Effect of Boron Content in LiOH Solutions on the Corrosion Behavior of Zr-Sn-Nb Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Alloy and Materials
2.2. Corrosion Experiment
2.3. Microscopic Analysis after Corrosion
3. Results
3.1. Effect of Boron Injection on Corrosion Behavior of Zr-Sn-Nb Alloy
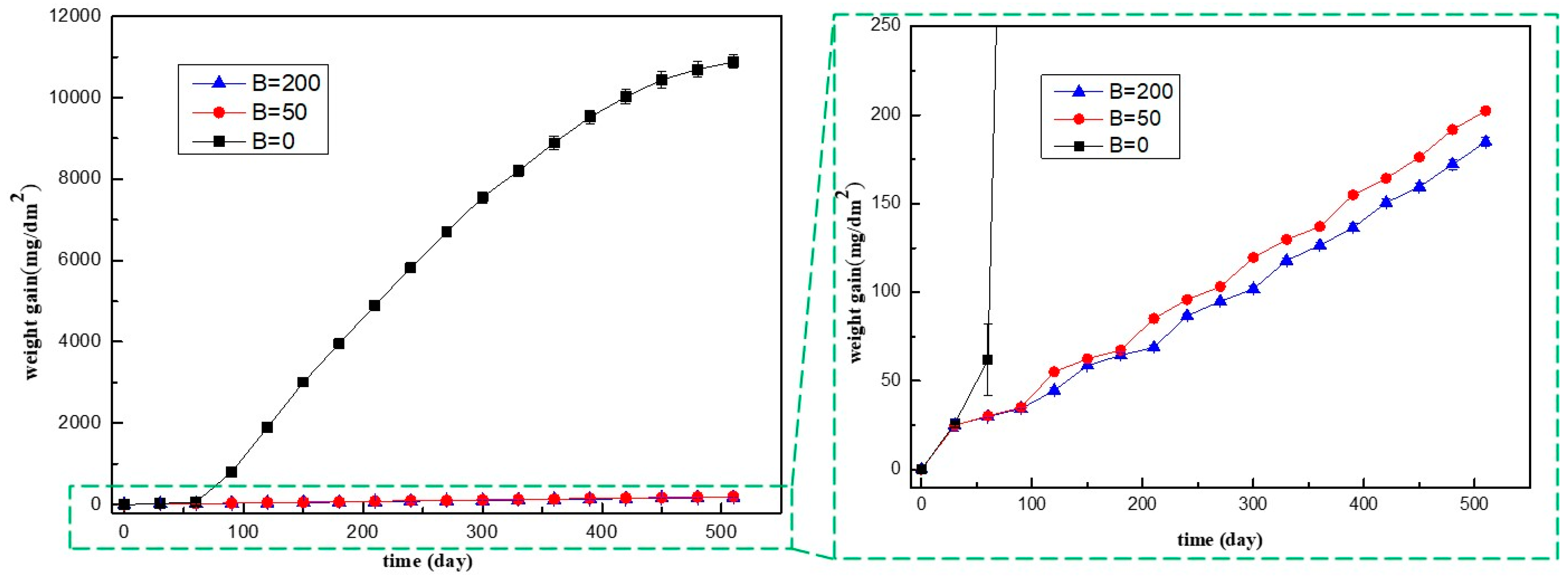
3.1.1. Corrosion Kinetics
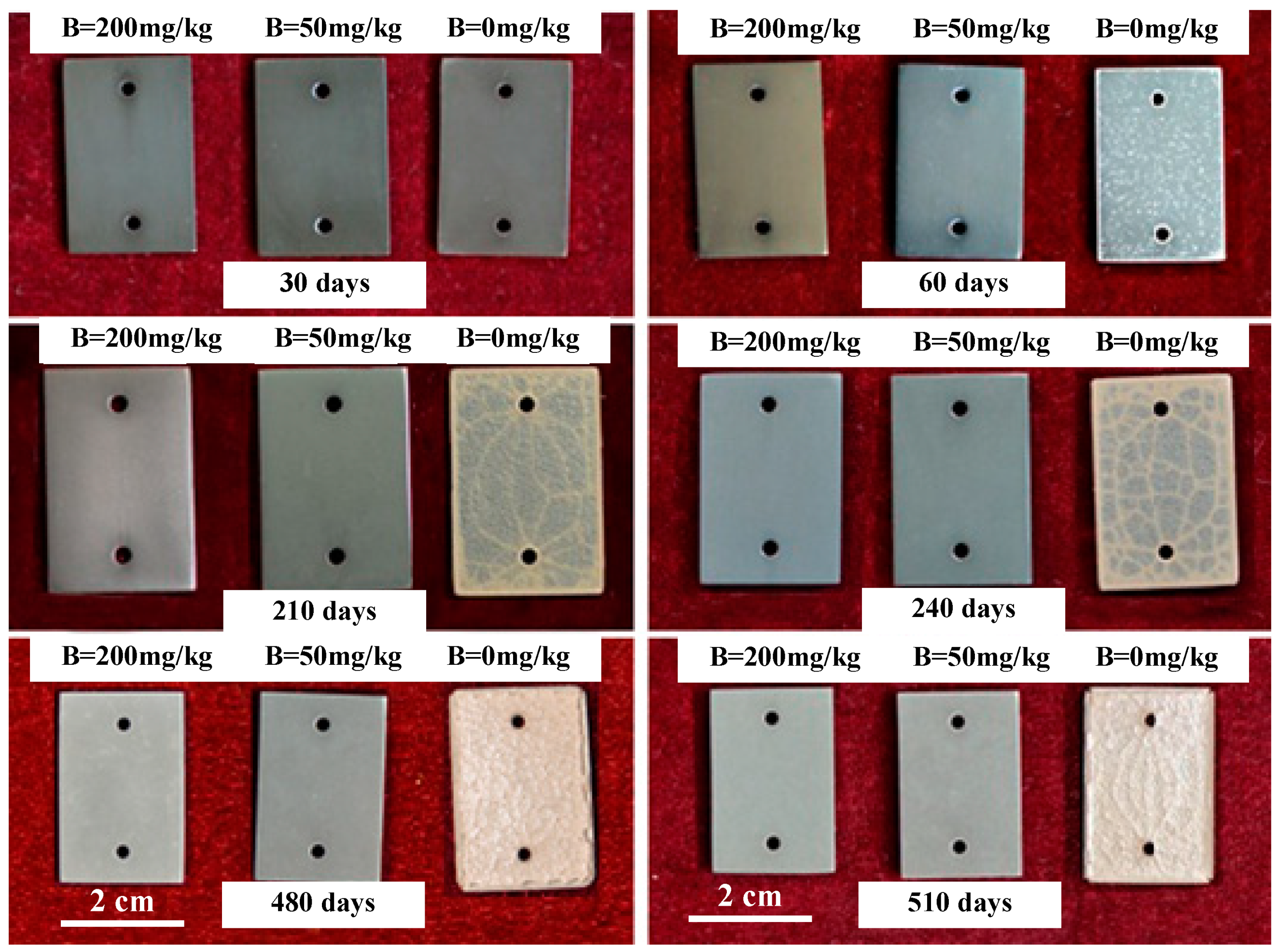
3.1.2. Top Appearance
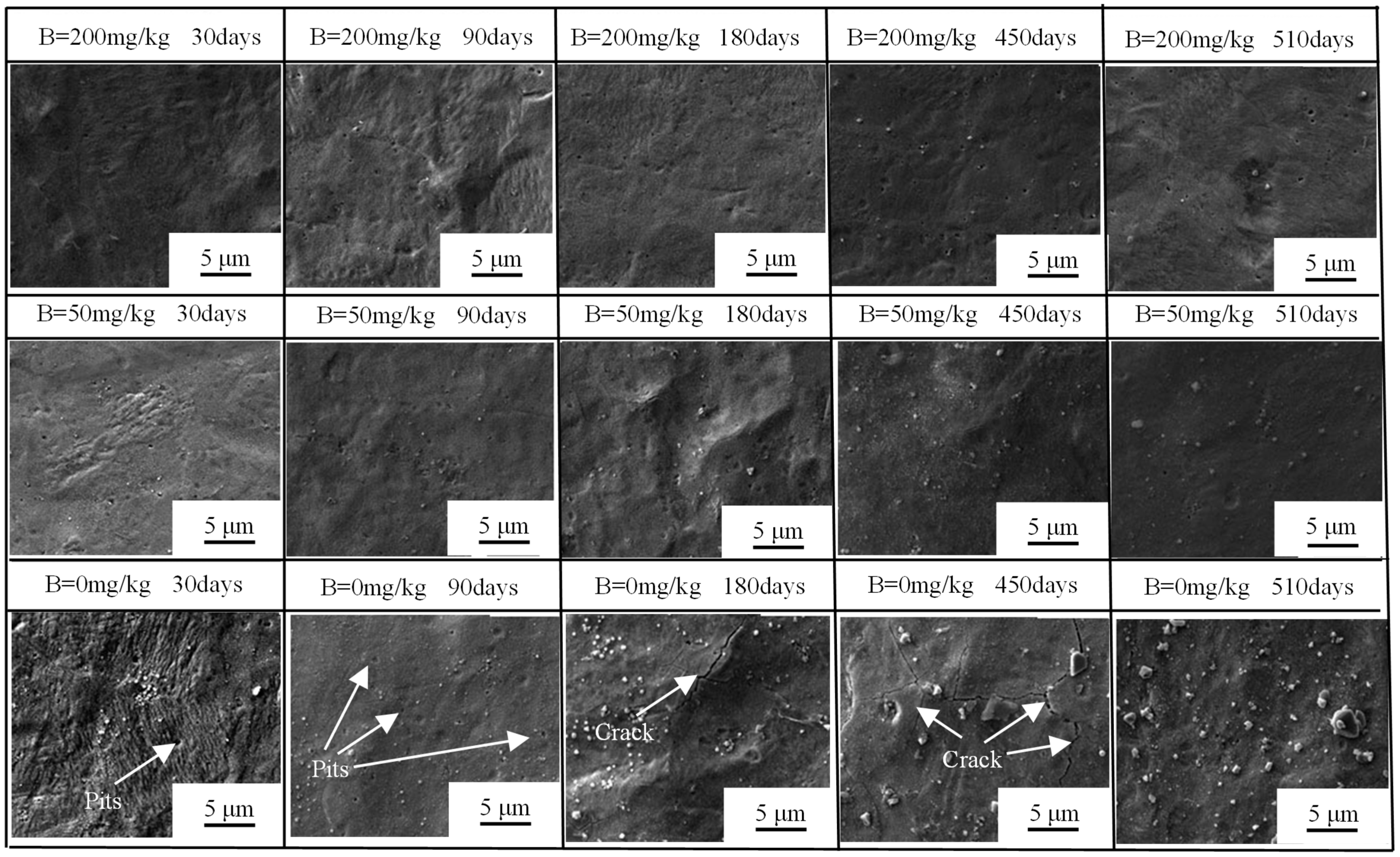
3.1.3. SEM Morphology of Oxide Films
3.1.4. Hydrogen Absorption Concentrations
3.1.5. Hydrides Morphology
3.2. Effect of Boron Injection on Microstructure of Oxide Films of Zr-Sn-Nb Alloys
