Designed Growth of Covalently Bonded WO3/PEDOT Hybrid Nanorods Array with Enhanced Electrochromic Performance
Abstract
1. Introduction
2. Materials and Methods
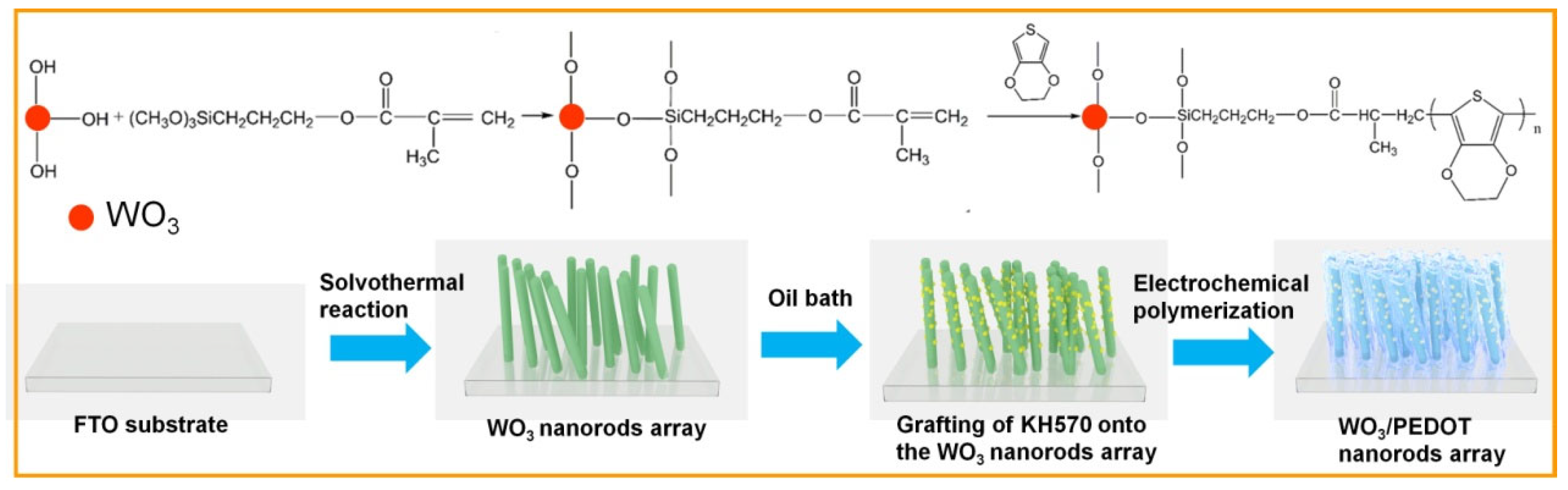
2.1. Fabrication and Chemical Modification of WO3 Nanorod Array Film
2.2. Fabrication of C-WO3/PEDOT Core/Shell Nanorod Array Film
2.3. Characterization
3. Results
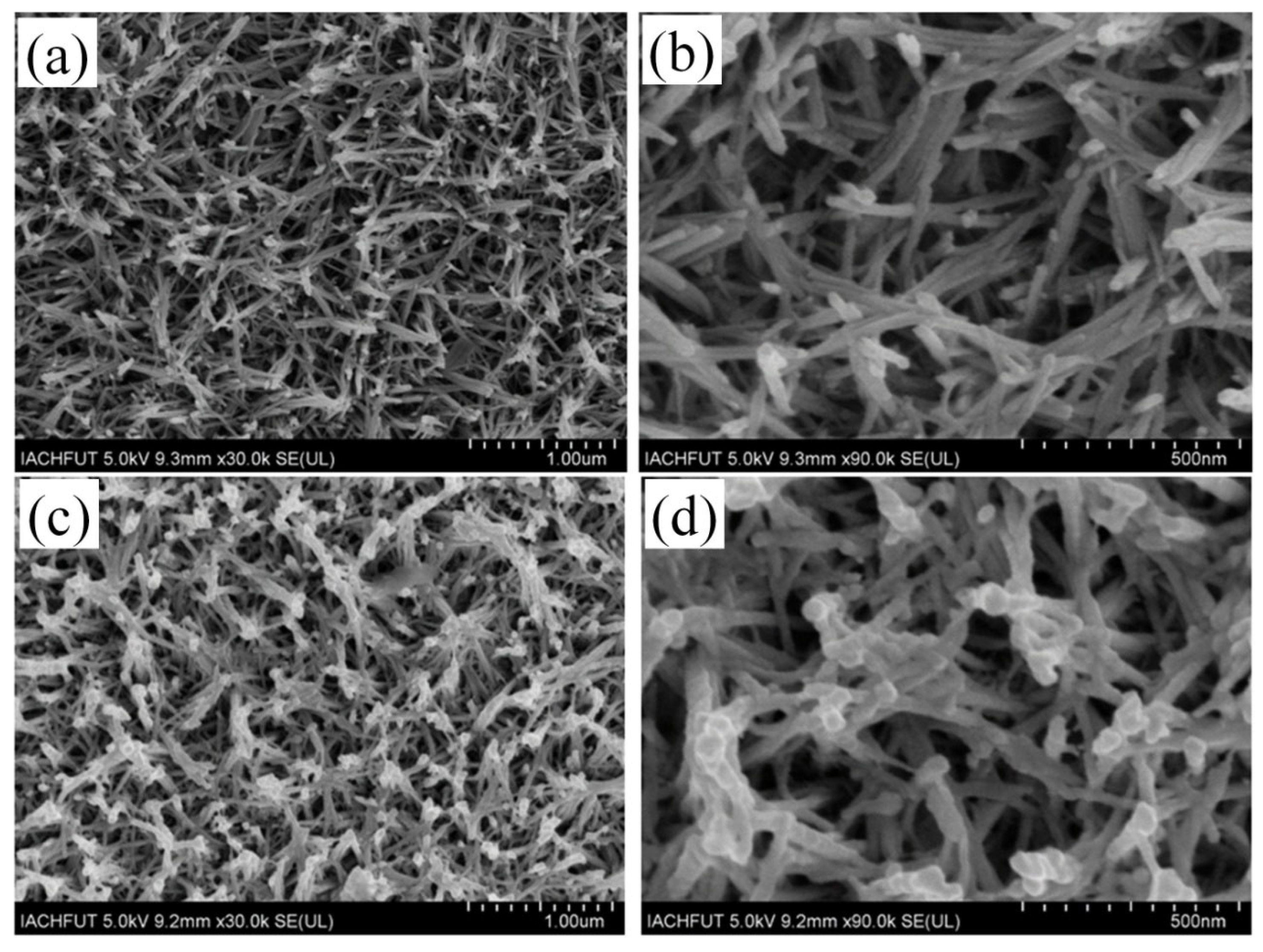
3.1. Morphology and Structure
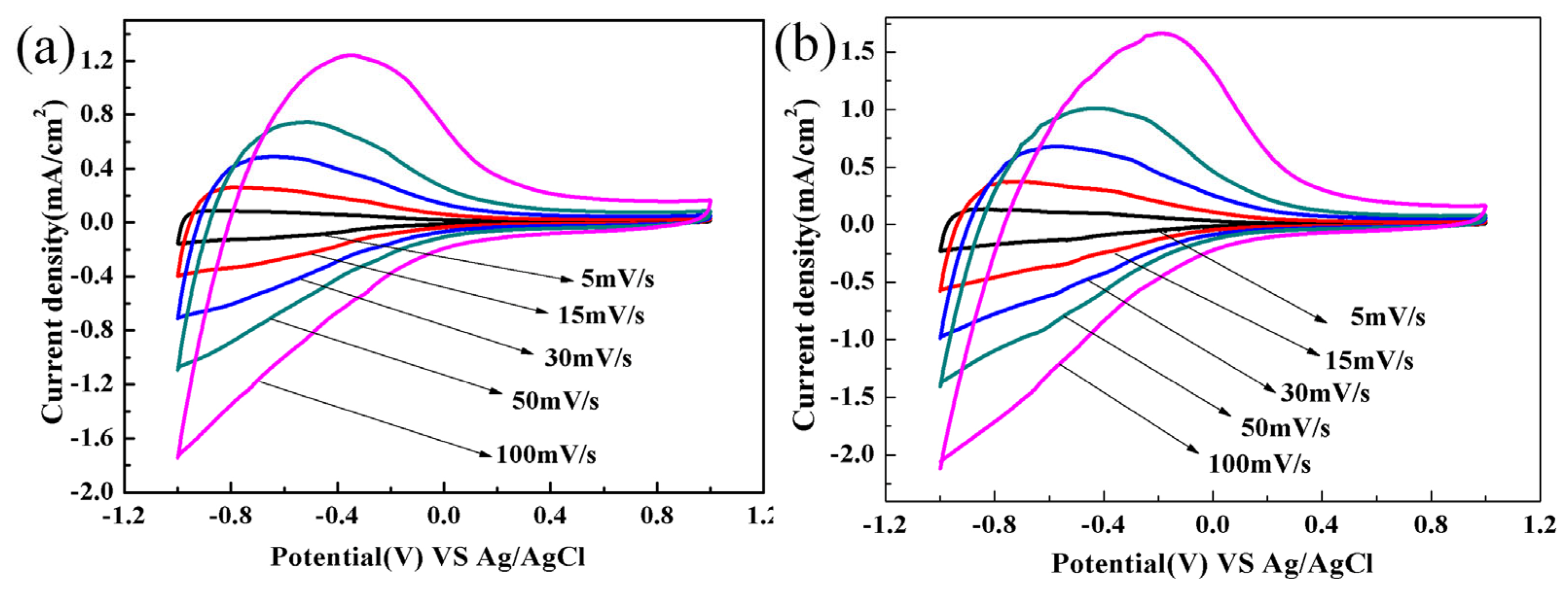
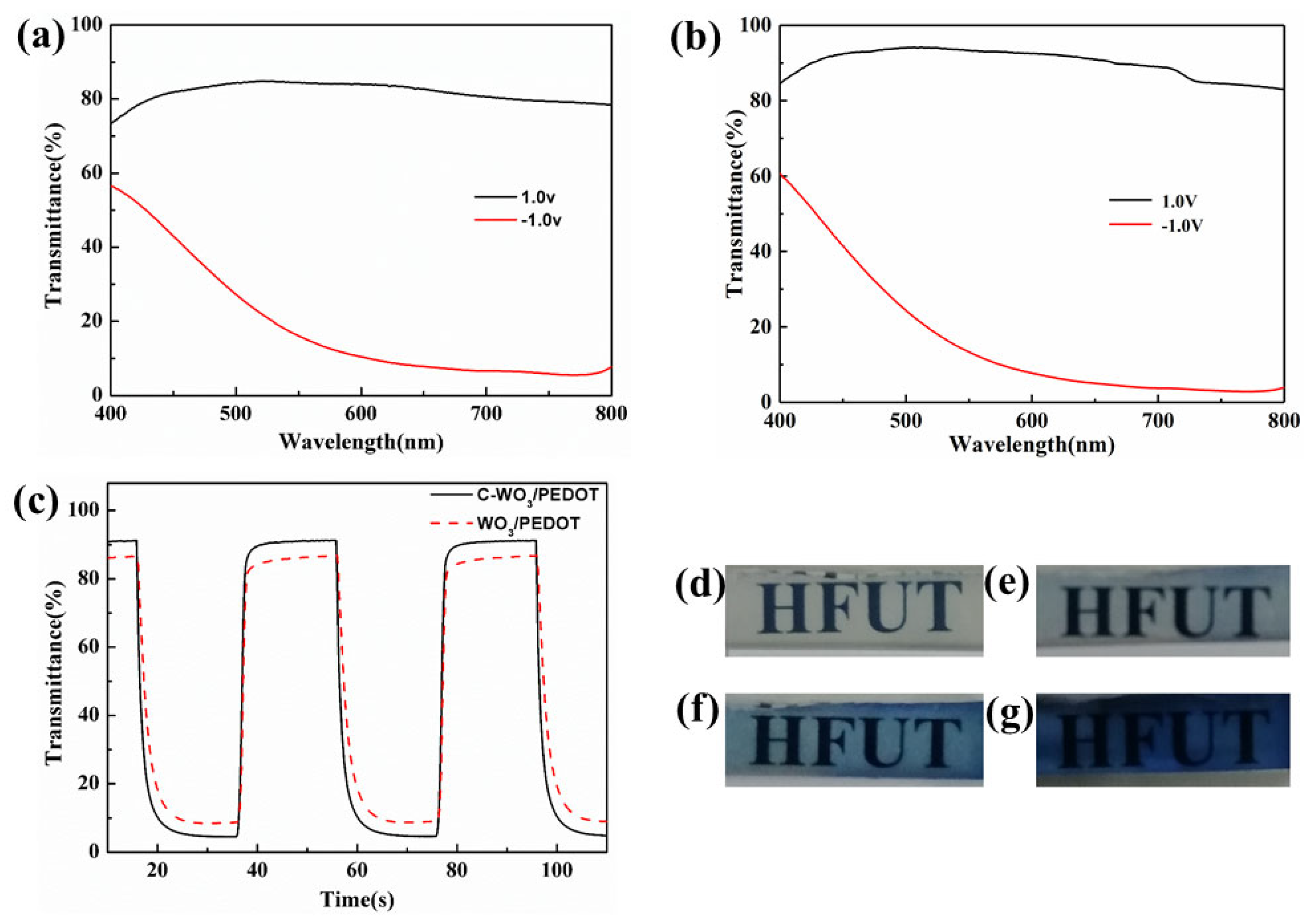
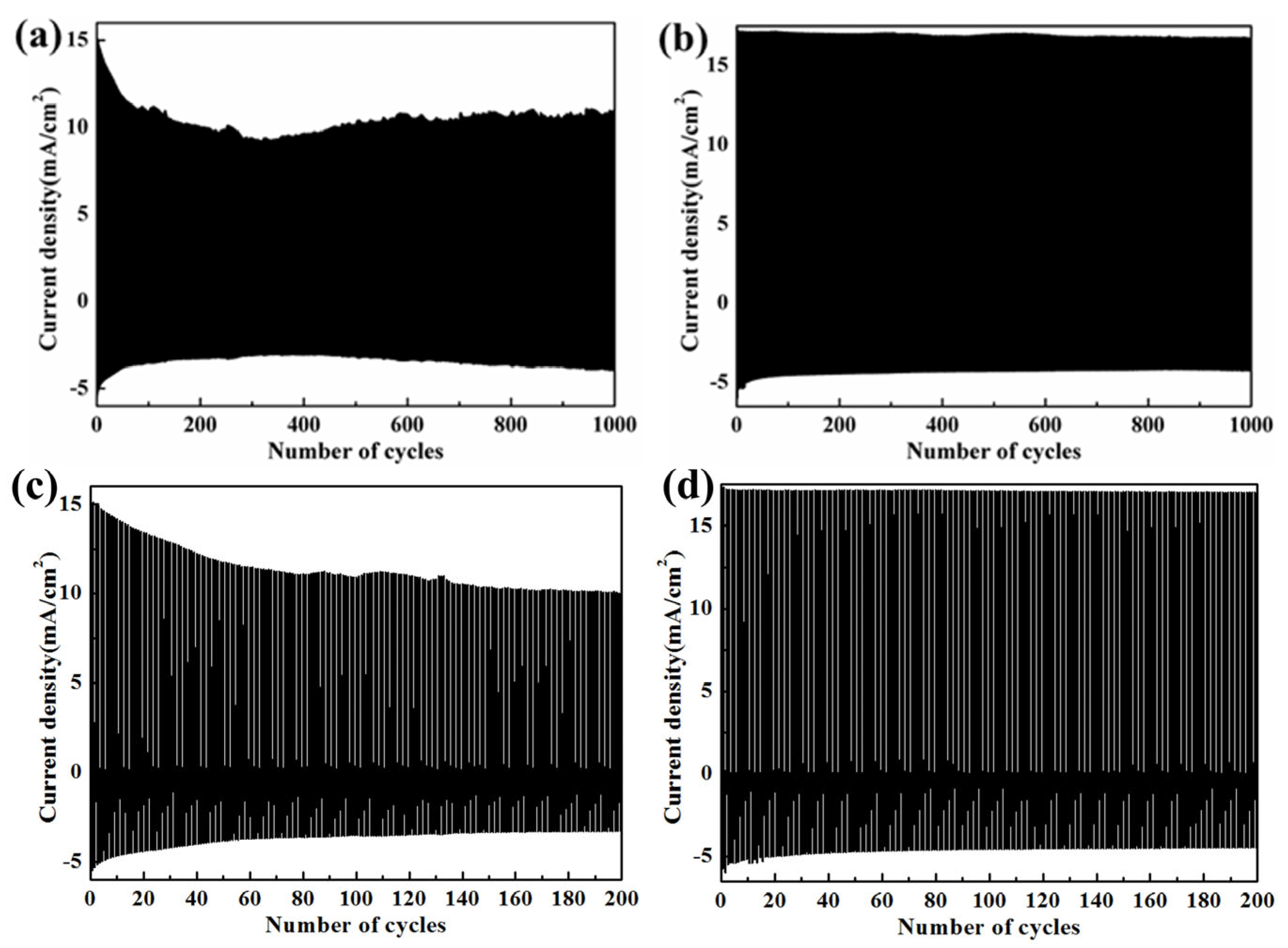
3.2. Electrochemical and Electrochromic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Granqvist, C.G. Electrochromics for energy efficiency and indoor comfort. Pure Appl. Chem. 2008, 80, 2489. [Google Scholar] [CrossRef]
- Cai, G.F.; Wang, J.X.; Lee, P.S. Next-Generation Multifunctional Electrochromic Devices. Acc. Chem. Res. 2016, 49, 1469. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Diao, X.G.; He, Z.B.; Yi, Y.; Wang, T.; Wang, M.Y.; Huang, J.L.; He, X.S.; Zhong, X.L.; Du, K. High-performance all-inorganic electrochromic Li-ion hybrid supercapacitors toward safe and smart energy storage. Energy Storage Mater. 2020, 33, 258. [Google Scholar] [CrossRef]
- Li, W.J.; Zhang, X.; Yan, D.K.; Wang, L.B.; Sun, W.H.; Li, Z.T.; Deng, J.B.; Zhao, J.P.; Li, Y. Rejuvenation of Electrochromic Devices. Small Methods 2024, 8, 2300850. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.H.; Sun, P.; Chai, Z.S.; Huang, L.H.; Cai, X.; Tan, S.Z.; Song, J.H.; Mai, W.J. Large-Scale Fabrication of Pseudocapacitive Glass Windows that Combine Electrochromism and Energy Storage. Angew. Chem. Int. Ed. 2014, 53, 11935. [Google Scholar] [CrossRef]
