PHB+aPHA Blends: From Polymer Bacterial Synthesis through Blend Preparation to Final Processing by Extrusion for Sustainable Materials Design
Abstract
1. Introduction
2. Materials
2.1. P(3HB) Synthesis
2.2. Solvent Casting (STEP I)
2.3. Extrusion Process (STEP II)
3. Methods
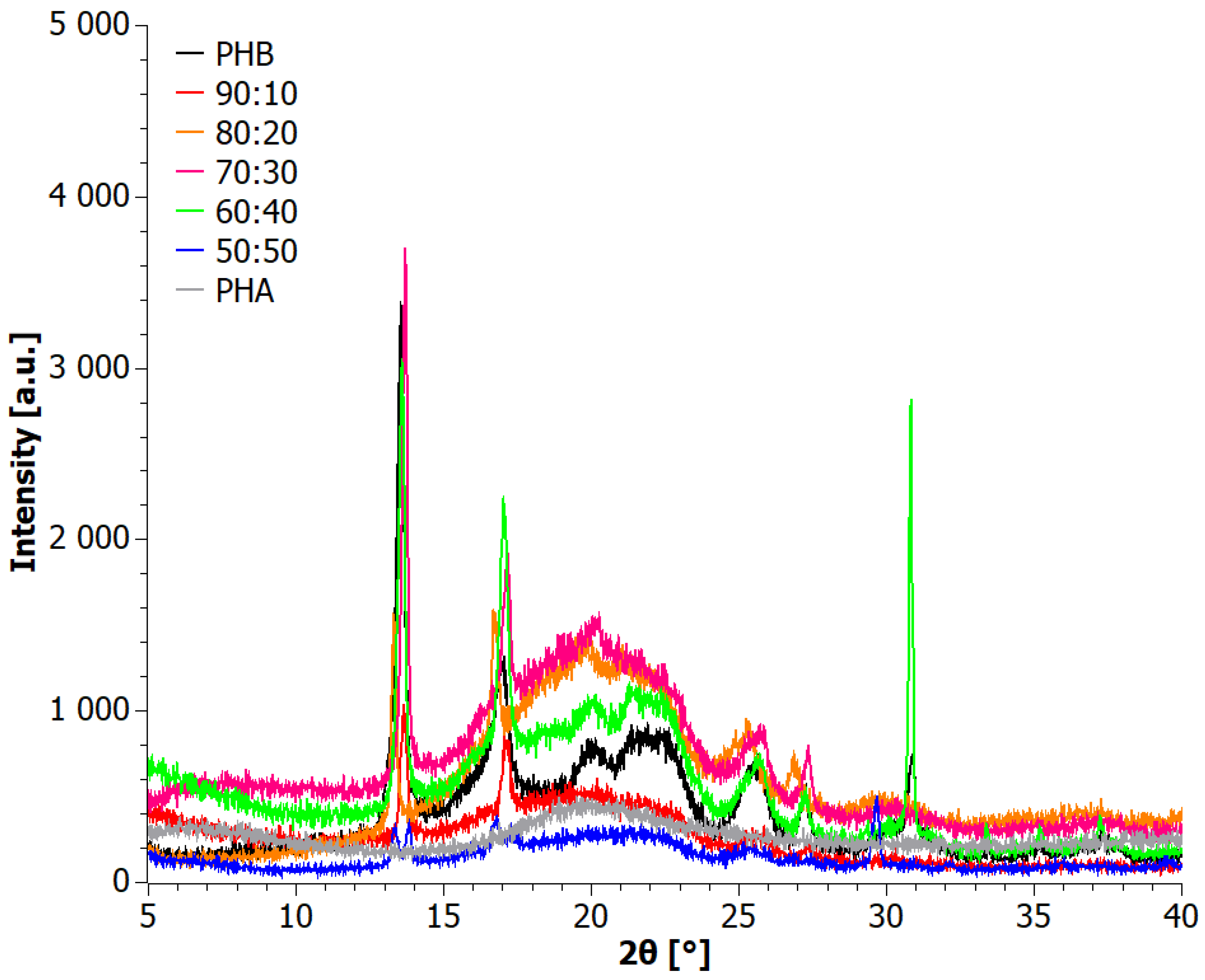
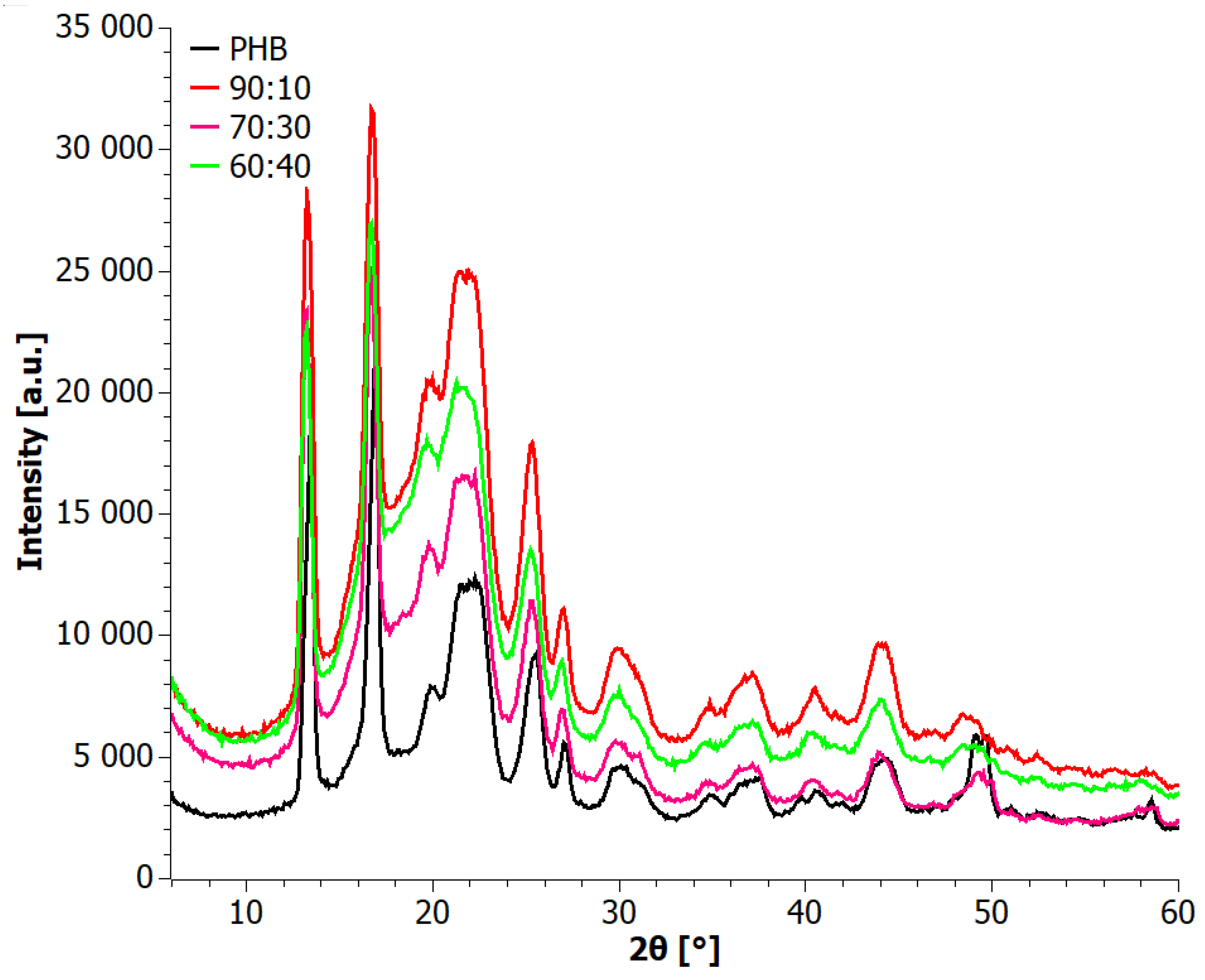
3.1. X-ray Diffraction (XRD) Analysis
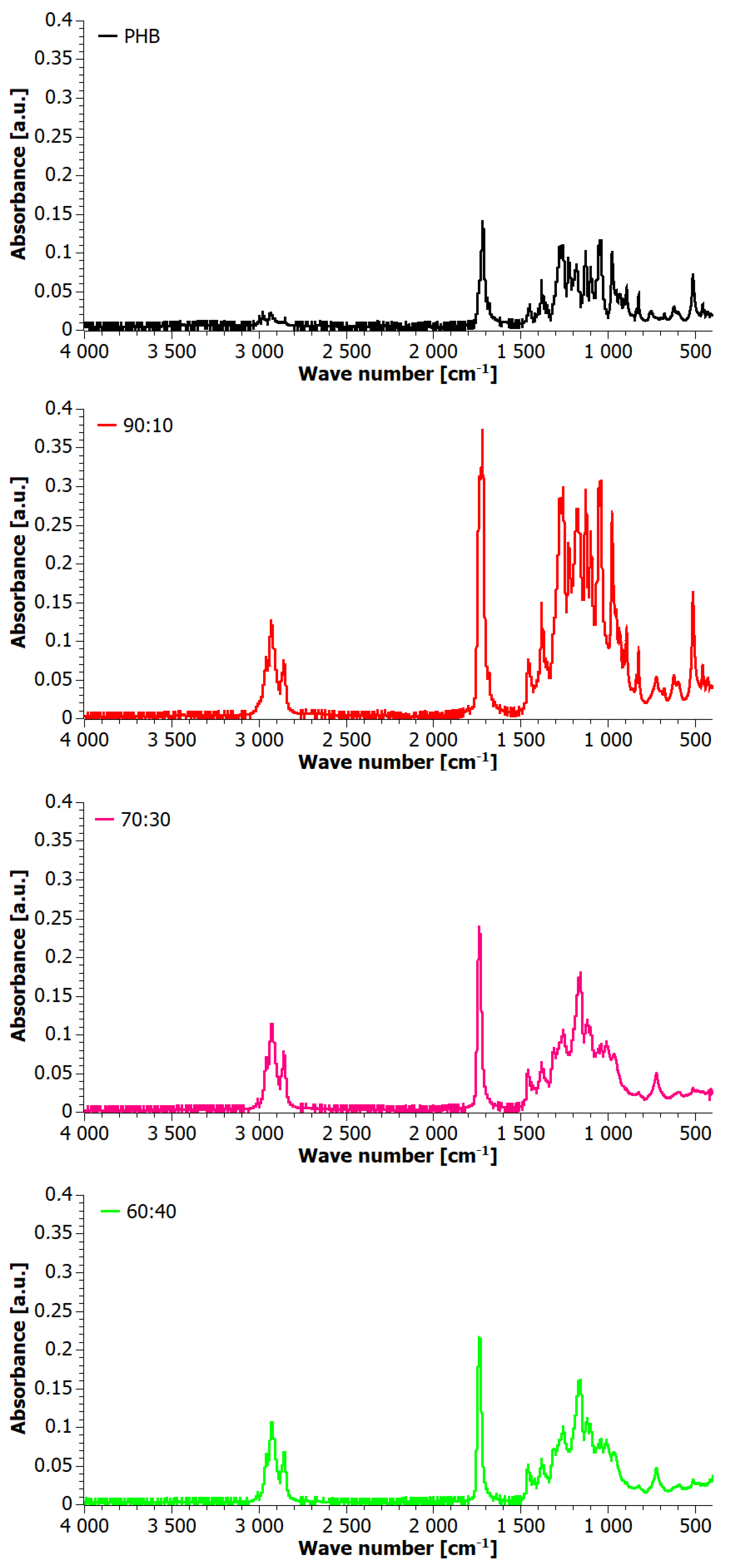
3.2. Infrared Spectrometry (FTIR)
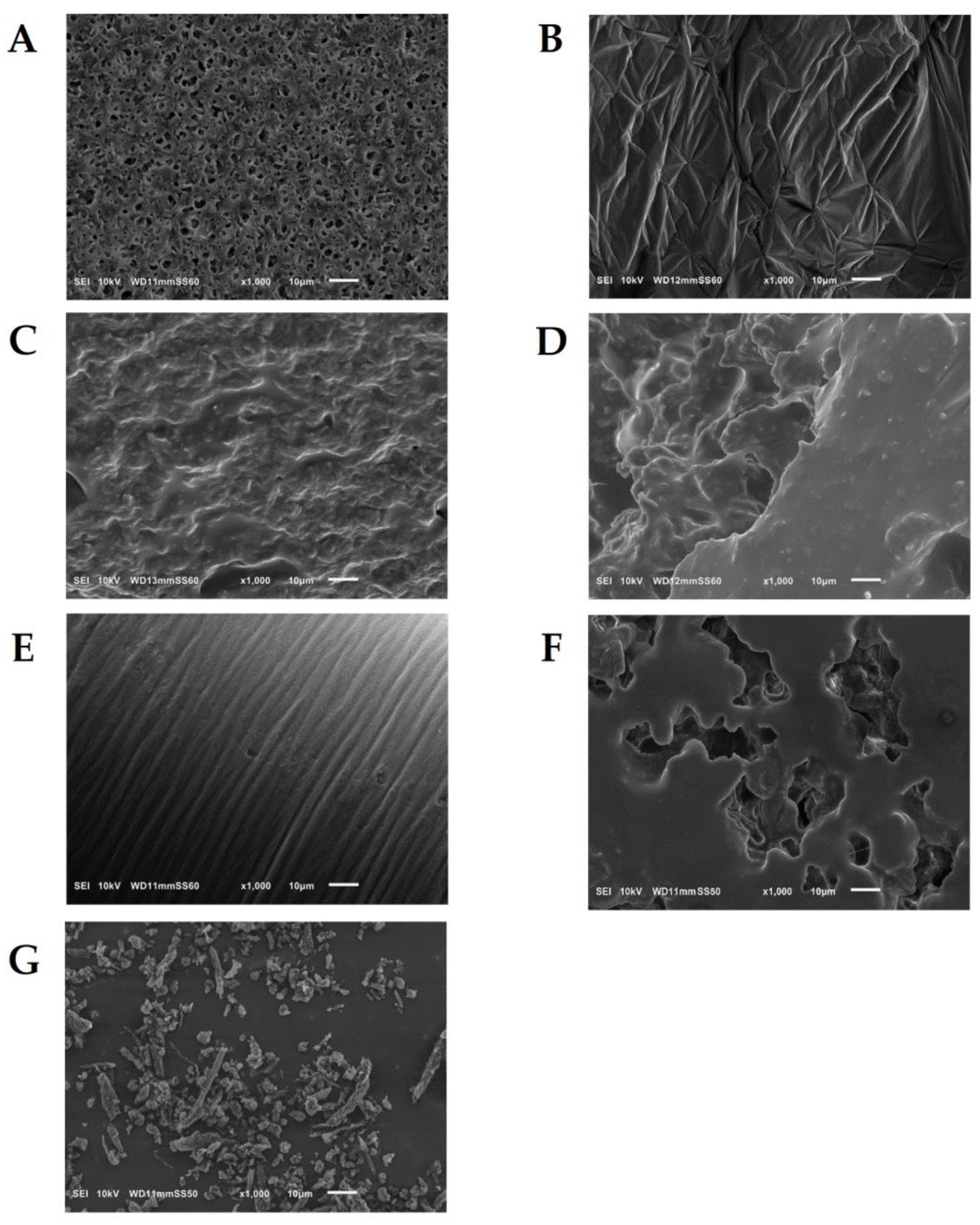
3.3. Scanning Electron Microscopy (SEM)