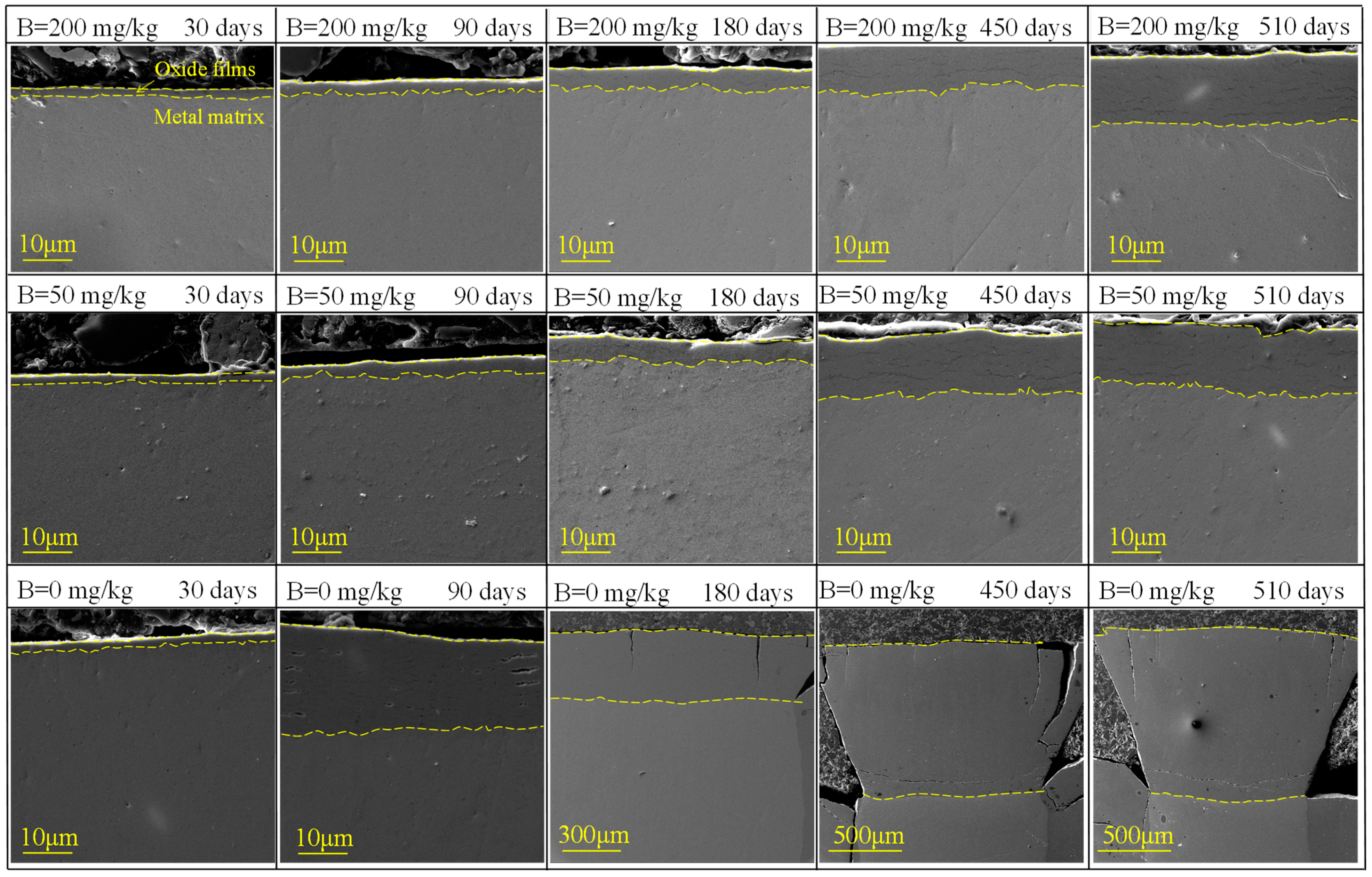
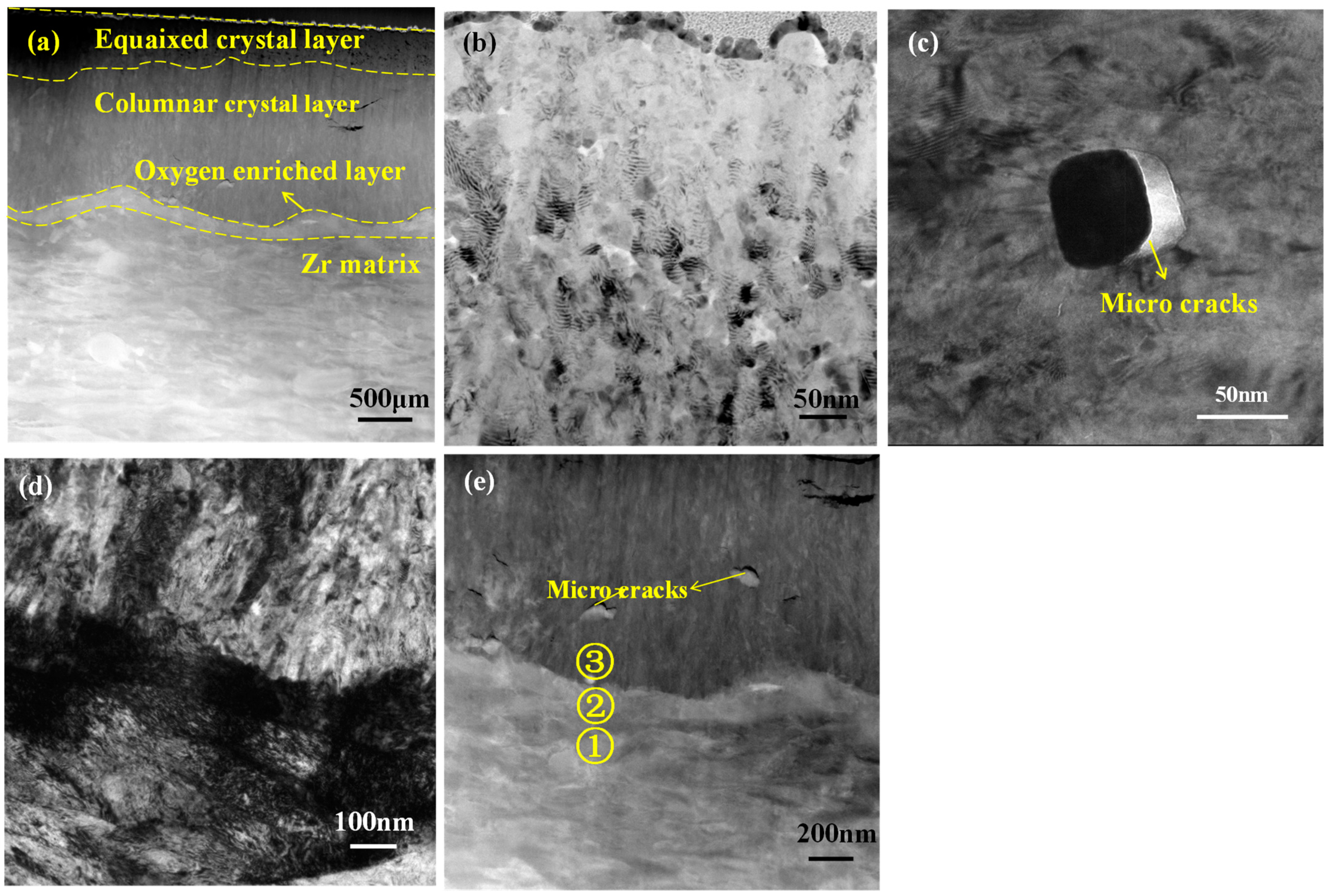
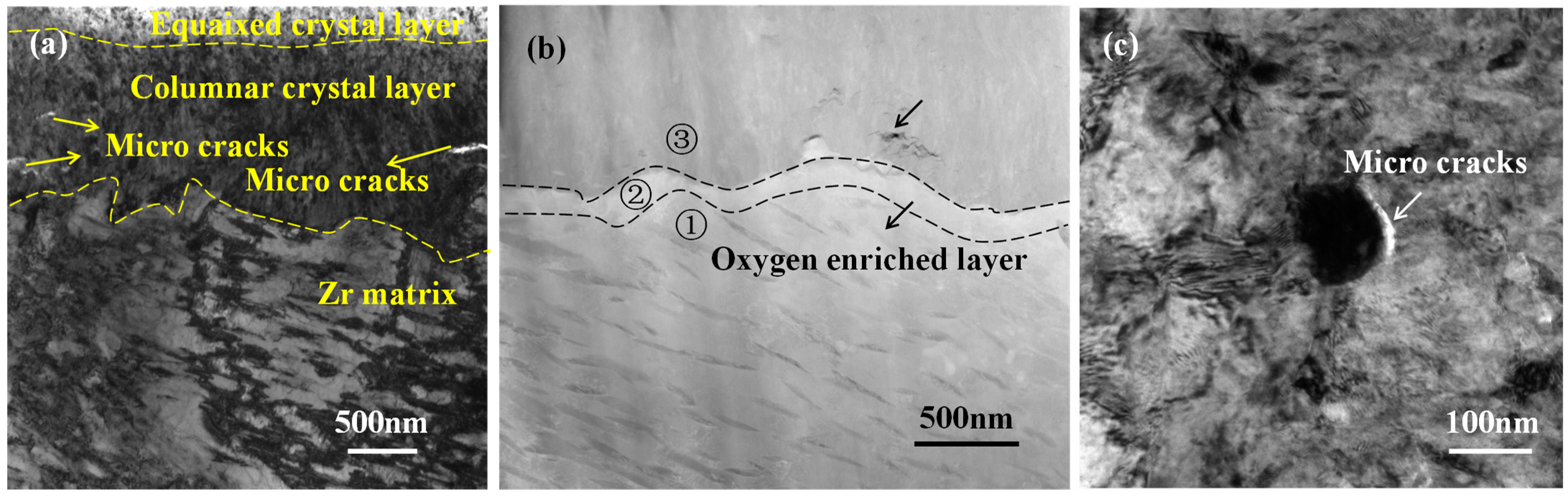
3.2.1. Cross-Sectional Microstructures of Oxide Films
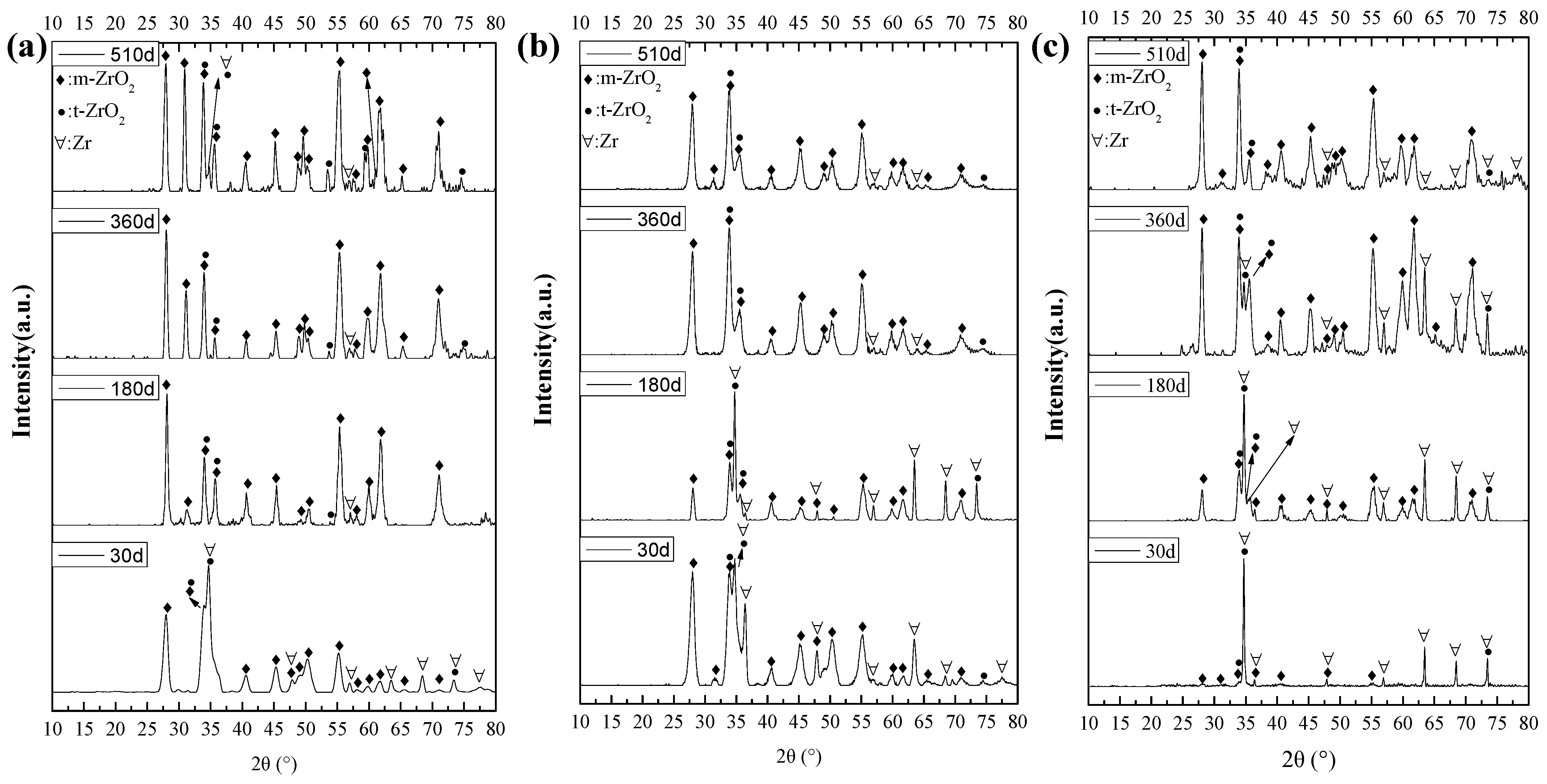
3.2.2. Crystal Structure of Oxide Films
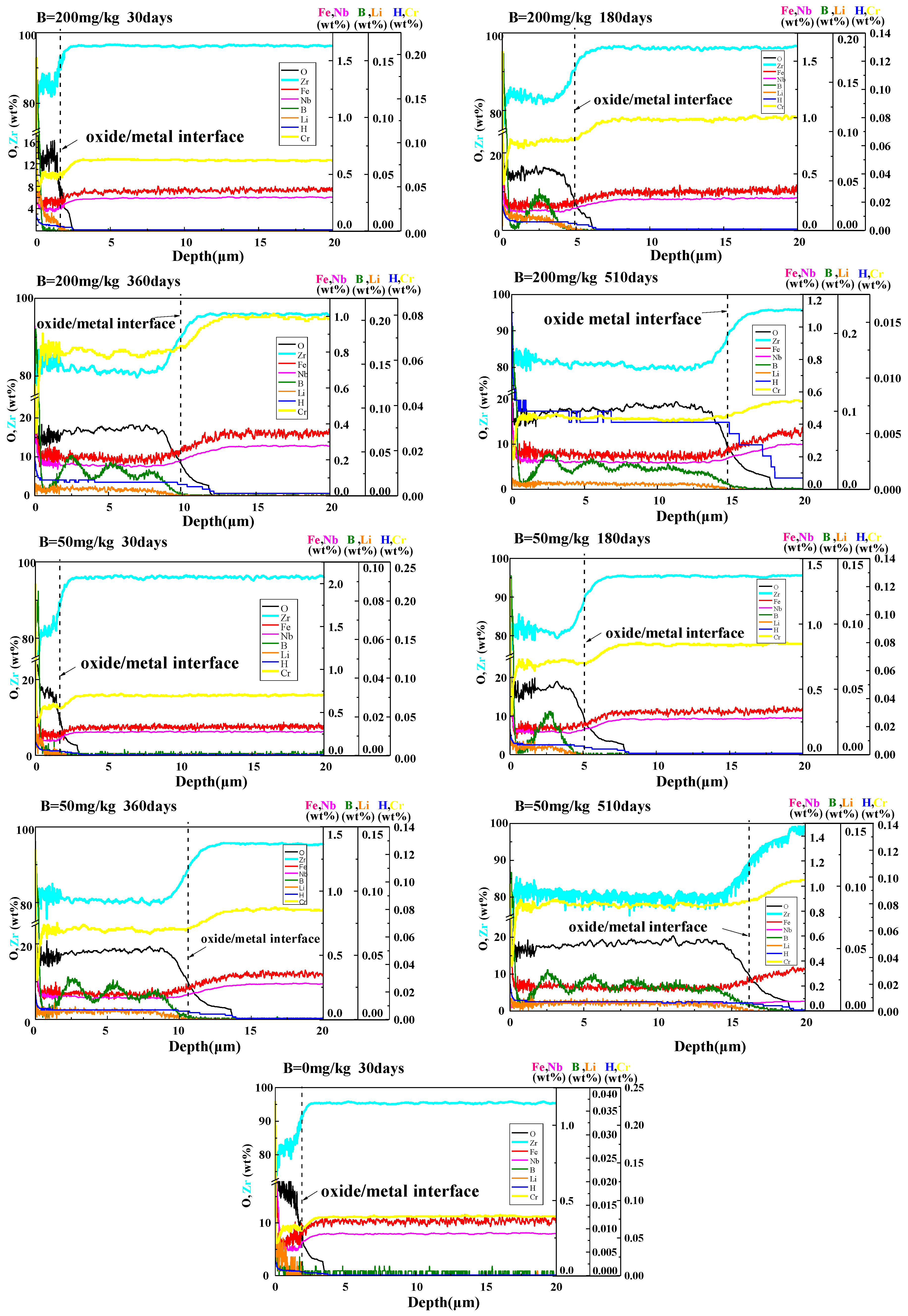
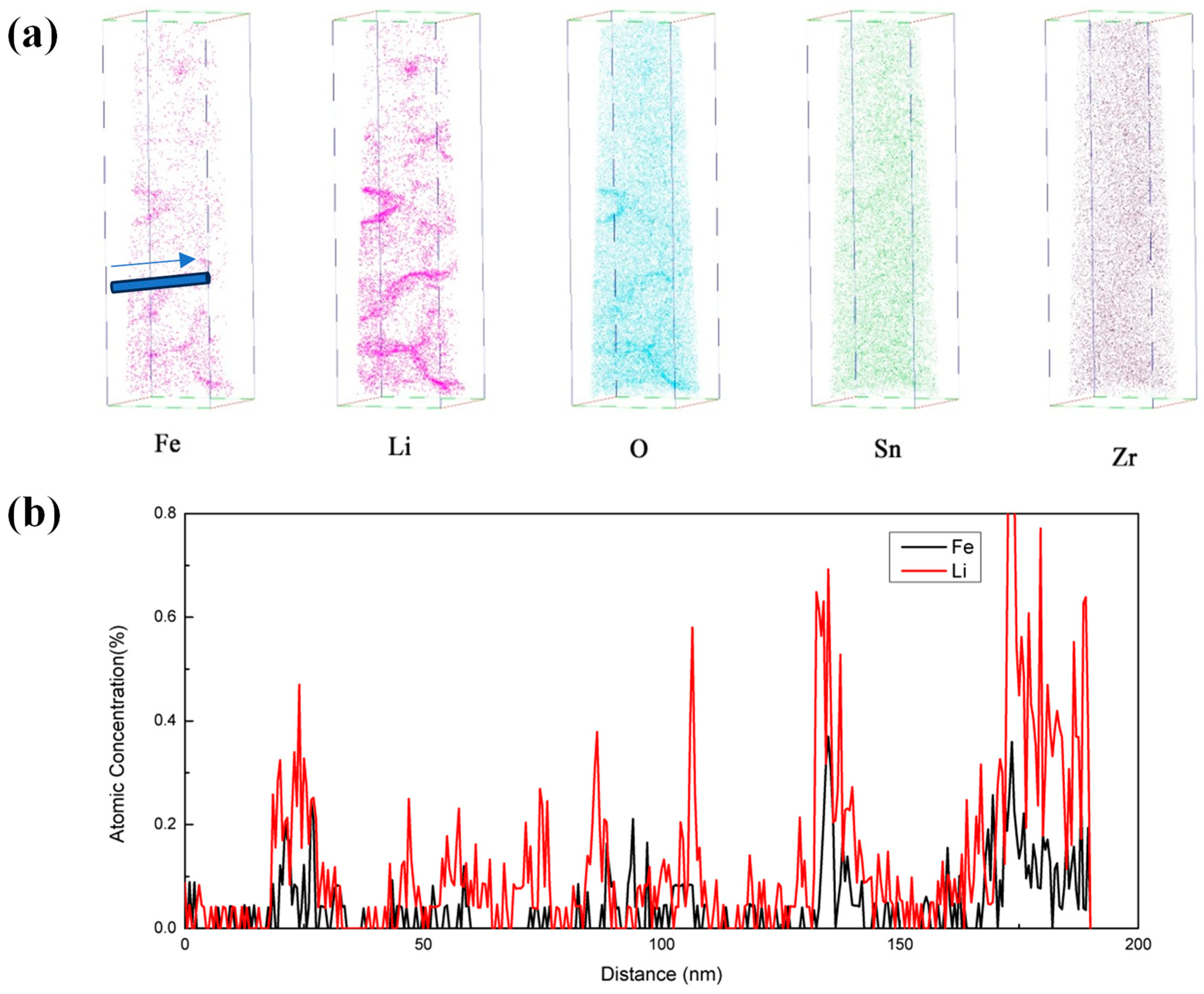
3.2.3. Distribution of Elements in Oxide Films
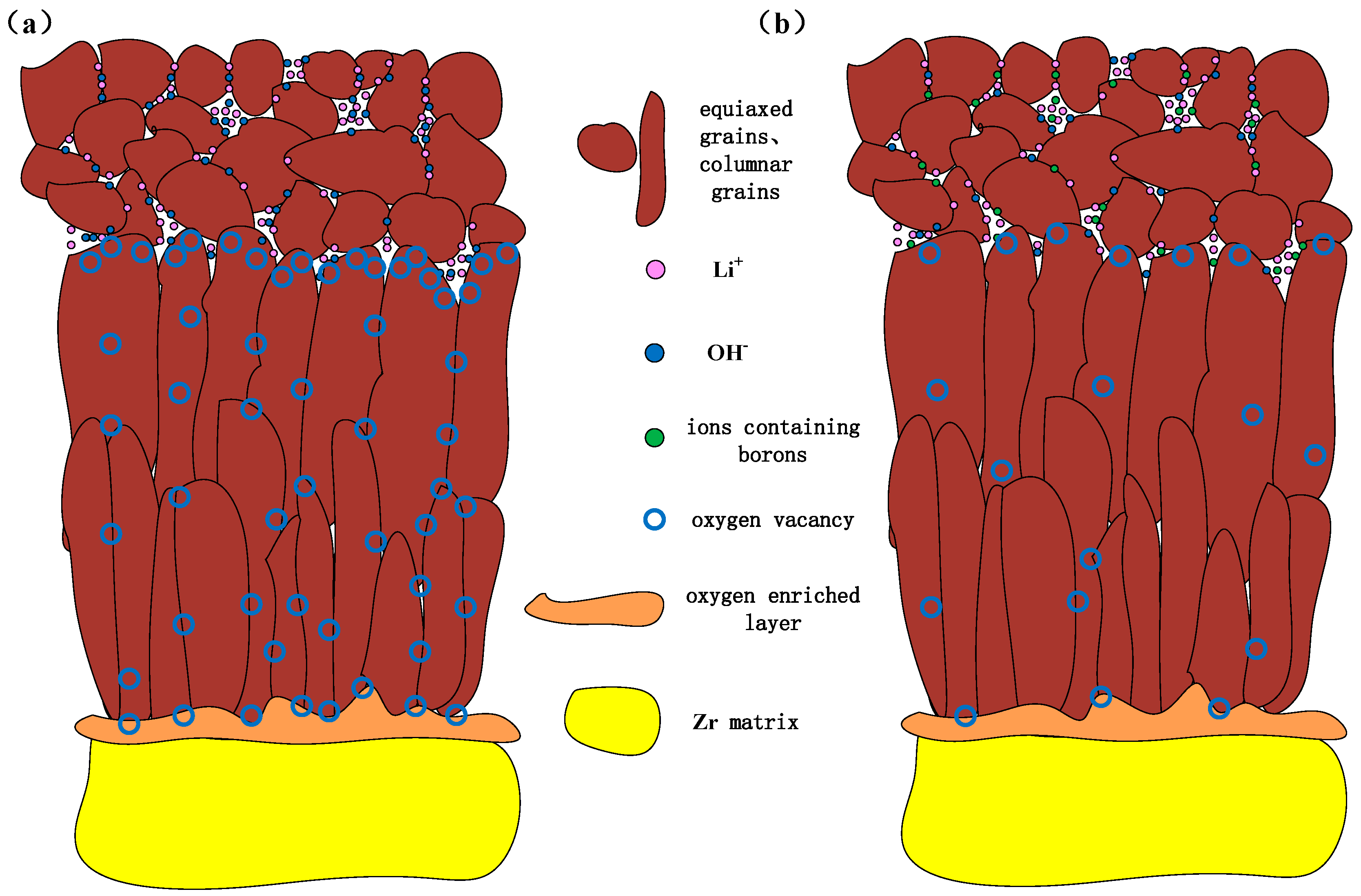
3.3. Accelerated Corrosion Mechanism Induced by Li and Corrosion Inhibition Mechanism of Injecting Boron
- Li+ and OH− diffuse from aqueous solutions to the top of columnar crystal through pores of equiaxed