- Li, C.A.; Ko, B.; Park, K.H.; Ahn, J.G.; Park, T.Y.; Lee, D.J.; Song, S.H. High-Performance Electrochromic Devices Based on Size-Controlled 2D WO3 Nanosheets Prepared Using the Intercalation Method. Materials 2024, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Tong, Z.B.; Xie, H.M.; Sun, J.T.; Chen, F.G. Effects of Additives on Electrochromic Properties of Nanocrystalline Tungsten Oxide Films Prepared by Complexation-Assisted Sol–Gel Method. Materials 2023, 16, 2681. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Yang, Y.; Jin, Q.Y.; Lou, Q.C.; Hu, Q.Z.; Xie, Z.L.; Song, W.J. A Scalable, Robust Polyvinyl-Butyral-Based Solid Polymer Electrolyte with Outstanding Ionic Conductivity for Laminated Large-Area WO3-NiO Electrochromic Devices. Adv. Funct. Mater. 2023, 33, 2214417. [Google Scholar] [CrossRef]
- Vernardou, D.; Drosos, H.; Spanakis, E.; Koudoumas, E.; Katsarakis, N.; Pemble, M.E. Electrochemical properties of amorphous WO3 coatings grown on polycarbonate by aerosol-assisted CVD. Electrochim. Acta 2012, 65, 185. [Google Scholar] [CrossRef]
- Chen, J.; Song, G.; Cong, S.; Zhao, Z.G. Resonant-Cavity-Enhanced Electrochromic Materials and Devices. Adv. Mater. 2023, 35, 2300179. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, Y.; Shi, Y.; Cui, J.; Shu, X.; Wang, Y.; Liu, J.; Wang, J.; Tan, H.H.; Wu, Y. Preparation of V2O5 dot-decorated WO3 nanorod arrays for high performance multi-color electrochromic devices. J. Mater. Chem. A 2018, 6, 12206. [Google Scholar] [CrossRef]
