Hydrophobization of Reduced Graphene Oxide Aerogel Using Soy Wax to Improve Sorption Properties
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of the rGO/Wax Aerogel
2.3. Characterization and Measurements
2.4. Study of Sorption Properties
3. Results and Discussion
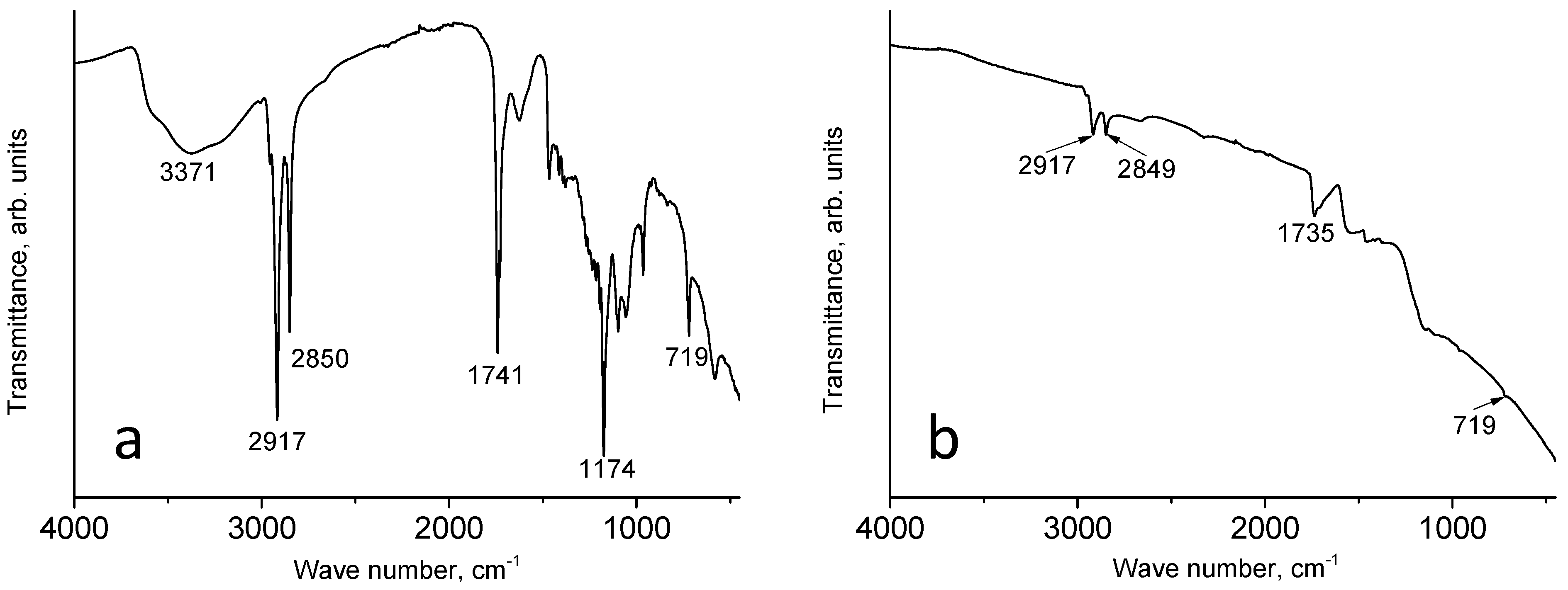
3.1. IR Spectra
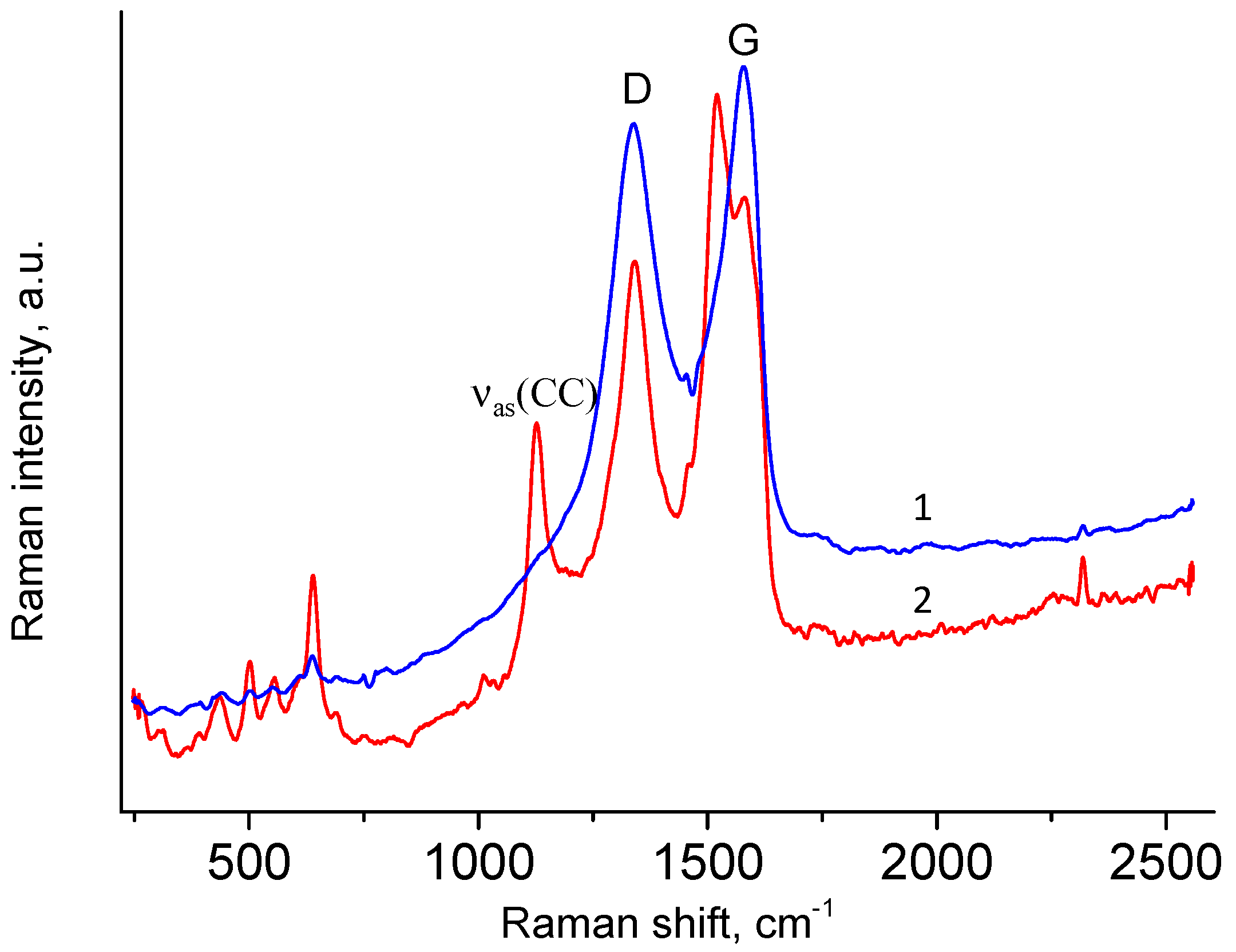
3.2. Raman Spectra
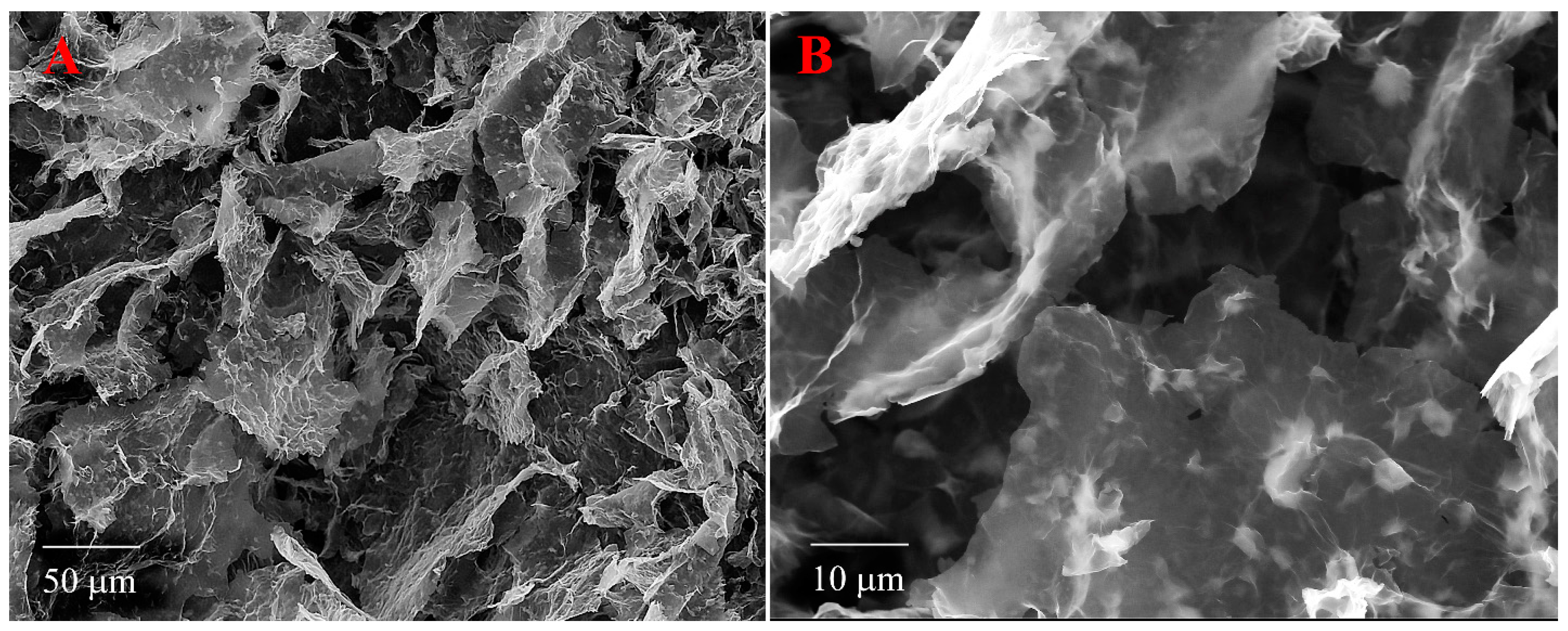
3.3. SEM Images
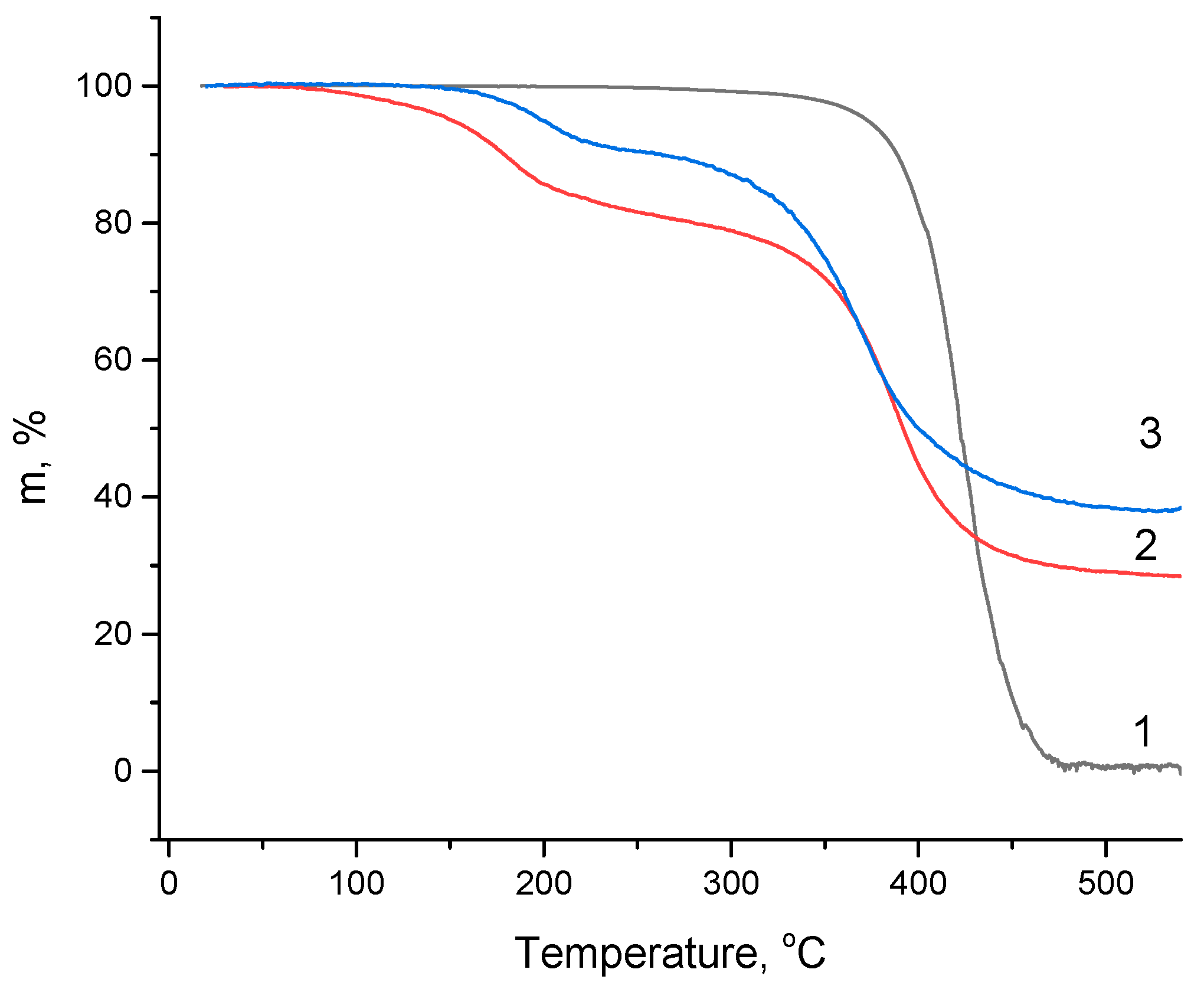
3.4. TGA Analysis
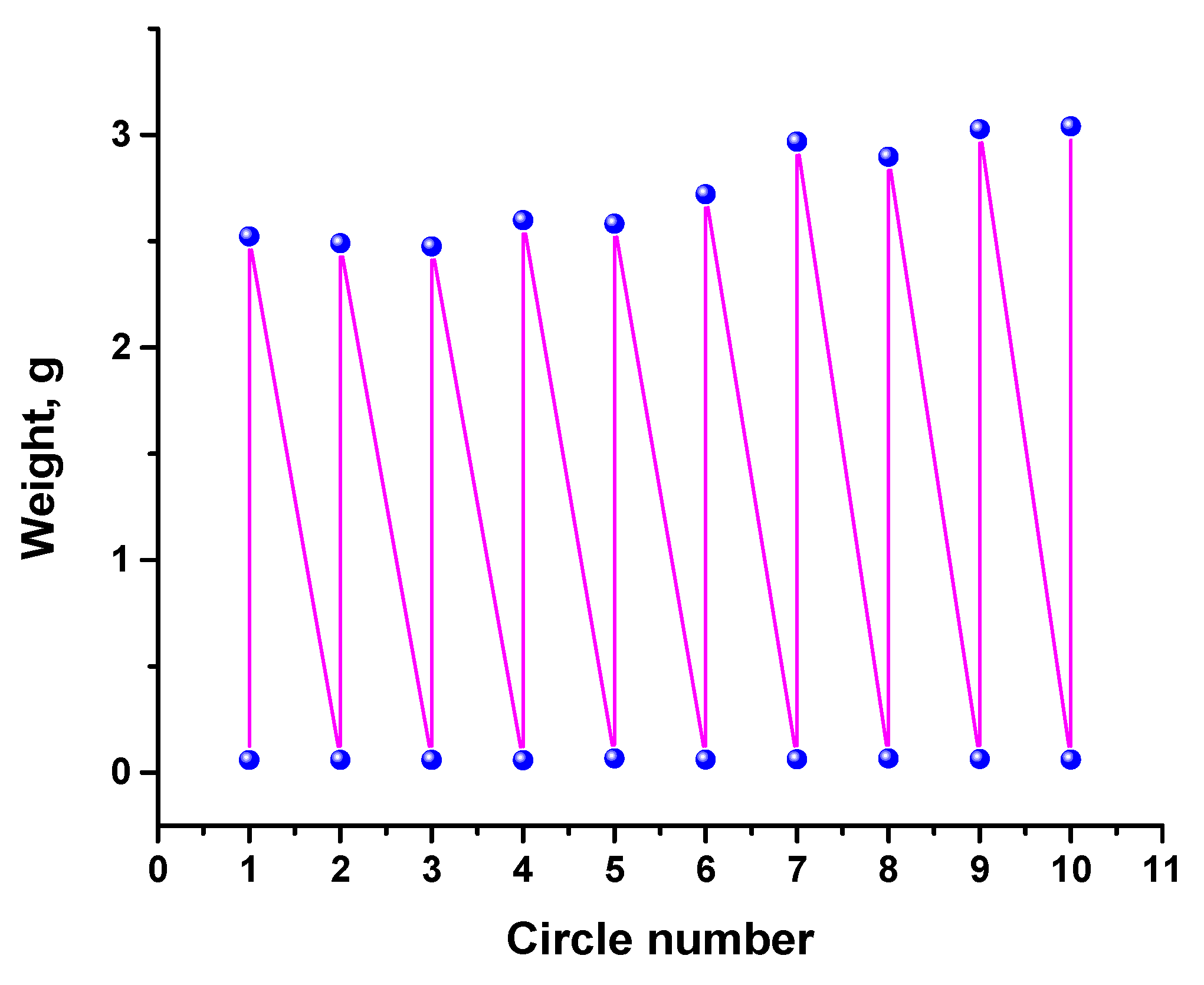
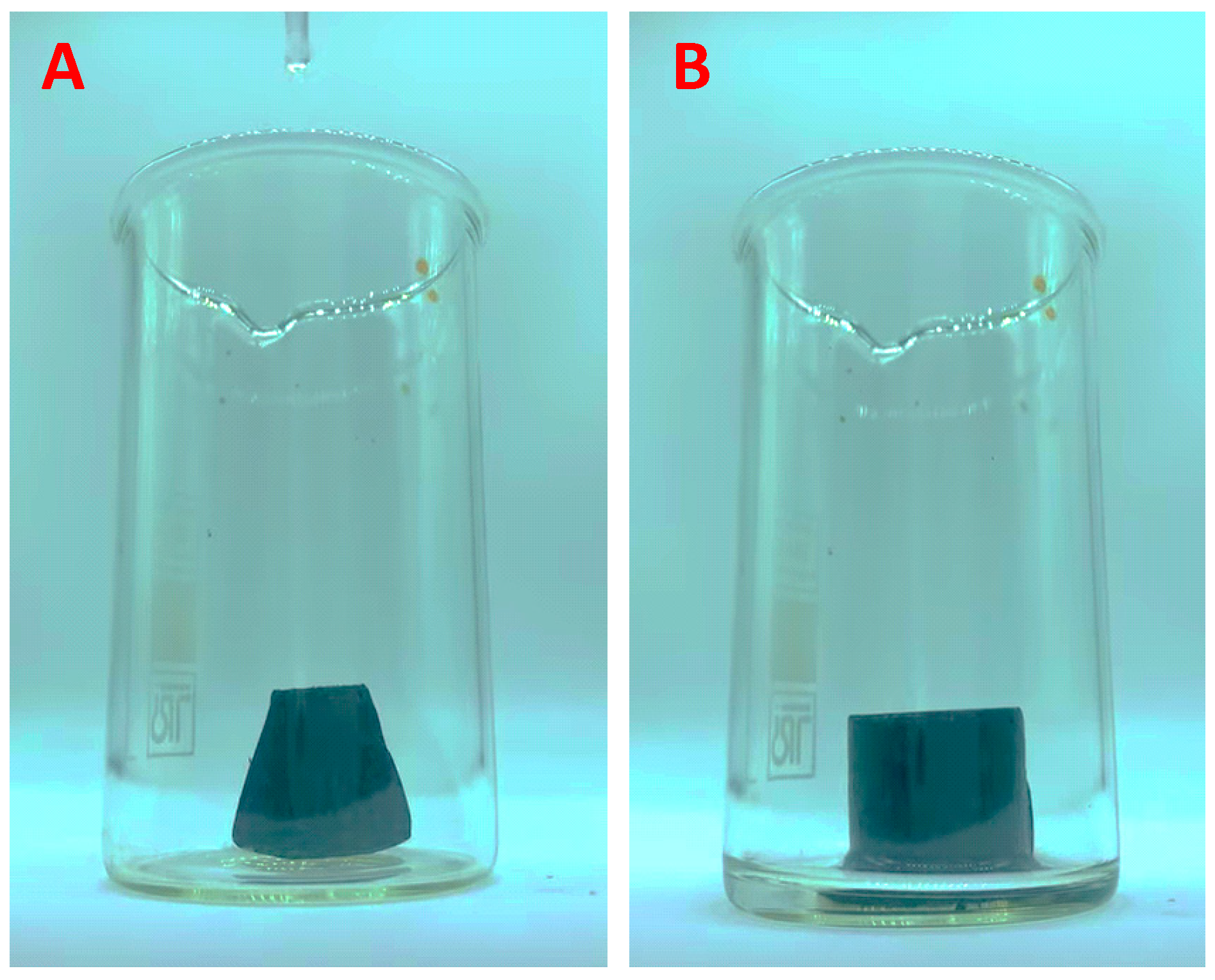
3.5. CWA Results
3.6. Adsorption Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Latthe, S.S.; Terashima, C.; Nakata, K.; Fujishima, A. Superhydrophobic Surfaces Developed by Mimicking Hierarchical Surface Morphology of Lotus Leaf. Molecules 2014, 19, 4256–4283. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Ensikat, H.-J. The hydrophobic coatings of plant surfaces: Epicuticular wax crystals and their morphologies, crystallinity and molecular self-assembly. Micron 2008, 39, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Guo, Z.; Liu, W. Interfacial Effects of Superhydrophobic Plant Surfaces: A Review. J. Bionic Eng. 2014, 11, 325–345. [Google Scholar] [CrossRef]
