Microstructural Dependence of the Impact Toughness of TP316H Stainless Steel Exposed to Thermal Aging and Room-Temperature Electrolytic Hydrogenation
Abstract
1. Introduction
2. Experimental Material and Procedures
3. Results and Discussion
3.1. The Effect of Thermal Aging on Microstructure and Phase Composition
3.2. Aging and Hydrogenation Effects on Impact Toughness and Fracture Behavior
4. Summary and Conclusions
- In the material aged at 700 °C for 2500 h, the precipitation behavior included the formation of densely distributed intergranular and intragranular secondary phase particles, specifically Cr23C6-based carbides and the Fe2Mo-based Laves phase. However, aging at 450 °C for 5000 h resulted in a much less pronounced precipitation of fine, mostly intergranular Cr23C6-based carbides.
- The matrix of the 700 °C aged material was formed of austenitic solid solution with an FCC crystal structure. Conversely, in the material aged at 450 °C, the additional formation of BCC-structured ferritic phase was found.
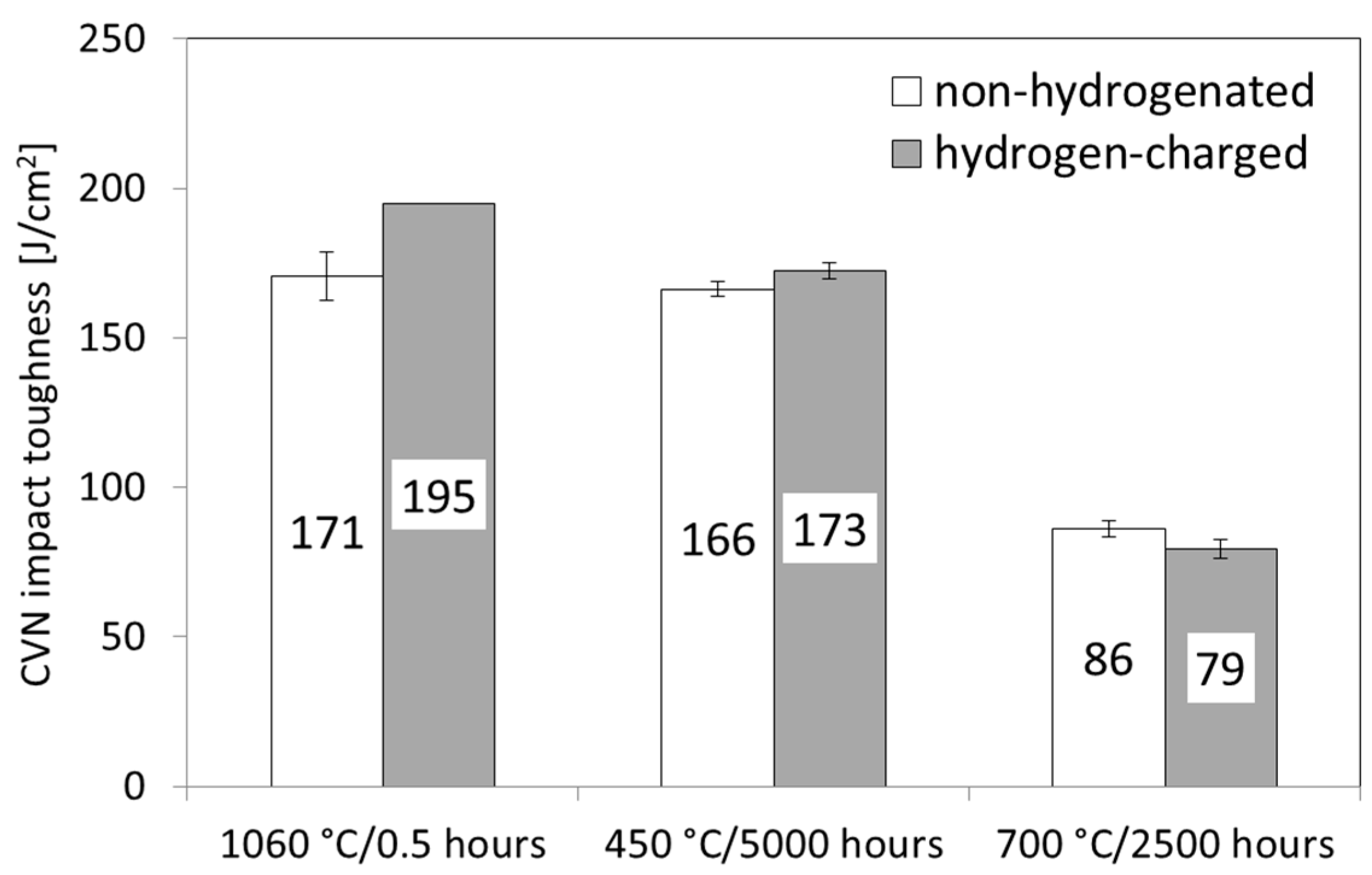
- The initial solution-annealed material exhibited high impact toughness under both the non-hydrogenated and hydrogen-charged conditions. The hydrogen-enhanced TWIP effect resulted in even higher CVN impact toughness, compared with the initial non-hydrogenated material. In contrast, both the thermally aged materials exhibited lower hydrogen embrittlement resistance, which was likely attributable to hydrogen trapping effects at the precipitate/matrix interfaces, leading to a reduced TWIP effect in the austenitic phase.
