Effect of Yttrium Additions on the High-Temperature Oxidation Behavior of GH4169 Ni-Based Superalloy
Abstract
1. Introduction
2. Materials and Methods
3. Results
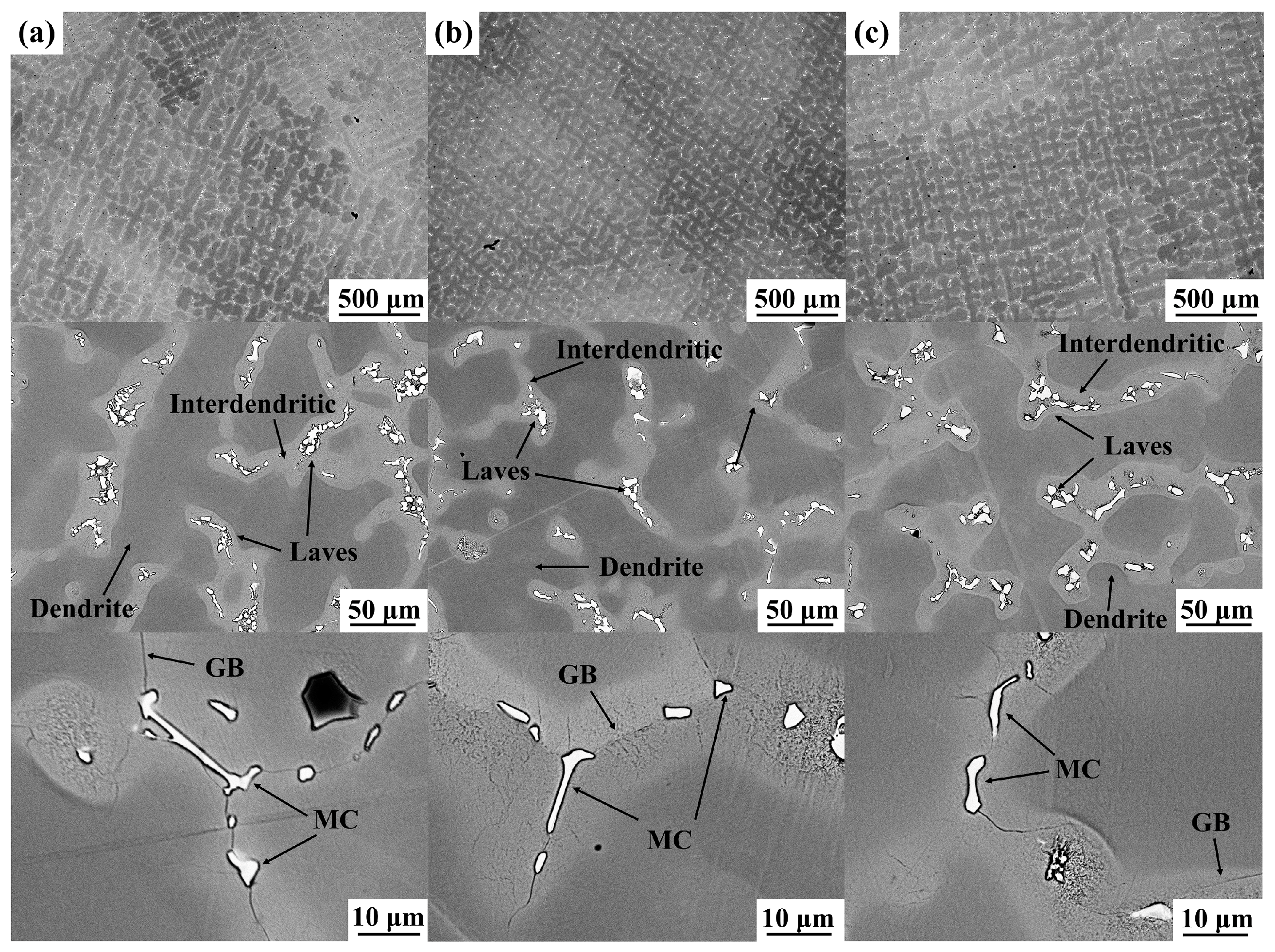
3.1. Microstructure of the As-Cast Alloys
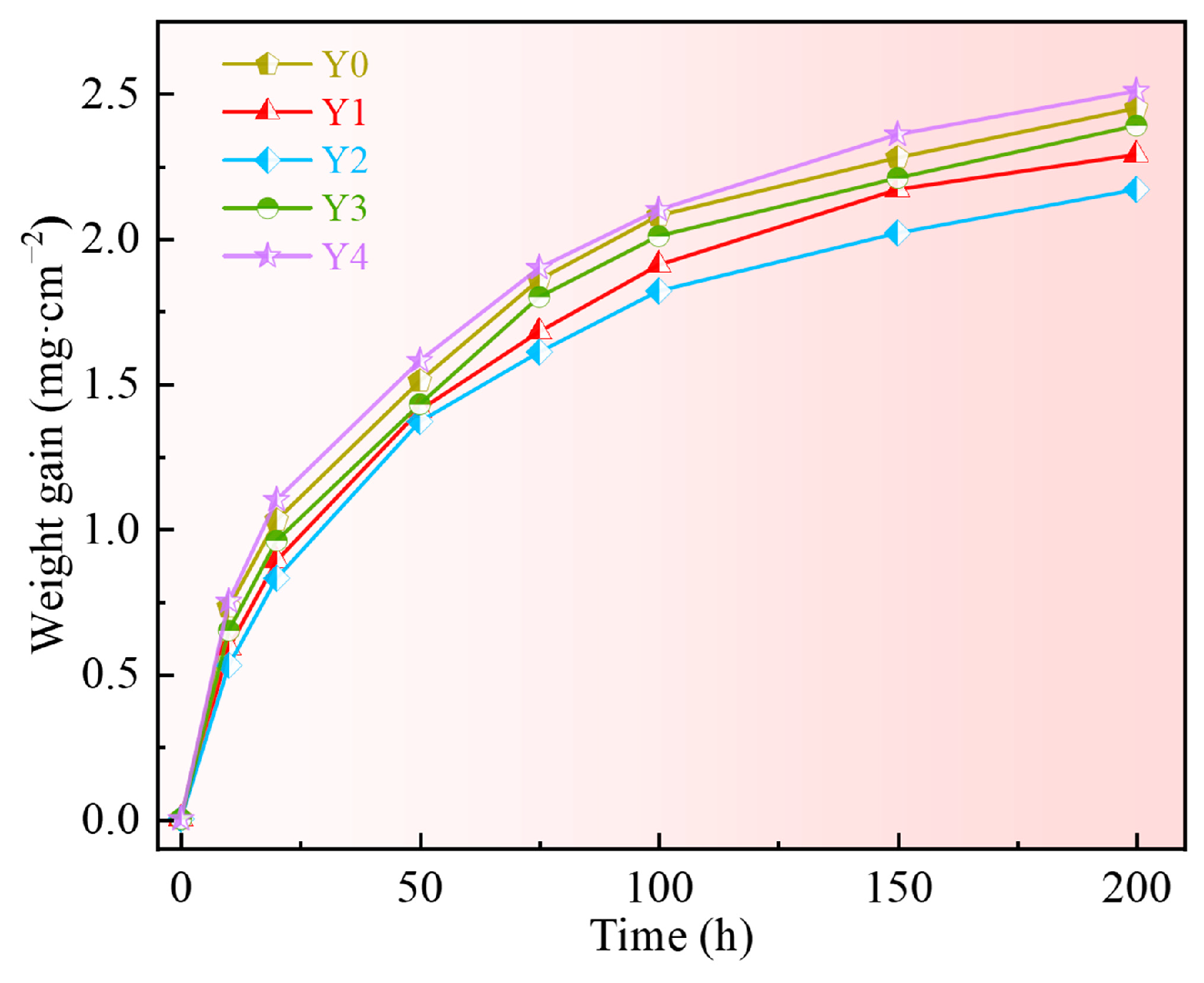
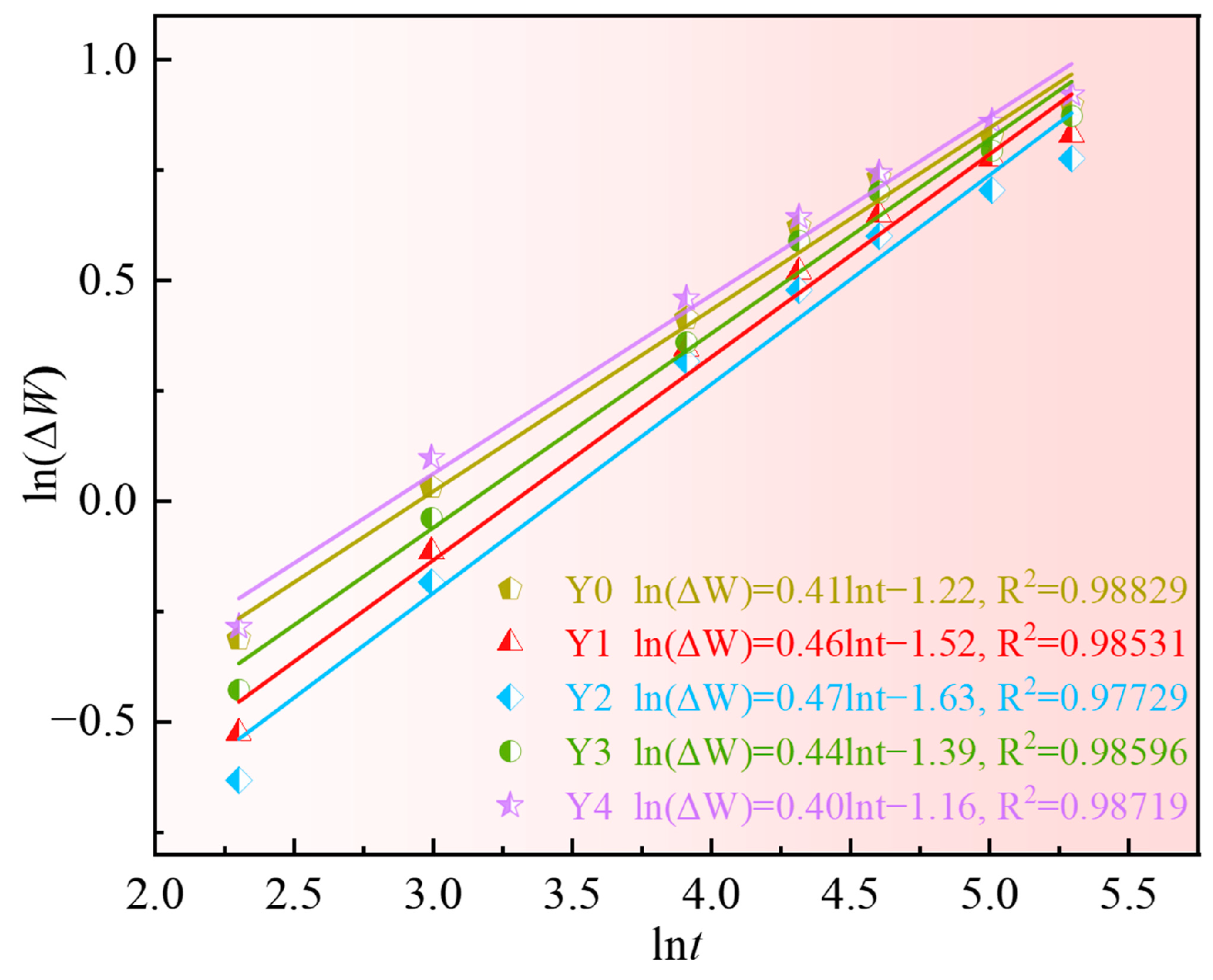
3.2. Oxidation Kinetics
3.3. Oxidation Products
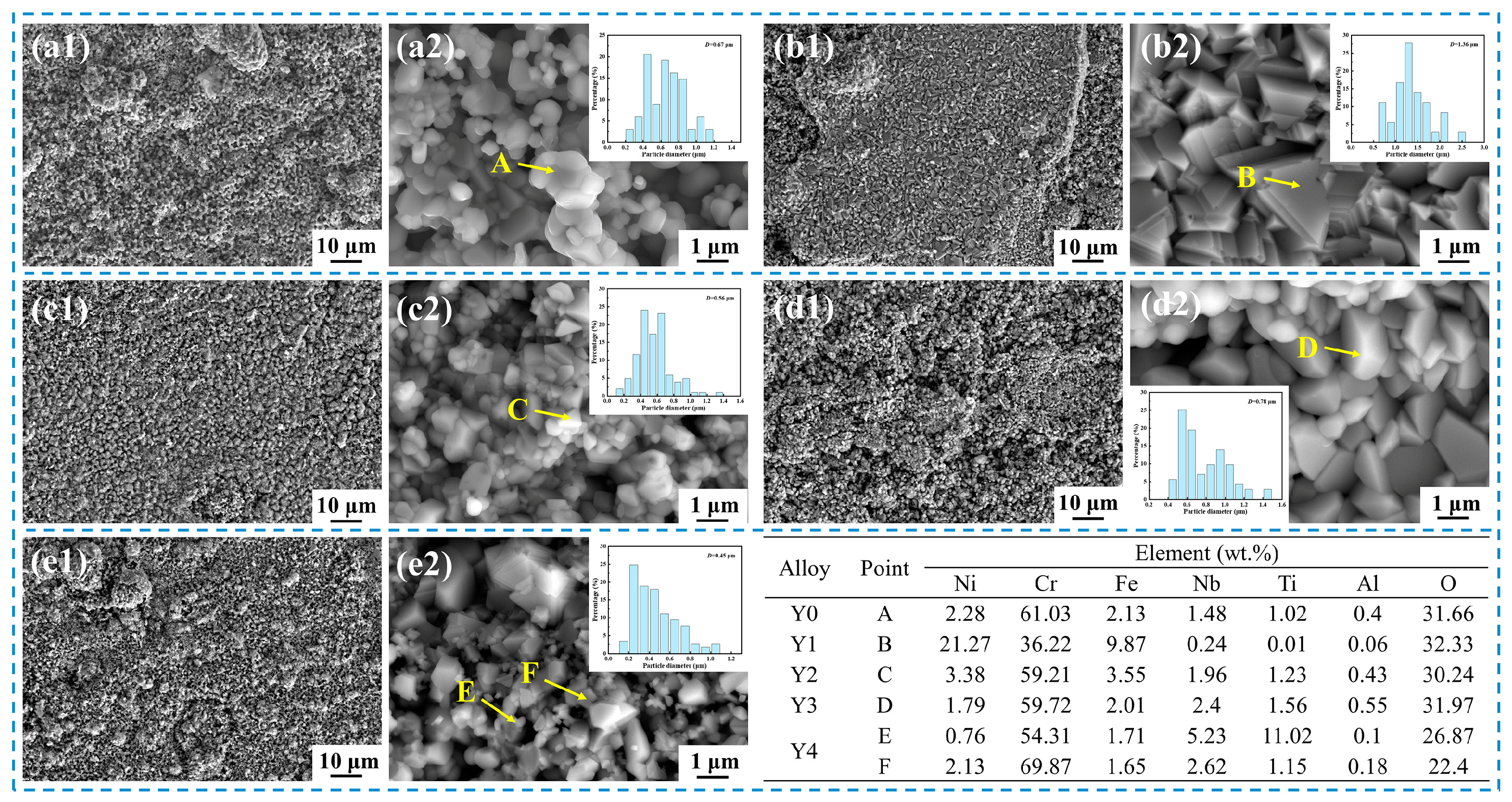
3.4. Morphology of the Surface Oxide Layer
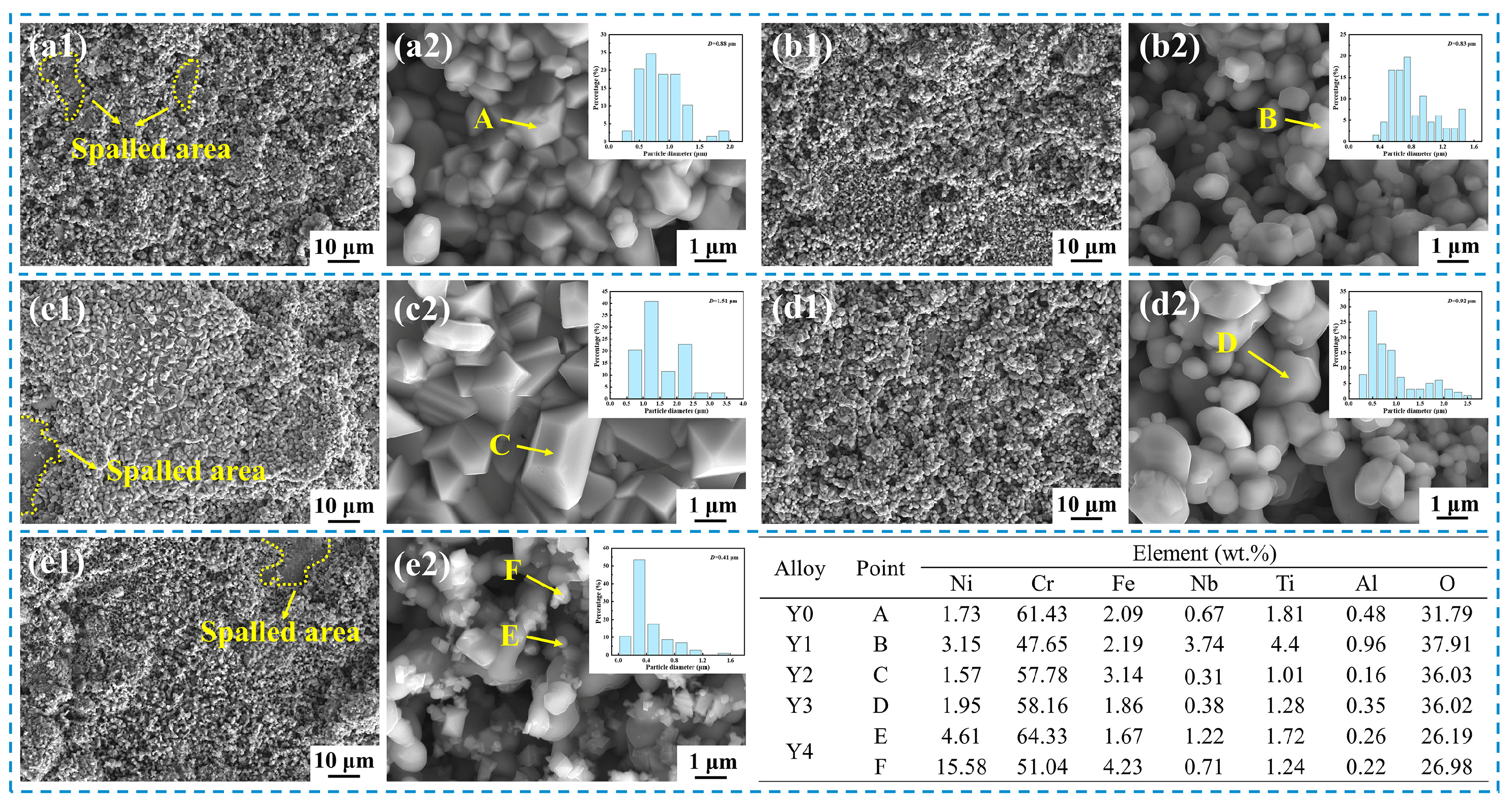
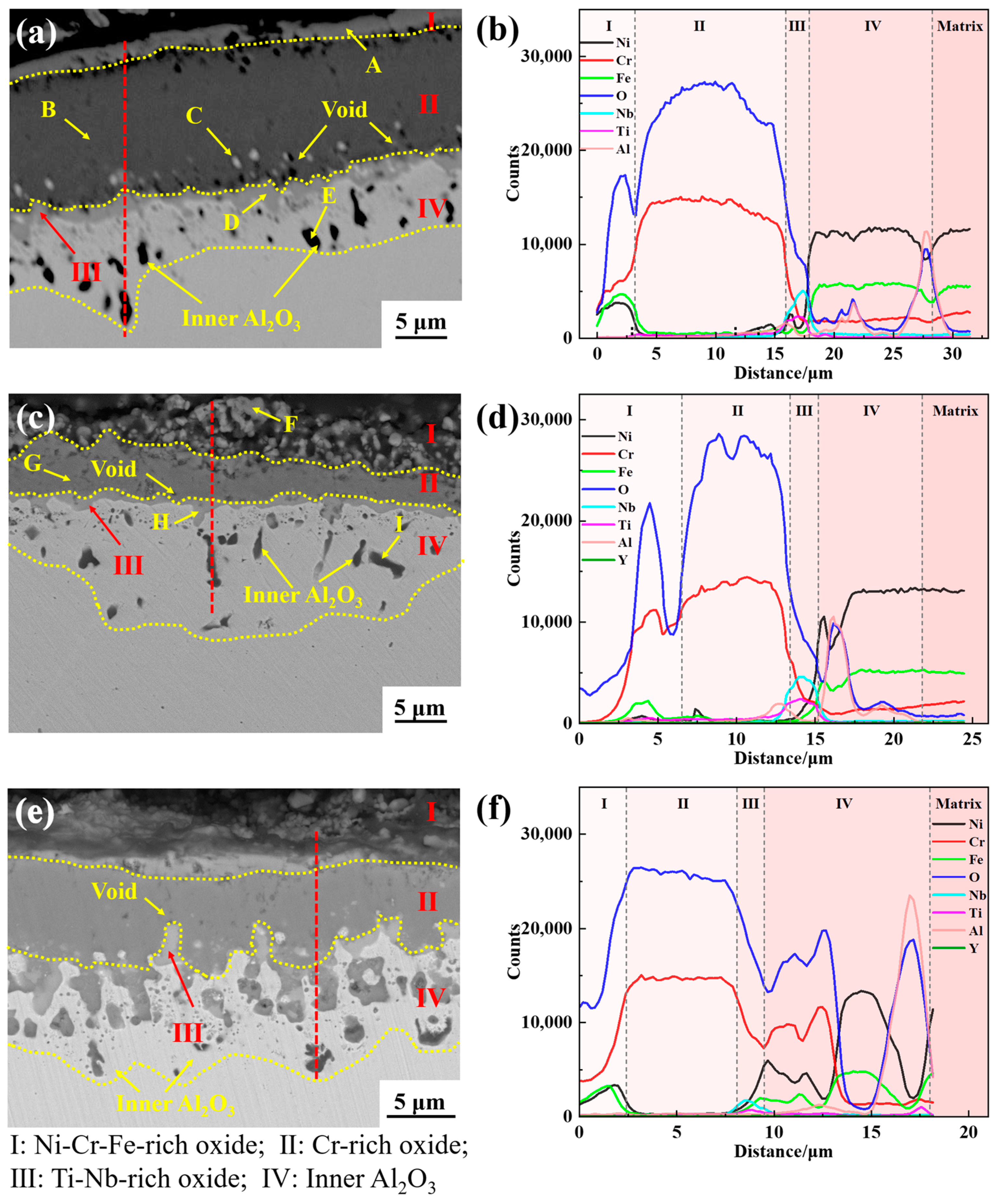
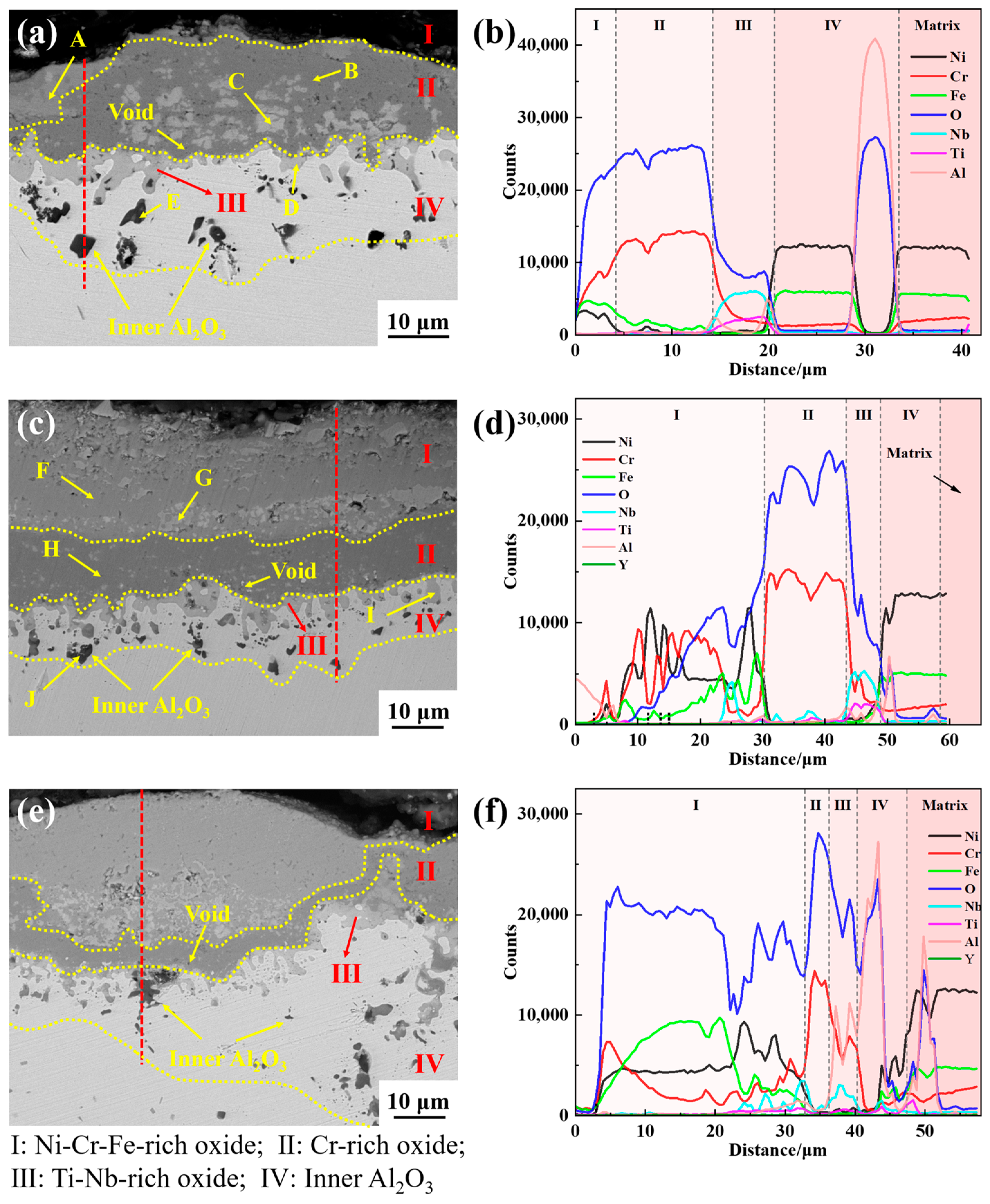
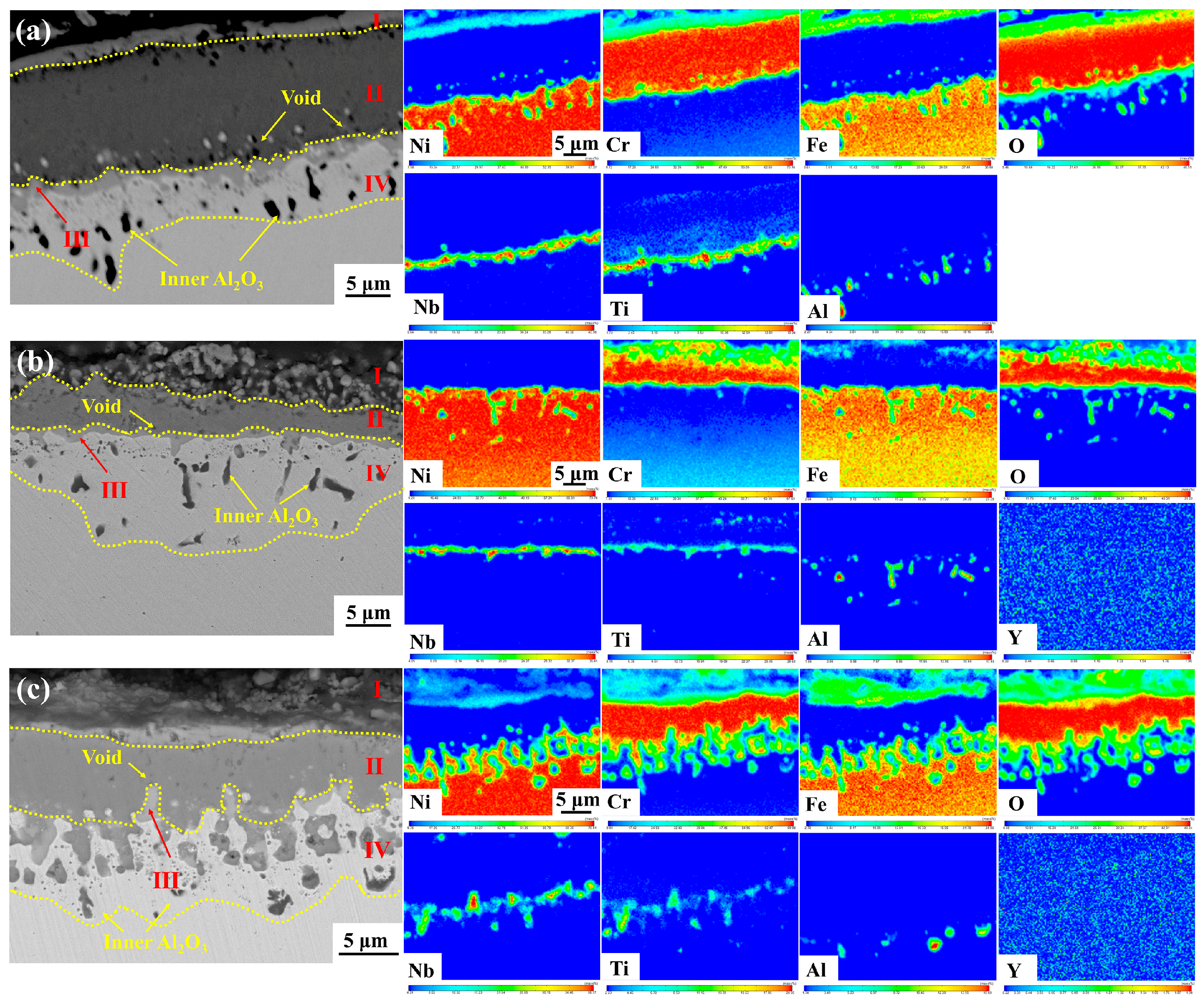
3.5. Cross-Sectional Analysis
4. Discussion
4.1. Effect of Yttrium on the Oxidation Rate
4.2. Effect of Yttrium on Scale Adhesion
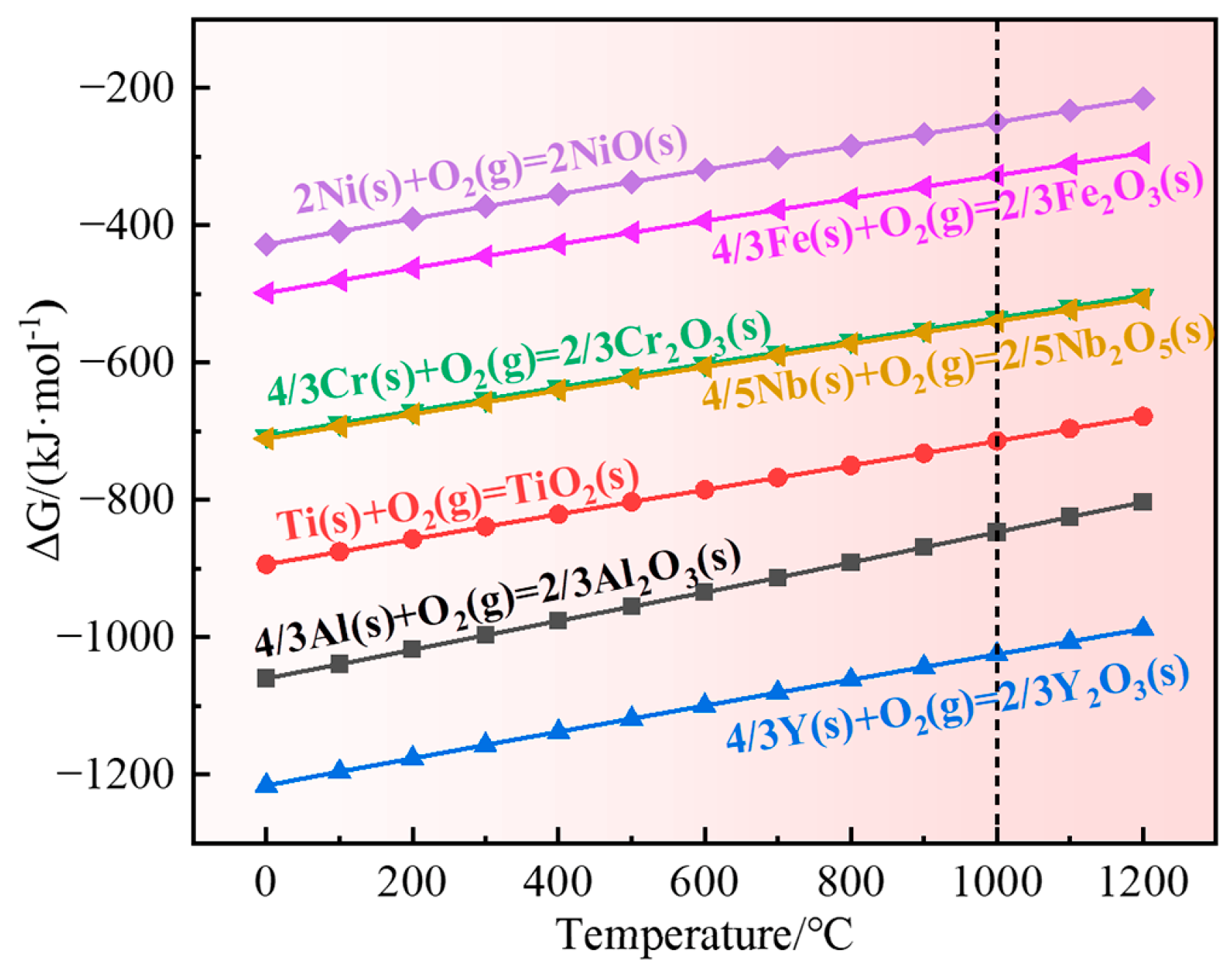
4.3. Oxidation Mechanism
5. Conclusions
- (1)
- The appropriate addition of yttrium can reduce the oxidation weight gain of the alloy, reduce the thickness of the oxide scale and the oxidation rate of GH4169 Ni-based superalloy, and improve the oxidation resistance of the alloy. Excessive addition of yttrium will worsen the high-temperature oxidation resistance of the alloy. The oxidation kinetics curve of alloys with different additions of yttrium at 1000 °C lies between a parabola and a cubic law;
- (2)
- The oxide scale of GH4169 is mainly divided into four layers. The outermost layer (I layer) has a relatively loose structure and is mainly composed of Cr2O3 and spinel NiCr2O4. The subouter layer (II layer) consists predominantly of Cr2O3 oxide with a dense structure; The subinner layer (III layer) comprises mainly Nb-Ti-O phase, composed of TiO2 and Nb2O5. The innermost layer (IV layer) is the inner oxide Al2O3;
- (3)
- The appropriate addition of yttrium can promote the formation of alloy Cr2O3 oxide scale, reduce the vacancy defect at the oxide scale/matrix interface, inhibit the formation of interfacial voids, and improve the anti-spallation ability of the alloy and the adhesion of oxide scale.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sato, A.; Chiu, Y.L.; Reed, R.C. Oxidation of nickel-based single-crystal superalloys for industrial gas turbine applications. Acta Mater. 2011, 59, 225–240. [Google Scholar] [CrossRef]