- Jiao, Z.; Sun, X.W.; Wang, J.; Ke LDemir, H.V. Hydrothermally grown nanostructured WO3 films and their electrochromic characteristics. J. Phys. D Appl. Phys. 2010, 43, 285501. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, J.P.; Xia, X.H.; Wang, X.L.; Gu, C.D. Hydrothermally synthesized WO3 nanowire arrays with highly improved electrochromic performance. J. Mater. Chem. 2011, 21, 5492. [Google Scholar] [CrossRef]
- Chen, T.; Chen, Q.; Liu, G.; Chen, G. High cycling stability and well printability poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate)/multi-walled carbon nanotube nanocomposites via in situ polymerization applied on electrochromic display. J. Appl. Polym. Sci. 2018, 135, 45943. [Google Scholar] [CrossRef]
- Ling, H.; Ding, G.Q.; Mandler, D.; Lee, P.S.; Xu, J.W.; Lu, X. Facile preparation of aqueous suspensions of WO3/sulfonated PEDOT hybrid nanoparticles for electrochromic applications. Chem. Commun. 2016, 52, 9379. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Li, H.; Li, K.; Wang, J.; Wang, H.; Zhang, Q.; Li, Y.; Chen, P. High-performance complementary electrochromic device based on WO3⋅0.33H2O/PEDOT and prussian blue electrodes. J. Phys. Chem. Solids 2017, 110, 284. [Google Scholar] [CrossRef]
- Chang-Jian, C.W.; Cho, E.C.; Yen, S.C.; Ho, B.C.; Lee, K.C.; Huang, J.H.; Hsiao, Y.S. Facile preparation of WO3/PEDOT:PSS composite for inkjet printed electrochromic window and its performance for heat shielding. Dye. Pigment. 2018, 148, 465. [Google Scholar] [CrossRef]
- Eren, E.; Aydın, M.F.; Oksuz, A.U. A practical approach for generation of WO3-based flexible electrochromic devices. J. Solid State Electrochem. 2020, 24, 1057. [Google Scholar] [CrossRef]