- The results of the impact toughness tests correlated well with the microstructural observations. The impact toughness deterioration of the “700 °C/2500 h” material state was predominantly caused by thermal embrittlement due to the precipitation of the intermetallic Fe2Mo-based Laves phase, occurring mainly on the grain boundaries. Conversely, the “450 °C/5000 h” material state did not show the precipitation of the brittle particles of the Laves phase within the timescale of the present investigation. Thus, thermal aging at 450 °C for 5000 h did not significantly affect impact toughness, whereas thermal aging at 700 °C for 2500 h resulted in significant thermal embrittlement.
- Regardless of the hydrogen charging application, fractographic observations after the Charpy impact bending tests revealed ductile dimple tearing fracture micro-mechanisms in both the solution-annealed and “450 °C/5000 h” thermally aged test specimens. In contrast, the fracture surfaces of the “700 °C/2500 h” thermally aged test specimens exhibited intergranular decohesion under both non-hydrogenated and hydrogen-charged conditions. The observed dimples on the surfaces of the intercrystalline fracture areas indicate the occurrence of micro-plastic behavior.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masuyama, F. History of Power Plants and Progress in Heat Resistant Steels. ISIJ Int. 2001, 41, 612–625. [Google Scholar] [CrossRef]
- Bhiogade, D.S. Ultra supercritical thermal power plant material advancements: A review. J. Alloys Metall. Syst. 2023, 3, 100024. [Google Scholar] [CrossRef]
- Barnard, P. 4—Austenitic steel grades for boilers in ultra-supercritical power plants. In Materials for Ultra-Supercritical and Advanced Ultra-Supercritical Power Plants; Di Gianfrancesco, A., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 99–119. [Google Scholar] [CrossRef]
- Wheeldon, J.; Shingledecker, J. 4—Materials for boilers operating under supercritical steam conditions. In Ultra-Supercritical Coal Power Plants, Materials, Technologies and Optimisation; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 81–103. [Google Scholar] [CrossRef]
- Yin, Y.; Faulkner, R.; Starr, F. 5—Austenitic Steels and Alloys for Power Plants. In Structural Alloys for Power Plants; Shirzadi, A., Jackson, S., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing: Sawston, UK, 2014; pp. 105–152. ISBN 978-0-85709-238-0. [Google Scholar] [CrossRef]
- Maloy, S.A.; Natesan, K.; Holcomb, D.E.; Fazio, C.; Yvon, P. Chapter 2—Overview of Reactor Systems and Operational Environments for Structural Materials in Gen-IV Fission Reactors. In Structural Alloys for Nuclear Energy Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 23–49. [Google Scholar] [CrossRef]
- Xu, L.; Li, C.; Zhao, L.; Han, Y.; Hao, K. Investigation on the creep-fatigue crack growth behavior of 316H welded joints in sodium-cooled fast reactors. Eng. Fail. Anal. 2022, 141, 106684. [Google Scholar] [CrossRef]
