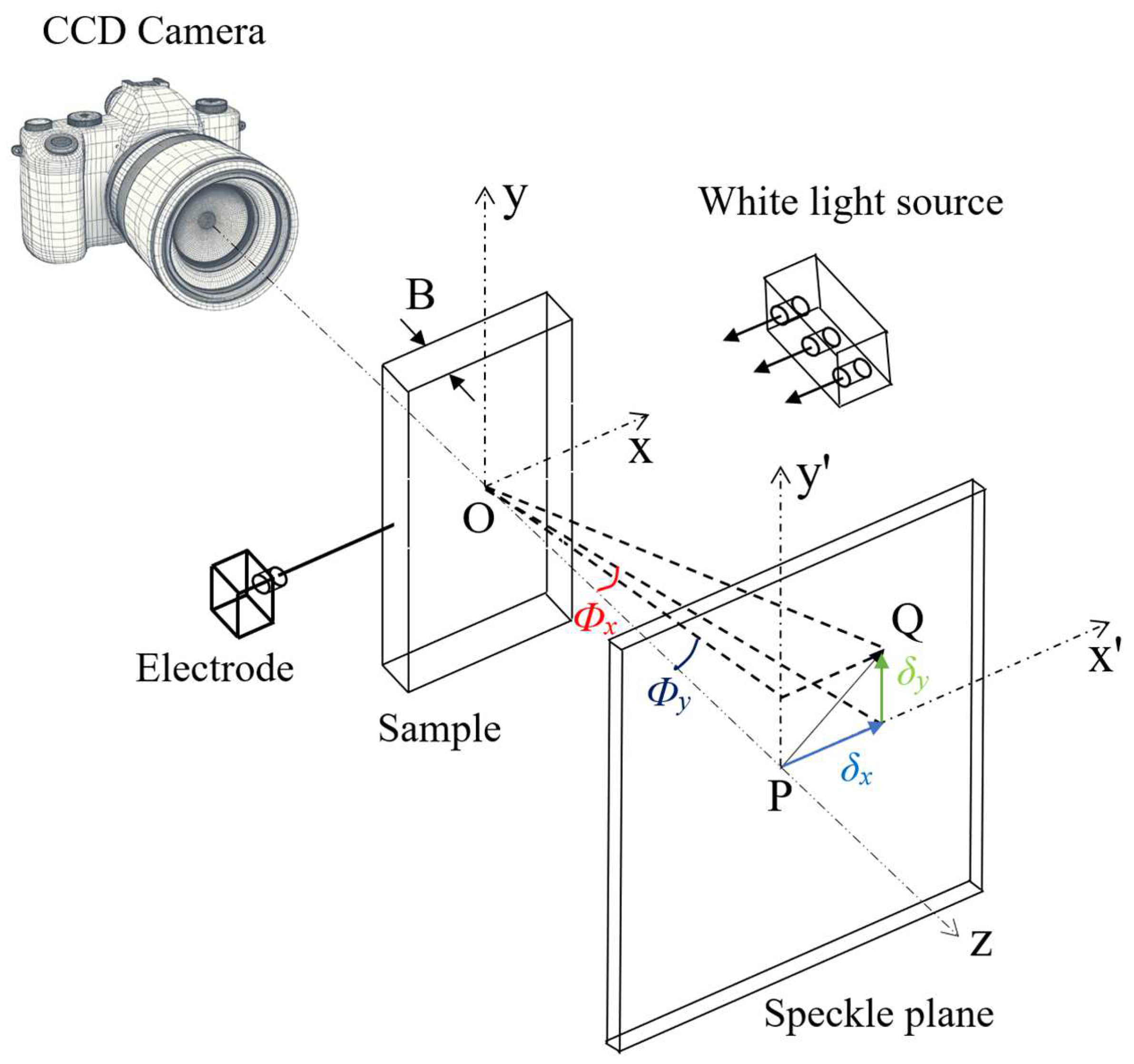
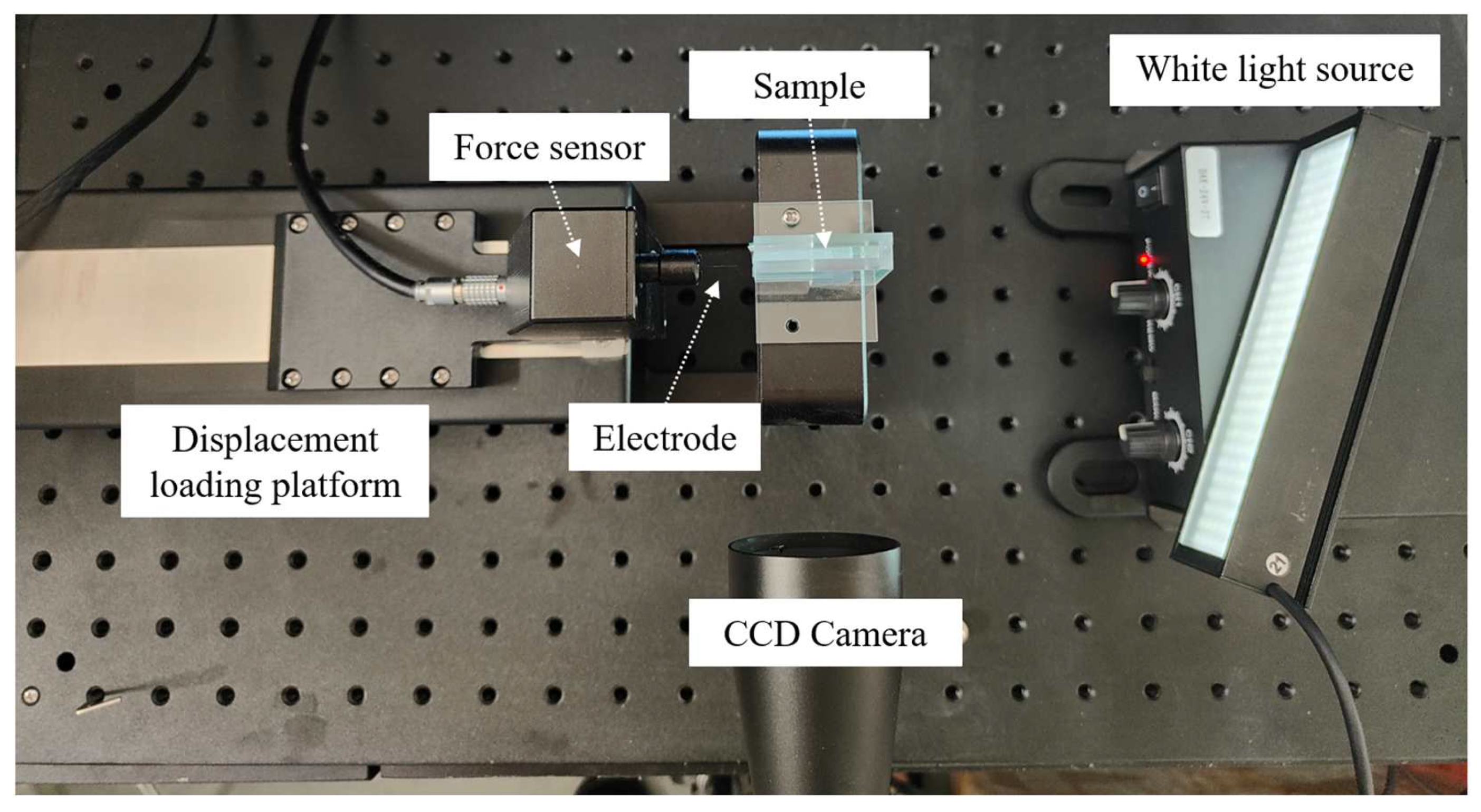
3.2. Single Electrode Implantation Experiments
Before determining the boundary conditions for experimental testing, we conducted a thorough finite element simulation analysis to assess the impact of boundary conditions such as the clamping method and the size of the agarose on the experimental outcomes. Our established finite element simulation model took into account the mechanical behavior of the probe and the agarose, under the same boundary conditions as the experimental tests. Based on the finite element simulation model, we calculated the deformation evolution under various implantation speeds. By comparing with the strain cloud map of the agarose shown in
Figure S1, it was observed that the material deformation near the boundaries of the agarose was minimal across different implantation speeds. This suggests that the selection of agarose size and the clamping method during the experimental testing process does not influence the deformation evolution of the brain tissue during implantation or the interpretation of the test results. Furthermore, the consistency between the experimental and simulation results further validates the rationality and reliability of our simulations, as depicted in
Figure S2.
It is worth noting that for electrode implantation experiments, the differences between resin electrodes and the silicon or metal electrodes commonly used in clinical practice are mainly reflected in their different moduli. However, the modulus of each type of electrode is much greater than that of agarose gel, and they can be approximated as rigid bodies. In clinical applications, to prevent signal interference between different electrodes, a layer of insulating material, such as Parylene, is commonly added to the surface of silicon or metal electrodes. Moreover, our study primarily focuses on the impact of electrode implantation on the macroscopic mechanical properties of agarose gel, where the conductivity of the electrode has a minimal effect on the tissue damage during the implantation process. Therefore, despite the differences in material properties between resin electrodes and silicon or metal electrodes, the resin electrode is still representative in studying the mechanical behavior of agarose gel during the implantation process.
