Impact of Superplasticizers on the Performance of Low-Grade Limestone-Based Cement Mixes
Abstract
1. Introduction
2. Materials and Methods
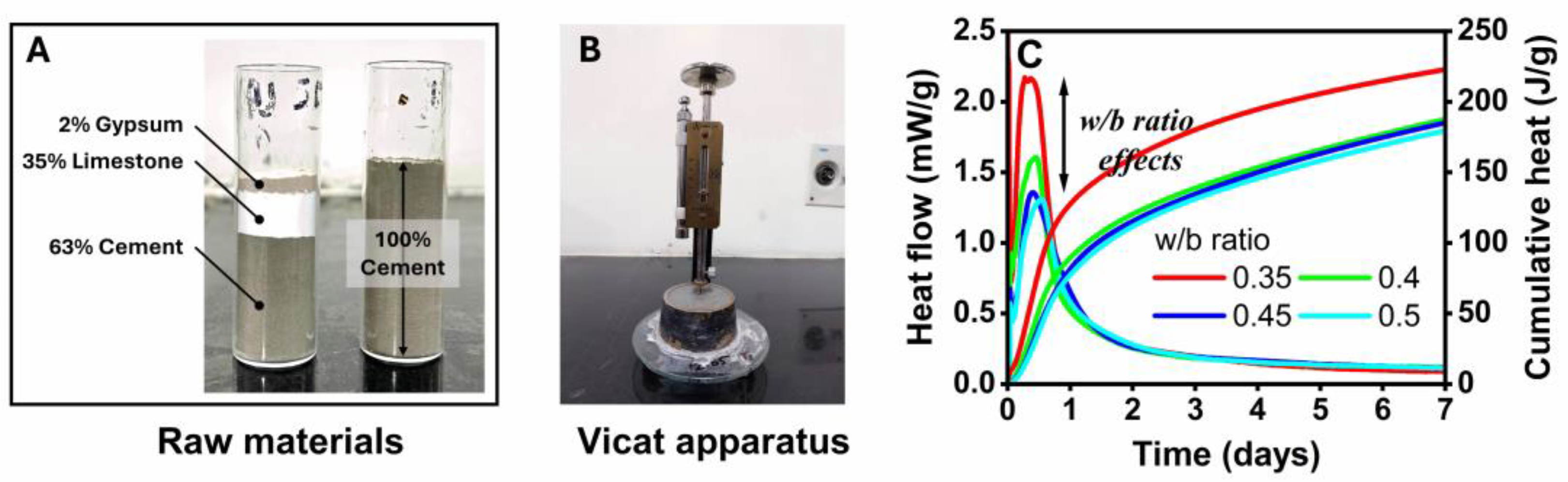
2.1. Sample Preparation
2.2. Isothermal Calorimetry and Vicat Apparatus
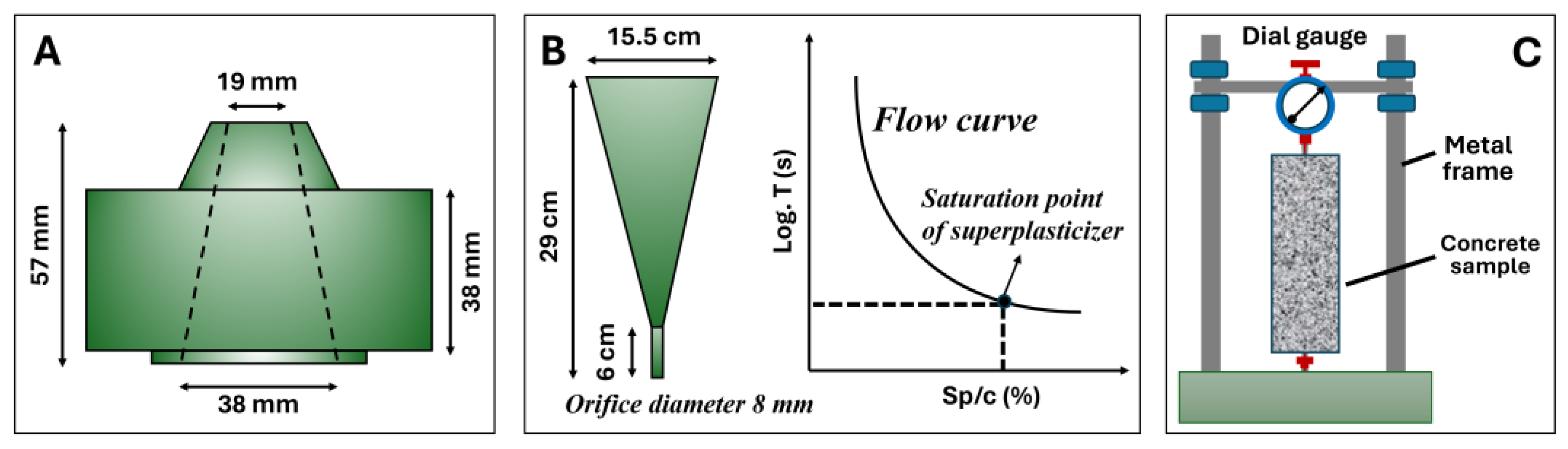
2.3. Mini Slump and Marsh Cone Tests
2.4. Flow Table, Compressive Strength, and Shrinkage Tests
2.5. Characterization Studies
3. Results and Discussion
3.1. Heat of Hydration and Setting Time
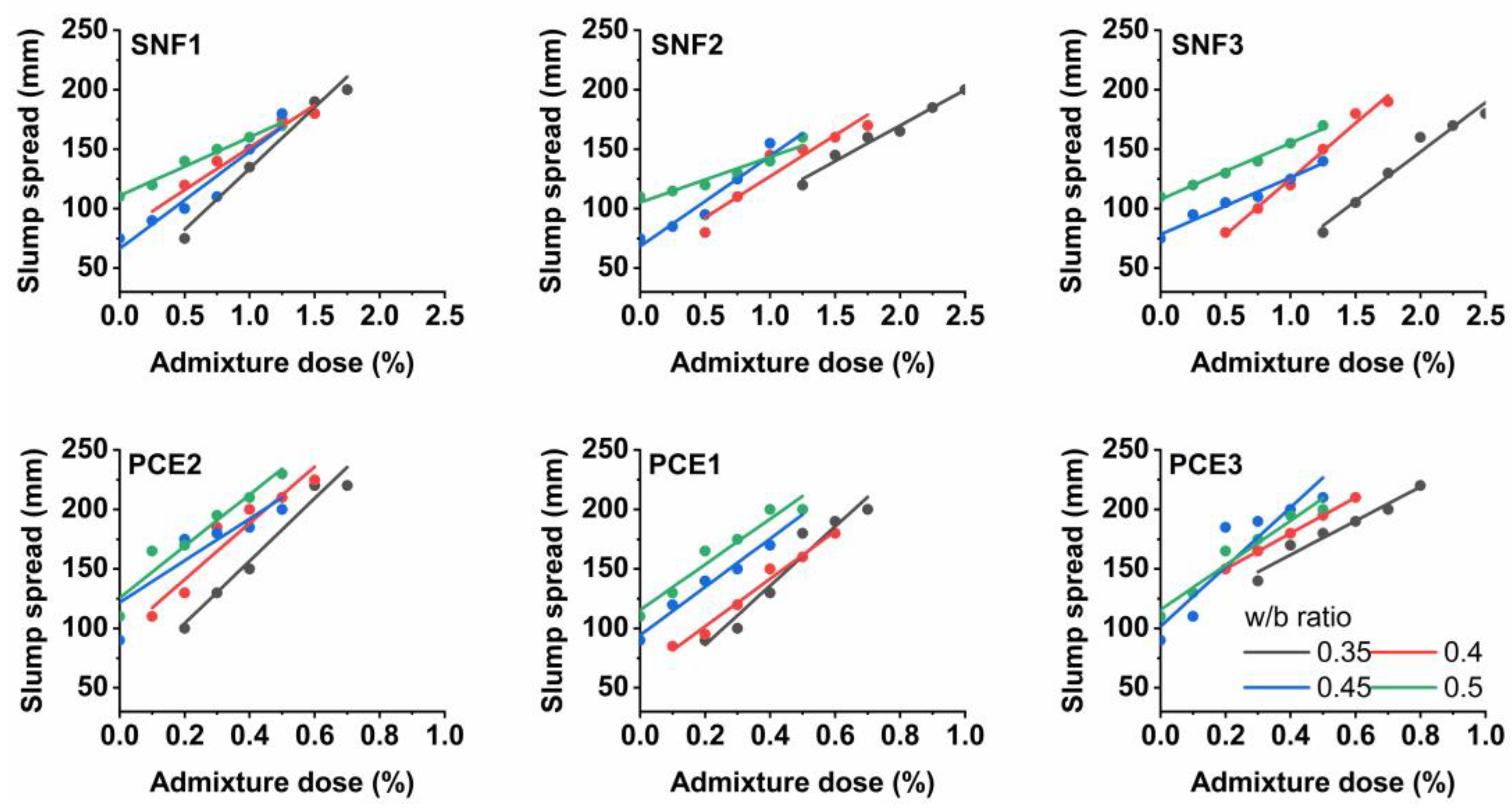
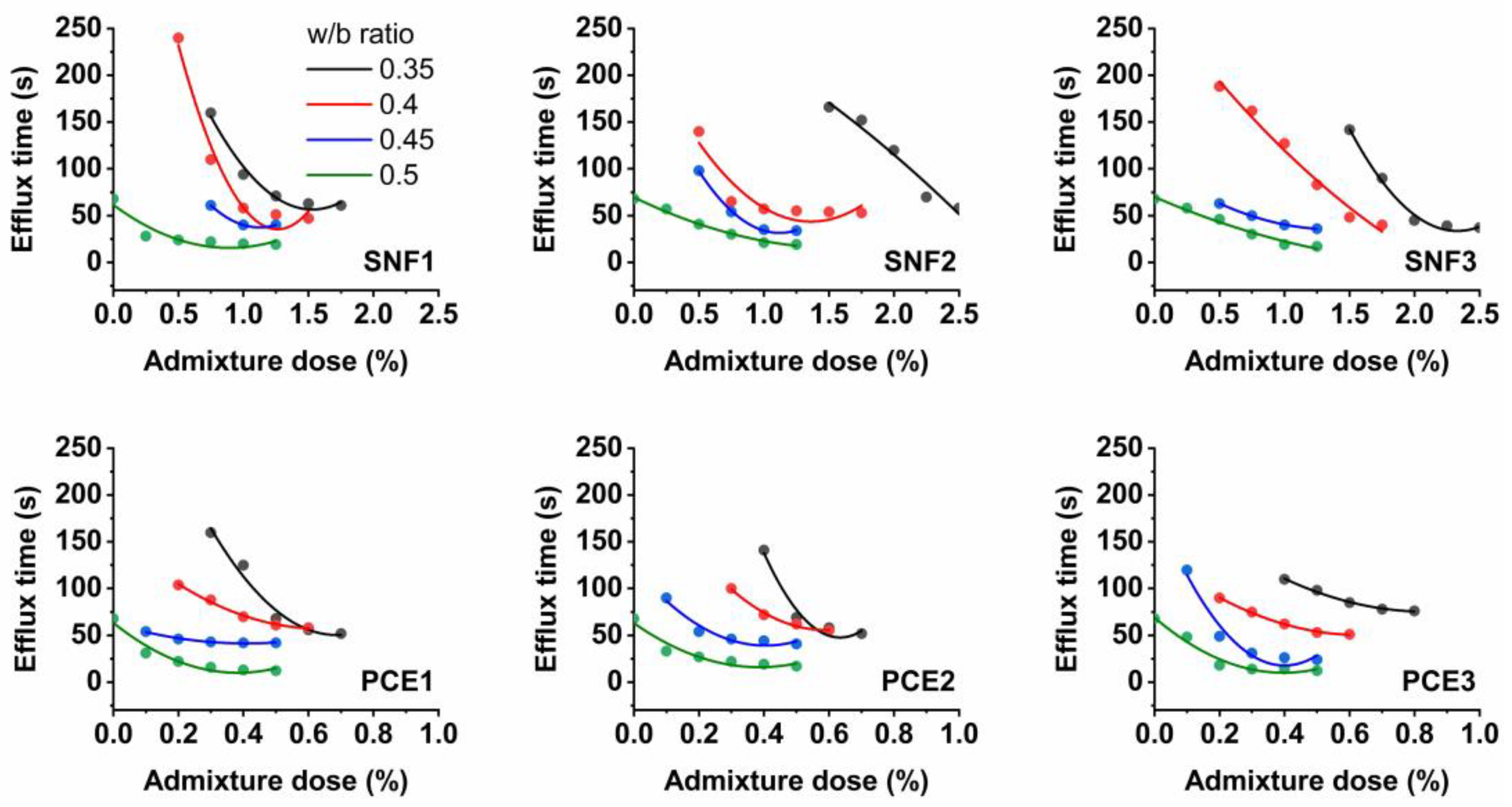
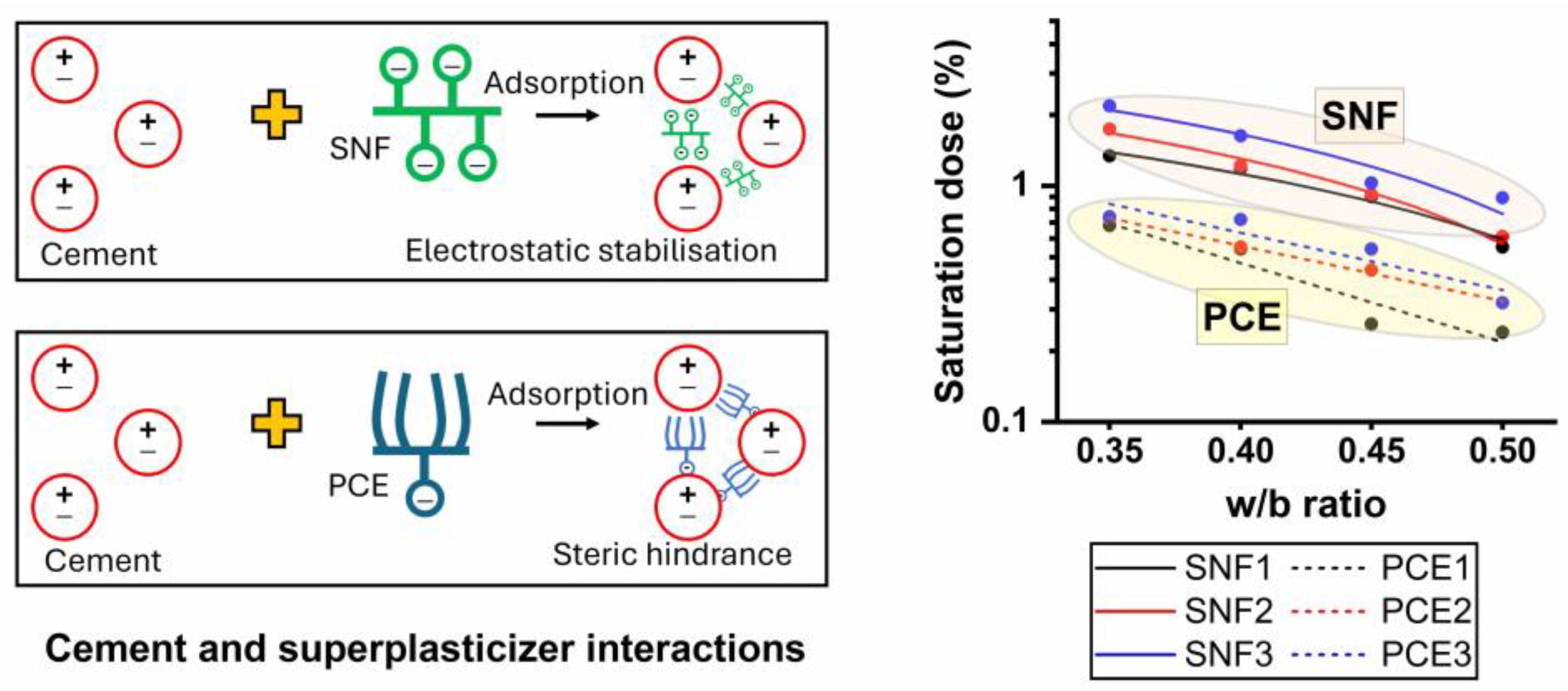
3.2. Mini-Slump Flow and Saturation Dose
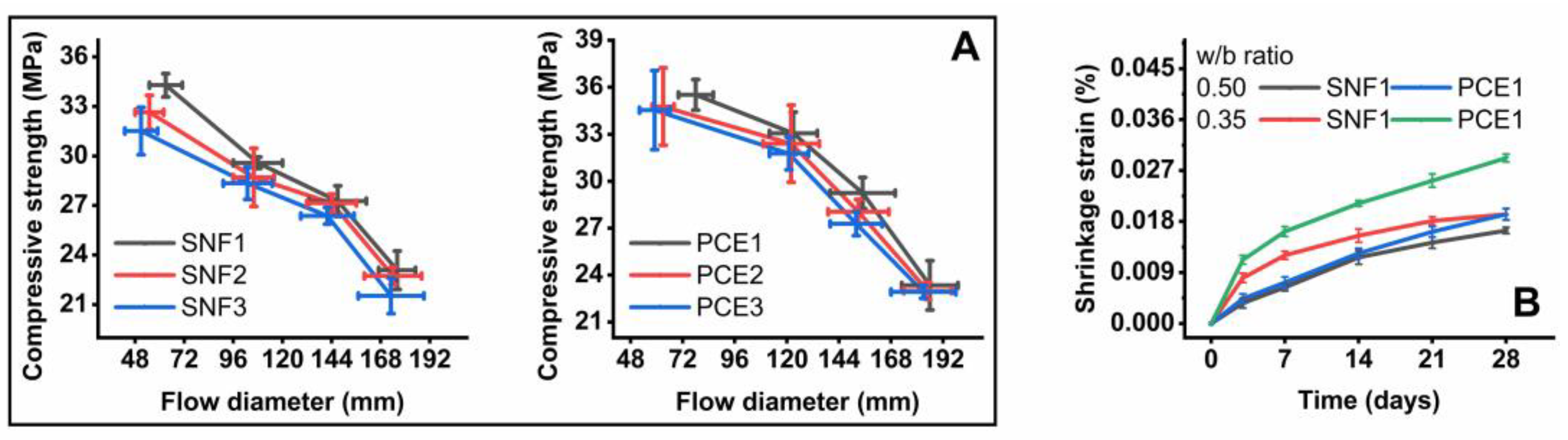
3.3. Mortar Performance
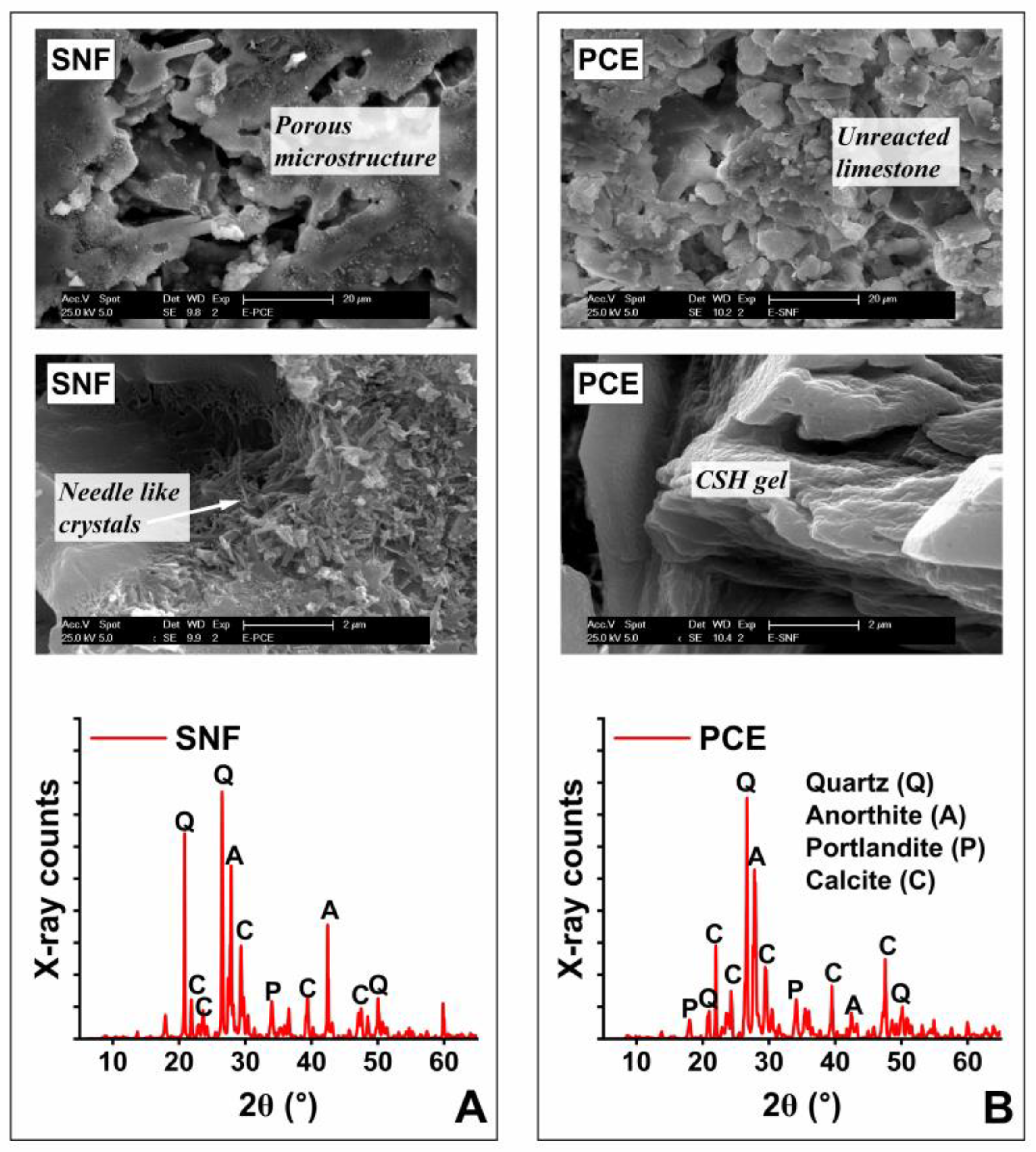
3.4. Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benhelal, E.; Shamsaei, E.; Rashid, M.I. Challenges against CO2 abatement strategies in cement industry: A review. J. Environ. Sci. 2021, 104, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Y.; Han, R.; Yu, B.; Wei, Y.