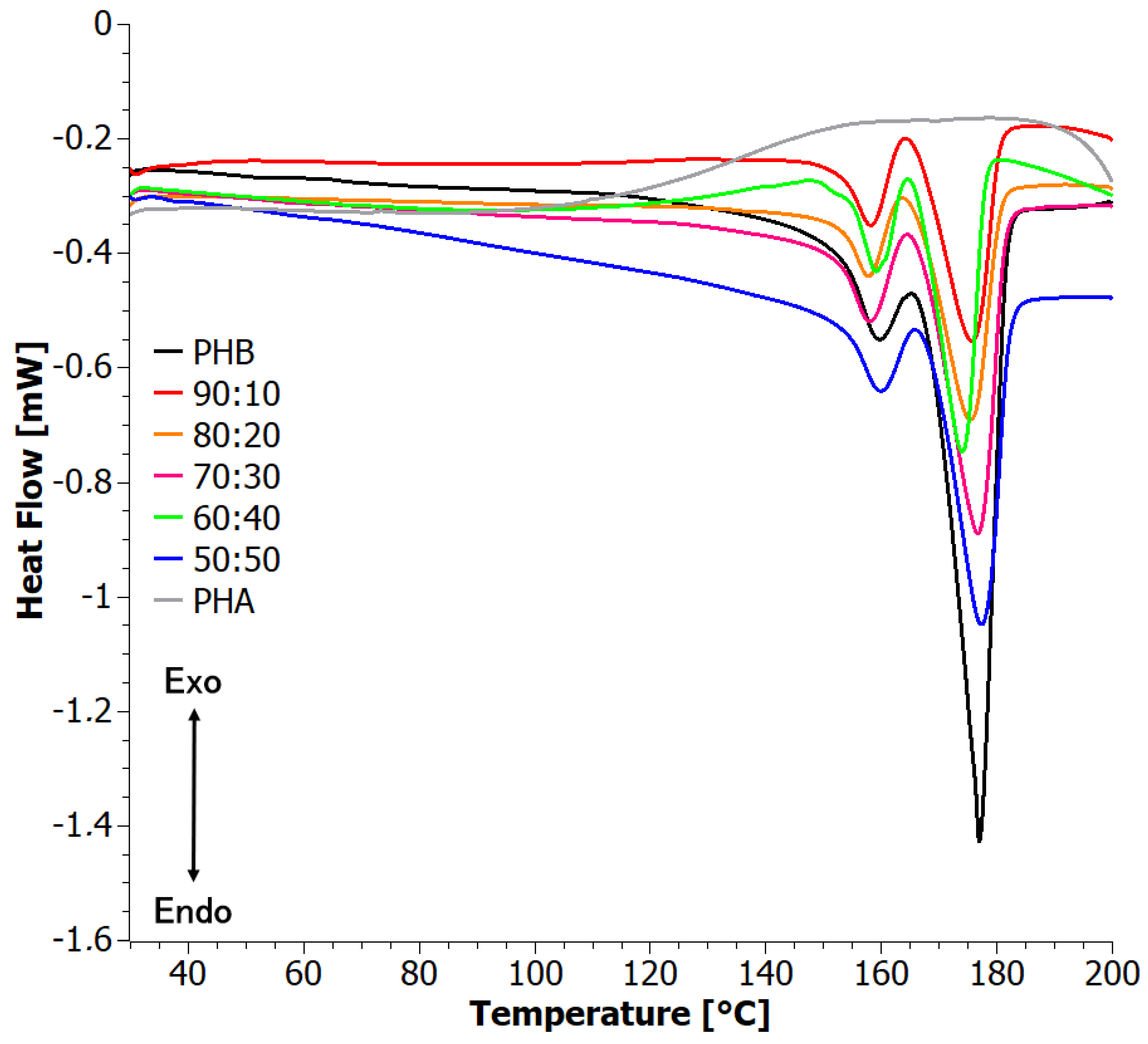
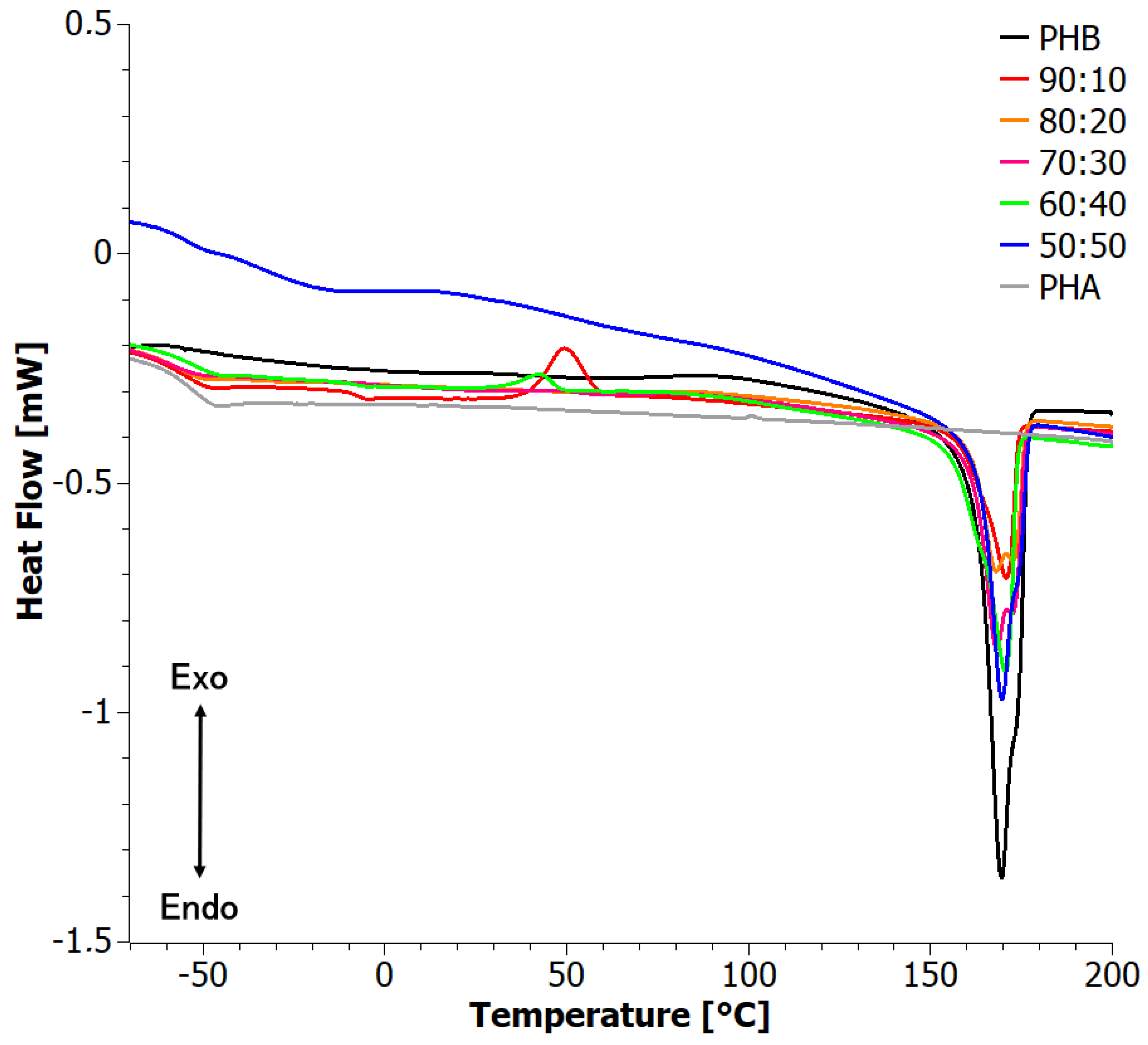
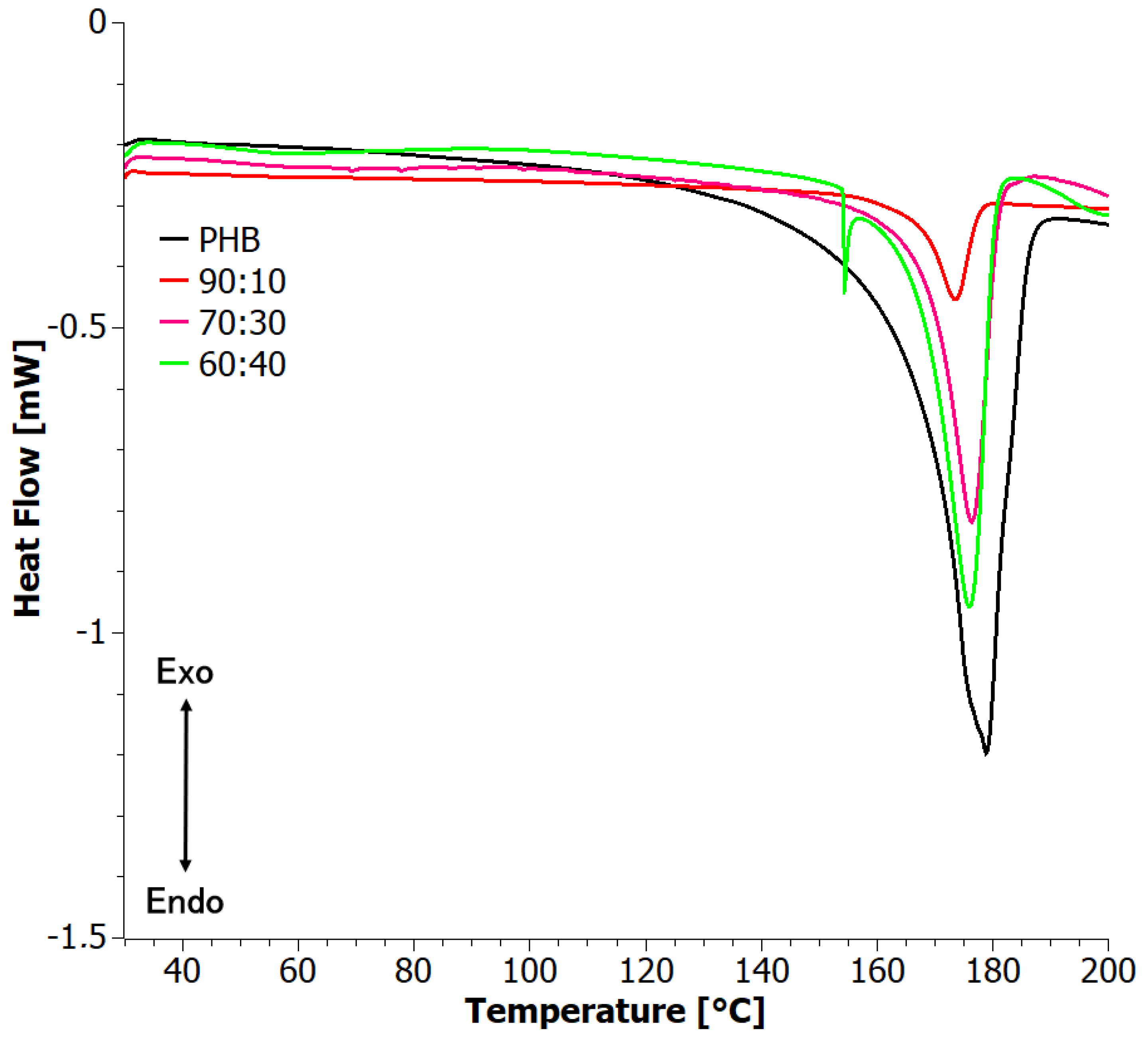
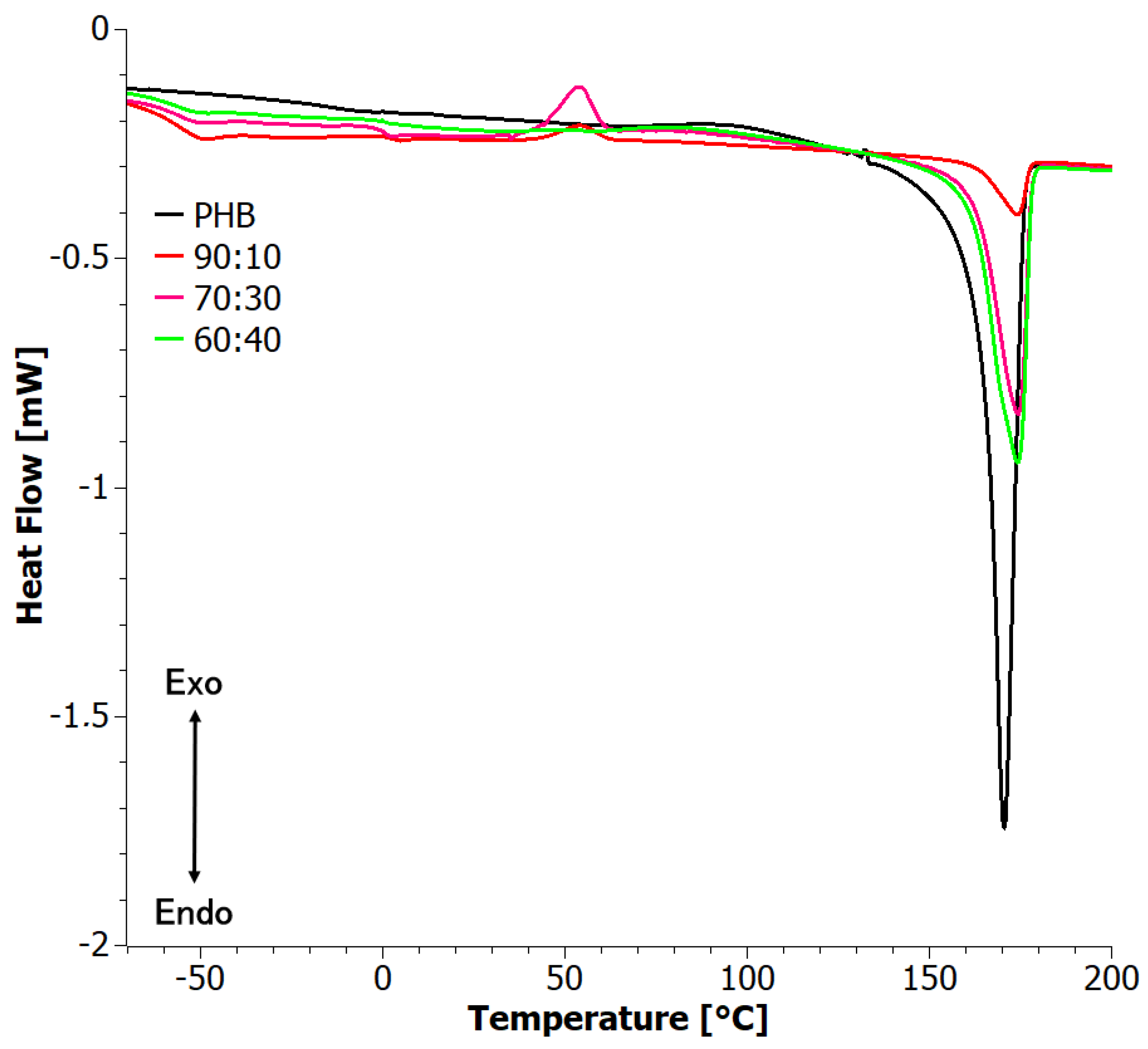
3.4. Difference Scanning Calorimetry (DSC)
- -
- is the degree of crystallinity of PHB, i.e., the mass of crystalline PHB of the total mass of PHB.
- -
- is the degree of crystallinity of the sample, i.e., the mass of crystalline PHB per unit mass of the sample.
- -
- is the melting enthalpy of the sample, normalized to its mass.
- -
- is the melting enthalpy of the 100% crystalline polymer, taken here as 146 J/g [36].