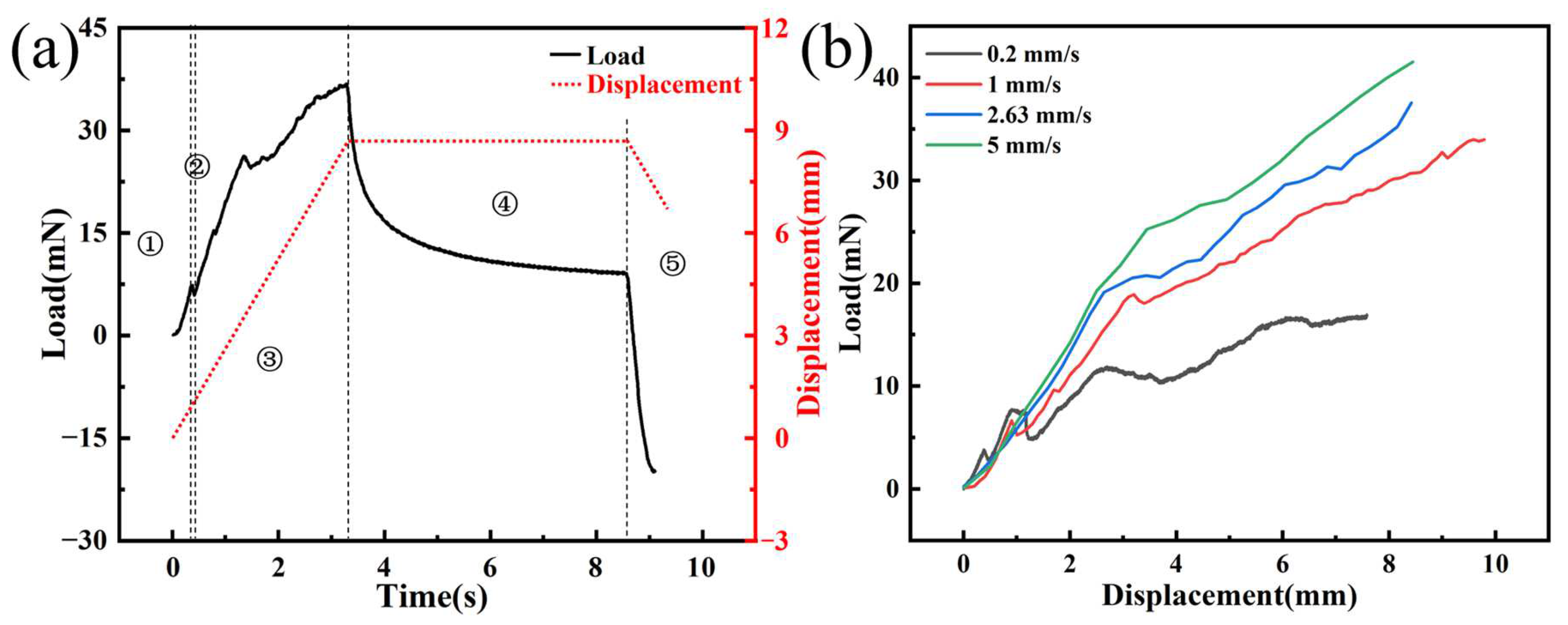
Figure 7a shows the time–load curve of a single electrode implanted into agarose gel at a speed of 2.63 mm/s, which reveals five distinct phases. Firstly, the initial phase is marked by the deformation of the agarose gel from the moment the needle tip makes contact until right before the puncture occurs. The load applied to the electrode in this phase originates from the elastic deformation of the agarose gel, and the electrode load steadily increases due to the gradual increase in the strain energy of it. Then, the punctured stage is entered, which is typically characterized by a sharp drop in the electrode load due to the release of strain energy from the agarose gel when it is punctured. The curve clearly shows a distinct peak, which is consistent with the findings of Welkenhuysen et al. [
20], indicating that the peak is caused by tissue penetration by the electrode. Meanwhile, we recorded the entire process of electrode implantation using a CCD camera. By comparing the curve with the images captured during the experiment, we confirmed that the peak phenomenon is indeed caused by the electrode puncturing the agarose gel. The third phase is the steady-state puncture stage when the load mainly comes from the friction between the needle axis and the agarose gel, the stiffness force of the tissue, and the puncture force. As the needle tip continues to puncture, the contact area between the needle axis and the agarose gel increases, resulting in a steady increase in load. Subsequently, the tissue relaxation phase begins when the electrode is implanted at the designated position and stops. Due to the viscoelasticity of the agarose gel, the release of strain energy leads to a rapid decrease in electrode load. Finally, in the electrode extraction phase, the electrode returns at a constant speed of 2.63 mm/s, and the load is mainly composed of friction.