- Milanovic, J.; Levic, S.; Djordjevi’c, V.; Nedovic, V.; Bugarski, B. Carnauba wax microparticles produced by melt dispersion technique. Chem. Pap. 2011, 65, 213–220. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, G.; Yue, Y. Fabrication of Fe3O4@SiO2@RGO nanocomposites and their excellent absorption properties with low filler content. RSC Adv. 2015, 5, 71718–71723. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, D.; Yin, X.; Xu, P.; Wu, F.; He, M. Hybrid of MoS2 and Reduced Graphene Oxide: A Lightweight and Broadband Electromagnetic Wave Absorber. ACS Appl. Mater. Interfaces 2015, 7, 26226–26234. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Shen, L.; Zhong, L.; Fu, H. Synthesis of silanized MoS2/Reduced graphene oxide for strong radar wave absorption. Ind. Eng. Chem. Res. 2017, 56, 10667–10677. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, Z.; Lv, X.; Zhang, X.; Zhang, Y.; Zhang, J.; Zhang, L.; Gong, C. Microwave absorption performance of reduced graphene oxide with negative imaginary permeability. J. Phys. D Appl. Phys. 2020, 53, 02LT01. [Google Scholar] [CrossRef]
- Baskakov, S.A.; Baskakova, Y.V.; Kabachkov, E.N.; Dremova, N.N.; Michtchenko, A.; Shulga, Y.M. Novel Superhydrophobic Aerogel on the Base of Polytetrafluoroethylene. ACS Appl. Mater. Interfaces 2019, 11, 32517–32522. [Google Scholar] [CrossRef]