-M. Accounting process-related CO2 emissions from global cement production under Shared Socioeconomic Pathways. J. Clean. Prod. 2018, 184, 451–465. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Mocharla, I.R.; Selvam, R.; Govindaraj, V.; Muthu, M. Performance and life-cycle assessment of high-volume fly ash concrete mixes containing steel slag sand. Constr. Build. Mater. 2022, 341, 127814. [Google Scholar] [CrossRef]
- Das, P.; Cheela, V.R.S.; Mistri, A.; Chakraborty, S.; Dubey, B.; Barai, S.V. Performance assessment and life cycle analysis of concrete containing ferrochrome slag and fly ash as replacement materials—A circular approach. Constr. Build. Mater. 2022, 347, 128609. [Google Scholar] [CrossRef]
- Panesar, D.K. 3—Supplementary cementing materials. In Developments in the Formulation and Reinforcement of Concrete, 2nd ed.; Mindess, S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 55–85. [Google Scholar]
- Ruiz-Sánchez, A.; Sánchez-Polo, M.; Rozalen, M. Waste marble dust: An interesting residue to produce cement. Constr. Build. Mater. 2019, 224, 99–108. [Google Scholar] [CrossRef]
- Woźniak, Z.Z.; Chajec, A.; Sadowski, Ł. Effect of the Partial Replacement of Cement with Waste Granite Powder on the Properties of Fresh and Hardened Mortars for Masonry Applications. Materials 2022, 15, 9066. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, Y.; Santhanam, M.; Kaladharan, G.; Ramanathan, S. Towards ternary binders involving limestone additions—A review. Cem. Concr. Res. 2021, 143, 106396. [Google Scholar] [CrossRef]
- Wang, D.; Shi, C.; Farzadnia, N.; Shi, Z.; Jia, H.; Ou, Z. A review on use of limestone powder in cement-based materials: Mechanism, hydration and microstructures. Constr. Build. Mater. 2018, 181, 659–672. [Google Scholar] [CrossRef]
- Lollini, F.; Redaelli, E.; Bertolini, L. Effects of portland cement replacement with limestone on the properties of hardened concrete. Cem. Concr. Compos. 2014, 46, 32–40. [Google Scholar] [CrossRef]
