Lithiophilic Reduced Graphene Oxide/Carbonized Zeolite Imidazolate Framework-8 Composite Host for Stable Li Metal Anodes
Abstract
:1. Introduction
2. Experimental Methods
2.1. Preparation of rGO/C-ZIF-8
2.2. Materials Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
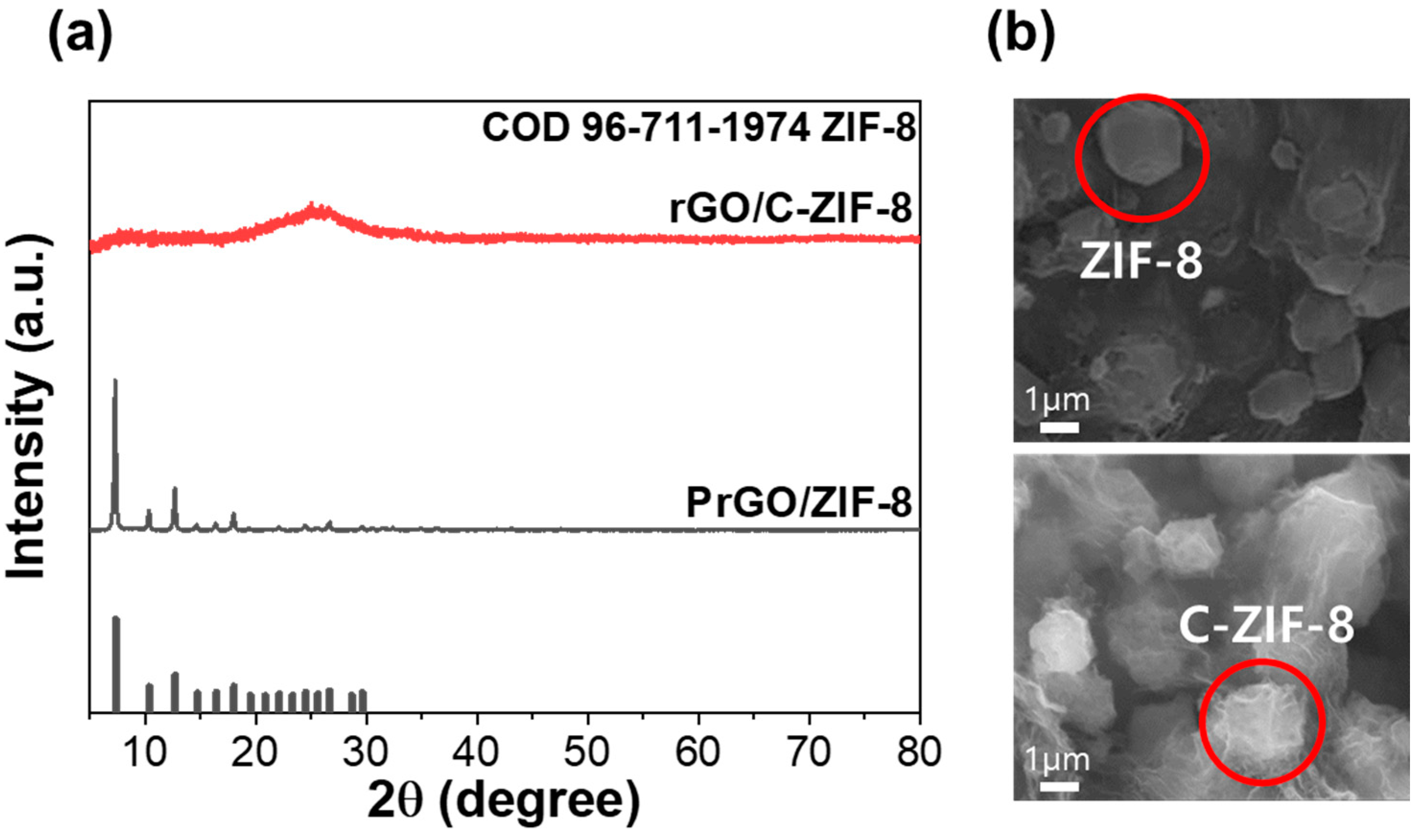
3.1. Preparation and Characterization of rGO/C-ZIF-8
3.2. Li Plating Process on the rGO/C-ZIF-8 Composite
3.3. Li Plating/Stripping Behavior of the rGO/C-ZIF-8-Li Electrode
3.4. Practical Applicability of rGO/C-ZIF-8-Li in Full Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, J.; Zhang, H.; Yang, R.; Li, X.; Qi, L. Morphology-controlled synthesis of SnO2 nanotubes by using 1D silica mesostructures as sacrificial templates and their applications in lithium-ion batteries. Small 2010, 6, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.W.; Yuan, C.; Archer, L.; Wang, Y.; Lee, J. Template-free synthesis of SnO2 hollow nanostructures with high lithium storage capacity. Adv. Mater. 2006, 18, 2325–2329. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Thirumalraj, B.; Hagos, T.T.; Huang, C.-J.; Teshager, M.A.; Cheng, J.-H.; Su, W.-N.; Hwang, B.-J. Nucleation and growth mechanism of lithium metal electroplating. J. Am. Chem. Soc. 2019, 141, 18612–18623. [Google Scholar] [CrossRef]
- An, Y.; Fei, H.; Zeng, G.; Xu, X.; Ci, L.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Vacuum distillation derived 3D porous current collector for stable lithium–metal batteries. Nano Energy 2018, 47, 503–511. [Google Scholar] [CrossRef]
- Yamaki, J.-I.; Tobishima, S.-I.; Hayashi, K.; Saito, K.; Nemoto, Y.; Arakawa, M. A consideration of the morphology of electrochemically deposited lithium in an organic electrolyte. J. Power Sources 1998, 74, 219–227. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Z.; Yan, X.; Hou, F.; Wen, L.; Luo, W.; Liang, J.; Dou, S.X. Engineering of lithium-metal anodes towards a safe and stable battery. Energy Storage Mater. 2018, 14, 22–48. [Google Scholar] [CrossRef]
- Yasin, G.; Arif, M.; Mehtab, T.; Lu, X.; Yu, D.; Muhammad, N.; Nazir, M.T.; Song, H. Understanding and suppression strategies toward stable Li metal anode for safe lithium batteries. Energy Storage Mater. 2020, 25, 644–678. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.-G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Cao, D.; Sun, X.; Li, Q.; Natan, A.; Xiang, P.; Zhu, H. Lithium dendrite in all-solid-state batteries: Growth mechanisms, suppression strategies, and characterizations. Matter 2020, 3, 57–94. [Google Scholar] [CrossRef]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, X.-B.; Huang, J.-Q.; Yuan, H.; Lu, Y.; Yan, C.; Zhu, G.-L.; Xu, R.; Zhao, C.-Z.; Hou, L.-P. Controlling dendrite growth in solid-state electrolytes. ACS Energy Lett. 2020, 5, 833–843. [Google Scholar] [CrossRef]
- Li, S.; Luo, Z.; Li, L.; Hu, J.; Zou, G.; Hou, H.; Ji, X. Recent progress on electrolyte additives for stable lithium metal anode. Energy Storage Mater. 2020, 32, 306–319. [Google Scholar] [CrossRef]
