1. Introduction
The search for novel high-performance and specific materials has intensified in recent years, driven by the need for enhanced efficiency and selectivity. These materials have often been the subject of research in various fields, particularly in the environment. The growing presence of pharmaceutical residues, a class of emerging environmental contaminants [
1,
2], has raised concerns due to their potential toxicity to both ecological and human health. These residues have been the focus of numerous scientific investigations.
Progesterone and ibuprofen, widely consumed drugs worldwide, are often found in surface water, municipal and hospital waste, and effluents.
Among the different methods used in the elimination of pharmaceutical residues [
3,
4,
5,
6,
7,
8,
9], the adsorption process is one of the most attractive methods due to its efficiency, ease of implementation, and the availability of different adsorbents [
10,
11,
12].
Biopolymers are considered innovative materials that can be modified physicochemically to improve their properties for specific applications.
Chitosan, a biopolymer derived from chitin, exhibits remarkable adsorption properties due to its numerous amino (–NH
2) and hydroxyl (–OH) groups that act as active adsorption sites [
13,
14,
15]. However, chitosan has limitations, such as its lack of selectivity, low mechanical strength, and solubility in acidic media, for practical application towards specific compounds [
16]. To overcome these limitations, modifications of the functional groups of chitosan by crosslinking and grafting, the generation of nanoparticles and hydrogels, and the association of chitosan with other materials have been developed [
17,
18,
19,
20,
21].
The grafting of cyclodextrins (CDs), cyclic oligosaccharides derived from starch, onto chitosan is a promising approach to improve its properties [
18]. Insoluble CD polymers prepared by crosslinking with crosslinking agents such as glutaraldehyde, epichlorohydrin (Ep), HMDI, and citric acid [
22,
23,
24] find many applications [
25,
26,
27,
28].
This study aims to develop a new bio-adsorbent material based on chitosan and three types of CDs (-α, -β, and -ɣ) crosslinked with citric acid for the selective removal of pharmaceutical pollutants in aqueous solution.
After characterization, the adsorption performance of the polymer was evaluated in the removal of two pharmaceutical contaminants, ibuprofen and progesterone, alone and in mixture. The effects of contact time, flow rate, pH of the solution, ionic strength, and temperature on the adsorption rate are studied.
Thermodynamic and kinetic studies were carried out to determine the nature of the adsorption reaction and the appropriate kinetic model. A thermogravimetric analysis (TGA) was undertaken to elucidate the mechanism of the adsorption of pollutants by the synthesized polymer.
The results of this research will contribute to the valorization of chitosan and the development of new solutions for water decontamination.
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
The progesterone and ibuprofen were obtained from UP John Company (Hastings, MI, USA) and Hubei Granules-biocause pharmaceutical Co., Ltd. (Wuhan, China), respectively, and were used without further purification.
The chitosan with molecular weight of 300 Da was obtained from SIGMA-ALDRICH and was put to use without further purification.
The insoluble alpha-beta-gamma-cyclodextrin polymer was procured from start-up, In-Cyclo®, Rouen, France.
All other reagents were of analytical grade.
2.1.2. Apparatus
An XRD analysis of the cyclodextrin–chitosan polymer was carried out with a diffractometer (kind Oxpert pro panalytical). The conditions for current and voltage were set at 20 mA and 40 kV, respectively. This analysis utilized a monochromatized X-ray beam from Cu Ka radiation with a wavelength of λ = 0.154 nm. The scan was performed over the range of 3–90° 2θ at a rate of 4° min−1.
Scanning electron microscopy (SEM) using a Jeol JSM-6031by with LFD mode was employed to investigate the morphology of the material.
A differential scanning calorimetry (DSC) analysis of ibuprofen, progesterone, and the cyclodextrin–chitosan polymer before and after adsorption was performed with a PERKIN ELMER instrument. Appropriate amounts of the samples were placed in perforated aluminum pans and were heated from 35 to 300 °C at a scanning rate of 10 °C/min, under a nitrogen purge gas flow of 25 mL/min.
A JASCO UV/VIS spectrophotometer was used to quantify progesterone and ibuprofen in aqueous solutions. Their respective maximum absorption wavelengths are 250 nm and 223 nm.
Size exclusion chromatography coupled with multi-angle laser light scattering (SEC-MALS) was employed to determine the molecular weight and the average molar mass.
pH measurements are made using a digital pH meter model, Inolab.
2.2. Methods
2.2.1. Synthesis of Cyclodextrin–Chitosan Polymer
The insoluble tetrapolymers (α-, β-, γ-), cyclodextrins–chitosan, were synthesized by a direct melt polycondensation with grafted chitosan (
Figure 1): Initially, 3 g of citric acid and 0.3 g of chitosan were introduced into a reactor maintained at a temperature of 140 °C. Then, a mixture of 1 g of α-cyclodextrin, 1.3 g of β-cyclodextrin, 1.5 g of γ-cyclodextrin, and 1 g of sodium phosphate dibasic (Na
2HPO
4) as a catalyst was added. The solution was stirred under vacuum for 30 min. The solid residue, obtained in accord with the invention [
24,
26], was washed successively with 20 mL of water three times.
2.2.2. Swelling Capacity of the Cyclodextrin–Chitosan Polymer
To determine the swelling capacity (SC) of this material, a sufficient amount of it is immersed in water, which is then removed at different times and wiped until it has a constant weight [
27].
The swelling capacity (%) is defined as the mass lost before and after swelling, and it was determined as follows [
28]:
where
and W
e are the mass of sample at dry state before and after swelling equilibrium (g), respectively.
2.2.3. Total Acidic Groups of the Cyclodextrin–Chitosan Polymer
The estimate of total acidic groups (TA), as well as ester and carboxylic groups, was carried out by a titration method. A total of 0.1 g of the polymer was introduced in a beaker, which contained 20 mL of 0.1 M of NaOH solution, the mixture was then blended for 15 h at 30 °C. The cyclodextrin–chitosan polymer was dissolved and hydrolyzed.
The titration of the final solution is carried out using a 0.1 M HCl solution until obtaining pH 7 [
22]. The following equation was used to determine the TA:
C: the concentration of HCl solution (mmol L−1);
V0: volume of HCl solution consumed by blank solution (L);
V1: volume of HCl solution consumed by sample solution (L);
W: weight of the cyclodextrin–chitosan polymer (g).
2.2.4. Determination of Molar Mass of Cyclodextrin–Chitosane Polymer
The molar mass (Mn) of the cyclodextrin–chitosan polymer is determined by SEC/MALS. This method allows the determination of mass distributions of polymers. Size Exclusion Chromatography (SEC) is used to separate macromolecules according to their size (their hydrodynamic volume in solution). For that, the polymer solutions are injected and then eluted onto columns filled with non-adsorbent porous material. At the outlet of the column, the fractions are separated according to their characteristics. Contrary to the techniques based on standard polymers and a simple detection of concentrations (usually with a differential refractometer), the addition of a second detection by diffusion of multiangle laser light, sensitive to molecular weights, gives access to instantaneous variations of the gyration radius and to the average molar mass (Mw) of the eluted species at each time of elution.
2.3. Adsorption Experiments
Adsorption experiments were performed using a fixed-bed column (inner diameter 35 mm, height 120 mm) containing varying amounts of adsorbent material. The column was filled with 60 mL of the solution to be treated, ensuring adequate contact time for efficient adsorption (
Figure 2). A peristaltic pump circulated the solution in a closed loop through the adsorbent in a downward direction at a constant, predetermined flow rate. Samples were collected at regular intervals using a pipette during the solution percolation process for subsequent analysis.
Equilibrium times were determined by conducting experiments for different durations: 6 h for ibuprofen and 2 h for progesterone. Each experiment was repeated three times to ensure reproducibility of the results. Stock solutions of ibuprofen (30 mg/L) and progesterone (20 mg/L) were prepared in deionized water and a deionized water/ethanol mixture 60/40 (v/v), respectively. Solutions at different concentrations were obtained by diluting the stock solutions.