- Li, H.; Pordesimo, L.; Weiss, J. High intensity ultrasound-assisted extraction of oil from soybeans. Food Res. Int. 2004, 37, 731. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, H.-Y.; Su, W.-H. Automatic Localization of Soybean Seedlings Based on Crop Signaling and Multi-View Imaging. Sensors 2024, 24, 3066. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, F.; Li, L.; Ma, S.; Yu, L.; Tang, C.; Zhao, K.; Song, Z.; Liu, C.; Chen, Q.; et al. QTL Mapping and Candidate Gene Mining for Stem Diameter Using Genetic Basis of Cultivated Soybean and Wild Soybean. Agronomy 2024, 14, 1019. [Google Scholar] [CrossRef]
- Caprita, R.; Caprita, A.; Ilia, G.; Cretescu, I.; Simulescu, V.O. Laboratory Procedures for Assessing Quality of Soybean Meal. In Proceedings of the World Congress on Engineering and Computer Science Vol II WCECS, San Francisco, CA, USA, 20–22 October 2010; pp. 791–794. [Google Scholar]
- Wu, S.; Wang, X.; Qin, J.; Tian, W.; Wang, M.; Yue, A.; Wang, L.; Du, W.; Zhao, J. Soybean CEP6 Signaling Peptides Positively Regulate Nodulation. Agronomy 2024, 14, 988. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Shulga, Y.M.; Baskakov, S.A.; Smirnov, V.A.; Shulga, N.Y.; Belay, K.G.; Gutsev, G.L. Graphene Oxide Films as Separators of Polyaniline-Based Supercapacitors. J. Power Sources 2014, 245, 33–36. [Google Scholar] [CrossRef]