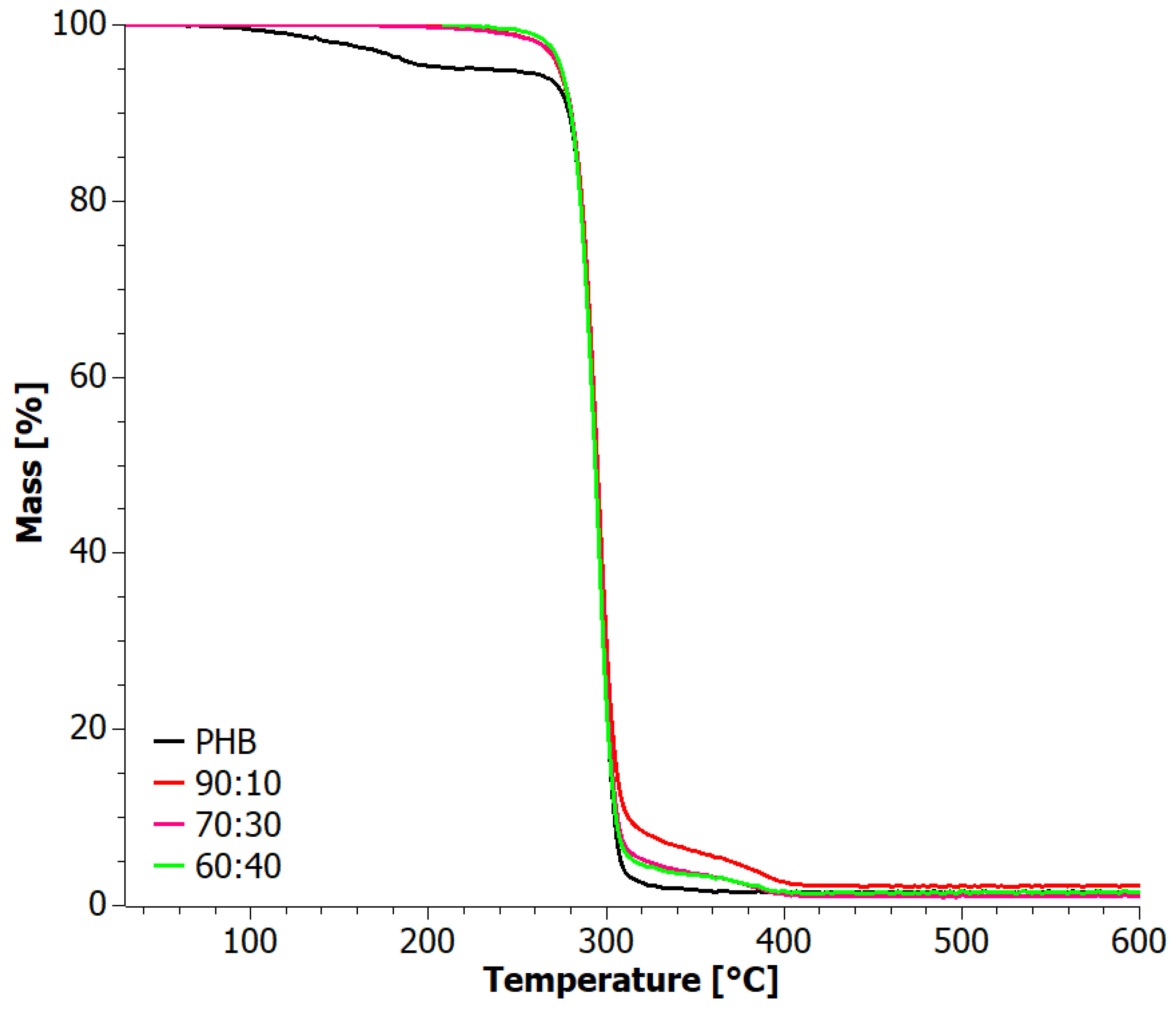
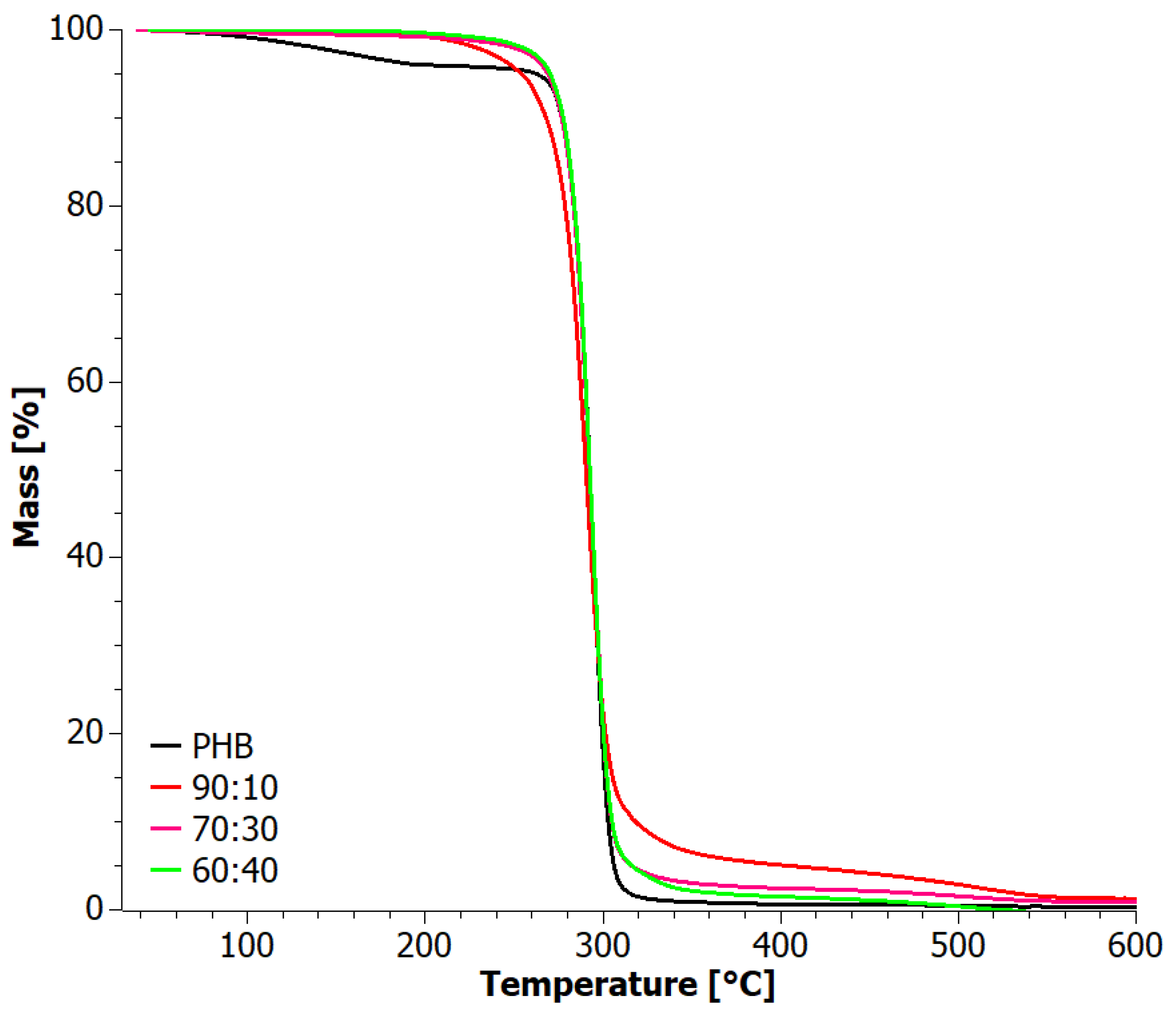
3.5. Thermograwimetry (TG)
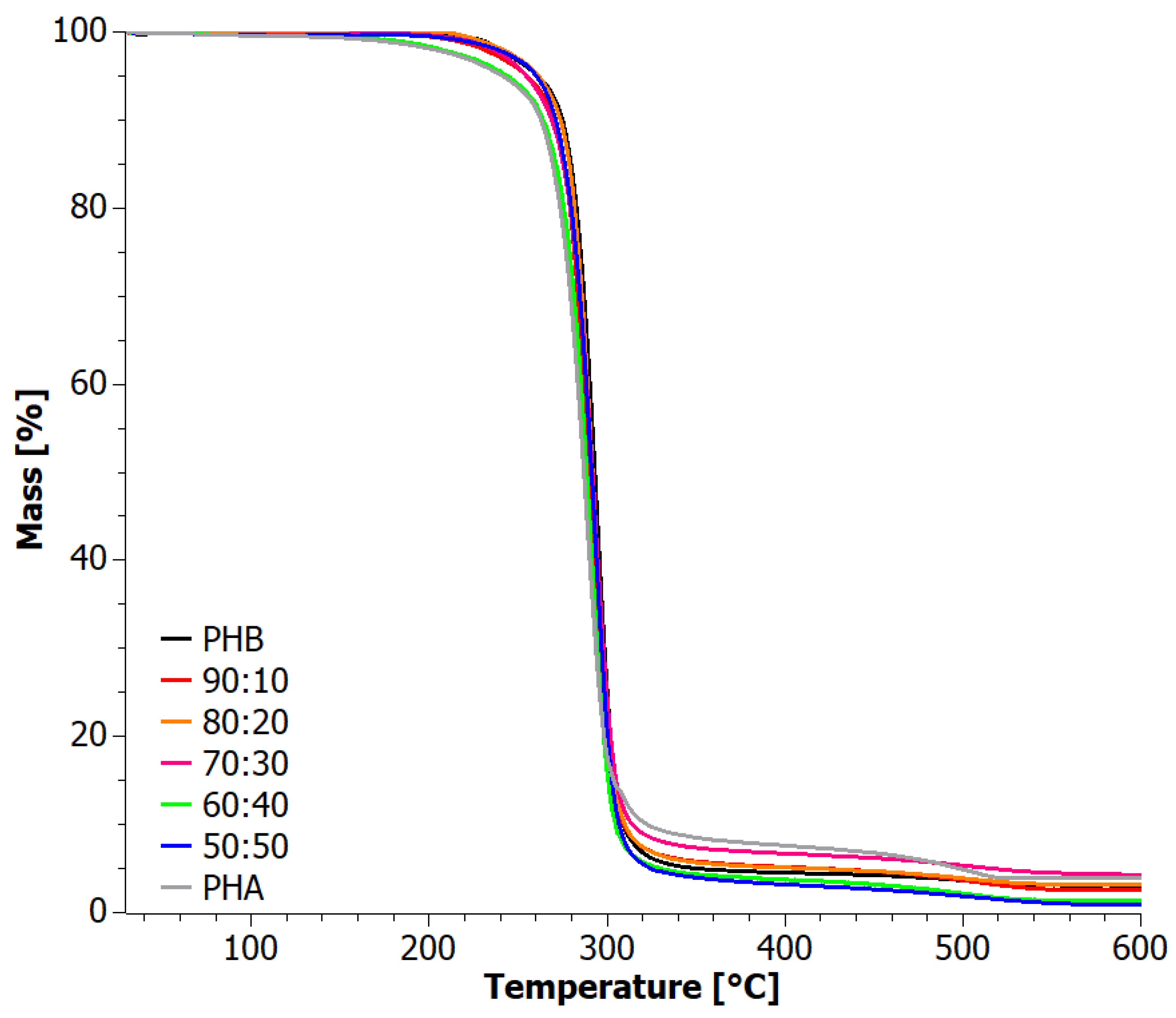
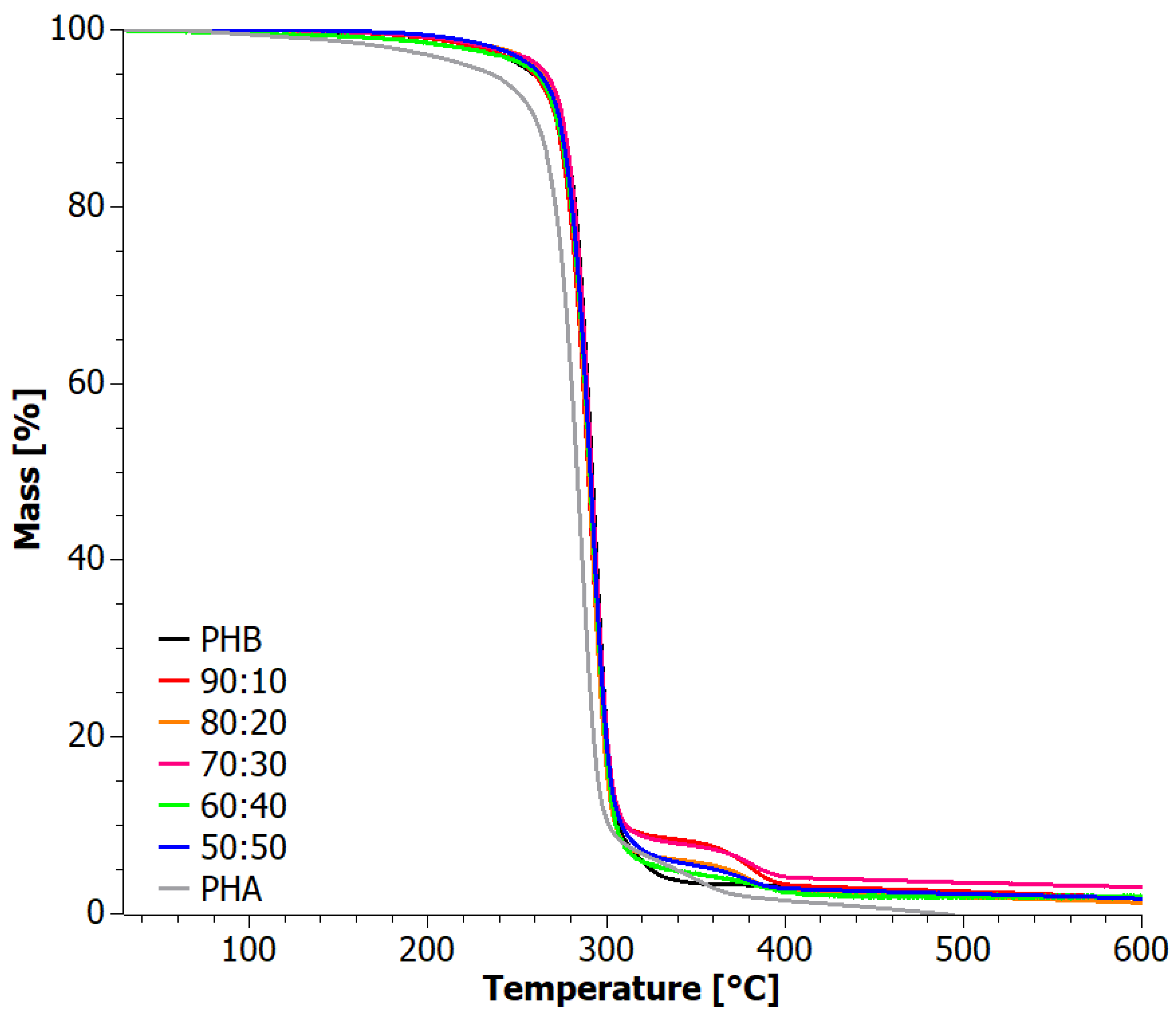
4. Results
4.1. Analysis of Materials Obtained in Step I
4.2. Analysis of Materials Obtained in Step II
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guzik, M.; Witko, T.; Steinbüchel, A.; Wojnarowska, M.; Sołtysik, M.; Wawak, S. What Has Been Trending in the Research of Polyhydroxyalkanoates? A Systematic Review. Front. Bioeng. Biotechnol. 2020, 8, 572988. [Google Scholar] [CrossRef]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current Progress on Bio-Based Polymers and Their Future Trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef]
- Yee, L.H.; Foster, L.J.R. Polyhydroxyalkanoates as Packaging Materials: Current Applications and Future Prospects. RSC Green Chem. 2014, 2015, 183–207. [Google Scholar] [CrossRef]
- Valappil, S.P.; Misra, S.K.; Boccaccini, A.; Roy, I. Biomedical Applications of Polyhydroxyalkanoates, an Overview of Animal Testing and in Vivo Responses. Expert Rev. Med. Devices 2006, 3, 853–868. [Google Scholar] [CrossRef]
- Nigmatullin, R.; Thomas, P.; Lukasiewicz, B.; Puthussery, H.; Roy, I. Polyhydroxyalkanoates, a Family of Natural Polymers, and Their Applications in Drug Delivery. J. Chem. Technol. Biotechnol. 2015, 90, 1209–1221. [Google Scholar] [CrossRef]
- Kuperkar, K.; Atanase, L.I.; Bahadur, A.; Crivei, I.C.; Bahadur, P. Degradable Polymeric Bio(Nano)Materials and Their Biomedical Applications: A Comprehensive Overview and Recent Updates. Polymers 2024, 16, 206. [Google Scholar] [CrossRef]
- Adamus, G.; Kurcok, P.; Radecka, I.; Kowalczuk, M. Bioactive Oligomers from Natural Polyhydroxyalkanoates and Their Synthetic Analogues. Polimery 2017, 62, 317–322. [Google Scholar] [CrossRef]
- Emaimo, A.J.; Olkhov, A.A.; Ragoubi, M.; Becquart, F.; Koubaa, A.; Emaimo, A.J.; Olkhov, A.A.; Iordanskii, A.L.; Vetcher, A.A. Polyhydroxyalkanoates Composites and Blends: Improved Properties and New Applications. J. Compos. Sci. 2022, 6, 206. [Google Scholar] [CrossRef]
- Hou, X.; Sun, W.; Liu, Z.; Liu, S.; Yeo, J.C.C.; Lu, X.; He, C. Tailoring Crystalline Morphology via Entropy-Driven Miscibility: Toward Ultratough, Biodegradable, and Durable Polyhydroxybutyrate. Macromolecules 2022, 55, 5527–5534. [Google Scholar] [CrossRef]
- Grassie, N.; Murray, E.J.; Holmes, P.A. The Thermal Degradation of Poly(-(d)-β-Hydroxybutyric Acid): Part 2—Changes in Molecular Weight. Polym. Degrad. Stab. 1984, 6, 95–103. [Google Scholar] [CrossRef]
- Han, L.; Han, C.; Zhang, H.; Chen, S.; Dong, L. Morphology and Properties of Biodegradable and Biosourced Polylactide Blends with Poly(3-Hydroxybutyrate-Co-4-Hydroxybutyrate). Polym. Compos. 2012, 33, 850–859. [Google Scholar] [CrossRef]