The adsorption kinetics were followed at regular time intervals. Solution samples of 800 µL were taken and analyzed by UV-Vis spectrometry at specific wavelengths: 223 nm for ibuprofen and 250 nm for progesterone. The amount of solute adsorbed by the insoluble cyclodextrin–chitosan polymer (qt, mg/g) and the removal efficiency (%) were calculated using the following equations:
C0 (mg/L): initial concentration of pharmaceuticals;
Ct (mg/L): concentration of pharmaceuticals at time t;
V (L): volume of solution;
m (g): mass of cyclodextrin–chitosan polymer.
3. Results
3.1. Characterization of Cyclodextrin–Chitosan Polymers
3.1.1. XRD Structural Analysis
Figure 3 presents the X-ray diffraction (XRD) patterns of chitosan and P–CD–chitosan. The XRD pattern of chitosan exhibits an intense peak at 2θ = 10°, characteristic of its semi-crystalline structure. In contrast, the XRD pattern of P–CD–chitosan shows a broad and diffuse peak at 2θ = 20°, accompanied by the absence of distinct diffraction peaks, indicating the amorphous nature of the synthesized polymer. This amorphous structure is attributed to the homogeneous dispersion of cyclodextrin molecules within the chitosan matrix.
3.1.2. Morphology Analysis
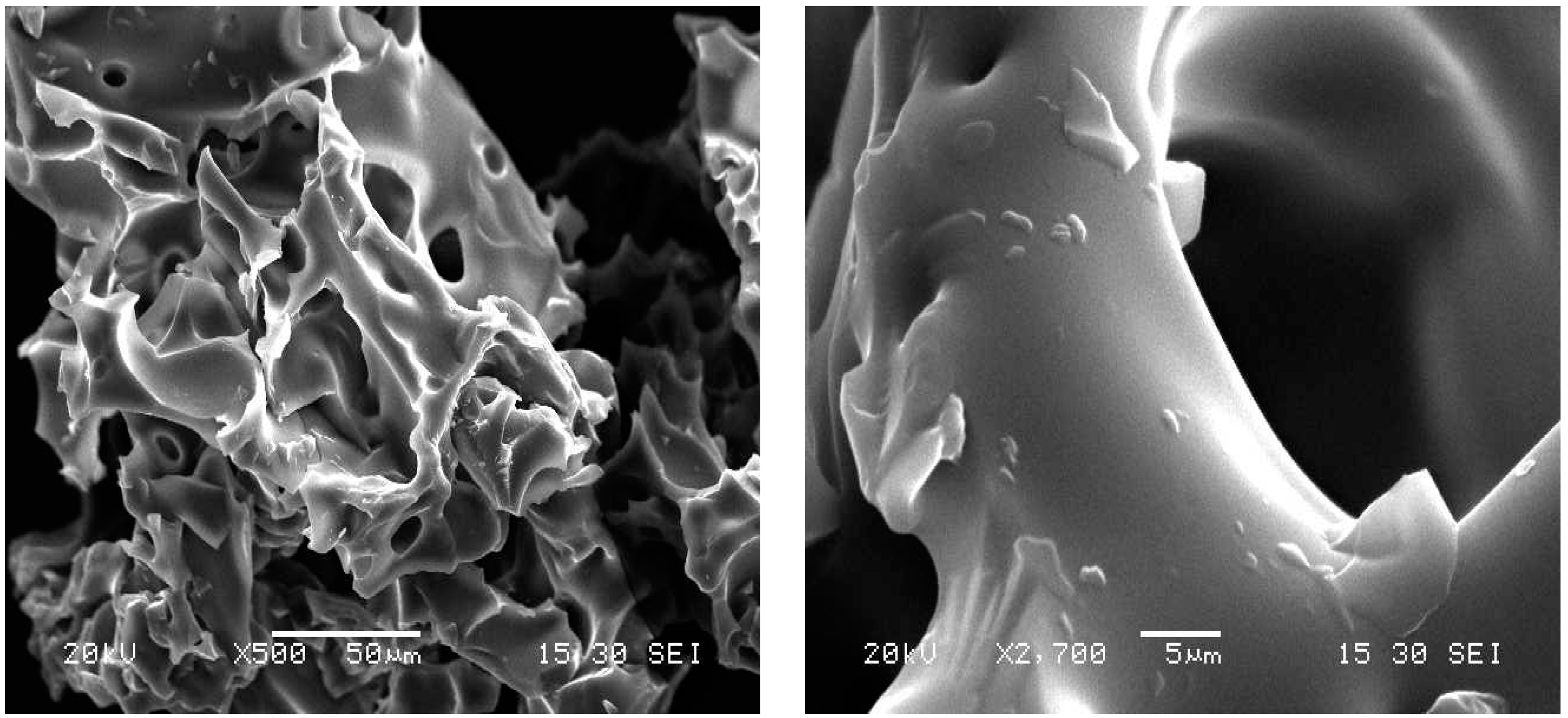
The scanning electron microscopy (SEM) images (
Figure 4) clearly reveal the morphology of the polymer surface in the dry state. The polymer has a sponge structure with thick, homogeneous, and smooth cavities. It has a porous structure with mesopores and some nanocavities. These properties allow it a high swelling capacity (
Table 1), which allows for the rapid diffusion of the adsorbates in the polymer matrix [
27].
3.1.3. Properties of Cyclodextrin–Chitosan Polymer
The swelling capacity (SC), total acidic (TA), molar mass (Mn) and average molar mass (Mw) results are reported in
Table 1. We note a high swelling capacity of the polymer.
3.2. Effects of Operating Parameters
The effects of the operating parameters (contact time, solution flow rate, pH, ionic strength, and temperature) on the adsorption rate of the two pharmaceutical molecules onto the synthesized cyclodextrin–chitosan polymer are investigated in a closed-loop fixed-bed column. The initial concentrations of ibuprofen and progesterone solutions, selected based on their solubility limits, are 10 mg/L and 30 mg/L, respectively. The different measurements were made on solution samples taken at different times.
3.2.1. Contact Time Effect
Samples of the ibuprofen and progesterone solutions are collected from the column and analyzed.
Figure 5 shows the adsorption rates of the samples by the cyclodextrin–chitosan polymer over time.
Adsorption yields increase with increasing contact time until an equilibrium is achieved. A rapid increase in the adsorption rate is observed during the first 10 min and then slows down to reach equilibrium after 50 min for progesterone and 250 min for ibuprofen.
3.2.2. Effect of Flow Rate
Figure 6 depicts the relationship between the flow rate and the progesterone adsorption rate. Higher flow rates enhance progesterone removal, likely due to two mechanisms: increased mass transfer of solute from the bulk solution to the solid surface and a thinning of the boundary layer surrounding the solid with increasing cycles.