- Theska, F.; Nomoto, K.; Godor, F.; Oberwinkler, B.; Stanojevic, A.; Ringer, S.P.; Primig, S. On the early stages of precipitation during direct ageing of Alloy 718. Acta Mater. 2020, 188, 492–503. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, L.; Wang, B.; Yao, L.; Jiang, B.; Luo, L.; Chen, R.; Su, Y.; Guo, J. Microstructure evolution and mechanical properties of wire-fed electron beam directed energy deposition repaired GH4169 superalloy. J. Mater. Res. Technol. 2023, 27, 7259–7270. [Google Scholar] [CrossRef]
- An, X.L.; Zhang, B.; Chu, C.L.; Zhou, L.; Paul, K.C. Evolution of microstructures and properties of the GH4169 superalloy during short-term and high-temperature processing. Mater. Sci. Eng. A 2019, 744, 255–266. [Google Scholar] [CrossRef]
- Lu, X.D.; Du, J.H.; Deng, Q. High temperature structure stability of GH4169 superalloy. Mater. Sci. Eng. A 2013, 559, 623–628. [Google Scholar] [CrossRef]
- Yang, X.; Ma, S.; Chu, Q.; Peng, C.; Su, Y.; Xiao, B.; Guo, Z.; Ma, T.; Li, W. Investigation of microstructure and mechanical properties of GH4169 superalloy joint produced by linear friction welding. J. Mater. Res. Technol. 2023, 24, 8373–8390. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Xu, Y.; Kang, M.; Wang, J.; Sun, B. Unveiling the mechanism of yttrium-related microstructure inhibiting or promoting high-temperature oxidation based on Ni-Al-Y alloys. Acta Mater. 2021, 211, 116879. [Google Scholar] [CrossRef]
- Liu, Y.L.; Hou, K.L.; Ou, M.Q.; Ma, Y.C.; Liu, K. Oxidation Behavior of K4750 Alloy at Temperatures between 750 °C and 1000 °C. Acta Metall. Sin. Engl. Lett. 2021, 34, 1657–1668. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Liang, Z.; He, J.; Qiu, J.; Pyczak, F.; Song, M. Effects of Ni and Cr on the high-temperature oxidation behavior and mechanisms of Co- and CoNi-base superalloys. Mater. Des. 2022, 224, 111291. [Google Scholar] [CrossRef]
- Li, M. High Temperature Corrosion of Metals, 1st ed.; Metallurgical Industry Press: Beijing, China, 2001; pp. 3–41. [Google Scholar]