- Nie, S.S.; Ning, C.Z.; Liu, Y.H.; Lian, Y.; Zhao, L.; Liu, Z.F. Enhanced electrochromic performance of WO3/PEDOT by π-electron conjugation system. Ceram. Int. 2024, 50, 12481. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Tang, K.; Cui, J.; Shu, X.; Wang, Y.; Liu, J.; Jiang, Y.; Tan, H.H.; Wu, Y. Designed growth of WO3/PEDOT core/shell hybrid nanorod arrays with modulated electrochromic properties. Chem. Eng. J. 2019, 355, 942. [Google Scholar] [CrossRef]
- Xiong, S.; Yin, S.; Wang, Y.; Kong, Z.; Lan, J.; Zhang, R.; Gong, M.; Wu, B.; Chu, J.; Wang, X. Organic/inorganic electrochromic nanocomposites with various interfacial interactions: A review. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2017, 221, 41. [Google Scholar] [CrossRef]
- Hu, J.; Chen, M.; Wu, L. Organic-inorganic nanocomposites synthesized via miniemulsion polymerization. Polym. Chem. 2011, 2, 760. [Google Scholar] [CrossRef]
- Yassin, A.; Oçafrain, M.; Blanchard, P.; Mallet, R.; Roncali, J. Synthesis of Hybrid Electroactive Materials by Low-Potential Electropolymerization of Gold Nanoparticles Capped with Tailored EDOT-Thiophene Precursor Units. ChemElectroChem 2014, 1, 1312. [Google Scholar] [CrossRef]
- Xu, J.; Tian, Y.; Peng, R.; Xian, Y.; Ran, Q.; Jin, L. Ferrocene clicked poly(3,4-ethylenedioxythiophene) conducting polymer: Characterization, electrochemical and electrochromic properties. Electrochem. Commun. 2009, 11, 1972. [Google Scholar] [CrossRef]
- Bu, X.H.; Feng, M.X.; Liu, Y.M.; Sun, B.W.; Zhou, Y.M.; Jiang, X.M. Novel WO3-PEDOT core–shell inverse opal films with enhanced electrochromic performance for smart windows. Funct. Mater. Lett. 2022, 3, 2250014. [Google Scholar]