3.2.3. Effect of Initial Solution pH
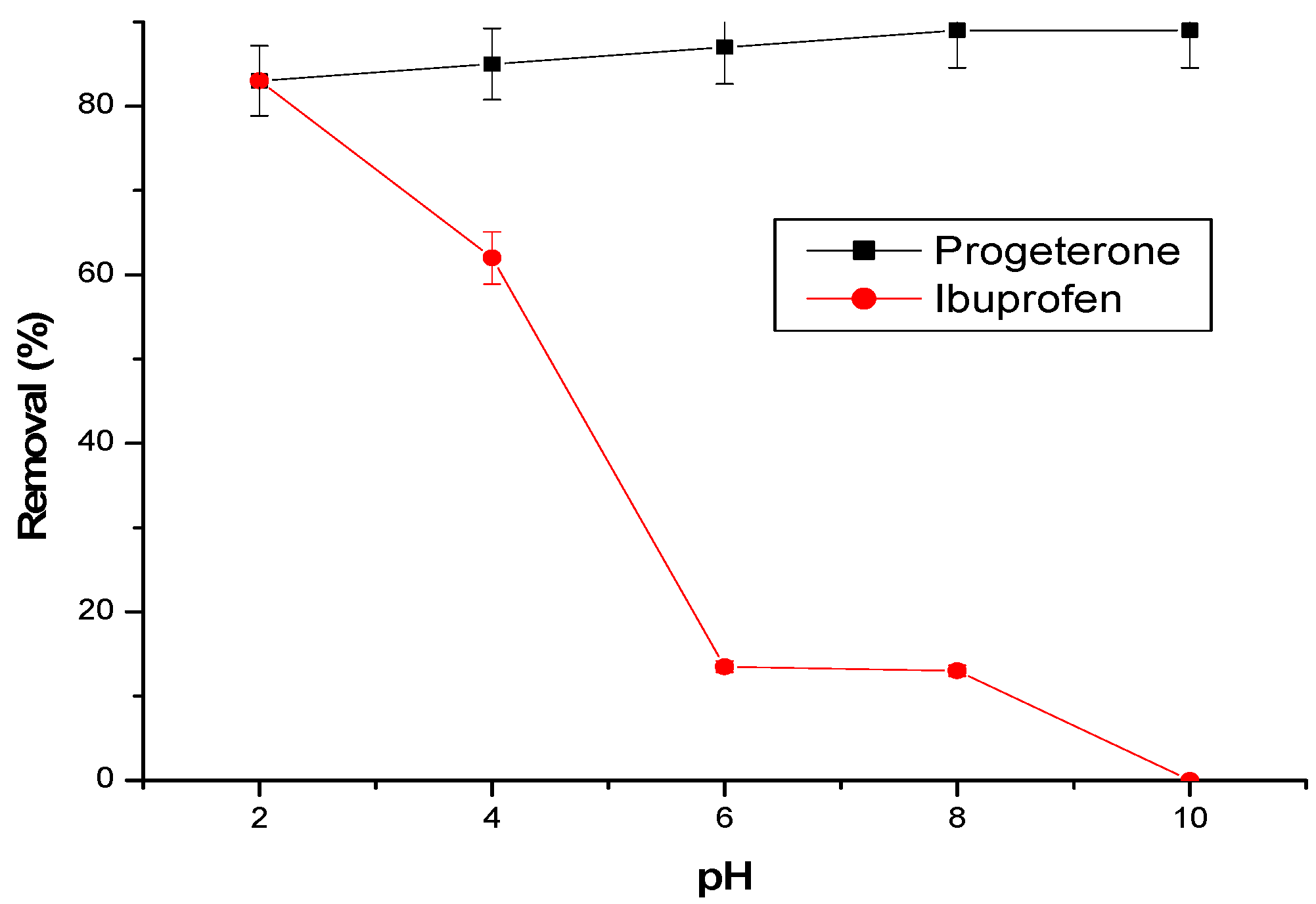
The effect of the initial pH is shown in
Figure 7. The initial pH significantly influences the adsorption of ibuprofen, but less so the adsorption of progesterone.
The best adsorption efficiency of the two molecules is observed at a pH of 2. However, the elimination of ibuprofen decreases with the increase in the initial pH. For a pH higher than the pKa = 4.91 of ibuprofen [
26,
29,
30], the molecule becomes deprotonated, becomes negatively charged, and, thus, prevents the formation of inclusion complexes with the cyclodextrin cavities. Additionally, there is an electrostatic repulsion with the negative charge of the acidic groups in the cyclodextrin–chitosan polymer formed at basic pH.
In the case of progesterone, the elimination rate remains practically constant (85%) at pH values below the pKa equal to 9.7; progesterone in molecular form has a greater affinity for cyclodextrin by forming inclusion complexes.
The results show that the elimination mechanism of ibuprofen and progesterone is mainly governed by the inclusion of the molecules in the cyclodextrin cavities.
3.2.4. Effect of Ionic Strength
The influence of ionic strength on the adsorption of the two pharmaceutical products is an interesting phenomenon.
Figure 8 illustrates these variations.
Progesterone adsorption shows minimal variation with increasing ionic strength. This suggests that progesterone is not strongly influenced by electrostatic interactions linked to ionic strength.
Adsorption of ibuprofen increases with ionic strength. This increase can be attributed to the solubility effect of ibuprofen on its negative charge. In other words, ibuprofen becomes less soluble as ionic strength increases, thus promoting electrostatic interactions.
Additionally, the formation of inclusion complexes with cyclodextrins may also contribute to this increased adsorption.
In summary, solubility and electrostatic interactions play a key role in the adsorption of these pharmaceuticals as a function of ionic strength.
3.2.5. Effect of Temperature
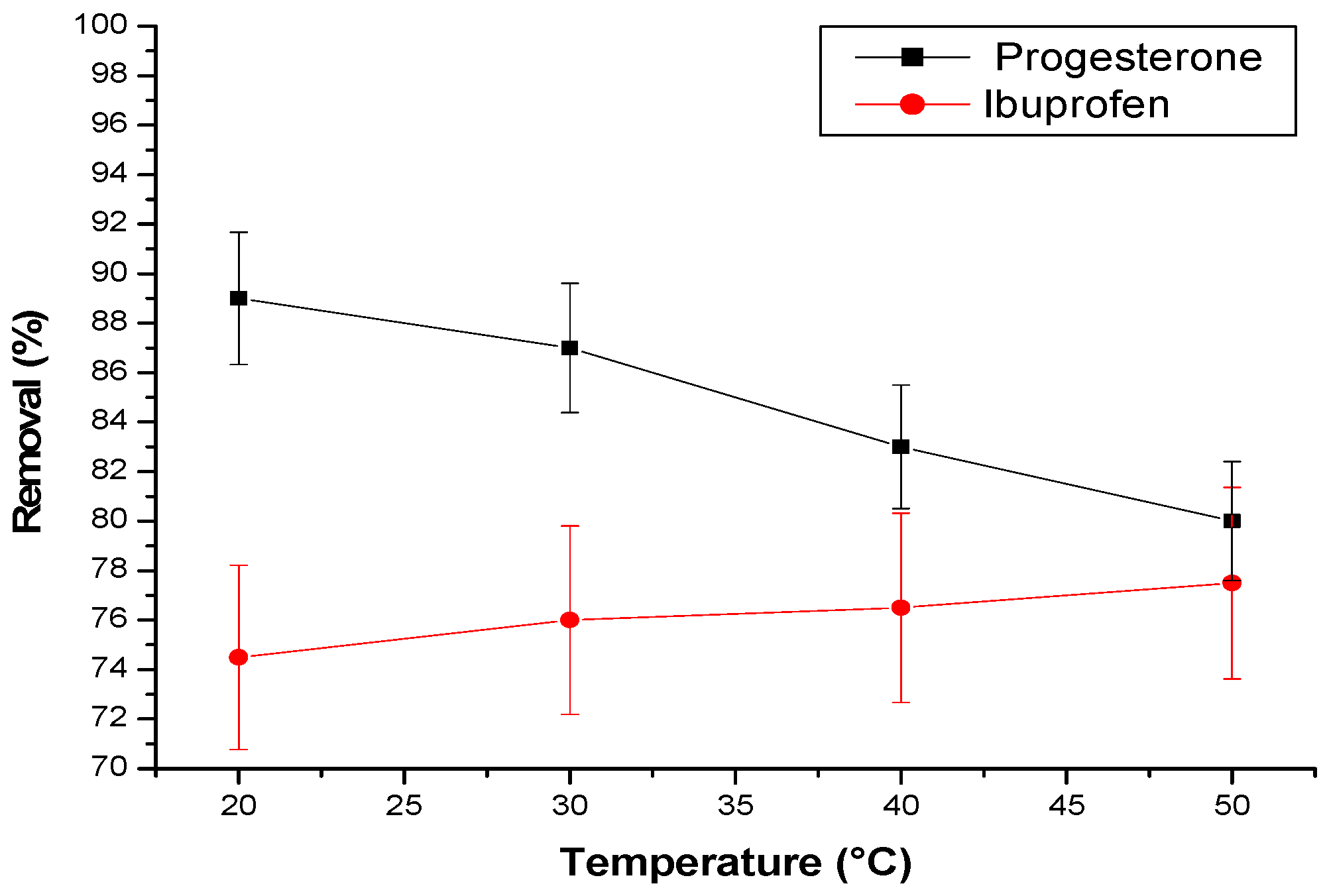
The influence of temperature on the elimination of progesterone and ibuprofen using a cyclodextrin–chitosan polymer was thoroughly investigated. The findings reveal a contrasting effect of temperature on the elimination of these two molecules (
Figure 9).
