Preparation of Expanded Graphite-VO2 Composite Cathode Material and Performance in Aqueous Zinc-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of Composite Cathode Materials
2.3. Characterization and Testing Methods
3. Results
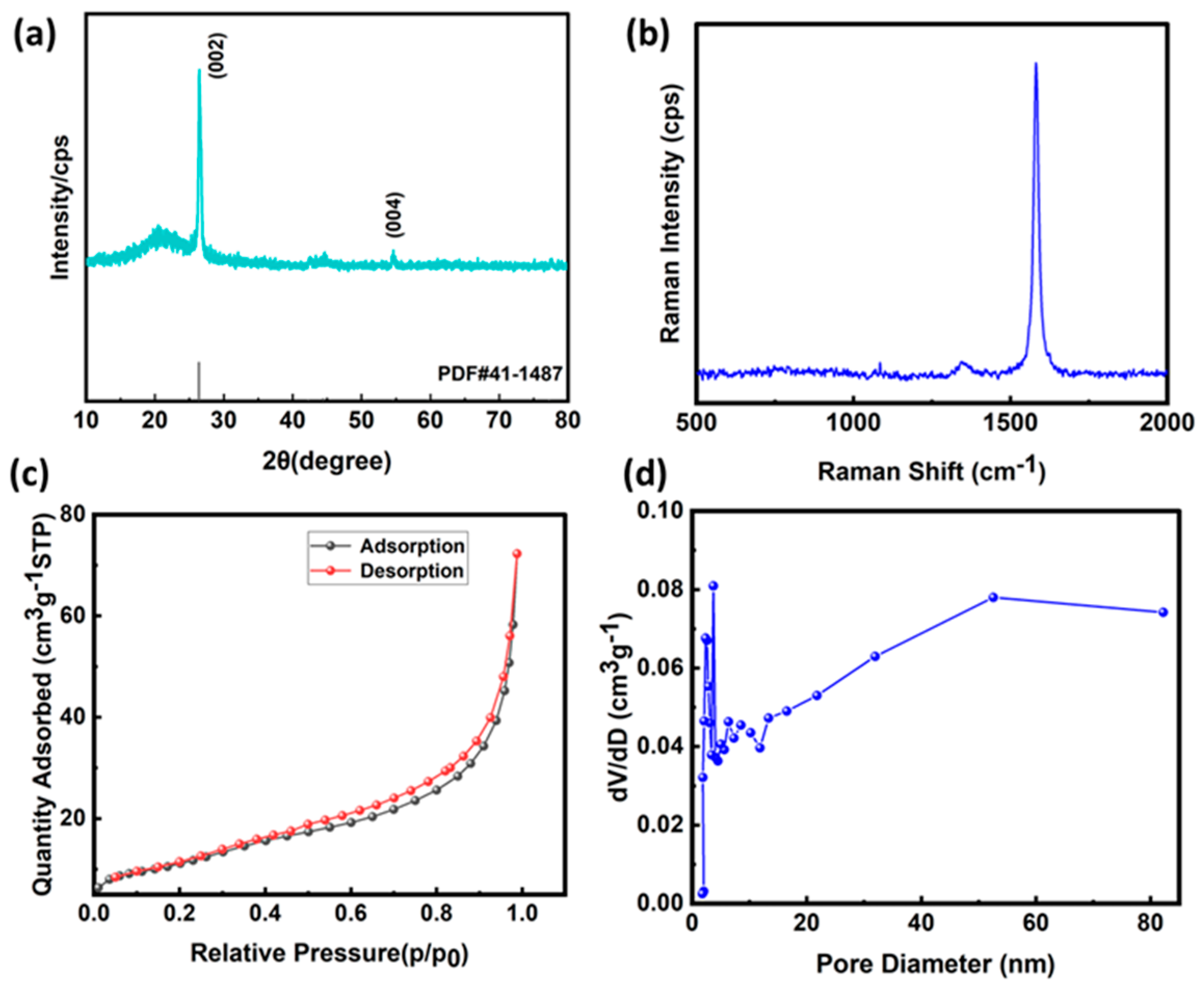
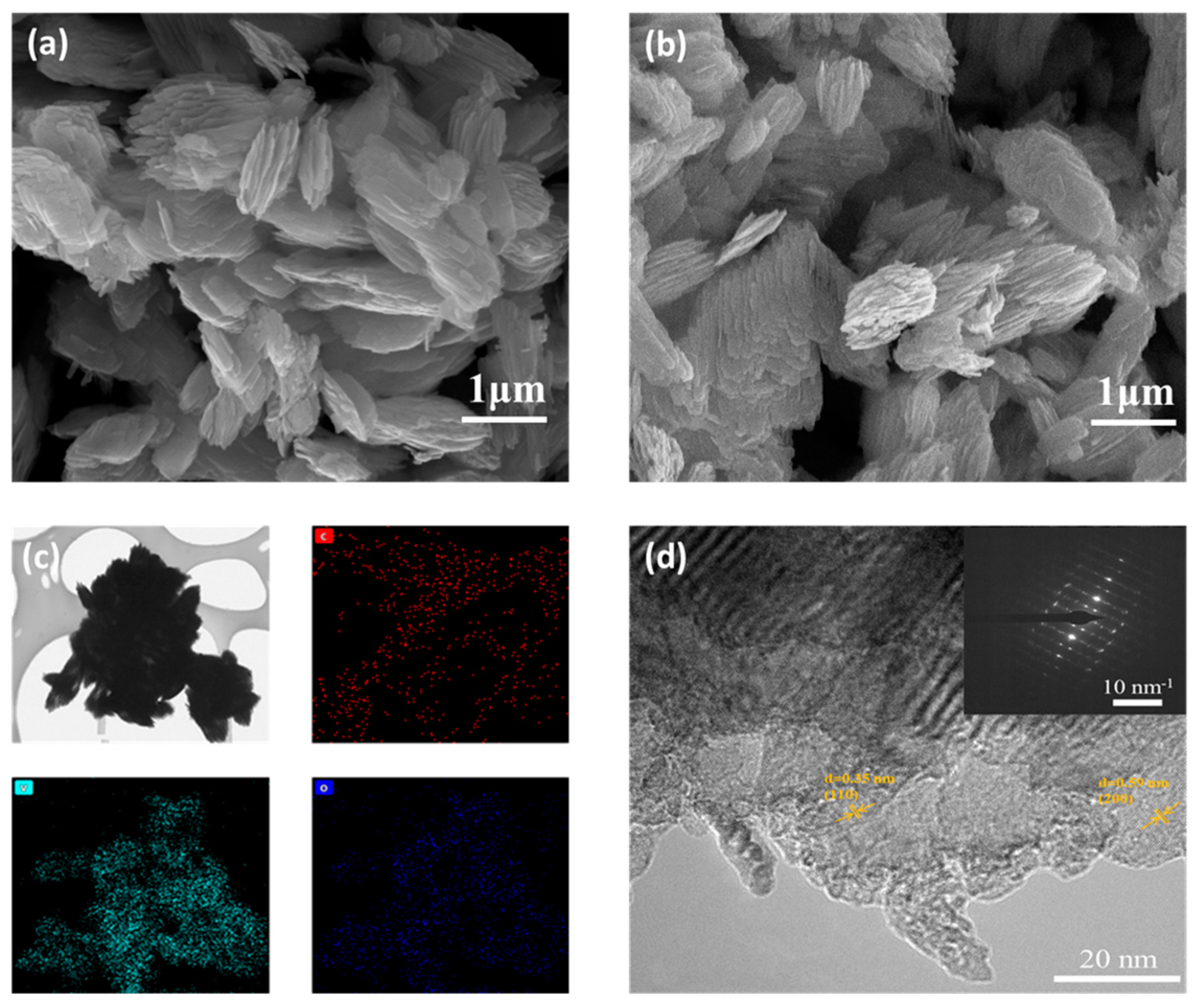
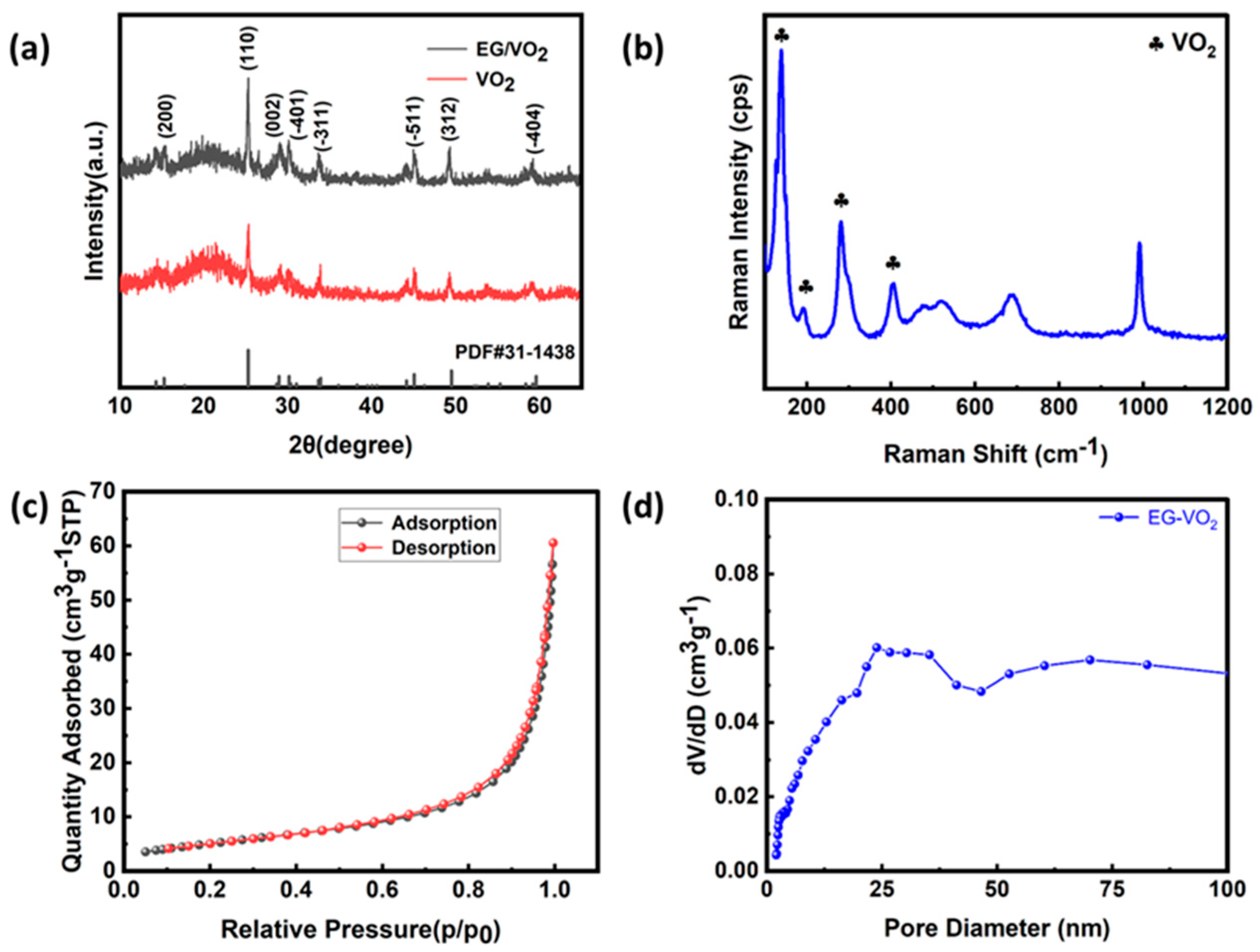
3.1. Characterization
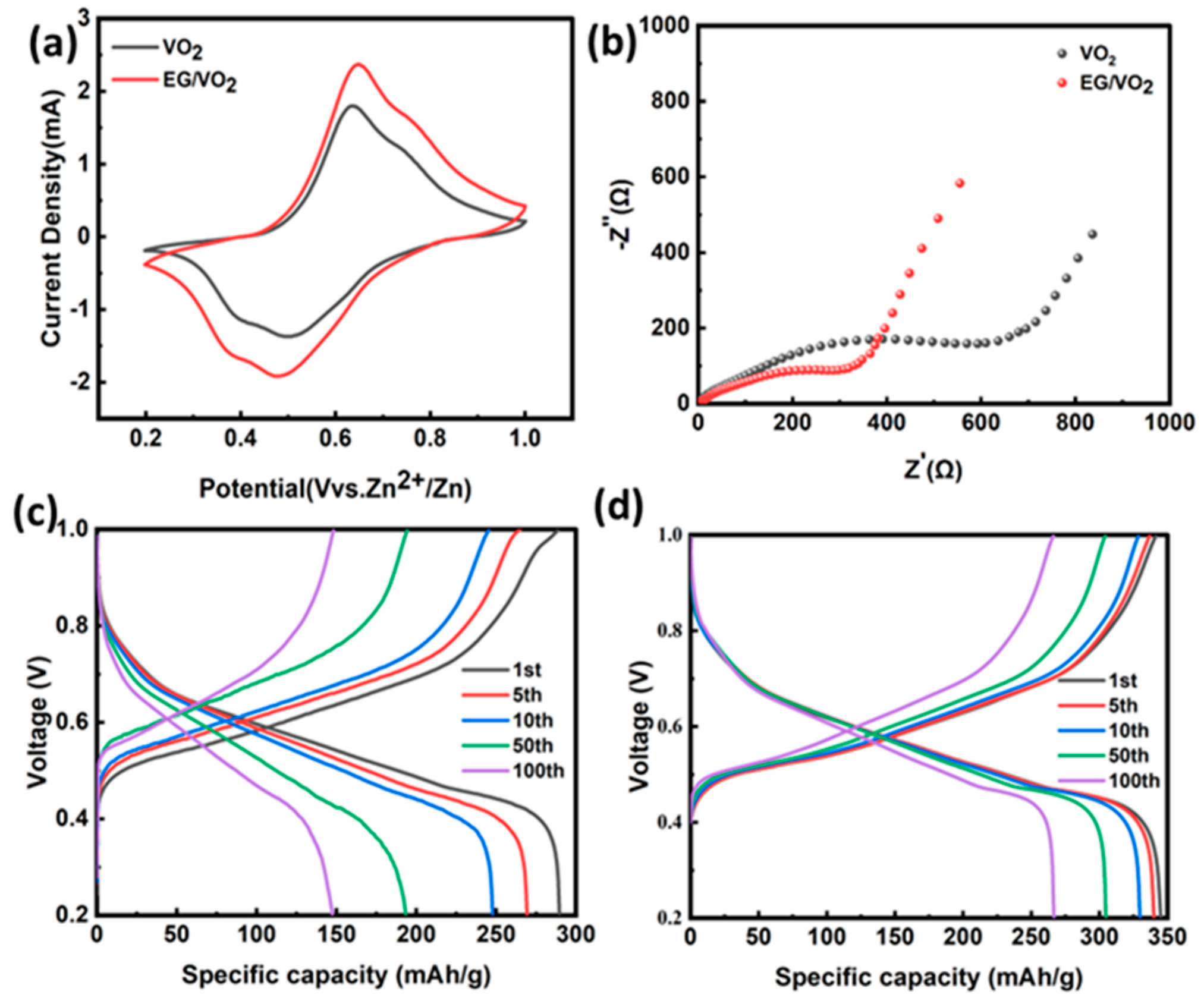
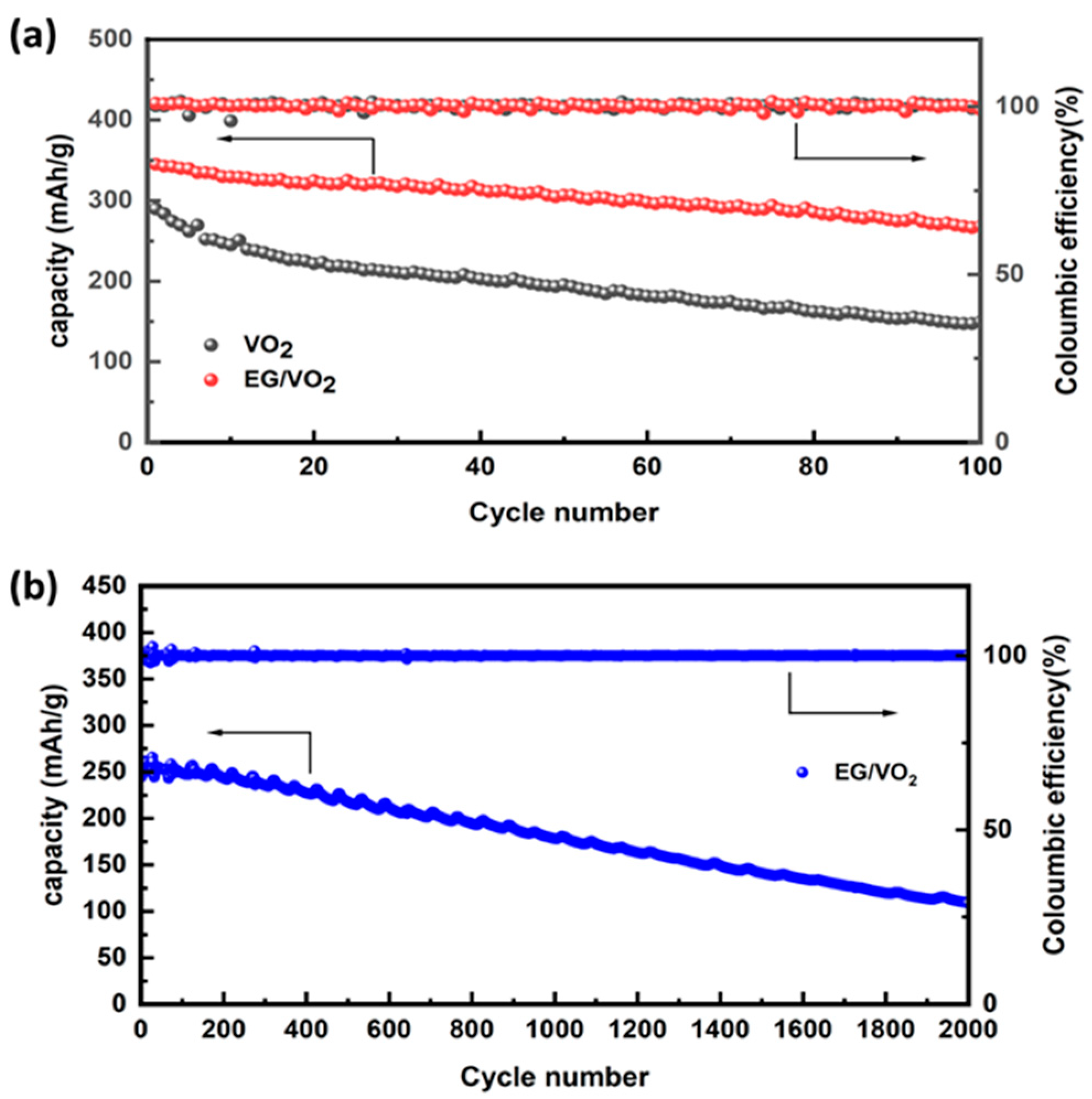
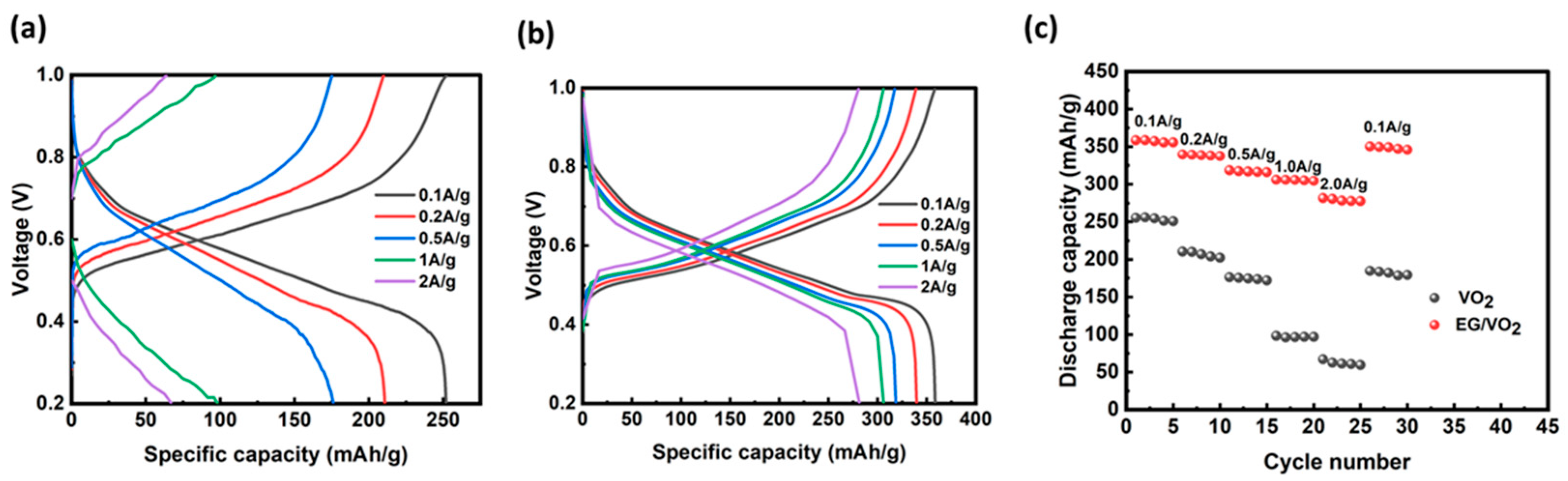
3.2. Electrochemical Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, Y.; Sang, Z.; Liu, D.; Zhang, T.; Feng, J.; Si, W.; Dou, S.X.; Liang, J.; Hou, F. Lithiophilic and conductive V2O3/VN nanosheets as regulating layer for high-rate, high-areal capacity and dendrite-free lithium metal anodes. Chem. Eng. J. 2021, 420, 129787. [Google Scholar]
- Song, M.; Tan, H.; Chao, D.; Fan, H.J. Recent advances in Zn-ion batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Cai, K.; Luo, S.; Feng, J.; Wang, J.; Zhan, Y.; Wang, Q.; Zhang, Y.; Liu, X. Recent Advances on Spinel Zinc Manganate Cathode Materials for Zinc-Ion Batteries. Chem. Rec. 2022, 22, e202100169. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; You, Z.; Wei, C.; Kang, F.; Wei, B. Rechargeable aqueous zinc-ion batteries: Mechanism, design strategies and future perspectives. Mater. Today 2021, 42, 73–98. [Google Scholar] [CrossRef]
- Park, S.; Shim, H.; Lee, W.C.; Song, H.J.; Park, I.J.; Kim, J.C.; Hong, K.S.; Kim, D.W. Tailoring uniform γ-MnO2 nanosheets on highly conductive three-dimensional current collectors for high-performance supercapacitor electrodes. Nano Res. 2015, 8, 990–1004. [Google Scholar]