- Xiong, S.; Phua, S.L.; Dunn, B.S.; Ma, J.; Lu, X. Covalently Bonded Polyaniline-TiO2 Hybrids: A Facile Approach to Highly Stable Anodic Electrochromic Materials with Low Oxidation Potentials. Chem. Mat. 2010, 22, 255. [Google Scholar] [CrossRef]
- Ma, L.; Cai, J.; Zhao, P.; Niu, H.; Wang, C.; Bai, X.; Wang, W. In situ preparation of composite from conjugated polyschiff bases and multiwalled carbon nanotube: Synthesis, electrochromic, acidochromic properties. Mater. Chem. Phys. 2012, 133, 333. [Google Scholar] [CrossRef]
- Ayranci, R.; Ak, M.; Karakus, M.; Cetisli, H. The effect of the monomer feed ratio and applied potential on copolymerization: Investigation of the copolymer formation of ferrocene-functionalized metallopolymer and EDOT. Des. Monomers Polym. 2016, 19, 545. [Google Scholar] [CrossRef]
- Ak, M.; Gacal, B.; Kiskan, B.; Yagci, Y.; Toppare, L. Enhancing electrochromic properties of polypyrrole by silsesquioxane nanocages. Polymer 2008, 49, 2202. [Google Scholar] [CrossRef]
- Cai, G.F.; Tu, J.P.; Zhou, D.; Zhang, J.H.; Wang, X.L.; Gu, C.D. Dual electrochromic film based on WO3/polyaniline core/shell nanowire array. Sol. Energy Mater. Sol. Cells 2014, 122, 51. [Google Scholar] [CrossRef]
- Yu, Y.H.; Lai, C.Y.; Chen, C.L.; Yeh, J.M. Durable electrochromic coatings prepared from electronically conductive poly(3HT-co-3TPP)-silica hybrid materials. J. Electron. Mater. 2006, 35, 1571. [Google Scholar] [CrossRef]
- Xiong, S.; Wei, J.; Jia, P.; Yang, L.; Ma, J.; Lu, X. Water-Processable Polyaniline with Covalently Bonded Single-Walled Carbon Nanotubes: Enhanced Electrochromic Properties and Impedance Analysis. ACS Appl. Mater. Interfaces 2011, 3, 782. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Yang, F.; Jiang, H.; Ma, J.; Lu, X. Covalently bonded polyaniline/fullerene hybrids with coral-like morphology for high-performance supercapacitor. Electrochim. Acta 2012, 85, 235. [Google Scholar] [CrossRef]