- Li, X.; Gao, F.; Jiao, J.; Cao, G.; Wang, Y.; Liu, Z. Influences of cooling rates on delta ferrite of nuclear power 316H austenitic stainless steel. Mater. Charact. 2021, 174, 111029. [Google Scholar] [CrossRef]
- Song, Y.; Pan, Z.; Li, Y.; Jin, W.; Gao, Z.; Wu, Z.; Ma, Y. Nanoindentation characterization on the temperature-dependent fracture mechanism of Chinese 316H austenitic stainless steel under creep-fatigue interaction. Mater. Charact. 2022, 186, 111806. [Google Scholar] [CrossRef]
- Petkov, M.P.; Chevalier, M.; Dean, D.; Cocks, A.C.F. Creep-Fatigue Interactions in Type 316H under Typical High-Temperature Power Plant Operating Conditions. Int. J. Press. Vessel. Pip. 2021, 194, 104500. [Google Scholar] [CrossRef]
- Guo, S.; Liu, L.; He, F.; Wang, S. Preparation of Cr-N coatings on 316H stainless steel via pack chromizing and gas nitriding, and their resistance to liquid metal corrosion in early stages. Surf. Coat. Technol. 2024, 481, 130665. [Google Scholar] [CrossRef]
- Raiman, S.S.; Kurley, J.M.; Sulejmanovic, D.; Willoughby, A.; Nelson, S.; Mao, K.; Parish, C.M.; Greenwood, M.S.; Pint, B.A. Corrosion of 316H stainless steel in flowing FLiNaK salt. J. Nucl. Mater. 2022, 561, 153551. [Google Scholar] [CrossRef]
- Manimozhi, S.; Suresh, S.; Muthupandi, V. HAZ hydrogen cracking of 9Cr-0.5 Mo-1.7W steels. Int. J. Adv. Manuf. Technol. 2010, 51, 217–223. [Google Scholar] [CrossRef]
- Slugeň, V.; Pecko, S.; Sojak, S. Experimental studies of irradiated and hydrogen implantation damaged reactor steels. J. Nucl. Mater. 2016, 468, 285–288. [Google Scholar] [CrossRef]
- Zhao, M.Z.; Liu, P.P.; Zhu, Y.M.; Wan, F.R.; He, Z.B.; Zhan, Q. Effects of hydrogen isotopes in the irradiation damage of CLAM steel. J. Nucl. Mater. 2015, 466, 491–495. [Google Scholar] [CrossRef]
- Seyedhabashi, M.M.; Shafiei, S.; Tafreshi, M.A.; Bidabadi, B.S. Study of surface damage and hydrogen distribution in irradiated tungsten by protons in plasma focus device. J. Vac. 2020, 175, 109249. [Google Scholar] [CrossRef]
- Yuan, X.; Huang, H.; Zhong, Y.; Cai, B.; Liu, Z.; Peng, Q. The Primary Irradiation Damage of Hydrogen-Accumulated Nickel: An Atomistic Study. Materials 2023, 16, 4296. [Google Scholar] [CrossRef]
- Shlimas, D.I.; Kozlovskiy, A.L.; Zdorovets, M.V.; Khametova, A.A. Study of Radiation Damage Processes Caused by Hydrogen Embrittlement in Lithium Ceramics under High-Temperature Irradiation. Ceramics 2022, 5, 447–458. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, D.; Jin, X.; Zhang, L.; Du, X.; Li, B. Design of non-equiatomic medium-entropy alloys. Sci. Rep. 2018, 8, 1236. [Google Scholar] [CrossRef] [PubMed]
- Padilha, A.F.; Rios, P.R. Decomposition of Austenite in Austenitic Stainless Steels—Review. ISIJ Int. 2002, 42, 325–337. [Google Scholar] [CrossRef]
- Li, X.; Chang, L.; Liu, C.; Leng, B.; Ye, X.; Han, F.; Yang, X. Effect of thermal aging on corrosion behavior of type 316H stainless steel in molten chloride salt. Corros. Sci. 2021, 191, 109784. [Google Scholar] [CrossRef]