- Larsson, M.; Hetherington, C.J.D.; Wallenberg, R.; Jannasch, P. Effect of Hydrophobically Modified Graphene Oxide on the Properties of Poly(3-Hydroxybutyrate-Co-4-Hydroxybutyrate). Polymer 2017, 108, 66–77. [Google Scholar] [CrossRef]
- Weng, Y.X.; Wang, L.; Zhang, M.; Wang, X.L.; Wang, Y.Z. Biodegradation Behavior of P(3HB,4HB)/PLA Blends in Real Soil Environments. Polym. Test. 2013, 32, 60–70. [Google Scholar] [CrossRef]
- Min Song, H.; Chan Joo, J.; Hyun Lim, S.; Jin Lim, H.; Lee, S.; Jae Park, S. Production of Polyhydroxyalkanoates Containing Monomers Conferring Amorphous and Elastomeric Properties from Renewable Resources: Current Status and Future Perspectives. Bioresour. Technol. 2022, 366, 128114. [Google Scholar] [CrossRef]
- Crétois, R.; Chenal, J.M.; Sheibat-Othman, N.; Monnier, A.; Martin, C.; Astruz, O.; Kurusu, R.; Demarquette, N.R. Physical Explanations about the Improvement of PolyHydroxyButyrate Ductility: Hidden Effect of Plasticizer on Physical Ageing. Polymer 2016, 102, 176–182. [Google Scholar] [CrossRef]
- Han, G.; Yoon, J.; Lee, C.; Lee, E.; Yoon, K.; Kwak, H.W.; Jin, H.J. Biodegradation Behavior of Amorphous Polyhydroxyalkanoate-Incorporated Poly(l-Lactic Acid) under Modulated Home-Composting Conditions. Polym. Test. 2023, 126, 108155. [Google Scholar] [CrossRef]
- El-Hadi, A.; Schnabel, R.; Straube, E.; Müller, G.; Henning, S. Correlation between Degree of Crystallinity, Morphology, Glass Temperature, Mechanical Properties and Biodegradation of Poly (3-Hydroxyalkanoate) PHAs and Their Blends. Polym. Test. 2002, 21, 665–674. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, P. Crystallization of Biodegradable and Biobased Polyesters: Polymorphism, Cocrystallization, and Structure-Property Relationship. Prog. Polym. Sci. 2020, 109, 101291. [Google Scholar] [CrossRef]
- Phongtamrug, S.; Tashiro, K. X-ray Crystal Structure Analysis of Poly(3-Hydroxybutyrate) β-Form and the Proposition of a Mechanism of the Stress-Induced α-to-β Phase Transition. Macromolecules 2019, 52, 2995–3009. [Google Scholar] [CrossRef]
- Popa, M.S.; Frone, A.N.; Panaitescu, D.M. Polyhydroxybutyrate Blends: A Solution for Biodegradable Packaging? Int. J. Biol. Macromol. 2022, 207, 263–277. [Google Scholar] [CrossRef]
- Shahzad, K.; Narodoslawsky, M.; Sagir, M.; Ali, N.; Ali, S.; Rashid, M.I.; Ismail, I.M.I.; Koller, M. Techno-Economic Feasibility of Waste Biorefinery: Using Slaughtering Waste Streams as Starting Material for Biopolyester Production. Waste Manag. 2017, 67, 73–85. [Google Scholar] [CrossRef]
- Koller, M.; Braunegg, G. Advanced Approaches to Produce Polyhydroxyalkanoate (PHA) Biopolyesters in a Sustainable and Economic Fashion. Eurobiotech J. 2018, 2, 89–103. [Google Scholar] [CrossRef]