crystal. Moreover, the concentration of corrosive medium is prone to occur in these pores, leading to the enrichment of Li+ and OH− at the top of columnar crystals.
- Li+ and OH− diffuse along the grain boundaries of columnar crystal towards by solid-state diffusion, resulting in a uniform distribution of Li in the depth direction. In oxide films, columnar crystals also contain micropores [30]. Therefore, Li+ and OH- also tend to be enriched near the micropores.
- Oxides exhibit acidity or neutrality in solutions, while their surfaces exhibit electronegativity in alkaline solutions [31], requiring the neutralization of cation in solutions. OH- enriched at the top of columnar crystal makes the local environment appear alkaline, promoting negative charge accumulate on the surface of the nearby columnar crystal. At this time, Li+ adsorbs on the surface of columnar crystal, which is conducive to its diffusion to the interior of columnar crystal.
- When Li+ is adsorbed on the surface of columnar crystal, it can easily replace Zr4+ in ZrO2 due to the very close size of Li+ and Zr4+. In addition, Li+ continuously diffuse along grain boundaries to the O/M interface.
- When Li+ replaces Zr4+, 1.5 oxygen vacancies form near it [32]. Therefore, due to the presence of a large amount of Li+ at the top of columnar crystals, grain boundaries and micropores, a large number of oxygen vacancies will also be generated nearby.
- Oxygen in solutions can occupy these oxygen vacancies easily through short-range diffusion, resulting in an excess of oxygen in ZrO2 lattice. These oxygen diffuse rapidly along grain boundaries to O/M interface through solid-state diffusion, accelerating corrosion [33].
4. Conclusions
- In B = 0 mg/kg solutions, Zr-Sn-Nb alloys were corroded severely, and oxide films showed significant cracking after 180 days. After 510 days, the weight gain was 10879.01 mg/dm2. After injecting 50 mg/kg and 200 mg/kg boron in LiOH solutions, the surface of oxide films maintained uniform and dense throughout the entire test. After 510 days, the weight gain increased to 202.38 mg/dm2 in B = 50 mg/kg solutions, and 184.77 mg/dm2 in B = 200 mg/kg solutions. Injecting boron significantly reduced the corrosion rate, hydrogen concentration, and length of Zr-Sn-Nb alloys.
- The accelerated corrosion mechanism induced by the Li is as follows: Li+ tends to be incorporated in oxides in an alkaline environment, leading to the generation of a large number of oxygen vacancies. Oxygen vacancies carry oxygen from the solutions to the O/M interface, accelerating corrosion.