- Kim, C.S. Variation of Mechanical Characteristics and Microstructural Evolution in AISI 316 Austenitic Stainless Steel Subjected to Long-Term Thermal Aging at Elevated Temperature. Strength Mater. 2017, 49, 263–271. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, F.; Tang, S.; Zhou, P.; Zhang, W.; Liu, Z. Effect of twin-related boundaries distribution on carbide precipitation and intergranular corrosion behavior in nuclear-grade higher carbon austenitic stainless steel. Corros. Sci. 2022, 209, 110791. [Google Scholar] [CrossRef]
- Hu, J.; Graham, G.; Hogg, S.; Higginson, R.; Cocks, A. Effect of microstructure evolution on the creep properties of a polycrystalline 316H austenitic stainless steel. Mater. Sci. Eng. 2020, 772, 138787. [Google Scholar] [CrossRef]
- Chen, B.; Flewitt, P.E.J.; Smith, D.J. Microstructural sensitivity of 316H austenitic stainless steel: Residual stress relaxation and grain boundary fracture. Mater. Sci. Eng. A 2010, 527, 7387–7399. [Google Scholar] [CrossRef]
- Warren, A.D.; Martinez-Ubeda, A.; Griffiths, I.; Flewitt, P.E.J. The Implications of Fabrication and Cast-to-Cast Variability on Thermal Aging in the Creep Range for AISI Type 316H Stainless Steel Components. Metall. Mater. Trans. A 2019, 50, 987–996. [Google Scholar] [CrossRef]
- Pan, Z.X.; Li, Y.B.; Song, Y.X.; Shen, R.; Xie, Y.C.; Jin, W.Y.; Zhang, K.; Gao, Z.L. Effects of strain rate on the tensile and creep-fatigue properties of 316H stainless steel. Int. J. Press. Vessel. Pip. 2022, 200, 104774. [Google Scholar] [CrossRef]
- He, S.; Shang, H.; Fernández-Caballero, A.; Warren, A.D.; Knowles, D.M.; Flewitt, P.E.J.; Martin, T.L. The role of grain boundary ferrite evolution and thermal aging on creep cavitation of type 316H austenitic stainless steel. Mater. Sci. Eng. A 2021, 807, 140859. [Google Scholar] [CrossRef]
- Nguyen, L.T.H.; Hwang, J.-S.; Kim, M.-S.; Kim, J.-H.; Kim, S.-K.; Lee, J.-M. Charpy impact properties of hydrogen-exposed 316L sainless steel at ambient and cryogenic temperatures. Metals 2019, 9, 625. [Google Scholar] [CrossRef]
- Li, J.; Mao, Q.; Nie, J.; Huang, Z.; Wang, S.; Li, Y. Impact property of high-strength 316L stainless steel with heterostructures. Mater. Sci. Eng. A 2019, 754, 457–460. [Google Scholar] [CrossRef]
- De Sonis, E.; Dépinoy, S.; Giroux, P.-F.; Maskrot, H.; Wident, P.; Hercher, O.; Villaret, F.; Gourgues-Lorenzon, A.-F. Microstructure—Toughness relationships in 316L stainless steel produced by laser powder bed fusion. Mater. Sci. Eng. A 2023, 877, 145179. [Google Scholar] [CrossRef]
- ISO 148-1:2016; Metallic Materials—Charpy Pendulum Impact Test—Part 1: Test Method. International Organization for Standardization: London, UK, 2016. Available online: https://www.iso.org/standard/63802.html (accessed on 5 August 2024).
- Lutterotti, L.; Scardi, P. Simultaneous structure and size-strain refinement by the Rietveld method. J. Appl. Cryst. 1990, 23, 246–252. [Google Scholar] [CrossRef]
- Lutterotti, L.; Matthies, S.; Wenk, H.-R. MAUD: A friendly Java program for material analysis using diffraction. IUCR Newsl. CPD 1999, 21, 14–15. [Google Scholar]
- Database “The Materials Project”, Berkeley Lab. Available online: https://next-gen.materialsproject.org (accessed on 5 July 2024).