- Panda, D.; Gotecha, S.; Roy, B. Marginal grade limestone resources upgradation: A challenge for indian cement industry. In Proceedings of the International Seminar on Mineral Processing Technology, Bhubaneswar, India, 19–21 February 2004. [Google Scholar]
- Lothenbach, B.; Le Saout, G.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Gupta, S.; Mohapatra, B.N.; Bansal, M. Development of Portland limestone cement in India using different composition of cement and marginal grade limestone: A sustainable approach. J. Indian Chem. Soc. 2022, 99, 100724. [Google Scholar] [CrossRef]
- Gyabaah, G.; Miyazawa, S.; Nito, N. Effects of gypsum and limestone powder on fresh properties and compressive strength of concrete containing ground granulated blast furnace slag under different curing temperatures. Constr. Mater. 2022, 2, 114–126. [Google Scholar] [CrossRef]
- Lushnikova, N.; Dvorkin, L. 25—Sustainability of gypsum products as a construction material. In Sustainability of Construction Materials, 2nd ed.; Khatib, J.M., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 643–681. [Google Scholar]
- Kim, J.; Na, S.; Zhang, W.; Sagawa, T.; Hama, Y. Effect of limestone powder and gypsum on the compressive strength mixture design of blast furnace slag blended cement mortar. J. Adv. Concr. Technol. 2017, 15, 67–80. [Google Scholar] [CrossRef]
- Gupta, S.; Mohapatra, B.N.; Bansal, M. A review on development of Portland limestone cement: A step towards low carbon economy for Indian cement industry. Curr. Res. Green Sustain. Chem. 2020, 3, 100019. [Google Scholar] [CrossRef]
- Nkinamubanzi, P.C.; Mantellato, S.; Flatt, R.J. 16—Superplasticizers in practice. In Science and Technology of Concrete Admixtures; Aïtcin, P.-C., Flatt, R.J., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 353–377. [Google Scholar]