- Nitkiewicz, T.; Wojnarowska, M.; Sołtysik, M.; Kaczmarski, A.; Witko, T.; Ingrao, C.; Guzik, M. How Sustainable Are Biopolymers? Findings from a Life Cycle Assessment of Polyhydroxyalkanoate Production from Rapeseed-Oil Derivatives. Sci. Total Environ. 2020, 749, 141279. [Google Scholar] [CrossRef]
- Czechowska, J.; Skibiński, S.; Guzik, M.; Zima, A. Silver Decorated ΒTCP-Poly(3hydroxybutyrate) Scaffolds for Bone Tissue Engineering. Materials 2021, 14, 4227. [Google Scholar] [CrossRef]
- Guzik, M.W.; Duane, G.F.; Kenny, S.T.; Casey, E.; Mielcarek, P.; Wojnarowska, M.; O’Connor, K.E. A Polyhydroxyalkanoates Bioprocess Improvement Case Study Based on Four Fed-Batch Feeding Strategies. Microb. Biotechnol. 2022, 15, 996–1006. [Google Scholar] [CrossRef]
- Tanikkul, P.; Sullivan, G.L.; Sarp, S.; Pisutpaisal, N. Biosynthesis of Medium Chain Length Polyhydroxyalkanoates (Mcl-PHAs) from Palm Oil. Case Stud. Chem. Environ. Eng. 2020, 2, 100045. [Google Scholar] [CrossRef]
- Jana, R.N.; Mukunda, P.G.; Nando, G.B. Thermogravimetric Analysis of Compatibilized Blends of Low Density Polyethylene and Poly(Dimethyl Siloxane) Rubber. Polym. Degrad. Stab. 2003, 80, 75–82. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Processing of Poly(Lactic Acid): Characterization of Chemical Structure, Thermal Stability and Mechanical Properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Sofińska, K.; Barbasz, J.; Witko, T.; Dryzek, J.; Haraźna, K.; Witko, M.; Kryściak-Czerwenka, J.; Guzik, M. Structural, Topographical, and Mechanical Characteristics of Purified Polyhydroxyoctanoate Polymer. J. Appl. Polym. Sci. 2019, 136, 47192. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Y.; Chen, G.Q. Medical Application of Microbial Biopolyesters Polyhydroxyalkanoates. Artif. Cells Blood Substit. Biotechnol. 2009, 37, 1–12. [Google Scholar] [CrossRef]
- Asrar, J.; Valentin, H.E.; Berger, P.A.; Tran, M.; Padgette, S.R.; Garbow, J.R. Biosynthesis and Properties of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) Polymers. Biomacromolecules 2002, 3, 1006–1012. [Google Scholar] [CrossRef]
- Fang, Q.; Hanna, M.A. Rheological Properties of Amorphous and Semicrystalline Polylactic Acid Polymers. Ind. Crops Prod. 1999, 10, 47–53. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, Mechanical and Morphological Characterization of Plasticized PLA–PHB Blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Narancic, T.; Verstichel, S.; Reddy Chaganti, S.; Morales-Gamez, L.; Kenny, S.T.; De Wilde, B.; Babu Padamati, R.; O’Connor, K.E. Biodegradable Plastic Blends Create New Possibilities for End-of-Life Management of Plastics but They Are Not a Panacea for Plastic Pollution. Environ. Sci. Technol. 2018, 52, 10441–10452. [Google Scholar] [CrossRef]
- Szczegóły PAT—P.434423. Available online: https://ewyszukiwarka.pue.uprp.gov.pl/search/pwp-details/P.434423?lng=pl (accessed on 10 April 2024).