- Falat, L.; Čiripová, L.; Petruš, O.; Puchý, V.; Petryshynets, I.; Kovaľ, K.; Džunda, R. The Effects of Electrochemical Hydrogen Charging on Charpy Impact Toughness and Dry Sliding Tribological Behavior of AISI 316H Stainless Steel. Crystals 2023, 13, 1249. [Google Scholar] [CrossRef]
- Park, J.H.; Seo, H.S.; Kim, K.Y. Alloy Design to Prevent Intergranular Corrosion of Low-Cr Ferritic Stainless Steel with Weak Carbide Formers. J. Electrochem. Soc. 2015, 162, C412–C418. [Google Scholar] [CrossRef]
- Ogawa, Y.; Nishida, H.; Takakuwa, O.; Tsuzaki, K. Hydrogen-enhanced deformation twinning in Fe-Cr-Ni-based austenitic steel characterized by in-situ EBSD observation. Mater. Today Commun. 2023, 34, 105433. [Google Scholar] [CrossRef]
- Luo, H.; Li, Z.; Raabe, D. Hydrogen enhances strength and ductility of an equiatomic high-entropy alloy. Sci. Rep. 2017, 7, 9892. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, X.; Song, X.; Gu, T.; Zhang, Y. Hydrogen Embrittlement of CoCrFeMnNi High-Entropy Alloy Compared with 304 and IN718 Alloys. Metals 2022, 12, 998. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, M.; Luo, Y.; Wang, L.; Wang, Z.; Wang, Z.; Li, F.; Wang, X. Immunity of Al0.25CoCrFeNi high-entropy alloy tohydrogen embrittlement. Mater. Sci. Eng. A 2021, 821, 141590. [Google Scholar] [CrossRef]
- Mohammadi, A.; Novelli, M.; Arita, M.; Bae, J.W.; Kim, H.S.; Grosdidier, T.; Edalati, K. Gradient-structured high-entropy alloy with improved combination of strength and hydrogen embrittlement resistance. Corros. Sci. 2022, 200, 110253. [Google Scholar] [CrossRef]
- Pu, Z.; Chen, Y.; Dai, L.H. Strong resistance to hydrogen embrittlement of high-entropy alloy. Mater. Sci. Eng. A 2018, 736, 156–166. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Won, J.W.; Park, S.-H.; Lee, J.H.; Lim, K.R.; Na, Y.S.; Lee, C.S. Ultrahigh-strength CoCrFeMnNi high-entropy alloy wire rod with excellent resistance to hydrogen embrittlement. Mater. Sci. Eng. A 2018, 732, 105–111. [Google Scholar] [CrossRef]
- Zhao, Y.; Lee, D.H.; Kim, W.J.; Seok, M.Y.; Kim, J.Y.; Han, H.N.; Suh, J.Y.; Ramamurty, U.; Jang, J.I. Influence of pre-strain on the gaseous hydrogen embrittlement resistance of a high-entropy alloy. Mater. Sci. Eng. A 2018, 718, 43–47. [Google Scholar] [CrossRef]