- Wang, G.; Xiong, X.; Xie, D.; Fu, X.; Ma, X.; Li, Y.; Liu, Y.; Lin, Z.; Yang, C.; Liu, M. Suppressing dendrite growth by a functional electrolyte additive for robust Li metal anodes. Energy Storage Mater. 2019, 23, 701–706. [Google Scholar] [CrossRef]
- Liu, D.; Xiong, X.; Liang, Q.; Wu, X.; Fu, H. An inorganic-rich SEI induced by LiNO3 additive for a stable lithium metal anode in carbonate electrolyte. Chem. Commun. 2021, 57, 9232–9235. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, J.; Lee, H.R.; Yan, K.; Li, Y.; Shi, F.; Huang, W.; Pei, A.; Chen, G.; Subbaraman, R. Engineering stable interfaces for three-dimensional lithium metal anodes. Sci. Adv. 2018, 4, eaat5168. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liao, J.; Luo, D.; Huang, Y.; Sun, F.; Nan, J. In situ formation of a lithiophilic surface on 3D current collectors to regulate lithium nucleation and growth for dendrite-free lithium metal anodes. Chem. Eng. J. 2023, 453, 139903. [Google Scholar] [CrossRef]
- Huang, G.; Lou, P.; Xu, G.-H.; Zhang, X.; Liang, J.; Liu, H.; Liu, C.; Tang, S.; Cao, Y.-C.; Cheng, S. Co3O4 nanosheet decorated nickel foams as advanced lithium host skeletons for dendrite-free lithium metal anode. J. Alloys Compd. 2020, 817, 152753. [Google Scholar] [CrossRef]
- Liu, F.; Xu, R.; Hu, Z.; Ye, S.; Zeng, S.; Yao, Y.; Li, S.; Yu, Y. Regulating lithium nucleation via CNTs modifying carbon cloth film for stable Li metal anode. Small 2019, 15, 1803734. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Liu, Y.; Liang, Z.; Lee, H.-W.; Sun, J.; Wang, H.; Yan, K.; Xie, J.; Cui, Y. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat. Nanotechnol. 2016, 11, 626–632. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, S.; Li, D.; Liao, J.; Ji, F.; Liu, H.; Ci, L. Commercial carbon cloth: An emerging substrate for practical lithium metal batteries. Energy Storage Mater. 2022, 48, 172–190. [Google Scholar] [CrossRef]
- Yan, X.; Lin, L.; Chen, Q.; Xie, Q.; Qu, B.; Wang, L.; Peng, D.L. Multifunctional roles of carbon-based hosts for Li-metal anodes: A review. Carbon Energy 2021, 3, 303–329. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, S.; Mei, J.; Qiu, L.; Zhang, P.; Xu, X.; Tu, J.; Xie, J.; Zhao, X. Lithiated carbon cloth as a dendrite-free anode for high-performance lithium batteries. Sustain. Energy Fuels 2020, 4, 5773–5782. [Google Scholar] [CrossRef]
- Liu, W.; Xia, Y.; Wang, W.; Wang, Y.; Jin, J.; Chen, Y.; Paek, E.; Mitlin, D. Pristine or highly defective? Understanding the role of graphene structure for stable lithium metal plating. Adv. Energy Mater. 2019, 9, 1802918. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, M.; Song, J.; Sun, D.; Qiao, Y.; Zhou, X.; Ye, C.; Liu, W.; Liu, W.; Wei, Z. Effect of the defect densities of reduced graphene oxide network on the stability of lithium-metal anodes. Mater. Today Commun. 2021, 27, 102276. [Google Scholar] [CrossRef]
- Xia, G.; Wang, C.; Jiang, P.; Lu, J.; Diao, J.; Chen, Q. Nitrogen/oxygen co-doped mesoporous carbon octahedrons for high-performance potassium-ion batteries. J. Mater. Chem. A 2019, 7, 12317–12324. [Google Scholar] [CrossRef]