- Giggins, C.S.; Pettit, F.S. Oxidation of Ni-Cr-Al Alloys between 1000° and 1200 °C. J. Electrochem. Soc. 1971, 118, 1782. [Google Scholar] [CrossRef]
- Sitek, R.; Bolek, T.; Dobosz, R.; Plocinski, T.; Mizera, J. Microstructure and oxidation resistance of aluminide layer produced on Inconel 100 nickel alloy by CVD method. Surf. Coat. Technol. 2016, 304, 584–591. [Google Scholar] [CrossRef]
- Khan, A.; Huang, Y.; Dong, Z.; Peng, X. Effect of Cr2O3 nanoparticle dispersions on oxidation kinetics and phase transformation of thermally grown alumina on a nickel aluminide coating. Corros. Sci. 2019, 150, 91–99. [Google Scholar] [CrossRef]
- Whittle, D.P.; Stringer, J. Improvements in high temperature oxidation resistance by additions of reactive elements or oxide dispersions. Philos. Trans. R. Soc. Lond. Ser. Math. Phys. Sci. 1980, 295, 309–329. [Google Scholar]
- Srivastava, M.; Balaraju, J.N.; Ravisankar, B.; Anandan, C.; William Grips, V.K. High temperature oxidation and corrosion behaviour of Ni/Ni–Co–Al composite coatings. Appl. Surf. Sci. 2012, 263, 597–607. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, M.; Chellali, R.; Dong, J. Investigations on the growing, cracking and spalling of oxides scales of powder metallurgy Rene95 nickel-based superalloy. Appl. Surf. Sci. 2011, 257, 9762–9767. [Google Scholar]
- Wongpromrat, P.; Galerie, A.; Thublaor, T.; Chandra-ambhorn, W.; Ponpo, P.; Watasuntornpong, P.; Yamanaka, K.; Chiba, A.; Tunthawiroon, P.; Siripongsakul, T.; et al. Evidence for chromium, cobalt and molybdenum volatilisations during high temperature oxidation of Co-27Cr-6Mo Alloy. Corros. Sci. 2022, 202, 110285. [Google Scholar] [CrossRef]
- Xu, K.D.; Ren, Z.M.; Li, C.J. Progress in application of rare metals in superalloys. Rare Met. 2014, 33, 111–126. [Google Scholar] [CrossRef]
- Jiang, C.; Qian, L.; Feng, M.; Liu, H.; Bao, Z.; Chen, M.; Zhu, S.; Wang, F. Benefits of Zr addition to oxidation resistance of a single-phase (Ni,Pt)Al coating at 1373 K. J. Mater. Sci. Technol. 2019, 35, 1334–1344. [Google Scholar] [CrossRef]
- Li, X.; He, S.; Liang, J.; Zhou, X. High-Temperature Oxidation Behavior and Oxide Scale Structure of Yttrium-Modified Ni–16Mo–7Cr–4Fe Superalloy at 1273 K. Oxid. Met. 2019, 92, 67–88. [Google Scholar] [CrossRef]