- Wu, B.; Zhang, G.; Yan, M.; Xiong, T.; He, P.; He, L.; Xu, X.; Mai, L. Graphene scroll-coated α-MnO2 nanowires as high-performance cathode materials for aqueous Zn-ion battery. Small 2018, 14, 1703850. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ba, D.; Li, Y.; Liu, J. Noninterference revealing of layered to layered zinc storage mechanism of δ-MnO2 toward neutral Zn-Mn batteries with superior performance. Adv. Sci. 2020, 7, 1902795. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.; Liu, J.; Wang, L.; Long, X.; Liu, X.; Li, F.; Chen, J. Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities. Nat. Commun. 2017, 8, 652–657. [Google Scholar] [CrossRef]
- Zhang, Z.; Shang, H.; Zhang, X.; Liu, C.; Li, S.; Wen, Z.; Ji, S.; Sun, J. Enhancing the electrochemical performances by wet ball milling to introduce structural water into an electrolytic MnO2/graphite nanocomposite cathode for zinc-ion batteries. ACS Appl. Energy Mater. 2021, 4, 5113–5122. [Google Scholar] [CrossRef]
- Solveig, K.; Iulian, D.; Aref, M.; Wagemaker, M.; Iversen, B.B.; Bentien, A. Strategies for synthesis of Prussian blue analogues. R. Soc. Open Sci. 2021, 8, 201779. [Google Scholar]
- Ding, J.; Gao, H.; Zhao, K.; Zheng, H.; Zhang, H.; Han, L.; Wang, S.; Wu, S.; Fang, S.; Cheng, F. In-situ electrochemical conversion of vanadium dioxide for enhanced zinc-ion storage with large voltage range. J. Power Sources 2021, 487, 229369. [Google Scholar] [CrossRef]