- Baskakov, S.A.; Baskakova, Y.V.; Lyskov, N.V.; Dremova, N.N.; Irzhak, A.V.; Kumar, Y.; Michtchenok, A.; Shulga, Y.M. Fabrication of Current Collector Using a Composite of Polylactic Acid and Carbon Nano-Material for Metal-Free Supercapacitors with Graphene Oxide Separators and Microwave Exfoliated Graphite Oxide Electrodes. Electrochim. Acta 2018, 260, 557–563. [Google Scholar] [CrossRef]
- Roach, J.A.G.; Mossoba, M.M.; Yurawecz, M.P.; Kramer, J.K.G. Chromatographic separation and identification of conjugated linoleic acid isomers. Anal. Chim. Acta 2002, 465, 207–226. [Google Scholar] [CrossRef]
- Smith, B.C. The Infrared Spectra of Polymers II: Polyethylene. Spectroscopy 2021, 36, 24–29. [Google Scholar] [CrossRef]
- Yoo, J.; Chang, S.J.; Wi, S.; Kim, S. Spent Coffee Grounds as Supporting Materials to Produce Bio-Composite PCM with Natural Waxes. Chemosphere 2019, 235, 626–635. [Google Scholar] [CrossRef]
- Dhal, S.; Alhamidi, A.; Al-Zahrani, S.M.; Anis, A.; Pal, K. The Influence of Emulsifiers on the Physiochemical Behavior of Soy Wax/Rice Bran Oil-Based Oleogels and Their Application in Nutraceutical Delivery. Gels 2023, 9, 47. [Google Scholar] [CrossRef]
- Si, Y.; Samulski, E.T. Synthesis of Water Soluble Graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.K.; Lee, Y.P.; Jin, M.H.; Kim, E.S.; Bae, J.J.; Lee, Y.H. Thermal stability of graphite oxide. Chem. Phys. Lett. 2009, 470, 255–258. [Google Scholar] [CrossRef]