- Tan, L.; Feng, S.; Li, X.; Wang, Z.; Peng, W.; Liu, T.; Yan, G.; Li, L.; Wu, F.; Wang, J. Oxygen-induced lithiophilicity of tin-based framework toward highly stable lithium metal anode. Chem. Eng. J. 2020, 394, 124848. [Google Scholar] [CrossRef]
- Du, Z.; Guan, W.; He, C.; Liu, Y.; Ai, W. Advances in graphene-based hosts for lithium metal anodes. Energy Storage Mater. 2024, 50, 103191. [Google Scholar] [CrossRef]
- Li, Z.; Ji, W.; Wang, T.-X.; Ding, X.; Han, B.-H.; Feng, W. Maximized lithiophilic carbonyl units in covalent organic frameworks as effective Li ion regulators for lithium metal batteries. Chem. Eng. J. 2022, 437, 135293. [Google Scholar] [CrossRef]
- Zhu, M.; Li, B.; Li, S.; Du, Z.; Gong, Y.; Yang, S. Dendrite-free metallic lithium in lithiophilic carbonized metal–organic frameworks. Adv. Energy Mater. 2018, 8, 1703505. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, R.; Liu, Z.; Wang, X.; Han, C.; Liu, H.; Li, B. A 3D lithiophilic ZIF-8@RGO free-standing scaffold with dendrite-free behavior enabling high-performance Li metal batteries. J. Mater. Chem. A 2023, 11, 12910–12917. [Google Scholar] [CrossRef]
- Wei, T.; Lu, J.; Wang, M.; Sun, C.; Zhang, Q.; Wang, S.; Zhou, Y.; Chen, D.; Lan, Y.Q. MOF-derived materials enabled lithiophilic 3D hosts for lithium metal anode—A Review. Chin. J. Chem. 2023, 41, 1861–1874. [Google Scholar] [CrossRef]
- Shichalin, O.; Papynov, E.; Ivanov, N.; Balanov, M.; Dran’kov, A.; Shkuratov, A.; Zarubina, N.; Fedorets, A.; Mayorov, V.Y.; Lembikov, A. Study of adsorption and immobilization of Cs+, Sr2+, Co2+, Pb2+, La3+ ions on Na-Faujasite zeolite transformed in solid state matrices. Sep. Purif. Technol. 2024, 332, 125662. [Google Scholar] [CrossRef]
- Dran’kov, A.; Shichalin, O.; Papynov, E.; Nomerovskii, A.; Mayorov, V.; Pechnikov, V.; Ivanets, A.; Buravlev, I.; Yarusova, S.; Zavjalov, A. Hydrothermal synthesis, structure and sorption performance to cesium and strontium ions of nanostructured magnetic zeolite composites. Nucl. Eng. Technol. 2022, 54, 1991–2003. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Yan, K.; Lu, Z.; Lee, H.-W.; Xiong, F.; Hsu, P.-C.; Li, Y.; Zhao, J.; Chu, S.; Cui, Y. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 2016, 1, 16010. [Google Scholar] [CrossRef]
- Zhuang, Z.; Liu, C.; Yan, Y.; Ma, P.; Tan, D.Q. Zn–CxNy nanoparticle arrays derived from a metal–organic framework for ultralow-voltage hysteresis and stable Li metal anodes. J. Mater. Chem. A 2021, 9, 27095–27101. [Google Scholar] [CrossRef]
- You, L.; Ju, S.; Liu, J.; Xia, G.; Guo, Z.; Yu, X. Synergistic effect of lithiophilic Zn nanoparticles and N-doping for stable Li metal anodes. J. Energy Chem. 2022, 65, 439–447. [Google Scholar] [CrossRef]
- Kim, H.-K.; Aravindan, V.; Mhamane, D.; Yoon, S.-B.; Park, S.-H.; Nazarian-Samani, M.; Han, J.T.; Park, H.S.; Roh, K.C.; Kim, K.-B. Bulk metal-derived metal oxide nanoparticles on oxidized carbon surface. J. Alloys Compd. 2018, 752, 198–205. [Google Scholar] [CrossRef]