- Meddah, M.S.; Lmbachiya, M.C.; Dhir, R.K. Potential use of binary and composite limestone cements in concrete production. Constr. Build. Mater. 2014, 58, 193–205. [Google Scholar] [CrossRef]
- Sanjuán, M.Á.; Andrade, C. Reactive powder concrete: Durability and applications. Appl. Sci. 2021, 11, 5629. [Google Scholar] [CrossRef]
- Kakali, G.; Tsivilis, S.; Aggeli, E.; Bati, M. Hydration products of C3A, C3S and Portland cement in the presence of CaCO3. Cem. Concr. Res. 2000, 30, 1073–1077. [Google Scholar] [CrossRef]
- Voglis, N.; Kakali, G.; Chaniotakis, E.; Tsivilis, S. Portland-limestone cements. Their properties and hydration compared to those of other composite cements. Cem. Concr. Compos. 2005, 27, 191–196. [Google Scholar]
- De Schutter, G.; Ezzat, M.; Lesage, K.; Hoogenboom, R. Responsive superplasticizers for active rheology control of cementitious materials. Cem. Concr. Res. 2023, 165, 107084. [Google Scholar] [CrossRef]
- IS 12269; Ordinary Portland Cement, 53 Grade—Specification. Bureau of Indian Standards: New Delhi, India, 2013; 10p.