- Weng, F.; Yu, H.; Chen, C.; Wan, K. High-temperature oxidation behavior of Ni-based superalloys with Nb and Y and the interface characteristics of oxidation scales: High-temperature oxidation behavior of Ni-based superalloys. Surf. Interface Anal. 2015, 47, 362–370. [Google Scholar] [CrossRef]
- Yu, P.; Wang, W.; Wang, F. Influence of cyclic frequency on oxidation behavior of K38 superalloy with yttrium additions at 1273 K. J. Rare Earths 2011, 29, 119–123. [Google Scholar] [CrossRef]
- Rehman, K.; Sheng, N.; Sang, Z.; Xun, S.; Wang, Z.; Xie, J.; Hou, G.; Zhou, Y.; Sun, X. Comparative study of the reactive elements effects on oxidation behavior of a Ni-based superalloy. Vacuum 2021, 191, 110382. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, S.; Han, M.; Li, J. Influence of yttrium addition on high temperature oxidation resistance of single crystal superalloy. J. Rare Earths 2013, 31, 795–799. [Google Scholar] [CrossRef]
- Muthu, S.M.; Veeman, D.; Paul, S.; Prem Kumar, M. Cyclic oxidation and hot corrosion performance of direct metal laser sintered and wrought alloy 718 at 800 °C in air and molten salts containing Na2SO4, V2O5 and NaCl. Corros. Eng. Sci. Technol. 2023, 58, 631–644. [Google Scholar] [CrossRef]
- Al-hatab, K.A.; Al-bukhaiti, M.A.; Krupp, U.; Kantehm, M. Cyclic Oxidation Behavior of IN 718 Superalloy in Air at High Temperatures. Oxid. Met. 2011, 75, 209–228. [Google Scholar]
- Luo, G.; Cheng, M.; Zhao, L.; Tang, Y.; Yao, J.; Cui, H.; Song, L. Preferential interdendritic oxidation of laser additively manufactured Inconel 718. Corros. Sci. 2021, 179, 109144. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, L.; Liu, R.; Cao, M.; Fan, L.; Li, Y.; Geng, S.; Wang, F. Corrosion Behavior of GH4169 Alloy under Alternating Oxidation at 900 °C and Solution Immersion. Materials 2019, 12, 1503. [Google Scholar] [CrossRef] [PubMed]
- Palleda, T.N.; Banoth, S.; Tanaka, M.; Murakami, H.; Kakehi, K. The role of yttrium micro-alloying on microstructure evolution and high-temperature mechanical properties of additively manufactured Inconel 718. Mater. Des. 2023, 225, 111567. [Google Scholar] [CrossRef]