- Kida, K.; Okita, M.; Fujita, K.; Tanaka, S.; Miyake, Y. Formation of high crystalline ZIF-8 in an aqueous solution. CrystEngComm 2013, 15, 1794–1801. [Google Scholar] [CrossRef]
- Sun, X.; Man, J.; Liu, K.; Liu, W.; Sun, J.; Zhang, N.; Zhou, Y.; Geng, Z.; Li, S.; Sun, J. Uniform lithium deposition enabled by a carbon nanotubes framework modified with nanosized ZIF-8 particles for dendrite-free lithium metal anode. Appl. Surf. Sci. 2023, 616, 156474. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.; Liu, Z.; Yang, W.; Liu, J. A highly conductive porous graphene electrode prepared via in situ reduction of graphene oxide using Cu nanoparticles for the fabrication of high performance supercapacitors. RSC Adv. 2015, 5, 54275–54282. [Google Scholar] [CrossRef]
- Luanwuthi, S.; Krittayavathananon, A.; Srimuk, P.; Sawangphruk, M. In situ synthesis of permselective zeolitic imidazolate framework-8/graphene oxide composites: Rotating disk electrode and Langmuir adsorption isotherm. RSC Adv. 2015, 5, 46617–46623. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Wang, Y.; Sun, M.H.; Dong, W.; Li, Y.; Su, B.L. Selenium confined in ZIF-8 derived porous carbon@MWCNTs 3D networks: Tailoring reaction kinetics for high performance lithium-selenium batteries. Chem Synth 2022, 2, 8. [Google Scholar] [CrossRef]
- Liu, S.; Wang, A.; Li, Q.; Wu, J.; Chiou, K.; Huang, J.; Luo, J. Crumpled graphene balls stabilized dendrite-free lithium metal anodes. Joule 2018, 2, 184–193. [Google Scholar] [CrossRef]
- Jin, C.; Sheng, O.; Luo, J.; Yuan, H.; Fang, C.; Zhang, W.; Huang, H.; Gan, Y.; Xia, Y.; Liang, C. 3D lithium metal embedded within lithiophilic porous matrix for stable lithium metal batteries. Nano Energy 2017, 37, 177–186. [Google Scholar] [CrossRef]
- Mohammadi, A.; Monconduit, L.; Stievano, L.; Younesi, R. Measuring the nucleation overpotential in lithium metal batteries: Never forget the counter electrode! J. Electrochem. Soc. 2022, 169, 070509. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.-W.; Oh, B.I.; Chang, E.S.; Park, J.-A.; Kim, H.-K. Lithiophilic Reduced Graphene Oxide/Carbonized Zeolite Imidazolate Framework-8 Composite Host for Stable Li Metal Anodes. Materials 2024, 17, 4300. https://doi.org/10.3390/ma17174300
Jeong S-W, Oh BI, Chang ES, Park J-A, Kim H-K. Lithiophilic Reduced Graphene Oxide/Carbonized Zeolite Imidazolate Framework-8 Composite Host for Stable Li Metal Anodes. Materials. 2024; 17(17):4300. https://doi.org/10.3390/ma17174300
Chicago/Turabian StyleJeong, Sang-Won, Byeong Il Oh, Eun Seo Chang, Jeong-Ann Park, and Hyun-Kyung Kim. 2024. "Lithiophilic Reduced Graphene Oxide/Carbonized Zeolite Imidazolate Framework-8 Composite Host for Stable Li Metal Anodes" Materials 17, no. 17: 4300. https://doi.org/10.3390/ma17174300
APA StyleJeong, S.-W., Oh, B. I., Chang, E. S., Park, J.-A., & Kim, H.-K. (2024). Lithiophilic Reduced Graphene Oxide/Carbonized Zeolite Imidazolate Framework-8 Composite Host for Stable Li Metal Anodes. Materials, 17(17), 4300. https://doi.org/10.3390/ma17174300