- Cote, L.J.; Cruz-Silva, R.; Huang, J. Flash Reduction and Patterning of Graphite Oxide and Its Polymer Composite. J. Am. Chem. Soc. 2009, 131, 11027–11032. [Google Scholar] [CrossRef] [PubMed]
- Karthika, P.; Rajalakshmi, N.; Dhathathreyan, K.S. Functionalized exfoliated graphene oxide as supercapacitor electrodes. Soft Nanosci. Lett. 2012, 2, 59–66. [Google Scholar] [CrossRef]
- Galande, C.; Mohite, A.D.; Naumov, A.V.; Gao, W.; Ci, L.; Ajayan, A.; Gao, H.; Srivastava, A.; Weisman, R.B.; Ajayan, P.M. Quasi-molecular fluorescence from graphene oxide. Sci. Rep. 2011, 1, 85. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Jiao, Q.-Z.; Zhao, Y.; Li, H.-S. Vapor diffusion synthesis of CoFe2O4 hollow sphere/graphene composites as absorbing materials. J. Mater. Chem. A 2014, 2, 735–744. [Google Scholar] [CrossRef]
- Shulga, Y.M.; Melezhik, A.V.; Kabachkov, E.N.; Milovich, F.O.; Lyskov, N.V.; Irzhak, A.V.; Dremova, N.N.; Gutsev, G.L.; Michtchenko, A.; Tkachev, A.G.; et al. Characterisation and Electrical Conductivity of Polytetrafluoroethylene/graphite Nanoplatelets Composite Films. Appl. Phys. A-Mater. 2019, 125, 460. [Google Scholar] [CrossRef]
- Childres, I.; Jauregui, L.; Park, W.; Cao, H.; Chen, Y. Raman Spectroscopy of Graphene and Related Materials. New Dev. Photon Mater. Res. 2013, 1, 1–20. [Google Scholar]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The Importance of Interbands on the Interpretation of the Raman Spectrum of Graphene Oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- López-Díaz, D.; López Holgado, M.