- Lai, Z.H.; Lin, Y.T.; Sun, Y.H.; Tu, J.F.; Yen, H.W. Hydrogen-induced ductilization in a novel austenitic lightweight TWIP steel. Scripta Materialia 2022, 213, 114629. [Google Scholar] [CrossRef]
- Murakami, Y.; Kanezaki, T.; Mine, Y. Hydrogen Effect against Hydrogen Embrittlement. Metall. Mater. Trans. A 2010, 41, 2548–2562. [Google Scholar] [CrossRef]
- Ueki, S.; Oura, R.; Mine, Y.; Takashima, K. Micro-mechanical characterisation of hydrogen embrittlement in nano-twinned metastable austenitic stainless steel. Int. J. Hydrogen Energy 2020, 45, 27950–27957. [Google Scholar] [CrossRef]
- Wang, R. Effects of hydrogen on the fracture toughness of a X70 pipeline steel. Corros. Sci. 2009, 51, 2803–2810. [Google Scholar] [CrossRef]
- Enomoto, M.; Cheng, L.; Mizuno, H.; Watanabe, Y.; Omura, T.; Sakai, J.; Yokohama, K.; Suzuki, H.; Okuma, R. Hydrogen Absorption into Austenitic Stainless Steels Under High-Pressure Gaseous Hydrogen and Cathodic Charge in Aqueous Solution. Metall. Mater. Transactions E 2014, 1, 331–340. [Google Scholar] [CrossRef]
- Hickel, T.; Nazarov, R.; McEniry, E.; Leyson, G.; Grabowski, B.; Neugebauer, J. Ab initio based understanding of the segregation and diffusion mechanisms of hydrogen in steels. JOM 2014, 66, 1399–1405. [Google Scholar] [CrossRef]
- Barrera, O.; Bombac, D.; Chen, Y.; Daff, T.; Galindo-Nava, E.; Gong, P.; Haley, D.; Horton, R.; Katzarov, I.; Kermode, J. Understanding and mitigating hydrogen embrittlement of steels: A review of experimental, modelling and design progress from atomistic to continuum. J. Mater. Sci. 2018, 53, 6251–6290. [Google Scholar] [CrossRef]
- Nagumo, M. Fundamentals of Hydrogen Embrittlement, 2nd ed.; Springer Nature: Singapore, 2023; ISBN 978-981-99-0991-9. [Google Scholar] [CrossRef]
- Turnbull, A. 4–Hydrogen diffusion and trapping in metals. In Gaseous Hydrogen Embrittlement of Materials in Energy Technologies; Woodhead Publishing: Cambridge, UK, 2012; pp. 89–128. [Google Scholar] [CrossRef]
- Li, H.Y.; Niu, R.M.; Li, W.; Lu, H.Z.; Cairney, J.L.; Chen, Y.S. Hydrogen in pipeline steels: Recent advances in characterization and embrittlement mitigation. J. Nat. Gas Sci. Eng. 2022, 105, 104709. [Google Scholar] [CrossRef]
- Song, E.J.; Bhadeshia, H.; Suh, D.-W. Effect of hydrogen on the surface energy of ferrite and austenite. Corros. Sci. 2013, 77, 379–384. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Chen, C.; Yu, H. Diffusion coefficient of hydrogen interstitial atom in α-Fe, γ-Fe and ε-Fe crystals by first-principle calculations. Int. J. Hydrogen Energy 2017, 42, 27438–27445. [Google Scholar] [CrossRef]