For progesterone, a decrease in elimination was observed as the temperature rose. This trend suggests that lower temperatures favor the adsorption of progesterone within the polymer. Conversely, the elimination of ibuprofen follows an inverse trajectory, increasing with temperature.
This differential behavior could be explained by two main mechanisms:
- -
Polymer pore expansion: At higher temperatures, the polymer pores expand, facilitating the diffusion of ibuprofen molecules into its matrix.
- -
Creation of new active sites: Elevated temperature could also lead to the creation of new active sites within the polymer, thereby increasing its capacity to adsorb ibuprofen.
In summary, temperature plays a crucial role in the interaction between these substances and the cyclodextrin–chitosan polymer, distinctly influencing their respective elimination.
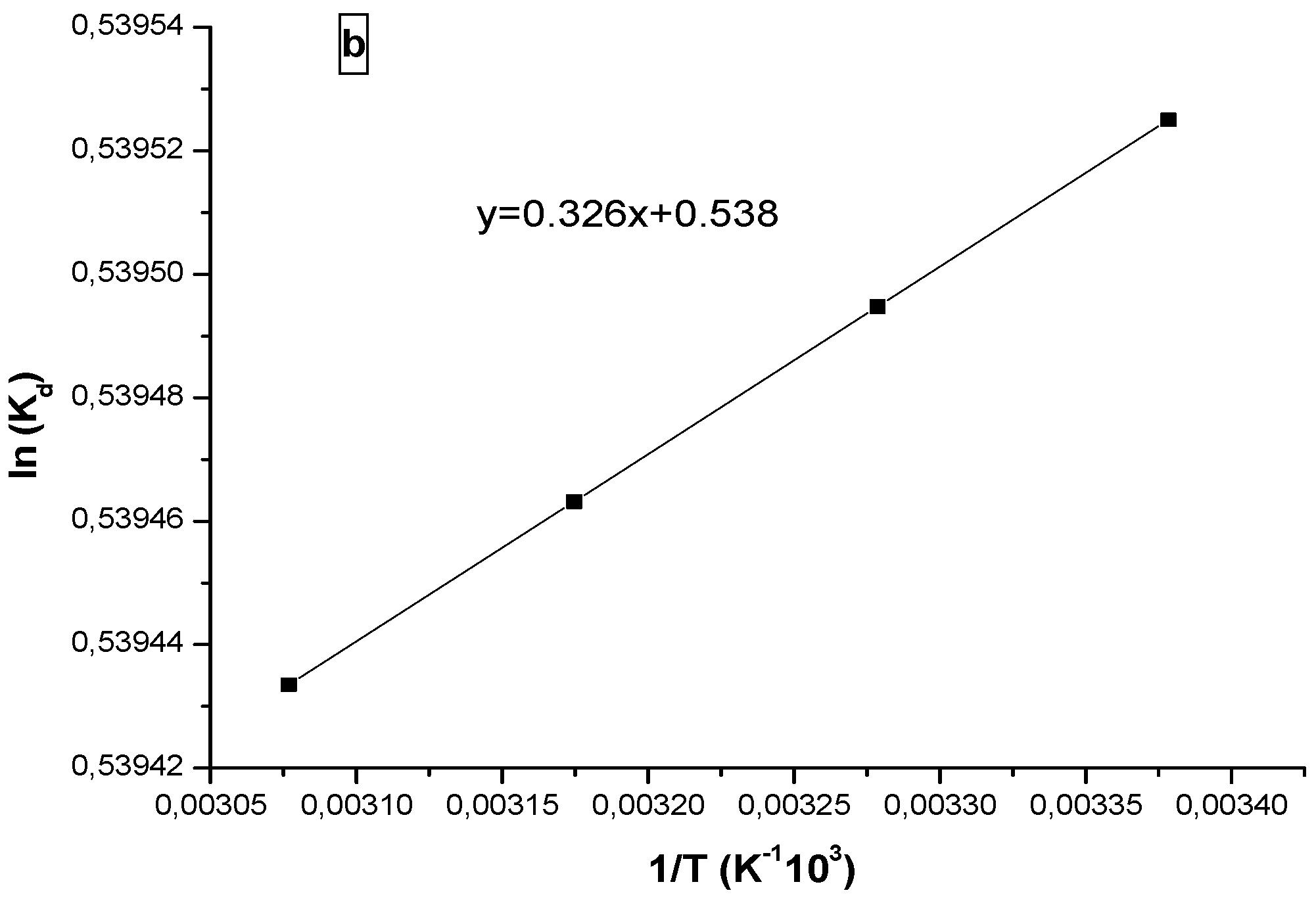
3.3. Thermodynamic Study
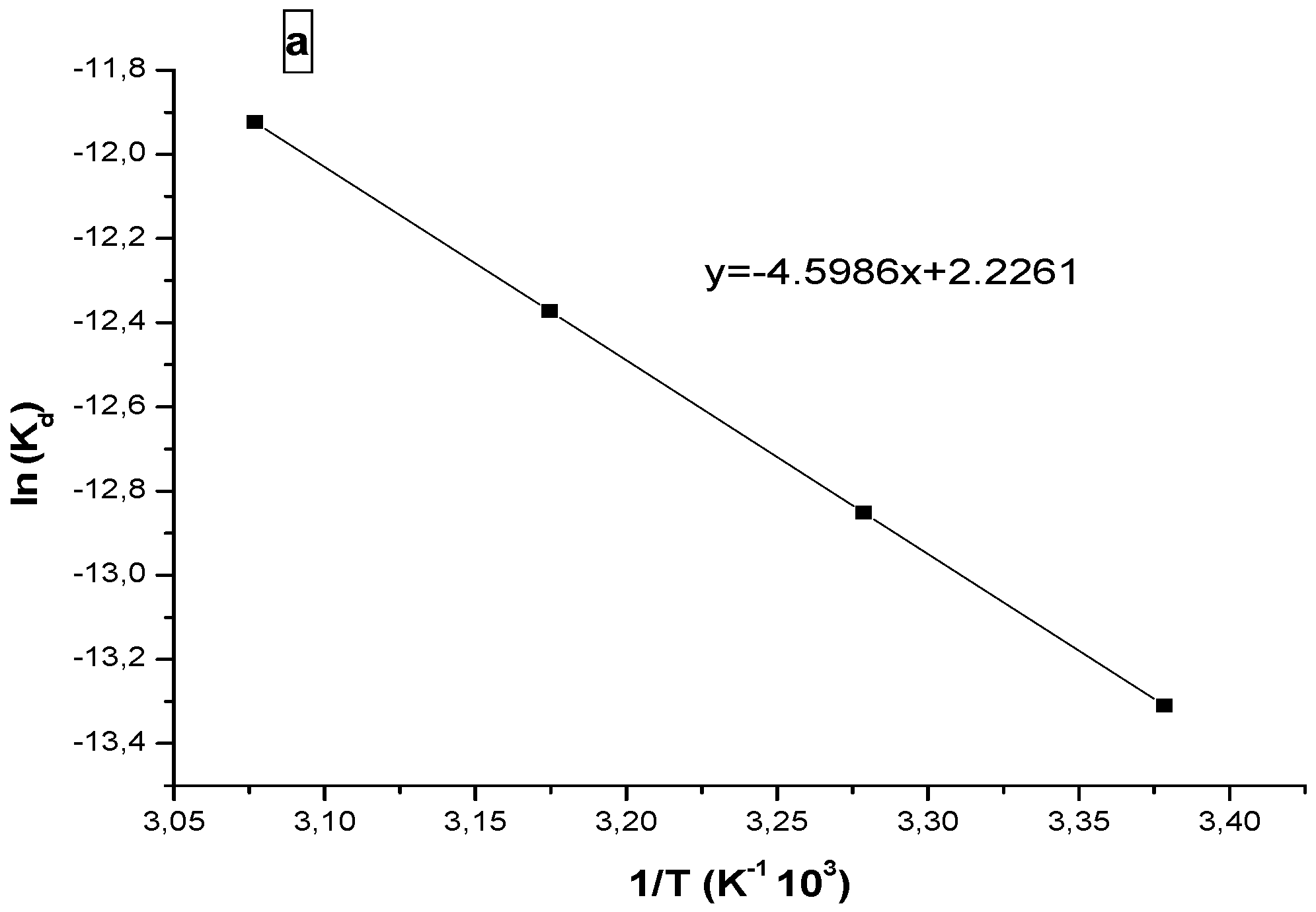
Thermodynamic parameters such as entropy (ΔS) and enthalpy (ΔH) are determined from the plot of ln (Kd) versus temperature (Van’t Hoff Equation (6)).