- The corrosion inhibition mechanism of injecting boron is as follows: after B3+ incorporated in oxides films, the generation of oxygen vacancies is inhibited. This leads to insufficiency of oxygen vacancies, thereby slowing down corrosion. However, when boron concentration exceeds the critical value, the B3+ incorporated in the ZrO2 lattice will reach saturation, and corrosion inhibition effect will reach the upper limit. Continuing to increase the boron concentration cannot significantly improve corrosion inhibition effect.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duan, Z.; Yang, H.; Satoh, Y.; Murakami, K.; Kano, S.; Zhao, S.; Shen, J.; Abe, H. Current status of materials development of nuclear fuel cladding tubes for light water reactors. Nucl. Eng. Des. 2017, 316, 131–150. [Google Scholar] [CrossRef]
- Wu, Z.; Jia, Y.; Dai, X.; Yi, W. Effect of Reprocess on Microstructure and Corrosion Resistance of Zr-Sn-Nb Alloy. Met. 2022, 12, 1822. [Google Scholar]
- Motta, A.; Couet, A.; Comstock, R. Corrosion of zirconium alloys used for nuclear fuel cladding. Annu. Rev. Mater. Res. 2015, 45, 311–343. [Google Scholar] [CrossRef]
- Yu, Z.; Werden, J.W.; Capps, N.A.; Linton, K.D.; Couet, A. (S)TEM/EDS study of native precipitates and irradiation induced Nb-rich platelets in high-burnup M5. J. Nucl. Mater. 2021, 554, 152667. [Google Scholar] [CrossRef]
- Zhou, B.; Yao, M.; Li, Z.; Wang, X.; Zhou, J.; Long, C.; Liu, Q.; Luan, B. Optimization of N18 zirconium alloy for fuel cladding of water reactors. J. Mater. Sci. Technol. 2012, 28, 606–613. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, Y.; Gong, B.; Tang, M.; Wu, Z. Influence of Ammonia on the Corrosion Behavior of a Zr-Sn-Nb Alloy in High Temperature Water. Front. Mater. 2022, 9, 910186. [Google Scholar] [CrossRef]
- Jeong, Y.; Baek, J.; Kim, S. Corrosion characteristics and oxide microstructure of Zircaloy-4 in aqueous alkali hydroxide solution. J. Nucl. Mater. 1999, 270, 322–333. [Google Scholar] [CrossRef]
- Han, J.; Rheem, K. The corrosion characteristics of Zircaloy-4 fuel cladding in LiOH-H3BO3 solutions. J. Nucl. Mater. 1994, 217, 197–199. [Google Scholar] [CrossRef]
- Cox, B.; Wu, C. Transient effects of lithium hydroxide and boric acid on zircaloy corrosion. J. Nucl. Mater. 1995, 22, 169–178. [Google Scholar] [CrossRef]
- Park, J.; Seung, J.; Choi, B.; Yong, H. Corrosion and oxide characteristics of Zr-1.5Nb-0.4Sn-0.2Fe-0.1Cr alloys in 360 °C pure water and LiOH solution. J. Nucl. Mater. 2008, 373, 343–350. [Google Scholar] [CrossRef]
- Yilmazbayhan, A.; Breval, E.; Motta, A.T.; Comstock, R.J. Transmission electron microscopy examination of oxide layers formed on Zr alloys. J. Nucl. Mater. 2006, 349, 265–281. [Google Scholar] [CrossRef]
- Yao, M.; Zhou, B.; Li, Q.; Liu, W.; Geng, X.; Lu, Y. A superior corrosion behavior of zircaloy-4 in lithiated water at 360 °C/18.6 mpa by beta-quenching. J. Nucl. Mater. 2008, 374, 197–203. [Google Scholar] [CrossRef]
- Oskarsson, M.; Ahlberg, E.; Pettersson, K. Oxidation of Zircaloy-2 and Zircaloy-4 in water and lithiated water at 360 °C. J. Nucl. Mater. 2001, 295, 97–108. [Google Scholar] [CrossRef]
- Xie, S.; Zhou, B.; Liang, X.; Li, Q.; Zhang, J. The distribution of Li ions in the oxide film formed on Zircaloy-4 corroded in lithiated water at 633 k. Materials 2020, 13, 873. [Google Scholar] [CrossRef]
- Wei, K.; Wang, X.; Zhu, M.; Guan, H.; Zhang, J. Effects of Li, B and H elements on corrosion property of oxide films on ZIRLO alloy in 300°C/14MPa lithium borate buffer solutions. Corros. Sci. 2020, 181, 109216. [Google Scholar] [CrossRef]
- Kim, T.; Choi, K.; Yoo, S.; Lee, Y. Influence of dissolved hydrogen on the early stage corrosion behavior of zirconium alloys in simulated light water reactor coolant conditions. Corros Sci. 2018, 131, 235–244. [Google Scholar] [CrossRef]
- Cox, B.; Wu, C. Dissolution of zirconium oxide films in 300 °C LiOH. J. Nucl. Mater. 1993, 199, 272–284. [Google Scholar] [CrossRef]
- ASTM G2/G2M-06(2011)e1.2011; Standard Test Method for Corrosion Testing of Products of Zirconium, Hafnium, and Their Alloys in Water at 680°F(360°C) or in Steam at 750°F (400°C). ASTM: West Conshohocken, PA, USA, 2011.
- Liu, J.; Yu, H.; Karamched, P.; Hu, J.; Grovenor, C. Mechanism of the α-Zr to hexagonal-ZrO transformation and its impact on the corrosion performance of nuclear Zr alloys. Acta Mater. 2019, 179, 328–341. [Google Scholar] [CrossRef]
- Polatidis, E.; Frankel, P.; Wei, J.; Klaus, M.; Comstock, R.; Ambard, A.; Lyon, S.; Cottis, R.; Preuss, M. Residual stresses and tetragonal phase fraction characterisation of corrosion tested zircaloy-4 using energy dispersive synchrotron x-ray diffraction. J. Nucl. Mater. 2013, 432, 102–112. [Google Scholar] [CrossRef]
- Pecheur, D.; Godlewski, J.; Billot, P.; Thomazet, J. Microstructure of oxide films formed during the waterside corrosion of the zircaloy-4 cladding in lithiated environment. In Zirconium in the Nuclear Industry: 15th International Symposium; ASTM STP 1505; Sunriver: Philadelphia, OR, USA, 1996. [Google Scholar]