- IS 383; Coarse and Fine Aggregates for Concrete—Specification. Bureau of Indian Standards: New Delhi, India, 2016; 21p.
- IS 2386; Methods of Test for Aggregates for Concrete—Specific Gravity, Density, Voids, Absorption and Bulking. Bureau of Indian Standards: New Delhi, India, 1963; p. 17.
- IS 9103; Concrete Admixtures—Specifications. Bureau of Indian Standards: New Delhi, India, 2004; p. 17.
- ASTM C1702; Standard Test Method for Measurement of Heat of Hydration of Hydraulic Cementitious Materials Using Isothermal Conduction Calorimetry. ASTM International: West Conshohocken, PA, USA, 2017; p. 9.
- IS 4031; Methods of Physical Tests for Hydraulic Cement. Bureau of Indian Standards: New Delhi, India, 1988; p. 5.
- IS 5513; Vicat Apparatus—Specification. Bureau of Indian Standards: New Delhi, India, 1996; p. 12.
- Tan, Z.; Bernal, S.A.; Provis, J.L. Reproducible mini-slump test procedure for measuring the yield stress of cementitious pastes. Mater. Struct. 2017, 50, 235. [Google Scholar] [CrossRef] [PubMed]
- EN 445; Grout for Prestressing Tendons—Test Methods. European Committee for Standardization: Brussels, Belgium, 2007; p. 15.
- IS 516-1; Hardened Concrete—Methods of Test. Compressive Flexural Split Tensile Strength. Bureau of Indian Standards: New Delhi, India, 2021; p. 20.
- ASTM C157; Standard Test Method for Length Change of Hardened Hydraulic-Cement Mortar and Concrete. ASTM International: West Conshohocken, PA, USA, 2017; p. 8.
- Livesey, P. Strength characteristics of Portland-limestone cements. Constr. Build. Mater. 1991, 5, 147–150. [Google Scholar] [CrossRef]
- Aïtcin, P.C. 1—The importance of the water–cement and water–binder ratios. In Science and Technology of Concrete Admixtures; Aïtcin, P.-C., Flatt, R.J., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 3–13. [Google Scholar]
- Hsu, K.-C.; Chiu, J.-J.; Chen, S.-D.; Tseng, Y.-C. Effect of addition time of a superplasticizer on cement adsorption and on concrete workability. Cem. Concr. Compos. 1999, 21, 425–430. [Google Scholar] [CrossRef]
- Lei, L.; Hirata, T.; Plank, J. 40 years of PCE superplasticizers—History, current state-of-the-art and an outlook. Cem. Concr. Res. 2022, 157, 106826. [Google Scholar] [CrossRef]
- Nair, N.; Mohammed Haneefa, K.; Santhanam, M.; Gettu, R. A study on fresh properties of limestone calcined clay blended cementitious systems. Constr. Build. Mater. 2020, 254, 119326. [Google Scholar] [CrossRef]
- Hewlett, P.C.; Justnes, H.; Edmeades, R.M. 14—Cement and concrete admixtures. In Lea’s Chemistry of Cement and Concrete, 5th ed.; Hewlett, P.C., Liska, M., Eds.; Butterworth-Heinemann: Oxford, UK, 2019; pp. 641–698. [Google Scholar]
- da Silva, O.; Gomes, P.; Morais, K. Analytical method for determining the saturation point of superplasticizers in cement pastes using the Marsh cone. Cerâmica 2019, 65, 252–260. [Google Scholar] [CrossRef]
- Gomes, P.C.; Gettu, R.; Agullo, L.; Bernad, C. Experimental optimization of high-strength self-compacting concrete. In Proceedings of the Second International Symposium on Self-compacting Concrete, Tokio, Japan, 23–25 October 2001; p. 377386. [Google Scholar]
- Zhang, J.; Han, G.; Shen, D.; An, X.; Mendomo Meye, S. A new model to predict the optimal mix design of self-compacting concrete considering powder properties and superplasticizer type. J. Mater. Res. Technol. 2022, 19, 3980–3993. [Google Scholar] [CrossRef]
- Schober, I.; Mader, U. Compatibility of polycarboxylate superplasticizers with cements and cementitious blends. In Proceedings of the SP-217, Seventh CANMET/ACI International Conference, Berlin, Germany, 20–23 October 2003. [Google Scholar]