- Xu, H.; Yang, S.F.; Wang, E.H.; Liu, Y.S.; Guo, C.Y.; Hou, X.M.; Zhang, Y.L. Competitive oxidation behavior of Ni-based superalloy GH4738 at extreme temperature. Int. J. Miner. Metall. Mater. 2024, 31, 138–145. [Google Scholar] [CrossRef]
- Brenneman, J.; Wei, J.; Sun, Z.; Liu, L.; Zou, G.; Zhou, Y. Oxidation behavior of GTD111 Ni-based superalloy at 900 °C in air. Corros. Sci. 2015, 100, 267–274. [Google Scholar] [CrossRef]
- Park, S.J.; Seo, S.M.; Yoo, Y.S.; Jeong, H.W.; Jang, H. Effects of Al and Ta on the high temperature oxidation of Ni-based superalloys. Corros. Sci. 2015, 90, 305–312. [Google Scholar] [CrossRef]
- Nychka, J.A.; Clarke, D.R.; Meier, G.H. Spallation and transient oxide growth on PWA 1484 superalloy. Mater. Sci. Eng. A 2008, 490, 359–368. [Google Scholar] [CrossRef]
- Mao, X.Y. SEM Investigation on High Temperature Oxidation of Fe-23Cr-6Al Alloys (With or Without La, Ce and Y Additions). Acta Metall. Sin. 1980, 16, 406–498. [Google Scholar]
- Naumenko, D.; Pint, B.A.; Quadakkers, W.J. Current Thoughts on Reactive Element Effects in Alumina-Forming Systems: In Memory of John Stringer. Oxid. Met. 2016, 86, 1–43. [Google Scholar] [CrossRef]
- Roy, T.K.; Balasubramaniam, R.; Ghosh, A. High-temperature oxidation of Ti3Al-based titanium aluminides in oxygen. Metall. Mater. Trans. A 1996, 27, 3993–4002. [Google Scholar] [CrossRef]
- Lin, J.P.; Zhao, L.L.; Li, G.Y.; Zhang, L.Q.; Song, X.P.; Ye, F.; Chen, G. Effect of Nb on oxidation behavior of high Nb containing TiAl alloys. Intermetallics 2011, 19, 131–136. [Google Scholar] [CrossRef]
- Ye, X.; Yang, B.; Nie, Y.; Yu, S.; Li, Y. Influence of Nb addition on the oxidation behavior of novel Ni-base superalloy. Corros. Sci. 2021, 185, 109436. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, T.; Shi, J.; Wang, B.; Zhang, X. Microstructural evolution during exposure in air and oxidation behavior of a nickel-based superalloy. Vacuum 2021, 183, 109801. [Google Scholar] [CrossRef]
- Ren, W.; Ouyang, F.; Ding, B.; Zhong, Y.; Yu, J.; Ren, Z.; Zhou, L. The influence of CrTaO4 layer on the oxidation behavior of a directionally-solidified nickel-based superalloy at 850–900 °C. J. Alloys Compd. 2017, 724, 565–574. [Google Scholar] [CrossRef]