- Hirata, K.; Iikubo, S.; Koyama, M.; Tsuzaki, K.; Ohtani, H. First-Principles Study on Hydrogen Diffusivity in BCC, FCC, and HCP Iron. Metall. Mater. Trans. A 2018, 49, 5015–5022. [Google Scholar] [CrossRef]
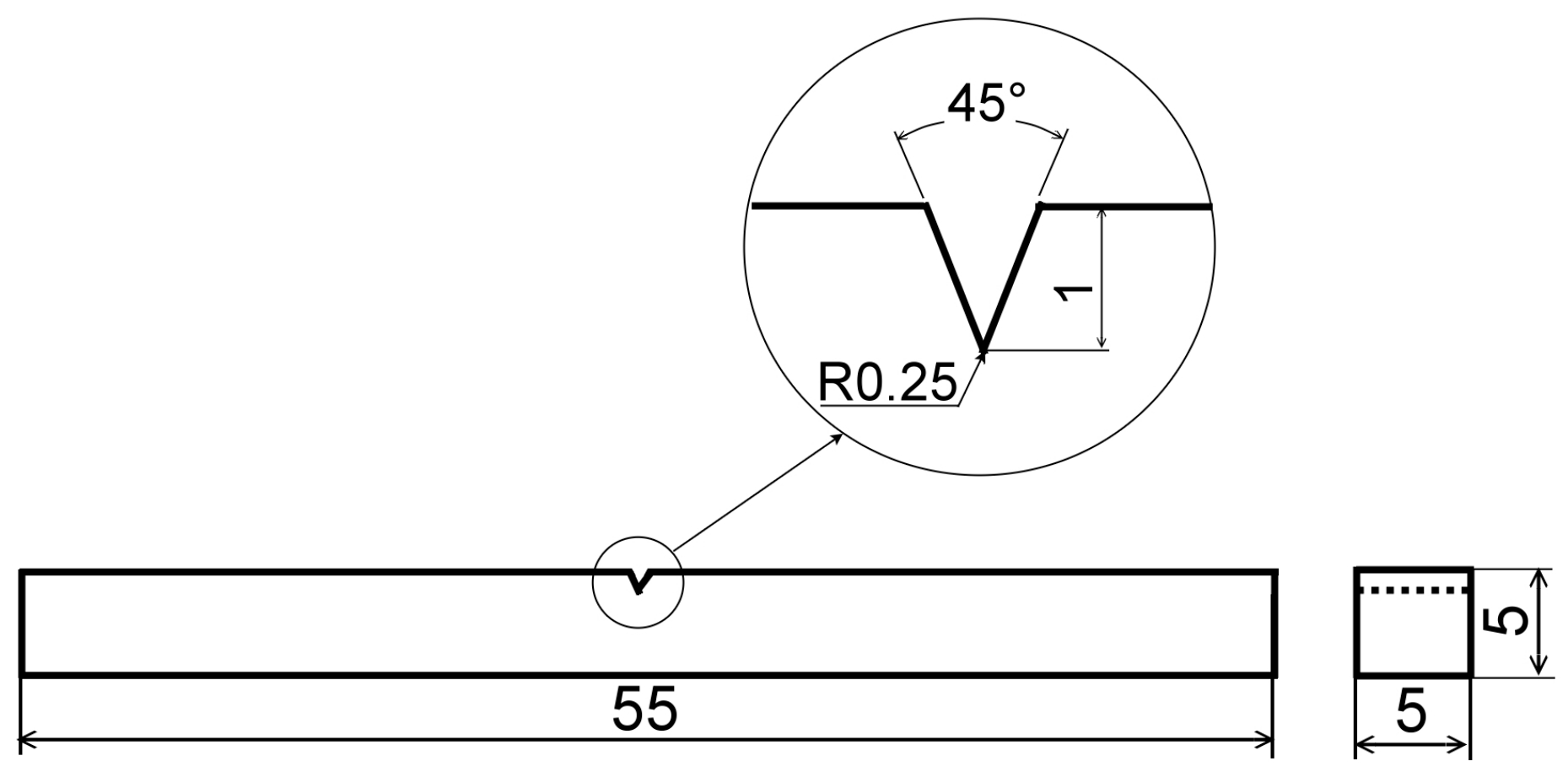












| Material | C | Si | Mn | Cr | Mo | Ni | Fe |
|---|---|---|---|---|---|---|---|
| TP316H | 0.052 | 0.51 | 1.77 | 16.76 | 2.05 | 11.13 | rest |
| Row | 0 | x | EI (0, x) [%] |
|---|---|---|---|
| 1 | 1060 °C/0.5 h | 1060 °C/0.5 h +H | −14.0 |
| 2 | 1060 °C/0.5 h + 450 °C/5000 h | 1060°C/0.5 h + 450 °C/5000 h +H | −4.2 |
| 3 | 1060 °C/0.5 h + 700 °C/2500 h | 1060°C/0.5 h + 700 °C/2500 h +H | 8.1 |
| 4 | 1060 °C/0.5 h | 1060°C/0.5 h + 450 °C/5000 h | 2.9 |
| 5 | 1060 °C/0.5 h | 1060 °C/0.5 h + 700 °C/2500 h | 49.7 |
| 6 | 1060 °C/0.5 h | 1060 °C/0.5 h + 450 °C/5000 h +H | −1.2 |
| 7 | 1060 °C/0.5 h | 1060 °C/0.5 h + 700 °C/2500 h +H | 53.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falat, L.; Čiripová, L.; Homolová, V.; Ďurčová, M.; Milkovič, O.; Petryshynets, I.; Džunda, R. Microstructural Dependence of the Impact Toughness of TP316H Stainless Steel Exposed to Thermal Aging and Room-Temperature Electrolytic Hydrogenation. Materials 2024, 17, 4303. https://doi.org/10.3390/ma17174303
Falat L, Čiripová L, Homolová V, Ďurčová M, Milkovič O, Petryshynets I, Džunda R. Microstructural Dependence of the Impact Toughness of TP316H Stainless Steel Exposed to Thermal Aging and Room-Temperature Electrolytic Hydrogenation. Materials. 2024; 17(17):4303. https://doi.org/10.3390/ma17174303
Chicago/Turabian StyleFalat, Ladislav, Lucia Čiripová, Viera Homolová, Miroslava Ďurčová, Ondrej Milkovič, Ivan Petryshynets, and Róbert Džunda. 2024. "Microstructural Dependence of the Impact Toughness of TP316H Stainless Steel Exposed to Thermal Aging and Room-Temperature Electrolytic Hydrogenation" Materials 17, no. 17: 4303. https://doi.org/10.3390/ma17174303
APA StyleFalat, L., Čiripová, L., Homolová, V., Ďurčová, M., Milkovič, O., Petryshynets, I., & Džunda, R. (2024). Microstructural Dependence of the Impact Toughness of TP316H Stainless Steel Exposed to Thermal Aging and Room-Temperature Electrolytic Hydrogenation. Materials, 17(17), 4303. https://doi.org/10.3390/ma17174303







