fang, T.; Shi, Y.; Zhang, R.; et al. Mechanisms and adsorption capacities of ball milled biomass fly ash/biochar composites for the adsorption of methylene blue dye from aqueous solution. J. Water Process. Eng. 2023, 53, 103713. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Wu, R.; Liu, Y.; Lin, X.; Kan, H.; Zheng, Y. Preparation of acid- and alkali-modified biochar for removal of methylene blue pigment. ACS Omega 2020, 5, 30906–30922. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lin, Q.; Gao, J.; Jia, X.; Cai, M.; Liang, Q. Adsorption properties and mechanisms of methylene blue and tetracycline by nano-silica biochar composites activated by KOH. Chemosphere 2023, 337, 139395. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, E.; Poonguzhali, E.; Nanditha, D.; Kapoor, A.; Arthanareeswaran, G.; Prabhakar, S. Current status and future prospects of membrane separation processes for value recovery from wastewater. Chemosphere 2022, 291, 132690. [Google Scholar] [CrossRef]
- Kanwal, F.; Batool, A.; Aziz, F.; Sandali, Y.; Li, C.; Ullah, H.M.N.; Qasim, M.; Irfan, A.; Sulaman, M. Facile synthesis of silver chloride-polyaniline nanocomposites and its photocatalytic activity for the degradation of methylene blue. Mater. Sci. Eng. B 2024, 299, 117026. [Google Scholar] [CrossRef]
- Akhter, P.; Ali, F.; Ali, A.; Hussain, M. TiO2 decorated cnts nanocomposite for efficient photocatalytic degradation of methylene blue. Diam. Relat. Mater. 2024, 141, 110702. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Du, W.; Liu, P.; Zhang, L.; Shi, F. Treatment of wastewater containing methyl orange dye by fluidized three dimensional electrochemical oxidation process integrated with chemical oxidation and adsorption. J. Environ. Manag. 2022, 311, 114775. [Google Scholar] [CrossRef]
- Adeyemo, A.A.; Adeoye, I.O.; Bello, O.S. Adsorption of dyes using different types of clay: A review. Appl. Water Sci. 2017, 7, 543–568. [Google Scholar] [CrossRef]
- Araújo, R.F.; Bezerra, L.C.A.; de Novais, L.M.R.; D’Oca, C.D.R.M.; Avelino, F. Unveiling the mechanistic aspects of methylene blue adsorption onto a novel phosphate-decorated coconut fiber lignin. Int. J. Biol. Macromol. 2023, 253, 127011. [Google Scholar] [CrossRef]
- Ifguis, O.; Ziat, Y.; Belkhanchi, H.; Ammou, F.; Moutcine, A.; Laghlimi, C. Adsorption mechanism of methylene blue from polluted water by Opuntia ficus indica of Beni Mellal and Sidi Bou Othmane areas: A comparative study. Chem. Phys. Impact 2023, 6, 100235. [Google Scholar] [CrossRef]
- Da Rosa, M.P.; Igansi, A.V.; Lütke, S.F.; Cadaval, T.R.S.A.; Santos, A.C.R.D.; de Oliveira Lopes Inacio, A.P.; de Almeida Pinto, L.A.; Beck, P.H. A new approach to convert rice husk waste in a quick and efficient adsorbent to remove cationic dye from water. J. Environ. Chem. Eng. 2019, 7, 103504. [Google Scholar] [CrossRef]
- Natrayan, L.; Niveditha, V.R.; Nadh, V.S.; Srinivas, C.; Dhanraj, J.A.; Saravanan, A. Application of response surface and artificial neural network optimization approaches for exploring methylene blue adsorption using luffa fiber treated with sodium chlorite. J. Water Process. Eng. 2024, 58, 104778. [Google Scholar] [CrossRef]
- Tahir, H.; Sultan, M.; Akhtar, N.; Hameed, U.; Abid, T. Application of natural and modified sugar cane bagasse for the removal of dye from aqueous solution. J. Saudi Chem. Soc. 2016, 20, S115–S121. [Google Scholar] [CrossRef]
- Coupal, B.; Lalancette, J. The treatment of waters with peat moss. Water Res. 1976, 10, 1071–1076. [Google Scholar] [CrossRef]
- Zhang, R.; Leiviskä, T.; Taskila, S.; Tanskanen, J. Iron-loaded sphagnum moss extract residue for phosphate removal. J. Environ. Manag. 2018, 218, 271–279. [Google Scholar] [CrossRef] [PubMed]
- González, A.G.; Jimenez-Villacorta, F.; Beike, A.K.; Reski, R.; Adamo, P.; Pokrovsky, O.S. Chemical and structural characterization of copper adsorbed on mosses (Bryophyta). J. Hazard. Mater. 2016, 308, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Kalmykova, Y.; Strömvall, A.; Steenari, B. Adsorption of Cd, Pb and Zn on Sphagnum peat from solutions with low metal concentrations. J. Hazard. Mater. 2008, 152, 885–891. [Google Scholar] [CrossRef]