The free energy change can be obtained by the following formula:
The free energy change can also be expressed as follows:
Figure 10 shows the plot of lnKd versus the reciprocal of absolute temperature (T); the thermodynamic parameters are calculated from the slope and the resulting right ordinate. The free energy variation can be obtained by Equation (5).
The thermodynamic parameters calculated are summarized in
Table 2. The positive value of enthalpy for progesterone indicates that the adsorption process on the polymer is endothermic. The negative value of the enthalpy in case of ibuprofen indicates an exothermic adsorption process.
The positives values of the entropy for the two pharmaceuticals show that the adsorbed molecules on the polymer surface are organized in a more random fashion compared to those in the aqueous phase [
31].
3.4. Adsorption Kinetic Modeling
Adsorption kinetics are of considerable practical interest for the implementation of the adsorbent. It allows us to highlight the specificity of the physicochemical interactions between the solute and the adsorbent and to obtain the adsorption rate and the amount adsorbed at equilibrium.
The adsorption kinetics of ibuprofen and progesterone on the surface of the synthesized polymer are studied at pH = 2 for ibuprofen, pH = 7 for progesterone, and a temperature of 25 °C, using the pseudo-first-order (Equation (7)), pseudo-second-order (Equation (8)), and Elovich (Equation (9)) models.
The linear form of pseudo-first-order equation is:
k1 (min−1): pseudo-first-order adsorption rate constant.
qe: amount adsorbed per unit mass at equilibrium;
qt: amount adsorbed per unit mass at any time t;
Linear form of pseudo second order equation [
29,
32]:
k2 (g/mg.min): pseudo-second-order adsorption rate constant.
The linear form of Elovich equation [
31]:
α and β are the Elovich coefficients.
The validity of each model could be verified by the linear regression value (correlation coefficient, R
2) and a normalized standard deviation Δq (%), which can be obtained by the following equation [
32]:
qexp: experimental adsorbed amount per adsorbent mass at equilibrium;
qcal: calculated adsorbed amount per adsorbent mass at equilibrium;
n: number of data points.
The R² values obtained from the linear form of the models are mentioned in
Table 3 and
Table 4.
Among the three models tested, the pseudo-second-order model appears to be the most favorable in the adsorption process of ibuprofen and progesterone, indicating strong interactions between the studied molecules and the synthesized polymer. Indeed, the values of the regression coefficients R² of the kinetic lines (a1) and (a2) (
Figure 11) for progesterone and ibuprofen, respectively, are close to 1. In addition, the low values of the normalized standard deviation ∆q confirm the validity of the model.
The higher goodness of fitting for the pseudo-second-order model could be ascribed to the nature of the cyclodextrin–chitosan polymer, with multiple adsorption sites, which are responsible for different adsorption steps [
33]. This model indicates that the adsorption process depends on the properties of the adsorbent and adsorbate.
3.5. Adsorption of Progesterone and Ibuprofen on Cyclodextrin–Chitosan Polymer: Alone and Mixed Systems
The adsorption of a mixture containing two molecules onto the cyclodextrin–chitosan polymer was investigated (
Figure 12).
Figure 12 shows the remarkable efficacy of the cyclodextrin–chitosan polymer as an adsorbent for progesterone, whether the latter is present alone or in a mixture with another molecule, namely, IB. This outstanding performance can be attributed to the molecular characteristics of progesterone, particularly its shape and size, which favorably predispose it for inclusion within the cavities of gamma CDs through physical interactions. Notably, the adsorption rates observed for progesterone and ibuprofen, alone or in a mixture, remain practically identical, highlighting the polymer’s remarkable ability to maintain its effectiveness even in the presence of another molecule.
3.6. Comparative Study of the Adsorption Efficiency of Chitosan, Cyclodextrin Polymer, and Cyclodextrin–Chitosan Polymer
This study aims to compare the adsorption efficiency of progesterone and ibuprofen by three polymers: chitosan, (α-, β-, γ-) cyclodextrin polymer, and cyclodextrin–chitosan polymer. Adsorption experiments were carried out using the experimental device illustrated in
Figure 2. The results obtained are presented in
Figure 13.
Chitosan exhibits the highest progesterone adsorption rates, reaching approximately 90%. Its adsorption kinetics are rapid, with equilibrium reached in about 50 min. This performance can be explained by the porous structure and large specific surface area of chitosan, which favor the physical adsorption of molecules to its surface.
The adsorption kinetics of progesterone on the cyclodextrin polymer are slower. After 200 min, the removal rate reaches 90%. This phenomenon can be explained by the time required for progesterone molecules to find their way into the cavities of the different CDs and form inclusion complexes.
The CDs–chitosan association does not significantly improve progesterone adsorption rates, which remain around 90% after 50 min.
The adsorption rates of ibuprofen by chitosan or by the cyclodextrin polymer are relatively low, reaching about 40%.
The combination of chitosan and CD polymer improves ibuprofen adsorption, with a rate reaching approximately 70%. This improvement can be explained by the creation of a composite material with a larger adsorption surface, favoring specific interactions between the polymers and the ibuprofen molecule.
In conclusion, chitosan proves to be the most effective polymer for progesterone adsorption, while the chitosan–cyclodextrin polymer composite is the most efficient for ibuprofen adsorption. The results of this study highlight the importance of the structure and properties of polymers in the adsorption process.
3.7. Physico-Chemical Methods
3.7.1. X-ray Powder Diffractometry (XRPD)
The X-ray powder diffractometry (XRPD) analysis revealed an amorphous structure for the cyclodextrins–chitosan polymer, in contrast to the crystalline nature of ibuprofen and progesterone, which exhibited a series of sharp, intense peaks (
Figure 14).
Upon extraction of ibuprofen and progesterone, the cyclodextrins–chitosan polymer maintained its amorphous structure, as evidenced by the absence of characteristic diffraction peaks for the drugs. This suggests the formation of a highly disordered inclusion complex between the pharmaceuticals and the polymer matrix, as corroborated by previous studies [
34,
35]. Alternatively, the lack of drug-related peaks could be attributed to a monomolecular dispersion of ibuprofen and progesterone within the polymeric matrix due to physical interactions [
36].