- Collepardi, M. Admixtures used to enhance placing characteristics of concrete. Cem. Concr. Compos. 1998, 20, 103–112. [Google Scholar] [CrossRef]
- Bhandari, I.; Kumar, R.; Sofi, A.; Nighot, N.S. A systematic study on sustainable low carbon cement—Superplasticizer interaction: Fresh, mechanical, microstructural and durability characteristics. Heliyon 2023, 9, e19176. [Google Scholar] [CrossRef]
- Ferrari, L.; Bernard, L.; Deschner, F.; Kaufmann, J.; Winnefeld, F.; Plank, J. Characterization of polycarboxylate-ether based superplasticizer on cement clinker surfaces. J. Am. Ceram. Soc. 2012, 95, 2189–2195. [Google Scholar] [CrossRef]
- Kondraivendhan, B.; Bhattacharjee, B. Strength and w/c ratio relationship of cement based materials through pore features. Mater. Charact. 2016, 122, 124–129. [Google Scholar] [CrossRef]
- Sua-Iam, G.; Makul, N. Effect of superplasticizer type and dosage on early-age shrinkage of portland cement and rice husk ash pastes. Adv. Mater. Res. 2012, 450, 407–412. [Google Scholar] [CrossRef]
- Bentz, D.P. Modeling the influence of limestone filler on cement hydration using CEMHYD3D. Cem. Concr. Compos. 2006, 28, 124–129. [Google Scholar] [CrossRef]
- Poppe, A.-M.; De Schutter, G. Cement hydration in the presence of high filler contents. Cem. Concr. Res. 2005, 35, 2290–2299. [Google Scholar] [CrossRef]
- Garrault, S.; Finot, E.; Lesniewska, E.; Nonat, A. Study of C-S-H growth on C3S surface during its early hydration. Mater. Struct. 2005, 38, 435–442. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Darweesh, H.H.M.; Abou-El-Anwar, E.A.; Mekky, H.S. Addition of limestone at the expense of gypsum in Portland cement. Int. Ceram. Rev. 2018, 67, 18–27. [Google Scholar] [CrossRef]
- Bonavetti, V.; Donza, H.; Menéndez, G.; Cabrera, O.; Irassar, E.F. Limestone filler cement in low w/c concrete: A rational use of energy. Cem. Concr. Res. 2003, 33, 865–871. [Google Scholar] [CrossRef]

| Sample (%) | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | Na2O | K2O | SO3 | Cl | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 61.77 | 20.01 | 4.97 | 4.4 | 1.22 | 0.14 | 0.28 | 2.56 | 0.01 | 4.64 |
| Limestone | 39.27 | 15.77 | 3.37 | 0.43 | 1.56 | 0.07 | 0.17 | 0.24 | - | 39.12 |
| Gypsum | 29.81 | 3.34 | 1.35 | 0.49 | 1.33 | - | - | 43.85 | 0.01 | 19.82 |
| Cement (g) | Limestone (g) | Gypsum (g) | Sand (g) |
|---|---|---|---|
| 630 | 350 | 20 | 2500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthu, M.; Kumar, B.G.; Ukrainczyk, N.; Sadowski, Ł.; Koenders, E. Impact of Superplasticizers on the Performance of Low-Grade Limestone-Based Cement Mixes. Materials 2024, 17, 2500. https://doi.org/10.3390/ma17112500
Muthu M, Kumar BG, Ukrainczyk N, Sadowski Ł, Koenders E. Impact of Superplasticizers on the Performance of Low-Grade Limestone-Based Cement Mixes. Materials. 2024; 17(11):2500. https://doi.org/10.3390/ma17112500
Chicago/Turabian StyleMuthu, Murugan, Boddapati Ganesh Kumar, Neven Ukrainczyk, Łukasz Sadowski, and Eddie Koenders. 2024. "Impact of Superplasticizers on the Performance of Low-Grade Limestone-Based Cement Mixes" Materials 17, no. 11: 2500. https://doi.org/10.3390/ma17112500
APA StyleMuthu, M., Kumar, B. G., Ukrainczyk, N., Sadowski, Ł., & Koenders, E. (2024). Impact of Superplasticizers on the Performance of Low-Grade Limestone-Based Cement Mixes. Materials, 17(11), 2500. https://doi.org/10.3390/ma17112500