- Xiong, S.; Wang, R.; Li, S.; Wu, B.; Chu, J.; Wang, X.; Zhang, R.; Gong, M. Electrochromic Behaviors of Water-Soluble Polyaniline with Covalently Bonded Acetyl Ferrocene. J. Electron. Mater. 2018, 47, 3974. [Google Scholar] [CrossRef]
- Yang, Z.; Pu, H.; Yin, J. Preparation and electrochromic property of covalently bonded WO3/polyvinylimidazole core-shell microspheres. J. Colloid Interface Sci. 2005, 292, 108. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Feng, X.; Sloppy, J.D.; Guo, L.; Grimes, C.A. Vertically Aligned WO3 Nanowire Arrays Grown Directly on Transparent Conducting Oxide Coated Glass: Synthesis and Photoelectrochemical Properties. Nano Lett. 2011, 11, 203. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Shi, G.; Wang, H.; Zhang, Q.; Li, Y. Morphology-tailored synthesis of vertically aligned 1D WO3 nano-structure films for highly enhanced electrochromic performance. J. Mater. Chem. A 2013, 1, 684. [Google Scholar] [CrossRef]
- Santato, C.; Odziemkowski, M.; Ulmann, M.; Augustynski, J. Crystallographically oriented mesoporous WO3 films: Synthesis, characterization, and applications. J. Am. Chem. Soc. 2001, 123, 10639. [Google Scholar] [CrossRef] [PubMed]
- Garreau, S.; Louarn, G.; Buisson, J.P.; Froyer, G.; Lefrant, S. In Situ Spectroelectrochemical Raman Studies of Poly(3,4-ethylenedioxythiophene) (PEDT). Macromolecules 1999, 32, 6807. [Google Scholar] [CrossRef]