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman Spectrum with the Chemical Composition of Graphene Oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Abaszade, R.G. Synthesis and analysis of flakes graphene oxide. J. Optoelectron. Biomed. Mater. 2022, 14, 107–114. [Google Scholar] [CrossRef]
- Nguyen, T.T.D.; Nguyen, D.; Doan, N.H.; Phu, P.V.; Huynh, V.T.; Hoang, V.H.; Phan, T.B.; Kinashi, K.; Nguyen, P.T. In-depth understanding of the photoreduction of graphene oxide to reduced-graphene oxide on TiO2 surface: Statistical analysis of X-ray photoelectron and Raman spectroscopy data. Appl. Surf. Sci. 2022, 581, 152325. [Google Scholar] [CrossRef]
- Amorim da Costa, A.M.; Marques, M.P.M.; Batista de Carvalho, L.A.E. Intra- versus interchain interactions in polyamines: A Raman spectroscopy study. Vib. Spec. 2004, 35, 165. [Google Scholar] [CrossRef]
- Zheng, M.; Du, W. Phase behavior, conformations, thermodynamic properties, and molecular motion of multicomponent paraffin waxes: A Raman spectroscopy study. Vib. Spectrosc. 2006, 40, 219–224. [Google Scholar] [CrossRef]
- Meksiarun, P.; Ishigaki, M.; Huck-Pezzei, V.A.; Huck, C.W.; Wongravee, K.; Sato, H.; Ozaki, Y. Comparison of multivariate analysis methods for extracting the paraffin component from the paraffin-embedded cancer tissue spectra for Raman imaging. Sci. Rep. 2017, 7, 44890. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hu, X.; Yang, L.; Xie, C.; Yang, L.; Geng, J.; Wang, X.; Yao, S.; Zhang, Z. Online Quantitative Analysis of Chlorine Contents in Chlorinated Paraffins by Facile Raman Spectroscopy. ACS Omega 2023, 8, 4711–4715. [Google Scholar] [CrossRef]
- Xu, L.; Xiao, G.; Chen, C.; Li, R.; Mai, Y.; Sun, G.; Yan, D. Superhydrophobic and superoleophilic graphene aerogel prepared by facile chemical reduction. J. Mater. Chem. A Mater. Energy Sustain. 2015, 3, 7498–7504. [Google Scholar] [CrossRef]


| Sorbate | Qw a, g/g | Qv a, % | |||
|---|---|---|---|---|---|
| rGO/Wax | rGO/PTFE b | rGO/Wax | rGO/PTFE b | ||
| 1 | Tetrahydrofuran | 64.38 | 23.23 | 99.91 | 82.9 |
| 2 | Toluene | 60.70 | 15.52 | 96.62 | 56.6 |
| 3 | Acetone | 52.31 | 23.15 | 91.39 | 94.1 |
| 4 | Dichlorobenzene | 85.83 | 0.25 | 91.12 | 6.20 |
| 5 | N-hexane | 41.86 | 18.97 | 87.99 | 92.5 |
| 6 | N-heptane | 48.58 | - | 98.68 | - |
| 7 | Methylpyrrolidone | 69.40 | - | 92.98 | - |
| 8 | Propanol-2 | 57.94 | 23.61 | 101.80 | 95.9 |
| 9 | Petroleum | 63.11 | - | 97.85 | - |
| 10 | Kerosene | 53.87 | - | 87.46 | - |
| 11 | White spirit | 52.43 | - | 91.59 | - |
| 12 | Machine oil | 56.55 | - | 91.81 | - |