3.7.2. Adsorption Mechanism
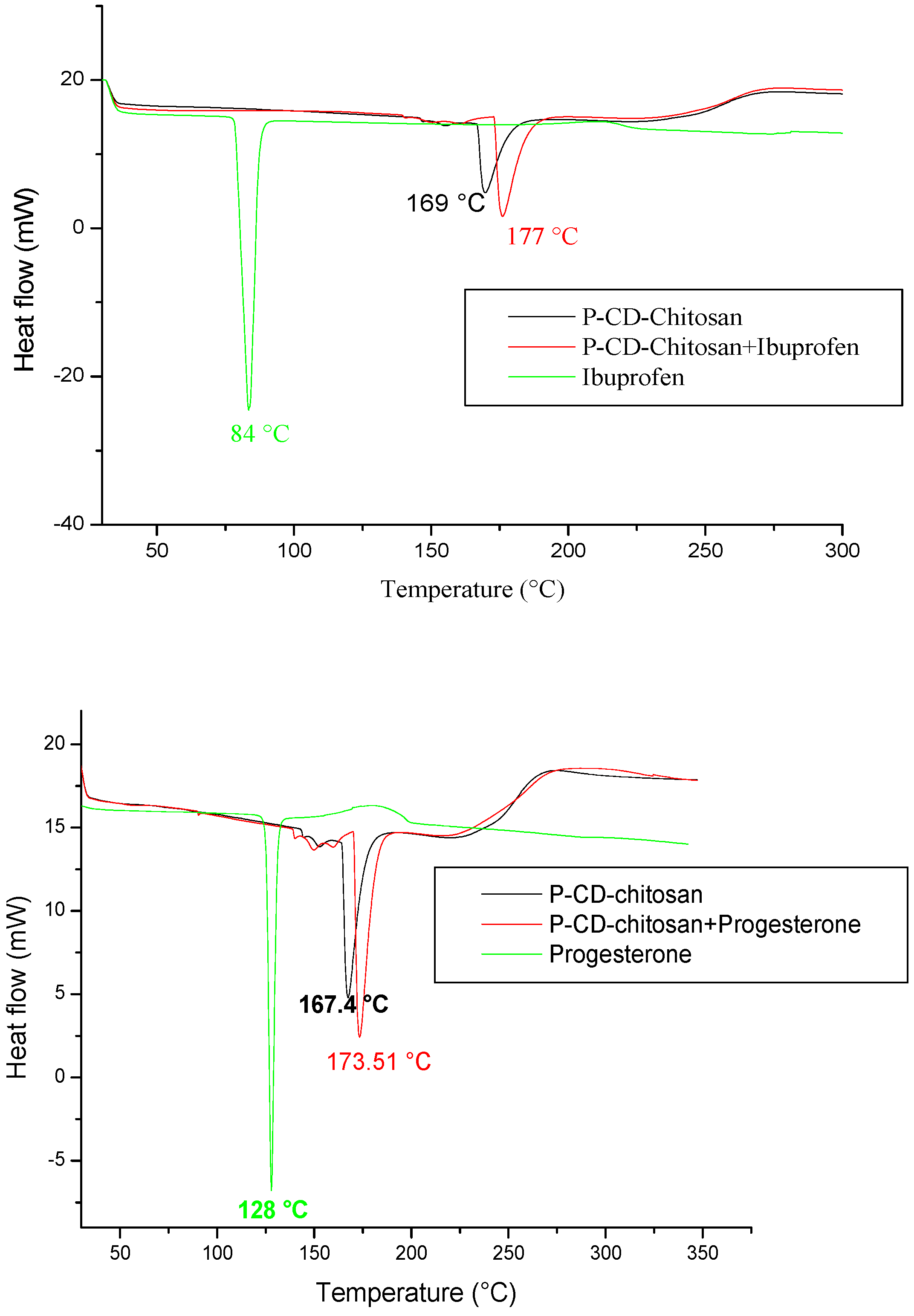
To elucidate the adsorption mechanism of the synthesized polymer, a differential scanning calorimetric (DSC) analysis of cyclodextrin–chitosan polymer, ibuprofen, and progesterone is performed before and after adsorption (
Figure 15).
The thermograms of ibuprofen and progesterone present endothermic peaks at 84 °C and 128 °C, respectively, corresponding to their melting point, while the cyclodextrin–chitosan polymer presents a broad endothermic peak corresponding to the loss of the crystalline water contained in the polymer.
After adsorption, the figure shows a shift and an increase in the amplitude of the endothermic peak of the polymer and the disappearance of the endothermic peaks, corresponding to the melting points of ibuprofen and progesterone. This can be explained by the formation of inclusion complexes under the effect of physical interactions between the molecules and the polymer network.
3.8. Regeneration of Cyclodextrin–Chitosan Polymer
The cyclodextrin–chitosan polymer was regenerated with a water/ethanol mixture in a proportion of (70/30).
Figure 16 shows the progesterone elimination rate as a function of the number of regeneration cycles (adsorption/desorption). The adsorption efficiency of the polymer remains constant after twenty-three cycles of use.
4. Conclusions
A novel biopolymer was synthesized by grafting three cyclodextrins (alpha, beta, and gamma) onto chitosan in the presence of a non-toxic cross-linker, citric acid. This polymer was characterized and evaluated for its adsorption and removal potential of two pharmaceutical molecules of different nature and size: ibuprofen and progesterone.
The synthesized polymer exhibits an amorphous and porous structure with a high swelling capacity (71.41%).
Ibuprofen adsorption is strongly influenced by the pH and ionic strength of the solution, while progesterone adsorption is not affected by these parameters.
The DSC analysis indicates that the adsorption of both molecules is mainly due to the formation of inclusion complexes and physical interactions within the polymer network.
The positive entropy values suggest random adsorption of pharmaceutical molecules onto the polymer surface.
Adsorption kinetics follows a pseudo-second-order model for both molecules.
The polymer exhibits a better adsorption capacity for ibuprofen (70%), compared to chitosan (45%) and alpha-, beta-, gamma-cyclodextrin-based polymer (38%).
The progesterone removal percentage reaches 90% with the polymer but does not show a significant improvement compared to chitosan or alpha-, beta-, or gamma-cyclodextrin-based polymer.
The polymer exhibits selective affinity for progesterone, adsorbing it even in the presence of ibuprofen.
The material can be regenerated up to 23 times without significant loss of efficiency, making it a major asset for repeated use.
Grafting cyclodextrins into the chitosan matrix increases the number of adsorption sites and improves the material’s selectivity for specific pharmaceutical molecules.
Its high adsorption capacity, selectivity, and efficient regeneration make this synthesized polymer a promising candidate for large-scale applications in drug delivery and pharmaceutical purification.
Overall, the synthesized biopolymer demonstrates remarkable properties and potential for various pharmaceutical applications.
Author Contributions
Conceptualization, M.S., L.M. and F.O.-H.; methodology, L.M., N.M., F.B. and F.O.-H.; software, N.M. and F.B.; validation, M.L.-S., M.S., L.M. and F.O.-H.; formal analysis, M.S., L.M. and F.O.-H.; investigation, L.M. and F.O.-H.; resources, M.L.-S. and M.S.; data curation, N.M. and F.B.; writing—original draft preparation, L.M. and F.O.-H.; writing—review and editing, M.S., L.M. and F.O.-H.; visualization, M.L.-S.; supervision, M.L.-S. and M.S.; project administration, M.S. and M.L.-S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors are grateful to the Start-Up In-Cyclo (France) for the financial and technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vinay, K.; Sivarama Krishna, L.; Neha, S.; Pritha, C.; Mridul, U.; Ritu, P.; Jithin, T.; Vishal, U.K.; Iyyappan, J.; Mukesh Kumar, A.; et al. A critical assessment of technical advances in pharmaceutical removal from wastewater—A critical review. Case Stud. Chem. Environ. Eng. 2023, 8, 100363. [Google Scholar]
- Tetiana, T.; Liubov, S.; Wojciech, M. Magnetic adsorbents for removal of pharmaceuticals: A review of adsorption properties. J. Mol. Liq. 2023, 384, 122174. [Google Scholar]
- Reoyo-Prats, B.; Hammadi, M.; Kim Lai, S.; Goetz, V.; Calas-Blanchard, C.; Joannis-Cassan, C.; Plantard, G. Implementation of an advanced photooxidation process to intensify pharmaceuticals removal by a membrane bioreactor. Chem. Eng. Process. Process Intensif. 2023, 191, 109460. [Google Scholar]
- Koyuncu, I.; Arikan, O.A.; Wiesner, M.R.; Rice, C. Removal of hormones and antibiotics by nanofiltration membranes. J. Membr. Sci. 2008, 309, 94–101. [Google Scholar] [CrossRef]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C.; Yoon, J. Removal of endocrine disrupting compounds and pharmaceuticals by nanofiltration and ultrafiltration membranes. Desalination 2007, 202, 16–23. [Google Scholar] [CrossRef]
- Broséus, R.; Vincent, S.; Aboulfadl, K.; Daneshvar, A.; Sauvé, S.; Barbeau, B.; Prévost, M. Ozone oxidation of pharmaceuticals, endocrine disruptors and pesticides during drinking water treatment. Water Res. 2009, 43, 4707–4717. [Google Scholar] [CrossRef]
- Banasiak, L.J.; Schäfer, A.I. Sorption of steroidal hormones by electrodialysis membranes. J. Membr. Sci. 2010, 365, 198–205. [Google Scholar] [CrossRef]
- Suarez, S.; Lema, J.M.; Omil, F. Pre-treatment of hospital wastewater by coagulation–flocculation and flotation. Bioresor. Technol. 2009, 100, 2138–2146. [Google Scholar] [CrossRef]
- Oughlis-Hammache, F.; Skiba, M.; Hallouard, F.; Moulahcene, L.; Kebiche-Senhadji, O.; Benamor, M.; Lahiani-Skiba, M. Synthesis and characterization of poly(vinyl-alcohol)-poly(ß-cyclodextrin) copolymer membranes for aniline extraction. Membr. Water Treat. 2016, 7, 223–240. [Google Scholar] [CrossRef]