- Zanfrognini, B.; Colina, A.; Heras, A.; Zanardi, C.; Seeber, R.; López-Palacios, J. A UV–Visible/Raman spectroelectrochemical study of the stability of poly(3,4-ethylendioxythiophene) films. Polym. Degrad. Stabil. 2011, 96, 2112. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Tang, K.; Song, Y.; Cui, J.; Shu, X.; Wang, Y.; Liu, J.; Wu, Y. In situ growth of PEDOT/graphene oxide nanostructures with enhanced electrochromic performance. RSC Adv. 2018, 8, 13679. [Google Scholar] [CrossRef] [PubMed]
- Phuruangrat, A.; Ham, D.J.; Hong, S.J.; Thongtem, S.; Lee, J.S. Synthesis of hexagonal WO3 nanowires by microwave-assisted hydrothermal method and their electrocatalytic activities for hydrogen evolution reaction. J. Mater. Chem. 2010, 20, 683. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Zia ud, D.; Fei, P.; Jin, W.; Xiong, H.; Wang, Z. Enhancing the performance of starch-based wood adhesive by silane coupling agent(KH570). Int. J. Biol. Macromol. 2017, 104, 137. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Qin, X.; Liang, H.; Huang, S.; Lian, Z. Covalent functionalization of carbon fiber with poly(acrylamide) by reversible addition–fragmentation chain transfer polymerization for improving carbon fiber/epoxy interface. Polym. Compos. 2017, 38, 27. [Google Scholar] [CrossRef]
- Ohlan, A.; Singh, K.; Chandra, A.; Dhawan, S.K. Microwave Absorption Behavior of Core/Shell Structured Poly(3,4-Ethylenedioxy Thiophene)-Barium Ferrite Nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 927. [Google Scholar] [CrossRef] [PubMed]


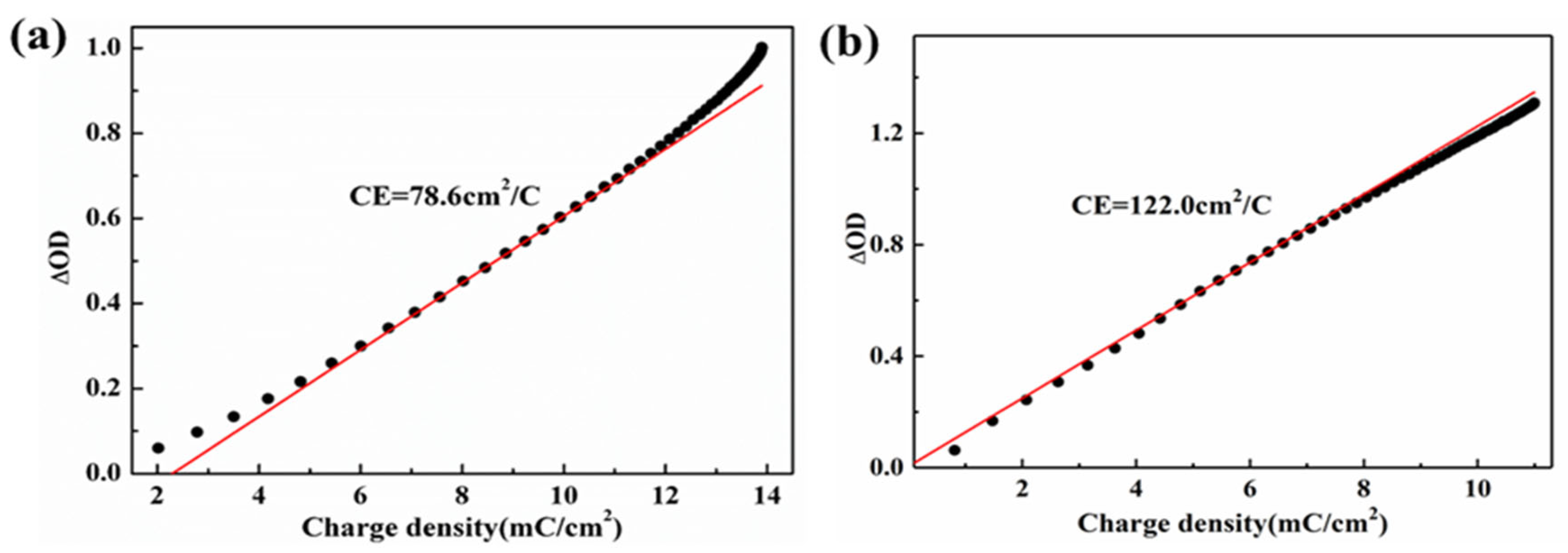
| Samples | Optical Modulation Range (%) 633 nm | Coloring Time (s) tc | Bleaching Time (s) tb |
|---|---|---|---|