- Proff, C.; Abolhassani, S.; Lemaignan, C. Oxidation behaviour of zirconium alloys and their precipitates—A mechanistic study. J. Nucl. Mater. 2013, 432, 222–238. [Google Scholar] [CrossRef]
- Ni, N. Study of Oxidation Mechanisms of Zirconium Alloys by Electron Microscopy. Ph.D. Thesis, Oxford University, Oxford, UK, 2011. [Google Scholar]
- Igawa, N.; Ishii, Y. Crystal structure of metastable tetragonal zirconia up to 1473 K. J. Am. Ceram. Soc. 2001, 84, 1169–1171. [Google Scholar] [CrossRef]
- Katz, G. X-ray diffraction powder pattern of metastable cubic ZrO2. J. Am. Ceram. Soc. 1971, 54, 531. [Google Scholar] [CrossRef]
- Cao, X.; Yao, M.; Peng, J.; Zhou, B. Corrosion behavior of Zr(Fex,Cr1-x)2 alloys in 400 °C superheated steam. Acta Metall. Sin. (Engl. Lett.) 2011, 47, 882–886. [Google Scholar]
- Hu, J.; Aarholt, T.; Setiadinata, B.; Li, K.; Grovenor, C. A multi-technique study of “barrier layer” nano-porosity in Zr oxides during corrosion and hydrogen pickup using (S)TEM, TKD, APT and Nano SIMS. Corros. Sci. 2019, 158, 108109. [Google Scholar] [CrossRef]
- Xie, S.; Zhou, B.; Liang, X.; Liu, W.; Li, H.; Li, Q.; Yao, M.; Zhang, J. A novel mechanism for nodular corrosion of zircaloy-4 corroded in 773 K superheated steam. Corros. Sci. 2017, 126, 44–54. [Google Scholar] [CrossRef]
- Macdonald, D. Some personal adventures in passivity—A review of the point defect model for film growth. Russ. J. Electrochem. 2012, 48, 235–258. [Google Scholar] [CrossRef]
- Hu, J.; Liu, J.; Lozano-Perez, S.; Grovenor, C.; Mader, E. Hydrogen pickup during oxidation in aqueous environments: The role of nano-pores and nano-pipes in zirconium oxide films. Acta Mater. 2019, 180, 105–115. [Google Scholar] [CrossRef]
- Wei, K.; Chen, L.; Qu, Y.; Zhang, Y.; Zhang, J. Zeta potential of microarc oxidation film on ZIRLO alloy in different aqueous solutions. Corros. Sci. 2018, 143, 129–135. [Google Scholar] [CrossRef]
- Krausová, A.; Macák, J.; Sajdl, P.; Novotny, R.; Reniuková, V.; Vrtílková, V. In-situ electrochemical study of Zr-1Nb alloy corrosion in high temperature Li+ containing water. J. Nucl. Mater. 2015, 467, 302–310. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, B.; Li, Q.; Yao, M. Detrimental role of LiOH on the oxide film formed on Zircaloy-4. Corros. Sci. 2005, 47, 1855–1860. [Google Scholar] [CrossRef]

| Element | Sn | Nb | Fe | Cr | Zr |
|---|---|---|---|---|---|
| Content | 0.9–1.2 | 0.25–0.35 | 0.3–0.4 | 0.05–0.10 | Bal. |
| Type of Solutions | Li Concentration | B Concentration | Dissolved Oxygen |
|---|---|---|---|
| B = 0 mg/kg | 100 ± 5 | 0 | ≤0.1 |
| B = 50 mg/kg | 100 ± 5 | 50 ± 5 | ≤0.1 |
| B = 200 mg/kg | 100 ± 5 | 200 ± 10 | ≤0.1 |
| Times | 30 Days | 90 Days | 180 Days | 270 Days | 360 Days | 450 Days | 510 Days | |
|---|---|---|---|---|---|---|---|---|
| Solutions | ||||||||
| B = 0 mg/kg | 18 | 400 | 2400 | 3700 | 3500 | 3800 | 3900 | |
| B = 50 mg/kg | 14 | 18 | 20 | 34 | 44 | 58 | 74 | |
| B = 200 mg/kg | 20 | 16 | 14 | 28 | 40 | 46 | 49 | |
| Position | O | Cr | Fe | Zr | Nb | Sn |
|---|---|---|---|---|---|---|
| 1 | 2.21 | 0.00 | 0.07 | 96.84 | 0.41 | 0.46 |
| 2 | 11.77 | 0.08 | 0.06 | 86.77 | 0.07 | 1.26 |
| 3 | 36.83 | 0.00 | 0.04 | 61.94 | 0.22 | 0.97 |
| Position | O | Cr | Fe | Zr | Nb | Sn |
|---|---|---|---|---|---|---|
| 1 | 2.59 | 0.43 | 2.10 | 93.11 | 1.77 | 0.00 |
| 2 | 5.78 | 0.03 | 0.03 | 91.34 | 0.39 | 2.43 |
| 3 | 23.39 | 0.00 | 0.03 | 75.48 | 0.19 | 0.92 |
| Serial Number | Li Concentration (mg/kg) | B Concentration (mg/kg) | pH360°C | Undissolved LiOH (mol/L) | Li+ Concentration (mg/kg) | Corrosion Accelerated? |
|---|---|---|---|---|---|---|
| 1 | 70 | 0 | 10.56 | 4.53−7 | ≈56 | yes |
| 2 | 70 | 100 | 10.44 | 3.41−7 | ≈70 | no |
| 3 | 70 | 1000 | 9.83 | −8 | ≈70 | no |
| 4 | 100 | 0 | 10.76 | 1.13−6 | ≈100 | yes |
| 5 | 100 | 50 | 10.71 | −6 | ≈100 | no |
| 6 | 100 | 200 | 10.56 | −7 | ≈100 | no |
| 7 | 300 | 0 | 11.05 | 4.22−6 | ≈300 | yes |
| 8 | 300 | 100 | 10.98 | 3.64−6 | ≈300 | no |
| 9 | 300 | 1000 | 10.44 | 1.0−6 | ≈300 | no |
| 10 | 700 | 0 | 11.30 | 1.33−5 | ≈700 | yes |
| 11 | 700 | 100 | 11.26 | 1.23−5 | ≈700 | yes |
| 12 | 700 | 200 | 11.23 | 1.13−5 | ≈700 | yes |
| 13 | 700 | 1000 | 10.90 | 5.27−6 | ≈700 | no |
| 14 | 700 | 2000 | 10.51 | 2.1−6 | ≈700 | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wu, Z.; Chen, Z.; Yin, Z.; Tang, M.; Xiong, J.; Deng, P. Effect of Boron Content in LiOH Solutions on the Corrosion Behavior of Zr-Sn-Nb Alloy. Materials 2024, 17, 2373. https://doi.org/10.3390/ma17102373
Zhao Y, Wu Z, Chen Z, Yin Z, Tang M, Xiong J, Deng P. Effect of Boron Content in LiOH Solutions on the Corrosion Behavior of Zr-Sn-Nb Alloy. Materials. 2024; 17(10):2373. https://doi.org/10.3390/ma17102373
Chicago/Turabian StyleZhao, Yongfu, Zongpei Wu, Zirui Chen, Zhaohui Yin, Min Tang, Jing Xiong, and Ping Deng. 2024. "Effect of Boron Content in LiOH Solutions on the Corrosion Behavior of Zr-Sn-Nb Alloy" Materials 17, no. 10: 2373. https://doi.org/10.3390/ma17102373
APA StyleZhao, Y., Wu, Z., Chen, Z., Yin, Z., Tang, M., Xiong, J., & Deng, P. (2024). Effect of Boron Content in LiOH Solutions on the Corrosion Behavior of Zr-Sn-Nb Alloy. Materials, 17(10), 2373. https://doi.org/10.3390/ma17102373