- Zampardi, G.; La Mantia, F. Prussian blue analogues as aqueous Zn-ion batteries electrodes: Current challenges and future perspectives. Curr. Opin. Electrochem. 2020, 21, 84–92. [Google Scholar] [CrossRef]
- Li, M.; Rosalinda, S.; Mariam, M.; Aquilanti, G.; Plaisier, J.; Berrettoni, M.; Giorgetti, M. Electrochemical performance of manganese hexacyanoferrate cathode material in aqueous Zn-ion battery. Electrochim. Acta 2021, 400, 139414. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, G.; Wang, X.; Liu, J.P.; Zeng, Q.Y.; Guo, X.T.; Wang, H.; Liu, C.S.; Pang, H. Tuning electronic structure of ultrathin V6O13 nanobelts via nickel doping for aqueous zinc-ion battery cathodes. Chem. Eng. J. 2022, 428, 132538. [Google Scholar] [CrossRef]
- Zhou, J.; Shan, L.; Wu, Z.; Fang, G.; Liang, S. Investigation of V2O5 as a low-cost rechargeable aqueous zinc ion battery cathode. Chem. Commun. 2018, 54, 4457–4460. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Singh, P.; Ansari, S.A.; Manippady, S.R.; Jaiswal, A.; Saxena, M. VO2 nanostructures for batteries and supercapacitors: A review. Small 2021, 17, 2006651. [Google Scholar]
- Li, Z.; Ren, Y.; Mo, L.; Hsu, K.; Ding, Y.; Zhang, X.; Li, X.; Hu, L.; Ji, D.; Cao, G. Impacts of oxygen vacancies on zinc ion intercalation in VO2. ACS Nano 2020, 14, 5581–5589. [Google Scholar] [CrossRef]
- Selvakumaran, D.; Pan, A.; Liang, S.; Cao, J. A review on recent developments and challenges of cathode materials for rechargeable aqueous Zn-ion batteries. J. Mater. Chem. A 2019, 7, 18209–18236. [Google Scholar] [CrossRef]
- Zhang, N.; Dong, Y.; Jia, M.; Zhang, N.; Dong, Y.; Jia, M.; Bian, X.; Wang, Y.; Qiu, M.; Xu, J.; et al. Rechargeable aqueous Zn–V2O5 battery with high energy density and long cycle life. ACS Energy Lett. 2018, 3, 1366–1372. [Google Scholar] [CrossRef]
