A Performance-Based Test for Mitigating the Risk of Geopolymer Concrete Surface Efflorescence Due to Alkali Leaching
Abstract
1. Introduction
2. Experimental Program
2.1. Geopolymer Paste Precursors
2.2. Geopolymer Paste Mixes and Fabrication
2.3. SEM-EDS Analysis
2.4. Ion Leaching and ICP Analysis
2.5. Efflorescence Tests
3. Results and Discussion
3.1. SEM/EDS Results
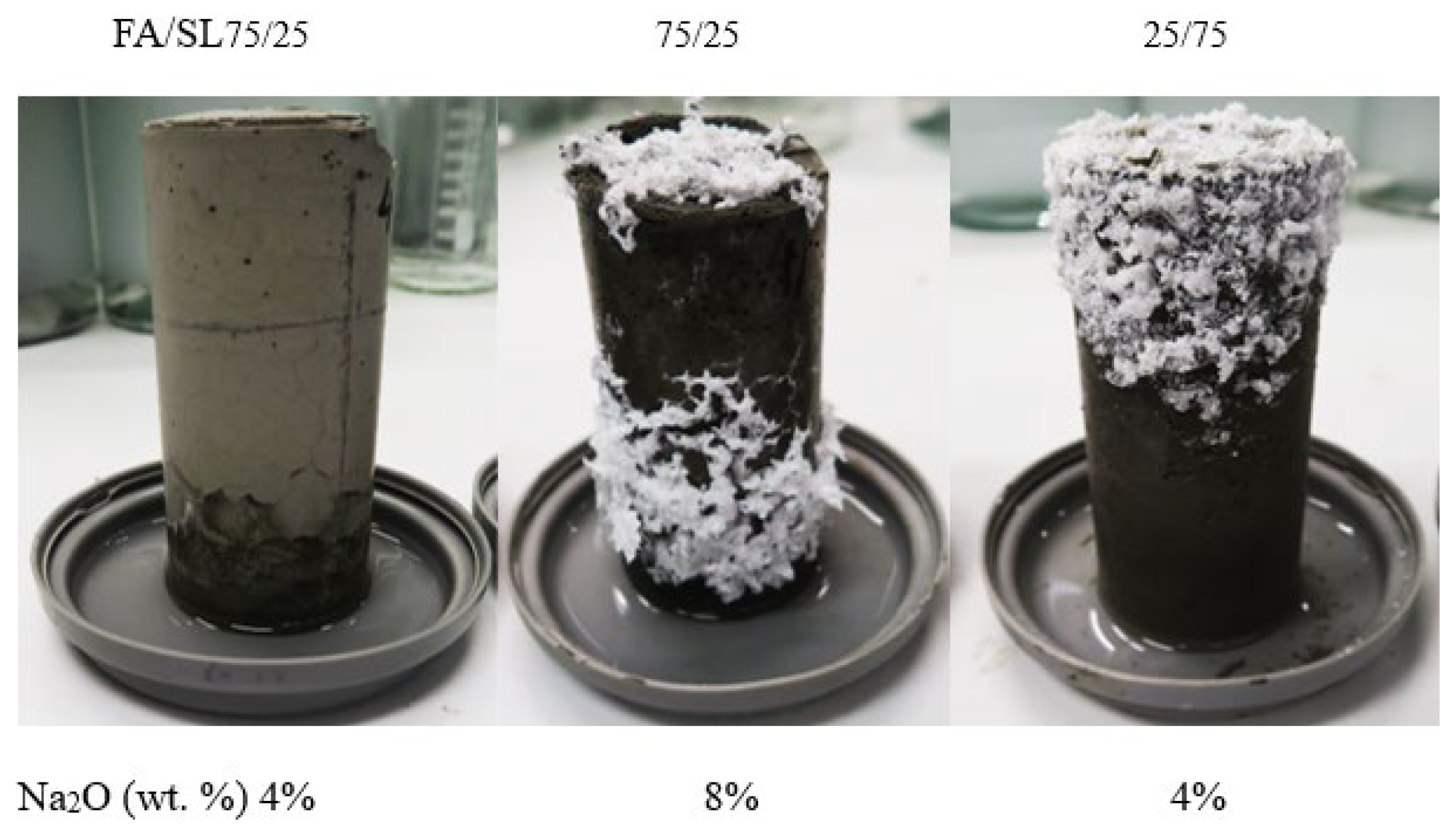
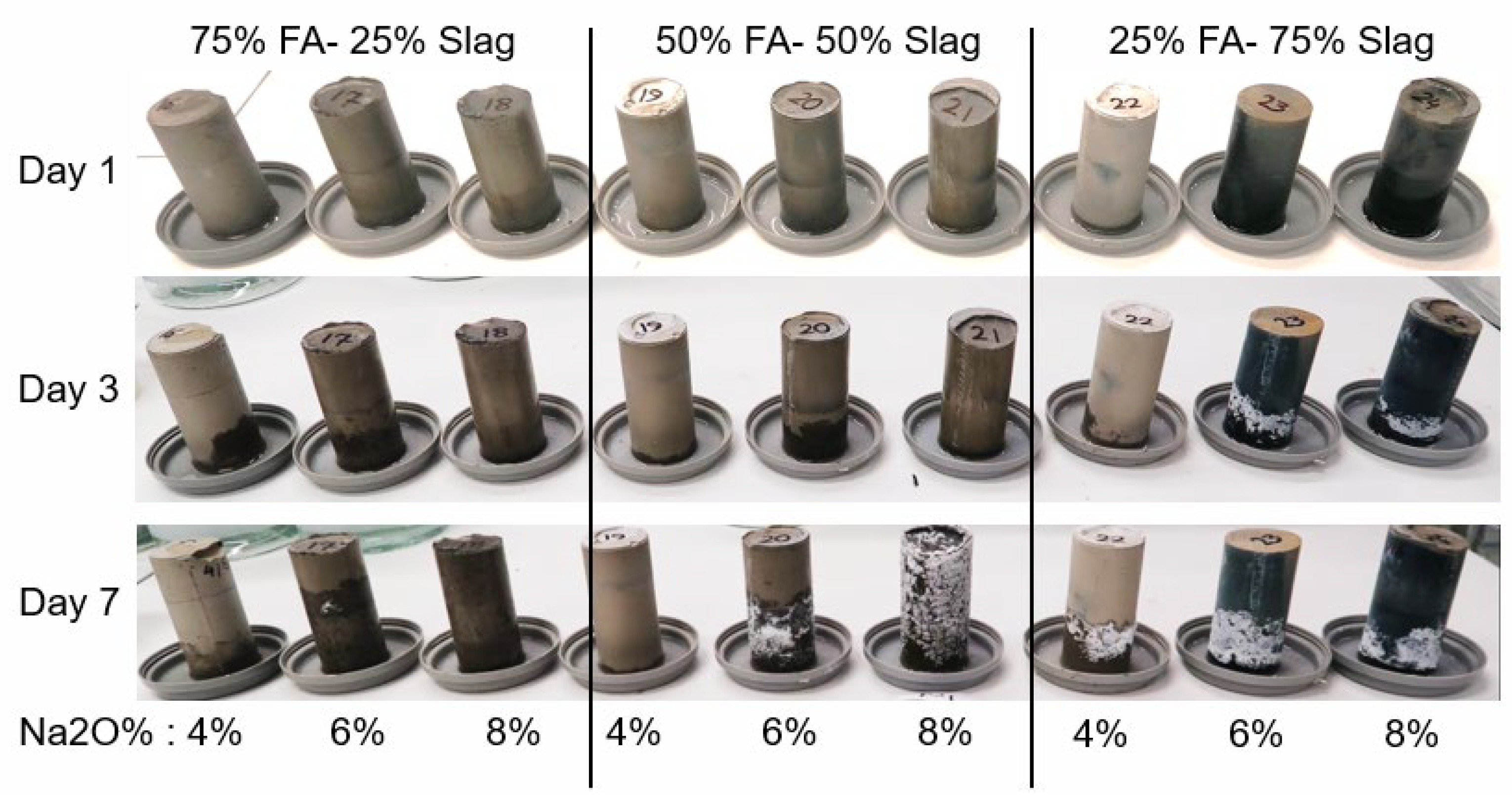
3.2. ICP Analysis and Efflorescence Results
3.3. Performance-Based Method to Control the Risk of Surface Efflorescence
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mehta, P.K. Reducing the Environmental Impact of Concrete. Concr. Int. 2001, 23, 61–66. [Google Scholar]
- Ali, M.B.; Saidur, R.; Hossain, M.S. A review on emission analysis in cement industries. Renew. Sustain. Energy Rev. 2011, 15, 2252–2261. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Rose, V.; de Gutiérrez, R.M. High-Resolution X-ray Diffraction and Fluorescence Microscopy Characterization of Alkali-Activated Slag-Metakaolin Binders. J. Am. Ceram. Soc. 2013, 96, 1951–1957. [Google Scholar] [CrossRef]
- Myers, R.J.; Bernal, S.A.; San Nicolas, R.; Provis, J.L. Generalized Structural Description of Calcium–Sodium Aluminosilicate Hydrate Gels: The Cross-Linked Substituted Tobermorite Model. Langmuir 2013, 29, 5294–5306. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Jennings, H.M. Pore solution chemistry of alkali-activated ground granulated blast-furnace slag. Cem. Concr. Res. 1999, 29, 159–170. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Glasser, F. Alkali sorption by C-S-H and C-A-S-H gels. Cem. Concr. Res. 2002, 32, 1101–1111. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Provis, J.L.; Myers, R.J.; White, C.E.; Rose, V.; van Deventer, J.S.J. X-ray microtomography shows pore structure and tortuosity in alkali-activated binders. Cem. Concr. Res. 2012, 42, 855–864. [Google Scholar] [CrossRef]
- De Burgh, J.M.; Al-Damad, I.M.A.; Foster, S.J. Experimental investigation of water vapour sorption isotherms and microstructure of low calcium geopolymer binders. In Mechanics of Structures and Materials XXIV; CRC Press: Boca Raton, FL, USA, 2017; pp. 472–477. [Google Scholar]
- Provis, J.; Bernal, S. Geopolymers and related alkali-activated materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Lloyd, R.R.; Provis, J.L.; van Deventer, J.S.J. Pore solution composition and alkali diffusion in inorganic polymer cement. Cem. Concr. Res. 2010, 40, 1386–1392. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Fly ash-based geopolymers: The relationship between composition, pore structure and efflorescence. Cem. Concr. Res. 2014, 64, 30–41. [Google Scholar] [CrossRef]
- Yao, X.; Yang, T.; Zhang, Z. Fly ash-based geopolymers: Effect of slag addition on efflorescence. J. Wuhan Univ. Technol. Sci. Ed. 2016, 31, 689–694. [Google Scholar] [CrossRef]
- Xiao, R.; Ma, Y.; Jiang, X.; Zhang, M.; Zhang, Y.; Wang, Y.; Huang, B.; He, Q. Strength, microstructure, efflorescence behavior and environmental impacts of waste glass geopolymers cured at ambient temperature. J. Clean. Prod. 2020, 252, 119610. [Google Scholar] [CrossRef]