- Gesmundo, F.; Hou, P.Y. Analysis of Pore Formation at Oxide–Alloy Interfaces—II: Theoretical Treatment of Vacancy Condensation for Immobile Interfaces. Oxid. Met. 2003, 59, 63–81. [Google Scholar] [CrossRef]
- Pint, B.A. The role of chemical composition on the oxidation performance of aluminide coatings. Surf. Coat. Technol. 2004, 188–189, 71–78. [Google Scholar] [CrossRef]
- Hou, P.Y.; Priimak, K. Interfacial segregation, pore formation, and scale adhesion on NiAl alloys. Oxid. Met. 2005, 63, 113–130. [Google Scholar] [CrossRef]
- Pint, B.A.; Wright, I.G.; Lee, W.Y.; Zhang, Y.; Prüßner, K.; Alexander, K.B. Substrate and bond coat compositions: Factors affecting alumina scale adhesion. Mater. Sci. Eng. A 1998, 245, 201–211. [Google Scholar] [CrossRef]
- Pérez-González, F.A.; Garza-Montes-de Oca, N.F.; Colás, R. High Temperature Oxidation of the Haynes 282© Nickel-Based Superalloy. Oxid. Met. 2014, 82, 145–161. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, X.; Gong, S. The influence of Nb diffusion on the oxidation behavior of TiNiAlNb alloys with different Ti/Ni ratio. Mater. Sci. Eng. A 2007, 458, 381–384. [Google Scholar] [CrossRef]
- Yu, F.H.; Yang, G.H.; Han, R.D.; Weng, H.M.; Shen, J.N. Effect of Reactive Elements Y and Ce on High Temperature Oxidation of Fe-25Cr-40Ni Alloy. Acta Metall. Sin. 1992, 28, 49–57. [Google Scholar]
- Calvarin, G.; Molins, R.; Huntz, A.M. Oxidation Mechanism of Ni–20Cr Foils and Its Relation to the Oxide-Scale Microstructure. Oxid. Met. 2000, 53, 25–48. [Google Scholar] [CrossRef]
- Zhai, Y.D.; Chen, Y.H.; Zhao, Y.S.; Long, H.B.; Li, X.Q.; Deng, Q.S.; Lu, H.; Yang, X.M.; Yang, G.; Li, W.; et al. Initial oxidation of Ni-based superalloy and its dynamic microscopic mechanisms: The interface junction initiated outwards oxidation. Acta Mater. 2021, 215, 116991. [Google Scholar] [CrossRef]
- Moricca, M.D.P.; Varma, S.K. Isothermal oxidation behaviour of Nb–W–Cr Alloys. Corros. Sci. 2010, 52, 2964–2972. [Google Scholar] [CrossRef]