| 13 | Water | 0.05 | - | 0.06 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baskakov, S.A.; Baskakova, Y.V.; Kabachkov, E.N.; Zhidkov, M.V.; Alperovich, A.V.; Krasnikova, S.S.; Chernyaev, D.A.; Shulga, Y.M.; Gutsev, G.L. Hydrophobization of Reduced Graphene Oxide Aerogel Using Soy Wax to Improve Sorption Properties. Materials 2024, 17, 2538. https://doi.org/10.3390/ma17112538
Baskakov SA, Baskakova YV, Kabachkov EN, Zhidkov MV, Alperovich AV, Krasnikova SS, Chernyaev DA, Shulga YM, Gutsev GL. Hydrophobization of Reduced Graphene Oxide Aerogel Using Soy Wax to Improve Sorption Properties. Materials. 2024; 17(11):2538. https://doi.org/10.3390/ma17112538
Chicago/Turabian StyleBaskakov, Sergey A., Yulia V. Baskakova, Eugene N. Kabachkov, Mikhail V. Zhidkov, Anastasia V. Alperovich, Svetlana S. Krasnikova, Dmitrii A. Chernyaev, Yury M. Shulga, and Gennady L. Gutsev. 2024. "Hydrophobization of Reduced Graphene Oxide Aerogel Using Soy Wax to Improve Sorption Properties" Materials 17, no. 11: 2538. https://doi.org/10.3390/ma17112538
APA StyleBaskakov, S. A., Baskakova, Y. V., Kabachkov, E. N., Zhidkov, M. V., Alperovich, A. V., Krasnikova, S. S., Chernyaev, D. A., Shulga, Y. M., & Gutsev, G. L. (2024). Hydrophobization of Reduced Graphene Oxide Aerogel Using Soy Wax to Improve Sorption Properties. Materials, 17(11), 2538. https://doi.org/10.3390/ma17112538