- Xiao, R.; Jiang, X.; Wang, Y.; He, Q.; Huang, B. Experimental and Thermodynamic Study of Alkali-Activated Waste Glass and Calcium Sulfoaluminate Cement Blends: Shrinkage, Efflorescence Potential, and Phase Assemblages. J. Mater. Civ. Eng. 2021, 33, 04021312. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications; Geopolymer Institute: Compiegne, France, 2008. [Google Scholar]
- Duxson, P.; Lukey, G.C.; Separovic, F.; Van Deventer, J.S.J. Effect of Alkali Cations on Aluminum Incorporation in Geopolymeric Gels. Ind. Eng. Chem. Res. 2005, 44, 832–839. [Google Scholar] [CrossRef]
- Yip, C.K.; van Deventer, J.S.J. Microanalysis of calcium silicate hydrate gel formed within a geopolymeric binder. J. Mater. Sci. 2003, 38, 3851–3860. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; Van Deventer, J.S.J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]


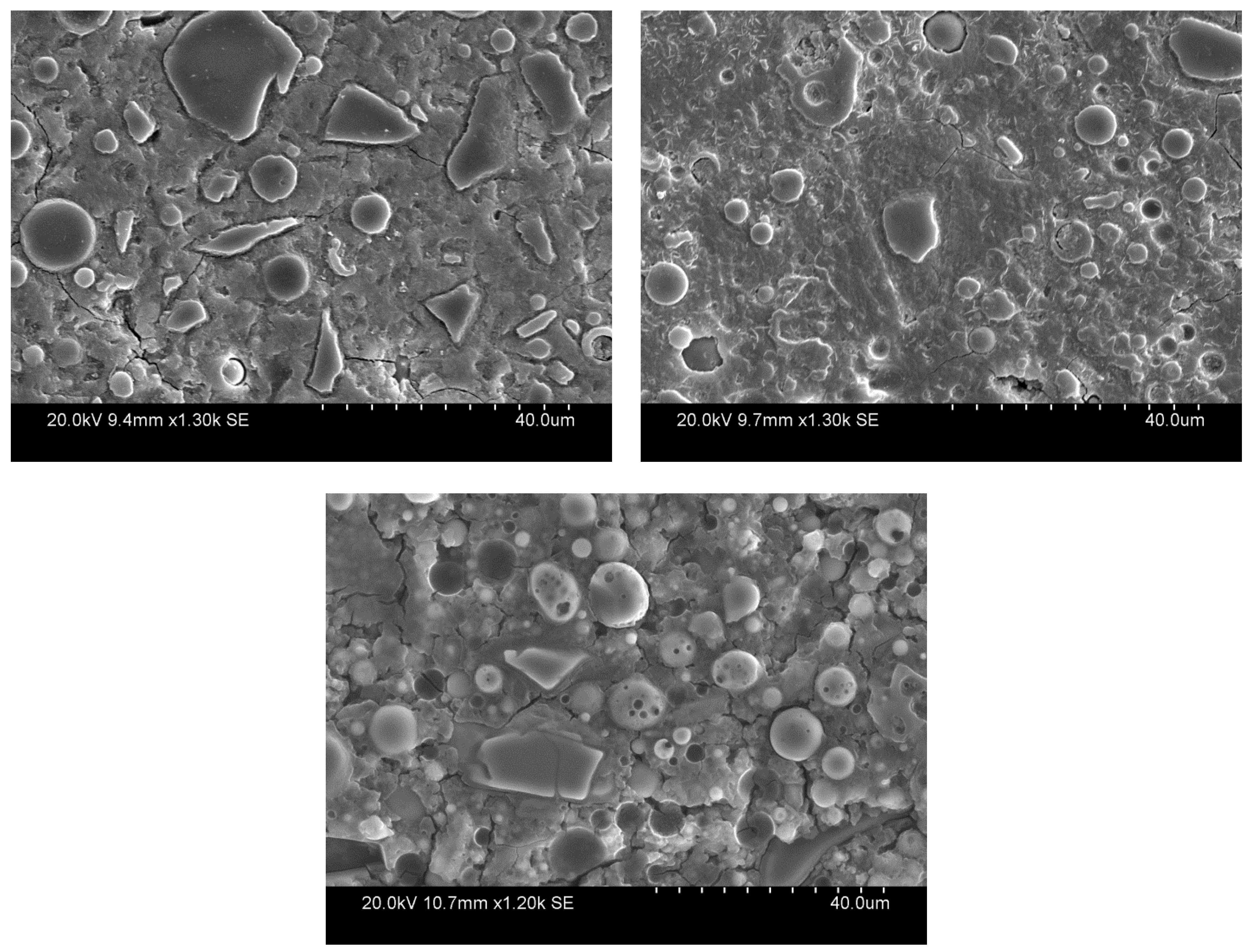


| Oxide | Gladstone FA [wt. %] | GGBS [wt. %] |
|---|---|---|
| SiO2 | 47.9 | 35.0 |
| Al2O3 | 25.7 | 14.1 |
| Fe2O3 | 14.7 | 0.36 |
| CaO | 4.11 | 40.9 |
| MgO | 1.36 | 5.51 |
| K2O | 0.67 | 0.30 |
| Na2O | 0.81 | 0.29 |
| TiO2 | 1.39 | 0.59 |
| P2O5 | 1.21 | 0.02 |
| Mn3O4 | 0.19 | 0.55 |
| SO3 | 0.19 | 1.15 |
| Loss of ignition (LOI) | 0.69 | 0.54 |
| Amorphous content | 79.1% | 100% |
| Mix | FA/GGBS | Ms | Na2O (wt. %) | Water/Binder * |
|---|---|---|---|---|
| Mix 1 | 25%/75% | 1.5 | 4 | 0.40 |
| Mix 2 | 75%/25% | 1.5 | 8 | 0.35 |
| Mix 3 | 75%/25% | 1.5 | 4 | 0.35 |
| Mix | FA/Slag | Ms | Na2O (wt. %) | Si/Al (wt. % Ratio) (Molar Ratio) | Na/Al (wt. % Ratio) (Molar Ratio) | Ca/Si (wt. % Ratio) (Molar Ratio) |
|---|---|---|---|---|---|---|
| 1 | 25/75 | 1.5 | 4 | 1.93(1.85) | 0.57 (0.67) | 0.54 (0.38) |