- Weng, F.; Yu, H.; Wan, K.; Chen, C. The influence of Nb on hot corrosion behavior of Ni-based superalloy at 800 °C in a mixture of Na2SO4–NaCl. J. Mater. Res. 2014, 29, 2596–2603. [Google Scholar] [CrossRef]
- Sanviemvongsak, T.; Monceau, D.; Desgranges, C.; Macquaire, B. Intergranular oxidation of Ni-base alloy 718 with a focus on additive manufacturing. Corros. Sci. 2020, 170, 108684. [Google Scholar] [CrossRef]
- Zhu, Z.; Cai, Y.; Gong, Y.; Shen, G.; Tu, Y.; Zhang, G. Isothermal oxidation behavior and mechanism of a nickel-based superalloy at 1000 °C. Int. J. Miner. Metall. Mater. 2017, 24, 776–783. [Google Scholar] [CrossRef]






| Alloy | Additive Designation | Actual Composition |
|---|---|---|
| Y0 | - | - |
| Y1 | 0.01 | 0.0052 |
| Y2 | 0.05 | 0.04 |
| Y3 | 0.15 | 0.11 |
| Y4 | 0.3 | 0.25 |
| Region | Alloy | Element (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ni | Cr | Fe | Mo | Nb | Ti | C | Y | ||
| Matrix | Y0 | 47.91 | 16.65 | 14.86 | 3.37 | 5.72 | 1.03 | 10.46 | - |
| Y2 | 48.96 | 17.17 | 15.43 | 3.16 | 5.19 | 0.95 | 9.11 | 0.03 | |
| Y4 | 48.79 | 17.36 | 15.91 | 3.12 | 4.67 | 0.91 | 9.22 | 0.02 | |
| Laves | Y0 | 31.52 | 11.04 | 9.20 | 5.90 | 28.79 | 0.87 | 12.67 | - |
| Y2 | 33.13 | 9.46 | 8.50 | 6.75 | 29.55 | 0.87 | 11.61 | 0.13 | |
| Y4 | 32.24 | 10.81 | 9.50 | 6.99 | 28.85 | 0.79 | 10.59 | 0.23 | |
| MC | Y0 | 1.77 | 0.64 | 0.60 | 2.66 | 69.05 | 6.76 | 18.52 | - |
| Y2 | 2.11 | 1.00 | 0.83 | 1.51 | 71.02 | 5.84 | 17.58 | 0.11 | |
| Y4 | 1.39 | 0.81 | 0.49 | 1.75 | 71.05 | 6.96 | 17.38 | 0.17 | |
| Alloy | Oxide Layer | Point | Element (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ni | Cr | Fe | Nb | Ti | Al | Y | O | |||
| Y0 | I | A | 21.88 | 26.81 | 21.7 | 0.25 | 0.07 | 0 | - | 29.29 |
| II | B | 0.52 | 62.75 | 2.77 | 0.38 | 0.65 | 0.03 | - | 32.9 | |
| C | 37.3 | 28.48 | 10.08 | 2.7 | 1.88 | 0.39 | - | 19.17 | ||
| III | D | 1.86 | 8.77 | 0.81 | 46.97 | 14.82 | 0.45 | - | 26.32 | |
| IV | E | 26.6 | 5.02 | 10.25 | 1.29 | 0.14 | 30.24 | - | 26.46 | |
| Y2 | I | F | 3.13 | 49.68 | 9.58 | 0.45 | 2.61 | 0.28 | - | 34.27 |
| II | G | 1.03 | 60.97 | 0.41 | 0.28 | 1.49 | 1.08 | - | 34.74 | |
| III | H | 0.43 | 8.45 | 0.96 | 51.6 | 10.32 | 0.36 | 0.76 | 27.12 | |