- Li, X.; Lei, S.; Wu, G.; Yu, Q.; Xu, K.; Ren, H.; Wang, Y.; Geng, J.C. Prediction of pharmaceuticals removal in activated sludge system under different operational parameters using an extended ASM-PhACs model. Sci. Total Environ. 2023, 871, 162065. [Google Scholar] [CrossRef]
- Adham, S.A.S.; Redding, A.M.; Cannon, F.S.; DeCarolis, J.; Oppenheimer, J.; Werta, E.C.; Yoon, Y. Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination 2007, 202, 156–181. [Google Scholar]
- Chen, Q.; Zhang, R.; Wang, J.; Li, L.; Guo, X. Spherical particles of α-, β- and γ-cyclodextrin polymers and their capability for phenol removal. Mater. Lett. 2012, 79, 156–158. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, J.; Liang, X.; Liu, Z. Adsorption of platinum (IV) and palladium(II) from aqueous solution by magnetic cross-linking chitosan nanoparticles modified with ethylenediamine. J. Hazard. Mater. 2010, 182, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Rêgo, T.V.; Cadaval, T.R.S., Jr.; Dotto, G.L.; Pinto, L.A.A. Statistical optimization, interaction analysis and desorption studies for the azo dyes adsorption onto chitosan films. J. Colloid Interface Sci. 2013, 411, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Alves, D.C.; Healy, B.; Pinto, L.A.d.A.; Cadaval, T.R.S., Jr.; Breslin, C.B. Recent Developments in Chitosan-Based Adsorbents for the Removal of Pollutants from Aqueous Environments. Molecules 2021, 26, 594. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowska, M.A.; Bożejewicz, D. The Application of Chitosan-Based Adsorbents for the Removal of Hazardous Pollutants from Aqueous Solutions—A Review. Sustainability 2024, 16, 2615. [Google Scholar] [CrossRef]
- Elbadawy, H.-A.; El-Dissouky, A.; Hussein, S.-M.; El-Kewaey, S.-R.; Elfeky, S.-A.; El-Ghannam, G. A novel terpolymer nanocomposite (carboxymethyl β-cyclodextrin–nano chitosan–glutaraldehyde) for the potential removal of a textile dye acid red 37 from water. Front. Chem. 2023, 1, 1115377. [Google Scholar] [CrossRef]
- Chai, K.; Ji, H. Dual functional adsorption of benzoic acid from wastewater by biological-based chitosan grafted β-cyclodextrin. Chem. Eng. J. 2012, 203, 309–318. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Bin, J.-P.; Bu, H.-T.; Jiang, G.-B.; Zeng, M.-H. Removal of anionic dye eosin Y from aqueous solution using ethylenediamine modified chitosan. Carbohydr. Polym. 2011, 84, 1350–1356. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Lazaridis, N.K.; Bikiaris, D.N. Optimisation of chitosan and β-cyclodextrin moleculary imprinted polymer synthesis for dye adsorption. Carbohydr. Polym. 2013, 91, 198–208. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X.; Hua, R.; Wang, Y.; Liu, Y.; Pang, C.; Wang, Y. Selective adsorption of uranyl ion on ion-imprinted chitosan/PVA cross-linked hydrogel. Hydrometallurgy 2010, 104, 150–155. [Google Scholar] [CrossRef]
- Bhaskar, M.; Aruna, P.; Jeevan, R.; Jeevan, G.; Radhakrishnan, G. β-cyclodextrin-polyurethane polymer as solid phase extraction material for the analysis of carcinogenic aromatic amines. Anal. Chim. Acta 2004, 509, 39–45. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, L.; Zhu, C.; Tian, Z.; Shen, X. Synthesis and properties of water-insoluble polymer crosslinked by citric acid with PEG-400 as modifier. Carbohydr. Polym. 2009, 78, 125–130. [Google Scholar] [CrossRef]
- Skiba, M.; Lahiani-Skiba, M. Novel method for preparation of cyclodextrin polymers: Physico-chemical characterization and cytotoxicity. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 341–349. [Google Scholar] [CrossRef]
- Moulahcene, L.; Skiba, M.; Milon, N.; Hammache, F.; Bounoure, F.; Lahiani-Skiba, M. Removal Efficiency of Insoluble ß-Cyclodextrin Polymer from Water–Soluble Carcinogenic Direct Azo Dyes. Polymers 2023, 15, 732. [Google Scholar] [CrossRef] [PubMed]
- Skiba, M. Method for Synthesizing Calixaren and/or Cyclodextrin Copolymers, Terpolymers and Tetrapolymers. Patent PCT-FR 2010 000875, 27 December 2010. [Google Scholar]
- Li, N.; Wei, X.; Mei, Z.; Xiong, X.; Chen, S.; Ye, M.; Ding, S. Synthesis and characterization of a novel polyamidoamine-cyclodextrin crosslinked copolymer. Carbohydr. Res. 2011, 346, 1721–1727. [Google Scholar]
- Lindqvist, N.; Tuhkanen, T.; Kronberg, L. Occurrence of acidic pharmaceuticals in raw and treated sewages and in receiving waters. Water Res. 2005, 39, 2219–2228. [Google Scholar] [CrossRef]
- Boukhris, T.; Lahiani-Skiba, M.; Martin, D.; Skiba, M. Pre-formulation of an oral cyclosporine free of surfactant. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 323–332. [Google Scholar] [CrossRef]
- Mestre, A.S.; Pires, J.; Nogueira, J.M.F.; Carvalho, A.P. Activated carbons for the adsorption of ibuprofen. Carbon 2007, 45, 1979–1988. [Google Scholar] [CrossRef]
- Purkait, M.K.; Maiti, A.; DasGupta, S.; De, S. Removal of congo red using activated carbon and its regeneration. J. Hazard. Mater. 2007, 145, 287–295. [Google Scholar] [CrossRef]
- Sen Gupta, S.; Bhattacharyya, K.G. Kinetics of adsorption of metal ions on inorganic materials: A review. Adv. Colloid Interface Sci. 2011, 162, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cai, X.; Wanga, Y.; Chen, J. Adsorption mechanism-based screening of cyclodextrin polymers for adsorption and separation of pesticides from water. Water Res. 2011, 45, 3499–3511. [Google Scholar] [CrossRef] [PubMed]
- Hirlekar, R.; Kadam, V. Preparation and characterization of inclusion complexes of carvedilol with methyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2009, 63, 219–224. [Google Scholar] [CrossRef]
- Ribeiro, A.; Figueiras, A.; Santos, D.; Veiga, F. Preparation and solid-state characterization of inclusion complexes formed between miconazole and methyl-β-cyclodextrin. AAPS PharmSciTech 2008, 9, 1102–1109. [Google Scholar] [CrossRef]
- Mura, P.; Faucci, M.T.; Maestrelli, F.; Furlanetto, S.; Pinzauti, S. Characterization of physico-chemical properties of naproxen systems with amorphous β-cyclodextrin-epichlorohydrin Polymers. J. Pharm. Biomed. Anal. 2002, 29, 1015–1024. [Google Scholar] [CrossRef]
Figure 1.
Crosslinking of cyclodextrin–chitosan polymer.
Figure 1.
Crosslinking of cyclodextrin–chitosan polymer.
Figure 2.
Experimental setup for pharmaceutical removal.