| 2 | 75/25 | 1.5 | 8 | 1.94 (1.86) | 0.83 (0.97) | 0.7 (0.49) |
| 3 | 75/25 | 1.5 | 4 | 1.71(1.64) | 0.41 (0.48) | 0.35 (0.245) |
| Mix | FA/Slag | Sand/Binder | Ms | Na2O (wt. %) | Compressive Strength (MPa) |
|---|---|---|---|---|---|
| 1 | 25/75 | 2.75 | 1.5 | 4 | 69.8 |
| 2 | 75/25 | 2.75 | 1.5 | 8 | 71 |
| 3 | 75/25 | 2.75 | 1.5 | 4 | 27.5 |
| Mix | FA/Slag | Ms | Na2O% | Leached Out [Na] mM (ppm) | Percentage of Leached Out [Na] Ions (%) (Theory) |
|---|---|---|---|---|---|
| 1 | 25/75 | 1.5 | 4 | 31.1 (715) | 25.0 |
| 2 | 75/25 | 1.5 | 8 | 40.3 (927) | 17.9 |
| 3 | 75/25 | 1.5 | 4 | 31.6 (726) | 24.3 |
| Density of Scrapped Efflorescence Products (mg/cm3) | Risk Level | Example |
|---|---|---|
| Density ≤ 1 | No risk |  |
| 1 < Density ≤ 2.5 | Low-to-medium Risk |  |
| 2.5 < Density ≤ 10 | Medium-to-high risk |  |
| Density > 10 | High risk |  |
| Cumulative Density of Efflorescence Products (Weight/Sample Volume) (mg/cm3) | Risk of Efflorescence | Guidance on Suitable Concrete Exposure Conditions |
|---|---|---|
| <1 | Low Risk | Geopolymer concrete suitable for all exposure conditions. |
| From 1 to 2.5 | Low to medium risk | Geopolymer concrete produces only a limited amount of efflorescence if intensively exposed to moisture. Geopolymer concrete suitable for all exposure conditions except Surfaces of members in above-ground exterior environments in areas that are in tropical climatic zone including industrial and non-industrial buildings as well as tidal zone and splash zone and surfaces of members in interior environments in industrial buildings where the member is subjected to repeated wetting and drying. |