- Yan, M.; He, P.; Mai, L.; Wang, S.; Wei, Q.; Zhao, K.; Xu, X.; An, Q.; Shuang, Y.; Shao, Y.; et al. Water-lubricated intercalation in V2O5·nH2O for high-capacity and high-rate aqueous rechargeable zinc batteries. Adv. Mater. 2018, 30, 1703725. [Google Scholar]
- Pang, Q.; He, W.; Yu, X.; Yang, S.; Zhao, H.; Fu, Y.; Xing, M.; Tian, Y.; Luo, X.; Wei, Y. Aluminium pre-intercalated orthorhombic V2O5 as high-performance cathode material for aqueous zinc-ion batteries. Appl. Surf. Sci. 2021, 538, 148043. [Google Scholar]
- Ding, J.; Du, Z.; Gu, L.; Li, B.; Wang, L.; Wang, S.; Gong, T.; Yang, S. Ultrafast Zn2+ intercalation and deintercalation in vanadium dioxide. Adv. Mater. 2018, 30, 1800762. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Chua, R.; Kou, Z.; Ren, H.; Yuan, D.; Huang, S.; Kumar, S.; Verma, V.; Amonpattaratkit, P.; Srinivasan, M. Boosting Zn-ion storage performance of bronze-type VO2 via Ni-mediated electronic structure engineering. ACS Appl. Mater. Interfaces 2020, 12, 36110–36118. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Z.; Yang, W.; Han, L.; Wei, M. One-step synthesis of porous V2O3@C hollow spheres as a high-performance anode for lithium-ion batteries. Chem. Commun. 2018, 54, 7346–7349. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Chu, H.; Zhang, H.; Ge, Y.; Clemente, R.; Dong, P.; Wang, L.; Shen, J.; Ye, M.; Ajayan, P.M. An in situ electrochemical oxidation strategy for formation of nanogrid-shaped V3O7·H2O with enhanced zinc storage properties. J. Mater. Chem. A 2019, 7, 25262–25267. [Google Scholar]