- Charazińska, S.; Lochyński, P.; Burszta-Adamiak, E. Removal of heavy metal ions form acidic electrolyte for stainless steel electropolishing via adsorption using polish peats. J. Water Process. Eng. 2021, 42, 102169. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Keskin, N.O.S.; Uyar, G. Methylene blue dye removal using Sphagnum palustre L. Bog-moss as a reusable biosorbent. Anatol. Bryol. 2019, 5, 1–7. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Wang, M.; Chen, B.; Zhang, Y.; Sun, Y.; Chen, K.; Du, Q.; Pi, X.; Wang, Y.; et al. Efficient adsorption of methylene blue in water by nitro-functionalized metal-organic skeleton-calcium alginate composite aerogel. Int. J. Biol. Macromol. 2023, 253, 126458. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Wang, M.; Chen, B.; Zhang, Y.; Sun, Y.; Chen, K.; Du, Q.; Jing, Z.; Jin, Y. Preparation of MIL-88A micro/nanocrystals with different morphologies in different solvents for efficient removal of Congo red from water: Synthesis, characterization, and adsorption mechanisms. Microporous Mesoporous Mater. 2022, 345, 112241. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Onukwuli, O.D.; Ighalo, J.O.; Okoye, P.U. Adsorption of cationic dyes on Dacryodes edulis seeds activated carbon modified using phosphoric acid and sodium chloride. Environ. Process. 2020, 7, 1151–1171. [Google Scholar] [CrossRef]
- Hachemaoui, M.; Mokhtar, A.; Ismail, I.; Mohamedi, M.W.; Iqbal, J.; Taha, I.; Bennabi, F.; Zaoui, F.; Bengueddach, A.; Hamacha, R.; et al. M (M: Cu, Cr or Fe) nanoparticles-loaded metal-organic framework MIL-101(Cr) material by sonication process: Catalytic activity and antibacterial properties. Microporous Mesoporous Mater. 2021, 323, 111244. [Google Scholar] [CrossRef]
- Lv, P.; Yang, S.; Ma, P. Bio-based oil gelling agent for effective removal of oil spills from the surface of water. Mater. Chem. Front. 2018, 2, 1784–1790. [Google Scholar] [CrossRef]
- Dong, Z.; Wei, Y.; Chen, Y.; Zhang, L.; Xu, D.; Lin, G.; Li, B.; Zhang, S.; Wei, J.; Akhtar, M.A.; et al. Nitrogen migration and transformation in chars and tars during co-pyrolysis of cellulose/xylan/lignin with urea formaldehyde. J. Anal. Appl. Pyrolysis 2024, 178, 106425. [Google Scholar] [CrossRef]
- Hemmati, F.; Norouzbeigi, R.; Sarbisheh, F.; Shayesteh, H. Malachite green removal using modified sphagnum peat moss as a low-cost biosorbent: Kinetic, equilibrium and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2016, 58, 482–489. [Google Scholar] [CrossRef]
- Karagozoglu, B.; Tasdemir, M.; Demirbas, E.; Kobya, M. The adsorption of basic dye (astrazon blue FGRL) from aqueous solutions onto sepiolite, fly ash and apricot shell activated carbon: Kinetic and equilibrium studies. J. Hazard. Mater. 2007, 147, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Bensedira, A.; Haddaoui, N.; Doufnoune, R.; Meziane, O.; Labidi, N.S. Study of methylene blue dye elimination from water using polyaniline (PANI) and PANI/SiO2 composite. Polym. Polym. Compos. 2022, 30, 09673911221141747. [Google Scholar] [CrossRef]
- Peretz, S.; Anghel, D.F.; Vasilescu, E.; Florea-Spiroiu, M.; Stoian, C.; Zgherea, G. Synthesis, characterization and adsorption properties of alginate porous beads. Polym. Bull. 2015, 72, 3169–3182. [Google Scholar] [CrossRef]
- Bai, R.; Feng, Y.; Wu, L.; Li, N.; Liu, Q.; Teng, Y.; He, R.; Zhi, K.; Zhou, H.; Qi, X. Adsorption mechanism of methylene blue by magnesium salt-modified lignite-based adsorbents. J. Environ. Manag. 2023, 344, 118514. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, H.; Hu, Y.; Yan, S.; Yang, J. Adsorption removal of cationic dyes from aqueous solutions using ceramic adsorbents prepared from industrial waste coal gangue. J. Environ. Manag. 2019, 234, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Abdulhameed, A.S.; Jawad, A.H.; Kashi, E.; Radzun, K.A.; ALOthman, Z.A.; Wilson, L.D. Insight into adsorption mechanism, modeling, and desirability function of crystal violet and methylene blue dyes by microalgae: Box-behnken design application. Algal Res. 2022, 67, 102864. [Google Scholar] [CrossRef]