| From 2.5 to 10 | Medium to high risk | Geopolymer concrete can produce large amounts of efflorescence if intensively exposed to moisture. Geopolymer concrete can only be used in interior environments, fully enclosed within a residential building except for a brief period of weather exposure during construction. |
| >10 | High risk | Geopolymer concrete is very likely to produce large amounts of fluorescence event if only briefly exposed to moisture. Geopolymer concrete can only be used in interior environments, fully enclosed within a residential building including during construction. Geopolymer concrete should not be used if exposed, even for a brief period, to the external environment during construction. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babaee, M.; Castel, A. A Performance-Based Test for Mitigating the Risk of Geopolymer Concrete Surface Efflorescence Due to Alkali Leaching. Materials 2024, 17, 3647. https://doi.org/10.3390/ma17153647
Babaee M, Castel A. A Performance-Based Test for Mitigating the Risk of Geopolymer Concrete Surface Efflorescence Due to Alkali Leaching. Materials. 2024; 17(15):3647. https://doi.org/10.3390/ma17153647
Chicago/Turabian StyleBabaee, Mahdi, and Arnaud Castel. 2024. "A Performance-Based Test for Mitigating the Risk of Geopolymer Concrete Surface Efflorescence Due to Alkali Leaching" Materials 17, no. 15: 3647. https://doi.org/10.3390/ma17153647
APA StyleBabaee, M., & Castel, A. (2024). A Performance-Based Test for Mitigating the Risk of Geopolymer Concrete Surface Efflorescence Due to Alkali Leaching. Materials, 17(15), 3647. https://doi.org/10.3390/ma17153647