| WO3 | 73.0 | 9.4 | 6.0 |
| PEDOT | 34.4 | 0.3 | 0.1 |
| WO3/PEDOT | 78.2 | 4.6 | 2.0 |
| c-WO3/PEDOT | 86.7 | 3.4 | 1.2 |
| Optical Contrast (%) | Coloring Time (s) | Bleaching Time (s) | Coloration Efficiency (cm2C−1) | Reference | |
|---|---|---|---|---|---|
| WO3/PEDOT nanoparticles | 58.0 | / | / | 84 | [15] |
| WO3·0.33H2O/PEDOT films | 50.9 | 32 | 12 | 74.6 | [16] |
| WO3/PEDOT printing films | 54.1 | 1.2 | 1.1 | 83.87 | [17] |
| WO3/PEDOT powders | 38.7 | 6.44 | 5.33 | / | [18] |
| WO3/PEDOT inverse opal films | 52.0 | 6.7 | 5.8 s | / | [25] |
| WO3/PEDOT nanowires | 68.2 | 22.4 | 26.0 | 109.9 | [19] |
| WO3/PEDOT nanowires | 72.0 | 3.8 | 3.6 | 163.5 | [20] |
| WO3/PEDOT nanowires | 86.7 | 3.4 | 1.2 | 122.0 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Cao, Y.; Chen, C.; Zhang, X. Designed Growth of Covalently Bonded WO3/PEDOT Hybrid Nanorods Array with Enhanced Electrochromic Performance. Materials 2024, 17, 3319. https://doi.org/10.3390/ma17133319
Zhang Q, Cao Y, Chen C, Zhang X. Designed Growth of Covalently Bonded WO3/PEDOT Hybrid Nanorods Array with Enhanced Electrochromic Performance. Materials. 2024; 17(13):3319. https://doi.org/10.3390/ma17133319
Chicago/Turabian StyleZhang, Qing, Yinhuan Cao, Chuansheng Chen, and Xueru Zhang. 2024. "Designed Growth of Covalently Bonded WO3/PEDOT Hybrid Nanorods Array with Enhanced Electrochromic Performance" Materials 17, no. 13: 3319. https://doi.org/10.3390/ma17133319
APA StyleZhang, Q., Cao, Y., Chen, C., & Zhang, X. (2024). Designed Growth of Covalently Bonded WO3/PEDOT Hybrid Nanorods Array with Enhanced Electrochromic Performance. Materials, 17(13), 3319. https://doi.org/10.3390/ma17133319