- Guan, K.; Duan, K.; Yang, G.; Tao, L.; Zhang, H.; Wan, H.; Yang, R.; Zhang, J.; Wang, H.; Wang, H. Ultra-long cycle H-doped VO2 (B) cathode for high capacity aqueous Zn-ion battery. Mater. Today Adv. 2022, 14, 100230. [Google Scholar]
- Zhang, N.; Chen, X.; Yu, M.; Niu, Z.; Cheng, F.; Chen, J. Materials chemistry for rechargeable zinc-ion batteries. Chem. Soc. Rev. 2020, 49, 4203–4219. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Niu, Z. Design strategies for vanadium-based aqueous zinc-ion batteries. Angew. Chem. 2019, 131, 16508–16517. [Google Scholar] [CrossRef]
- Dai, X.; Wan, F.; Zhang, L.; Cao, H.; Niu, Z. Freestanding graphene/VO2 composite films for highly stable aqueous Zn-ion batteries with superior rate performance. Energy Storage Mater. 2018, 17, 143–150. [Google Scholar] [CrossRef]
- Zhang, L.; Miao, L.; Zhang, B.; Wang, J.; Liu, J.; Tan, Q.; Wan, H.; Jiang, J. A durable VO2(M)/Zn battery with ultrahigh rate capability enabled by pseudocapacitive proton insertion. J. Mater. Chem. A 2019, 8, 1731–1740. [Google Scholar] [CrossRef]
- Liu, Y.; He, G.; Jiang, H.; Parkin, I.P.; Shearing, P.R.; Brett, D.J. Cathode design for aqueous rechargeable multivalent ion batteries: Challenges and opportunities. Adv. Funct. Mater. 2021, 31, 2010445. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Kim, J.M.; Chun, Y.T.; Zhang, J.; Jun, S.C. Recent advances in vanadium-based aqueous rechargeable zinc-ion batteries. Adv. Energy Mater. 2020, 10, 2000477. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, A.; Sun, J. Promise and challenge of vanadium-based cathodes for aqueous zinc-ion batteries. J. Energy Chem. 2021, 54, 655–667. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.F.; Liu, W.L.; Ren, M.M.; Kong, F.G.; Wang, S.J.; Wang, X.Q.; Zhao, H.; Bao, J.M. A high-capacity and long-life aqueous rechargeable zinc battery using a porous metal–organic coordination polymer nanosheet cathode. Inorg. Chem. Front. 2018, 5, 3067–3073. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, X.; Liu, X.Q.; Sun, L.B.; Chen, F.; Liu, C.; Wu, Z.; Chen, Z. Synthesis of mesoporous manganese dioxide/expanded graphite composite and its lithium-storage performance. Bull. Mater. Sci. 2020, 43, 73. [Google Scholar] [CrossRef]
- Liu, N.; Wu, X.; Fan, L.; Gong, S.; Guo, Z.; Chen, A.; Zhao, C.; Mao, Y.; Zhang, N.; Sun, K. Intercalation pseudocapacitive Zn2+ storage with hydrated vanadium dioxide toward ultrahigh rate performance. Adv. Mater. 2020, 32, 1908420. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mou, J.; Zhong, L.; Liu, T.; Xu, Y.; Pan, W.; Huang, J.; Wang, J.; Liu, M. Porous ultrathin W-doped VO2 nanosheets enable boosted Zn2+ (De) intercalation kinetics in VO2 for high-performance aqueous Zn-ion batteries. ACS Sustain. Chem. Eng. 2021, 9, 14193–14201. [Google Scholar] [CrossRef]
- Wu, J.; Yang, L.; Wang, S.; Jian, J.; Abliz, A.; Xie, X.; Zhao, F.; Li, H. Adjusting the V5+ content of vanadium oxide cathodes for high-performance Zn-ion batteries by aging. J. Alloys Compd. 2022, 926, 166773. [Google Scholar] [CrossRef]