- Barham, P.J.; Keller, A.; Otun, E.L.; Holmes, P.A. Crystallization and Morphology of a Bacterial Thermoplastic: Poly-3-Hydroxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. [Google Scholar] [CrossRef]
- Mottina, A.C.; Ayres, E.; Orefice, R.L.; Câmara, J.J.D. What Changes in Poly(3-Hydroxybutyrate) (PHB) When Processed as Electrospun Nanofibers or Thermo-Compression Molded Film? Mater. Res. 2016, 19, 57–66. [Google Scholar] [CrossRef]
- Canetti, M.; Urso, M.; Sadocco, P. Influence of the Morphology and of the Supermolecular Structure on the Enzymatic Degradation of Bacterial Poly(3-Hydroxybutyrate). Polymer 1999, 40, 2587–2594. [Google Scholar] [CrossRef]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The Chemomechanical Properties of Microbial Polyhydroxyalkanoates. Prog. Polym. Sci. 2014, 39, 397–442. [Google Scholar] [CrossRef]
- Lauritzen, J.I.; Hoffman, J.D. Theory of Formation of Polymer Crystals with Folded Chains in Dilute Solution. J. Res. Natl. Bur. Stand. A Phys. Chem. 1960, 64A, 73. [Google Scholar] [CrossRef]
- El-Taweel, S.H.; Höhne, G.W.H.; Mansour, A.A.; Stoll, B.; Seliger, H. Glass Transition and the Rigid Amorphous Phase in Semicrystalline Blends of Bacterial Polyhydroxybutyrate PHB with Low Molecular Mass Atactic R, S-PHB-Diol. Polymer 2004, 45, 983–992. [Google Scholar] [CrossRef]
- Klonos, P.; Terzopoulou, Z.; Koutsoumpis, S.; Zidropoulos, S.; Kripotou, S.; Papageorgiou, G.Z.; Bikiaris, D.N.; Kyritsis, A.; Pissis, P. Rigid Amorphous Fraction and Segmental Dynamics in Nanocomposites Based on Poly(l–Lactic Acid) and Nano-Inclusions of 1–3D Geometry Studied by Thermal and Dielectric Techniques. Eur. Polym. J. 2016, 82, 16–34. [Google Scholar] [CrossRef]
- El-Hadi, A.M.; El-Hadi, A.; Schnabel, R.; Straube, E.; Müller, G.; Riemschneider, M. Effect of Melt Processing on Crystallization Behavior and Rheology of Poly(3-Hydroxybutyrate) (PHB) and Its Blends. Macromol. Mater. Eng. 2002, 287, 363–372. [Google Scholar] [CrossRef]
- Furukawa, T.; Sato, H.; Murakami, R.; Zhang, J.; Duan, Y.X.; Noda, I.; Ochiai, S.; Ozaki, Y. Structure, Dispersibility, and Crystallinity of Poly(Hydroxybutyrate)/Poly(l-Lactic Acid) Blends Studied by FT-IR Microspectroscopy and Differential Scanning Calorimetry. Macromolecules 2005, 38, 6445–6454. [Google Scholar] [CrossRef]
| First Heating | Second Heating | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Tm1 (°C) | Tm2 (°C) | ΔH (J/g) | χBlend | χPHB | Tg,PHB (°C) | Tg,PHA (°C) | Tm (°C) | ΔHm (J/g) | χBlend | χPHB |
| PHB | 159.5 | 176.9 | 68.6 | 0.47 | 0.47 | --- | --- | 169.1 | 63.7 | 0.44 | 0.44 |
| 90:10 | 158.2 | 175.7 | 25.9 | 0.18 | 0.20 | −6.5 | −55.2 | 171.1 | 18.6 | 0.13 | 0.14 |
| 80:20 | 157.8 | 175.5 | 25.7 | 0.18 | 0.22 | --- | −56.8 | 168.2 | 23.3 | 0.16 | 0.20 |
| 70:30 | 157.8 | 176.8 | 35.2 | 0.24 | 0.34 | --- | −58.8 | 168.4 | 32.3 | 0.22 | 0.32 |
| 60:40 | 159.3 | 174.0 | 30.4 | 0.21 | 0.35 | −7.9 | −51.8 | 170.2 | 29.2 | 0.20 | 0.33 |
| 50:50 | 159.7 | 177.3 | 38.3 | 0.26 | 0.52 | --- | −55.3 | 170.3 | 31.9 | 0.22 | 0.44 |
| PHA | --- | --- | --- | --- | --- | --- | -54.0 | --- | --- | --- | --- |
| Sample | TOnset (°C) | TEndset (°C) | DTG First Peak (°C) | DTG Second Peak (°C) | Residue (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ox | Inert | Ox | Inert | Ox | Inert | Ox | Inert | Ox | Inert | |
| PHA | 271.6 | 269.4 | 299.6 | 295.1 | 289.1 | 286.1 | 509.1 | 348.0 | 3.89 | 0 |
| 50:50 | 277.6 | 278.6 | 303.6 | 302.1 | 293.5 | 292.2 | --- | 376.8 | 0.85 | 1.69 |
| 60:40 | 274.3 | 279.6 | 301.2 | 307.1 | 291.3 | 292.2 | --- | 382.4 | 1.40 | 2.00 |
| 70:30 | 276.6 | 279.3 | 304.2 | 302.1 | 293.7 | 292.6 | --- | 383.8 | 4.27 | 2.99 |
| 80:20 | 277.7 | 277.0 | 303.9 | 300.9 | 293.1 | 291.5 | --- | 383.8 | 3.22 | 1.28 |
| 90:10 | 275.5 | 276.3 | 302.9 | 300.7 | 291.7 | 291.1 | --- | 381.2 | 2.66 | 1.78 |
| PHB | 289.6 | 279.7 | 304.8 | 303.5 | 294.8 | 292.9 | --- | --- | 3.09 | 1.72 |
| Sample | First Heating | Second Heating | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tm (°C] | ΔHm (J/g) | χblend | χPHB | Tg,PHB (°C) | Tg,PHA (°C) | Tm (°C) | ΔHm (J/g) | χblend | χPHB | |
| 90:10 | 167.2 | 7.3 | 0.05 | 0.06 | 1.0 | −56.2 | 174.0 | 6.5 | 0.04 | 0.05 |
| 70:30 | 176.4 | 38.2 | 0.26 | 0.37 | −0.1 | −56.2 | 174.2 | 35.6 | 0.24 | 0.35 |
| 60:40 | 175.9 | 43.8 | 0.30 | 0.50 | 1.3 | −56.5 | 174.1 | 45.0 | 0.31 | 0.51 |
| Sample | TOnset (°C) | TEndset (°C) | DTG 1st Peak (°C) | DTG 2nd Peak (°C) | Residue (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ox | Inert | Ox | Inert | Ox | Inert | Ox | Inert | Ox | Inert | |
| 60:40 | 281.3 | 282.4 | 303.6 | 304.1 | 293.2 | 296.0 | 509.6 | 387.8 | 0.0 | 1.5 |
| 70:30 | 280.7 | 282.7 | 303.7 | 304.6 | 293.9 | 296.1 | 493.3 | 387.5 | 0.9 | 1.1 |
| 90:10 | 275.4 | 283.0 | 303.3 | 305.1 | 291.6 | 296.0 | 508.8 | 387.6 | 1.3 | 2.3 |
| PHB | 282.7 | 284.4 | 303.0 | 304.7 | 149.5 | 138.6 | 294.0 | 296.3 | 0.3 | 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majka, T.M.; Raftopoulos, K.N.; Hebda, E.; Szeligowski, A.; Zastawny, O.; Guzik, M.; Pielichowski, K. PHB+aPHA Blends: From Polymer Bacterial Synthesis through Blend Preparation to Final Processing by Extrusion for Sustainable Materials Design. Materials 2024, 17, 3105. https://doi.org/10.3390/ma17133105
Majka TM, Raftopoulos KN, Hebda E, Szeligowski A, Zastawny O, Guzik M, Pielichowski K. PHB+aPHA Blends: From Polymer Bacterial Synthesis through Blend Preparation to Final Processing by Extrusion for Sustainable Materials Design. Materials. 2024; 17(13):3105. https://doi.org/10.3390/ma17133105
Chicago/Turabian StyleMajka, Tomasz M., Konstantinos N. Raftopoulos, Edyta Hebda, Adam Szeligowski, Olga Zastawny, Maciej Guzik, and Krzysztof Pielichowski. 2024. "PHB+aPHA Blends: From Polymer Bacterial Synthesis through Blend Preparation to Final Processing by Extrusion for Sustainable Materials Design" Materials 17, no. 13: 3105. https://doi.org/10.3390/ma17133105
APA StyleMajka, T. M., Raftopoulos, K. N., Hebda, E., Szeligowski, A., Zastawny, O., Guzik, M., & Pielichowski, K. (2024). PHB+aPHA Blends: From Polymer Bacterial Synthesis through Blend Preparation to Final Processing by Extrusion for Sustainable Materials Design. Materials, 17(13), 3105. https://doi.org/10.3390/ma17133105