| IV | I | 24.74 | 3.68 | 8.79 | 0.6 | 0.52 | 33.88 | - | 27.79 | |
| Alloy | Oxide Layer | Point | Element (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ni | Cr | Fe | Nb | Ti | Al | Y | O | |||
| Y0 | I | A | 24.4 | 26.29 | 22.95 | 0.63 | 0.24 | 0.01 | - | 25.48 |
| II | B | 0.48 | 63.64 | 4.51 | 0.28 | 0.4 | 0 | - | 30.69 | |
| C | 0.12 | 22.69 | 0.37 | 38.73 | 7.21 | 0.1 | - | 30.78 | ||
| III | D | 1.47 | 7.47 | 0.07 | 52.49 | 10.82 | 0.13 | - | 27.55 | |
| IV | E | 6.44 | 6.83 | 3 | 0.71 | 3.21 | 43.36 | - | 36.45 | |
| Y2 | I | F | 27.52 | 32.76 | 16.76 | 0.31 | 0.04 | 0.29 | - | 22.32 |
| G | 57.63 | 4.44 | 15.57 | 10.93 | 1.18 | 0 | 0.12 | 10.13 | ||
| II | H | 0.75 | 65.68 | 1.95 | 0.58 | 0.84 | 0.61 | - | 29.59 | |
| III | I | 0.91 | 8.31 | 2.37 | 49.26 | 12.85 | 0.21 | 0.72 | 25.37 | |
| IV | J | 9.54 | 4.46 | 5.21 | 0.57 | 0.81 | 43.22 | 0 | 36.19 | |
| Alloy | n | Kp (mgn·cm−2n·h−1) |
|---|---|---|
| Y0 | 2.43 | 5.21 × 10−2 |
| Y1 | 2.17 | 3.70 × 10−2 |
| Y2 | 2.13 | 3.19 × 10−2 |
| Y3 | 2.27 | 4.31 × 10−2 |
| Y4 | 2.47 | 5.76 × 10−2 |
| NiO | Cr2O3 | TiO2 | Al2O3 | Y2O3 | Fe2O3 | Nb2O5 |
|---|---|---|---|---|---|---|
| −250.0 | −535.4 | −714.2 | −847.0 | −1024.8 | −327.6 | −539.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Liu, W.; Yang, S.; Li, J.; Zhao, P.; Xue, H. Effect of Yttrium Additions on the High-Temperature Oxidation Behavior of GH4169 Ni-Based Superalloy. Materials 2024, 17, 2733. https://doi.org/10.3390/ma17112733
Wang T, Liu W, Yang S, Li J, Zhao P, Xue H. Effect of Yttrium Additions on the High-Temperature Oxidation Behavior of GH4169 Ni-Based Superalloy. Materials. 2024; 17(11):2733. https://doi.org/10.3390/ma17112733
Chicago/Turabian StyleWang, Tiantian, Wei Liu, Shufeng Yang, Jingshe Li, Peng Zhao, and Hui Xue. 2024. "Effect of Yttrium Additions on the High-Temperature Oxidation Behavior of GH4169 Ni-Based Superalloy" Materials 17, no. 11: 2733. https://doi.org/10.3390/ma17112733
APA StyleWang, T., Liu, W., Yang, S., Li, J., Zhao, P., & Xue, H. (2024). Effect of Yttrium Additions on the High-Temperature Oxidation Behavior of GH4169 Ni-Based Superalloy. Materials, 17(11), 2733. https://doi.org/10.3390/ma17112733