- Yang, G.; Yang, S.; Sun, J.; Duan, G.; Cao, B.; Liu, Z. W-doped VO2 for high-performance aqueous Zn-ion batteries. Phys. Chem. Chem. Phys. 2023, 25, 25435–25441. [Google Scholar] [CrossRef]
- Jia, D.; Zheng, K.; Song, M.; Tan, H.; Zhang, A.; Wang, L.; Yue, L.; Li, D.; Li, C.W.; Liu, J. VO2·0.2H2O nanocuboids anchored onto graphene sheets as the cathode material for ultrahigh capacity aqueous zinc ion batteries. Nano Res. 2020, 13, 215–224. [Google Scholar]
- Li, H.; Zhang, F.; Xian, Q.; Yang, L. In-situ sintering induced VO2/V2O5 heterostructure as cathode for high-capacity reversible aqueous Zn-ion batteries. Ionics 2024, 30, 2697–2704. [Google Scholar] [CrossRef]
- Vincent, M.; Avvaru, V.S.; Haranczyk, M.; Etacheri, V. Fast-charging and long-lasting Mg-Na hybrid batteries based on extremely pseudocapacitive bronze TiO2 nanosheet cathodes. Chem. Eng. J. 2022, 433, 133810. [Google Scholar] [CrossRef]
- Vincent, M.; Avvaru, V.S.; Haranczyk, M.; Etacheri, V. High-Performance Mg–Li Hybrid Batteries Based on Pseudocapacitive Anatase Ti1−xCoxO2–y Nanosheet Cathodes. ChemSusChem 2022, 15, e202102562. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Lv, T.T.; Wang, H.; Guo, X.T.; Liu, C.S.; Pang, H. Nsutite-type VO2 microcrystals as highly durable cathode materials for aqueous zinc-ion batteries. Chem. Eng. J. 2021, 417, 128408. [Google Scholar]
- Pang, Q.; Zhao, H.; Lian, R.; Fu, Q.; Wei, Y.; Sarapulova, A.; Sun, J.; Wang, C.; Chen, G.; Ehrenberg, H. Understanding the mechanism of byproduct formation with in operando synchrotron techniques and its effects on the electrochemical performance of VO2 (B) nanoflakes in aqueous rechargeable zinc batteries. J. Mater. Chem. A 2020, 8, 9567–9578. [Google Scholar]