- Kubra, K.T.; Salman, M.S.; Hasan, M.N. Enhanced toxic dye removal from wastewater using biodegradable polymeric natural adsorbent. J. Mol. Liq. 2021, 328, 115468. [Google Scholar] [CrossRef]
- Noori, M.; Tahmasebpoor, M.; Foroutan, R. Enhanced adsorption capacity of low-cost magnetic clinoptilolite powders/beads for the effective removal of methylene blue: Adsorption and desorption studies. Mater. Chem. Phys. 2022, 278, 125655. [Google Scholar] [CrossRef]
- Yardımcı, B.; Kanmaz, N. An effective-green strategy of methylene blue adsorption: Sustainable and low-cost waste cinnamon bark biomass enhanced via MnO2. J. Environ. Chem. Eng. 2023, 11, 110254. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Li, D.; Liu, C.; Lu, Y.; Lin, X.; Zheng, Z. Insight into the KOH/KMnO4 activation mechanism of oxygen-enriched hierarchical porous biochar derived from biomass waste by in-situ pyrolysis for methylene blue enhanced adsorption. J. Anal. Appl. Pyrolysis 2021, 158, 105269. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Wilson, L.D.; Syed-Hassan, S.S.A.; ALOthman, Z.A.; Khan, M.R. High surface area and mesoporous activated carbon from KOH-activated dragon fruit peels for methylene blue dye adsorption: Optimization and mechanism study. Chin. J. Chem. Eng. 2021, 32, 281–290. [Google Scholar] [CrossRef]
- Liao, Y.; Ge, W.; Liu, M.; Bi, W.; Jin, C.; Chen, D.D.Y. Eco-friendly regeneration of lignin with acidic deep eutectic solvent for adsorption of pollutant dyes for water cleanup. Int. J. Biol. Macromol. 2024, 260, 129677. [Google Scholar] [CrossRef]
- Tran, H.N.; Wang, Y.; You, S.; Chao, H. Insights into the mechanism of cationic dye adsorption on activated charcoal: The importance of π–π interactions. Process Saf. Environ. Protect. 2017, 107, 168–180. [Google Scholar] [CrossRef]






| Adsorption Isotherm | Parameter | 298 K | 308 K | 318 K |
|---|---|---|---|---|
| Langmuir | /(·) | 195.3125 | 184.5018 | 221.3142 |
| /(L·m) | 0.0381 | 0.0432 | 0.0535 | |
| R2 | 0.9341 | 0.9551 | 0.8999 | |
| Freundlich | () | 14.2116 | 16.3634 | 17.5152 |
| 0.5891 | 0.5453 | 0.6182 | ||
| R2 | 0.9344 | 0.9711 | 0.9181 |
() | Concentration () | () | k1 (min−1) | R2 | |
| 88.76748 | Pseudo-first-order | 150 | 12.8689 | 0.0278 | 0.8453 |
| 116.89756 | 200 | 21.6427 | 0.0180 | 0.7285 | |
| Concentration () | () | k2 (g·mg−1·min−1) | R2 | ||
| Pseudo-second-order | 150 | 90.9090 | 0.0047 | 0.9988 | |
| 200 | 116.2791 | 0.0029 | 0.9998 | ||
| Concentration () | () | C () | R2 | ||
| Intra-particle diffusion model | 150 | 1.6260 | 71.3677 | 0.6361 | |
| 200 | 1.9789 | 92.4940 | 0.6041 |
| 298 K | 308 K | 318 K | ||
|---|---|---|---|---|
| 94.3884 | 359.4830 | −4080.5043 | −4700.6398 | −5358.9559 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Zhang, S.; Wang, Y.; Yang, H. Adsorption Properties and Mechanisms of Methylene Blue by Modified Sphagnum Moss Bio-Based Adsorbents. Materials 2024, 17, 4329. https://doi.org/10.3390/ma17174329
Ren J, Zhang S, Wang Y, Yang H. Adsorption Properties and Mechanisms of Methylene Blue by Modified Sphagnum Moss Bio-Based Adsorbents. Materials. 2024; 17(17):4329. https://doi.org/10.3390/ma17174329
Chicago/Turabian StyleRen, Junpeng, Shijiang Zhang, Yu Wang, and Hengxiu Yang. 2024. "Adsorption Properties and Mechanisms of Methylene Blue by Modified Sphagnum Moss Bio-Based Adsorbents" Materials 17, no. 17: 4329. https://doi.org/10.3390/ma17174329
APA StyleRen, J., Zhang, S., Wang, Y., & Yang, H. (2024). Adsorption Properties and Mechanisms of Methylene Blue by Modified Sphagnum Moss Bio-Based Adsorbents. Materials, 17(17), 4329. https://doi.org/10.3390/ma17174329