Figure 2.
Experimental setup for pharmaceutical removal.
Figure 3.
X-ray diffraction pattern of chitosan and cyclodextrin–chitosan polymer (P–CD–chitosan).
Figure 3.
X-ray diffraction pattern of chitosan and cyclodextrin–chitosan polymer (P–CD–chitosan).
Figure 4.
SEM images of cyclodextrin–chitosan polymer with different magnifications.
Figure 4.
SEM images of cyclodextrin–chitosan polymer with different magnifications.
Figure 5.
Effect of contact time on the adsorption of pharmaceuticals. Conditions: flow rate (1.5 L/h), temperature 25 °C; initial concentration of progesterone and ibuprofen are, respectively, 10 ppm and 30 ppm; pH = 2 (ibuprofen) and pH = 7 (progesterone).
Figure 5.
Effect of contact time on the adsorption of pharmaceuticals. Conditions: flow rate (1.5 L/h), temperature 25 °C; initial concentration of progesterone and ibuprofen are, respectively, 10 ppm and 30 ppm; pH = 2 (ibuprofen) and pH = 7 (progesterone).
Figure 6.
Effect of flow rate (F) on progesterone removal. Conditions: amount of adsorbent (25 mg), temperature (25 °C), initial concentration of progesterone (10 ppm), pH = 7.
Figure 6.
Effect of flow rate (F) on progesterone removal. Conditions: amount of adsorbent (25 mg), temperature (25 °C), initial concentration of progesterone (10 ppm), pH = 7.
Figure 7.
Effect of pH on pharmaceuticals adsorption. Conditions: adsorbent amount = 25 mg; flow rate = 3 L/h; temperature 25 °C; initial concentration of progesterone and ibuprofen are, respectively, 10 ppm and 30 ppm.
Figure 7.
Effect of pH on pharmaceuticals adsorption. Conditions: adsorbent amount = 25 mg; flow rate = 3 L/h; temperature 25 °C; initial concentration of progesterone and ibuprofen are, respectively, 10 ppm and 30 ppm.
Figure 8.
Effect of NaCl concentration on pharmaceuticals removal. Conditions: amount of adsorbent = 25 mg; flow rate = 3 L/h, temperature 25 °C; initial concentration of progesterone and ibuprofen are, respectively, 10 ppm and 30 ppm; pH = 2 in case of ibuprofen and pH = 7 in case of progesterone.
Figure 8.
Effect of NaCl concentration on pharmaceuticals removal. Conditions: amount of adsorbent = 25 mg; flow rate = 3 L/h, temperature 25 °C; initial concentration of progesterone and ibuprofen are, respectively, 10 ppm and 30 ppm; pH = 2 in case of ibuprofen and pH = 7 in case of progesterone.
Figure 9.
Effect of temperature (T) on pharmaceuticals. Conditions: adsorbent amount is 25 mg; flow rate = 3 L/h; initial concentration of progesterone and ibuprofen are, respectively, 10 ppm and 30 ppm; pH = 2 in case of ibuprofen and pH = 7 in case of progesterone.
Figure 9.
Effect of temperature (T) on pharmaceuticals. Conditions: adsorbent amount is 25 mg; flow rate = 3 L/h; initial concentration of progesterone and ibuprofen are, respectively, 10 ppm and 30 ppm; pH = 2 in case of ibuprofen and pH = 7 in case of progesterone.
Figure 10.
Vant’ Hoff plots of ln(Kd) versus 1/ T for (a) progesterone and (b) ibuprofen.
Figure 10.
Vant’ Hoff plots of ln(Kd) versus 1/ T for (a) progesterone and (b) ibuprofen.
Figure 11.
Curve-fitting plot of a pseudo-second-order model for adsorption kinetics of progesterone (a) and ibuprofen (b), respectively.
Figure 11.
Curve-fitting plot of a pseudo-second-order model for adsorption kinetics of progesterone (a) and ibuprofen (b), respectively.
Figure 12.
Removal rates of ibuprofen and progesterone alone and in a mixture by the P–CD–chitosan. Conditions: adsorbent mass = 25 mg; flow rate = 3 L/h; temperature 25 °C; initial concentration of progesterone and ibuprofen are, respectively, 10 ppm and 30 ppm; pH = 2.
Figure 12.
Removal rates of ibuprofen and progesterone alone and in a mixture by the P–CD–chitosan. Conditions: adsorbent mass = 25 mg; flow rate = 3 L/h; temperature 25 °C; initial concentration of progesterone and ibuprofen are, respectively, 10 ppm and 30 ppm; pH = 2.
Figure 13.
Adsorption rates of ibuprofen and progesterone by the three polymers. Adsorbent mass (25 mg); flow rate (3 L/h); temperature (25 °C); initial concentration of progesterone and ibuprofen are, respectively, 10 ppm and 30 ppm; pH = 2 (ibuprofen) and pH = 7 (progesterone).
Figure 13.
Adsorption rates of ibuprofen and progesterone by the three polymers. Adsorbent mass (25 mg); flow rate (3 L/h); temperature (25 °C); initial concentration of progesterone and ibuprofen are, respectively, 10 ppm and 30 ppm; pH = 2 (ibuprofen) and pH = 7 (progesterone).
Figure 14.
X-ray diffractogram of (a) ibuprofen, (b) progesterone, and cyclodextrins–chitosan polymers before and after extraction.
Figure 14.
X-ray diffractogram of (a) ibuprofen, (b) progesterone, and cyclodextrins–chitosan polymers before and after extraction.
Figure 15.
Differential scanning calorimetry (DSC) of ibuprofen, progesterone, and cyclodextrin–tosan polymer before and after extraction of ibuprofen and progesterone.
Figure 15.
Differential scanning calorimetry (DSC) of ibuprofen, progesterone, and cyclodextrin–tosan polymer before and after extraction of ibuprofen and progesterone.
Figure 16.
Cycle of adsorption–desorption of progesterone with chitosan–cyclodextrin polymer.
Figure 16.
Cycle of adsorption–desorption of progesterone with chitosan–cyclodextrin polymer.
Table 1.
Properties of cyclodextrin–chitosan polymer.
Table 1.
Properties of cyclodextrin–chitosan polymer.
| SC (%) | TA (mmol/g) | Mn
(g/mol) | Mw
(g/mol) |
|---|
| 71.41 | 9.50 | 43,000 | 310,000 |
Table 2.
Thermodynamic parameters of ibuprofen and progesterone adsorption on cyclodextrin–chitosan polymer.
Table 2.
Thermodynamic parameters of ibuprofen and progesterone adsorption on cyclodextrin–chitosan polymer.
| Molecule | C0 (mg/L) | T (K°) | ΔH°
(J/mol) | ΔS◦
(J/°K.mol) |
|---|
| Progesterone | 10 | 296 | 38.2319 | 18.5077 |
| 305 |
| 315 |
| 325 |
| Ibuprofen | | 296 | | |
| 30 | 305 | −2.7103 | 4.4770 |
| | 315 | | |
| | 325 | | |
Table 3.
Results of R2, k, and Δq (%) for different equations used to model the kinetic adsorption of progesterone by cyclodextrin–chitosan polymer.
Table 3.
Results of R2, k, and Δq (%) for different equations used to model the kinetic adsorption of progesterone by cyclodextrin–chitosan polymer.
| Equation | (5) | (6) | (7) |
|---|
| R2 | 0.856 | 0.9998 | 0.932 |
| k1 (g/mg.min) | - | 0.0020 | - |
| Δq (%) | 95.08 | 0.52 | 9.53 |
Table 4.
Results of R2, k, and Δq (%) for different equations used to model the kinetic extraction of ibuprofen by cyclodextrin–chitosan polymer.
Table 4.
Results of R2, k, and Δq (%) for different equations used to model the kinetic extraction of ibuprofen by cyclodextrin–chitosan polymer.
| Equation | (5) | (6) | (7) |
|---|
| R2 | 0.692 | 0.9997 | 0.8300 |
| k2 (g/mg.min) | - | 0.0003 | - |
| Δq (%) | 85.87 | 5.94 | 8.91 |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).