| Material | Current Density | Discharge-Specific Capacity | Reference |
|---|---|---|---|
| V2O5 | 0.2 A g−1 | 470 mAh g−1 | [20] |
| V2O5/(VOG) | 0.3 A g−1 | 144 Wh kg−1 | [21] |
| Al0.2V2O | 0.1 A g−1 | 448.4 mAh g−1 | [22] |
| VO2(B) | 0.05 A g−1 | 357 mAh g−1 | [23] |
| (Ni)VO2 | 5 A g−1 | 182 mAh g−1 | [24] |
| V2O3@C | 2 A g−1 | 853 mAh g−1 | [25] |
| V3O7·H2O | 1 C | 375 mAh g−1 | [26] |
| NixV6−xO13 | 1 A g−1 | 302.6 mAh g−1 | [27] |
| VO2 | 0.1 A g−1 | 289.7 mAh g−1 | This text |
| EG/VO2 | 0.1 A g−1 | 345 mAh g−1 | This text |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhao, J.; Wang, Z.; Liu, H.; Wen, Q.; Yin, J.; Wang, G. Preparation of Expanded Graphite-VO2 Composite Cathode Material and Performance in Aqueous Zinc-Ion Batteries. Materials 2024, 17, 2817. https://doi.org/10.3390/ma17122817
Li J, Zhao J, Wang Z, Liu H, Wen Q, Yin J, Wang G. Preparation of Expanded Graphite-VO2 Composite Cathode Material and Performance in Aqueous Zinc-Ion Batteries. Materials. 2024; 17(12):2817. https://doi.org/10.3390/ma17122817
Chicago/Turabian StyleLi, Jiaye, Jing Zhao, Zebin Wang, Huan Liu, Qing Wen, Jinling Yin, and Guiling Wang. 2024. "Preparation of Expanded Graphite-VO2 Composite Cathode Material and Performance in Aqueous Zinc-Ion Batteries" Materials 17, no. 12: 2817. https://doi.org/10.3390/ma17122817
APA StyleLi, J., Zhao, J., Wang, Z., Liu, H., Wen, Q., Yin, J., & Wang, G. (2024). Preparation of Expanded Graphite-VO2 Composite Cathode Material and Performance in Aqueous Zinc-Ion Batteries. Materials, 17(12), 2817. https://doi.org/10.3390/ma17122817