Figure 7b illustrates the load–displacement curves at different insertion speeds, revealing that with increasing velocity, the implant load also increases correspondingly. When the implantation depth is 7 mm, the implantation forces of the electrode are 16.46, 27.82, 31.12, and 36.13 mN, with implantation velocities of 0.2, 1, 2.63, and 5 mm/s, respectively. This trend can be attributed to the rate-dependent stiffening behavior of the material, where the material’s resistance augments as the insertion velocity is enhanced. At higher application speeds, agarose gel predominantly exhibits its elastic behavior, leading to an increased resistance to deformation and, consequently, a more pronounced rigidity characteristic. Specifically, as the implantation speed increases, the viscoelastic response of agarose gel relatively weakens. This is primarily because, under rapid application of force, the gel’s viscous component struggles to absorb the applied force through internal flow or energy dissipation, making the elastic component the dominant response mechanism. This shift in mechanism enhances the gel’s resistance to penetration attempts, reflecting its adjustment to mechanical properties under high-speed implantation conditions. However, we observed that when the implantation speed was 0.2 mm/s, the load–displacement curve was relatively flat within the range of approximately 2.5–5 mm. Due to the viscoelasticity and stick-slip effect of the agarose gel, there is a sudden drop in load at the moment the electrode punctures the agarose gel. As the electrode continues to be implanted, the load gradually increases. Thus, the initial decrease followed by a rise in load depicted in the graph is caused by the continuous puncture of the agarose gel by the electrode tip. The phenomenon is more pronounced when the implantation speed is low, but it diminishes as the implantation speed increases.
The puncture angle refers to the angle formed at the tissue surface the instant the electrode pierces through it. To minimize tissue damage and reduce bleeding, inflammation, and cell death caused by the implantation process [
31], the ideal scenario is to pursue the smallest possible puncture angle.
Figure 8 illustrates the moment when the agarose gel is penetrated at different implantation speeds, clearly demonstrating a trend: as the implantation speed increases, the puncture angle gradually decreases. The puncture angles are 129.54°, 97.43°, 84.36°, and 66.89°, at implantation speeds of 0.2, 1, 2.63, and 5 mm/s, respectively. This is due to the viscoelastic properties of the material; at lower implantation speeds, strain typically affects a wider area. However, as the implantation speed increases, the range of strain gradually narrows, resulting in more localized damage. It is worth noting that in brain–computer interface research, the issue of electrode implantation is an important research direction that spans multiple disciplines. The “damage” defined in our study primarily considers macroscopic injury to brain tissue, which is different from the microscopic injuries discussed in the field of neurology, such as blood vessel rupture, bleeding, neuronal cell death, and tissue necrosis. Therefore, based on our research focus, which is on the mechanical macroscopic injury issues during the implantation process, we have chosen agarose gel with mechanical properties similar to real brain tissue to simulate the macroscopic injury issues of real brain tissue during the electrode implantation process. The internal biological microscopic injuries caused by macroscopic damage to brain tissue during the implantation process require further analysis and characterization through multidisciplinary research methods.
Through the utilization of the digital image correlation method, we conducted a quantitative analysis of the implantation process, which not only corroborated the visual observations but also facilitated a detailed examination of the stress distribution.
Figure 9 illustrates the results of angular deflection measurements of the agarose gel in the X and Y directions during implantation with a single electrode at various speeds. The data reveal significant angular deflection near the needle tip and along its axis, indicating stress concentration phenomena. Conversely, minimal angular deflections are observed at the plot boundaries, suggesting the absence of boundary effects. This finding underscores the reliability of our experimental setup.
To provide a comprehensive understanding,
Supplementary Video S1 records the complete implantation process at an insertion speed of 2.63 mm/s. The video vividly showcases the deformation process, offering valuable insights into the experimental procedure. Combined with the observable depression of the electrode during steady-state implantation in the 3 mm thick agarose gel, the validation of our experimental setup suggests that the selected dimensions are suitable for this study. This observation provides valuable insights for future research in this field.
Furthermore, under the same implant displacement, the degree of depression in the agarose gel was observed to increase progressively with the enhancement of implantation velocity. The angular deflection, as indicated by the graph, exhibits a radial distribution, with the radiating phenomenon becoming more distinct as the implantation speed increases. This can be attributed to the increased kinetic energy at the needle tip with rising velocities, which amplifies its impact on the agarose gel. However, the regions of damage within the agarose gel appear to decrease as the implantation speed escalates which is a common reason for preferring higher speeds in electrode implantation practices [
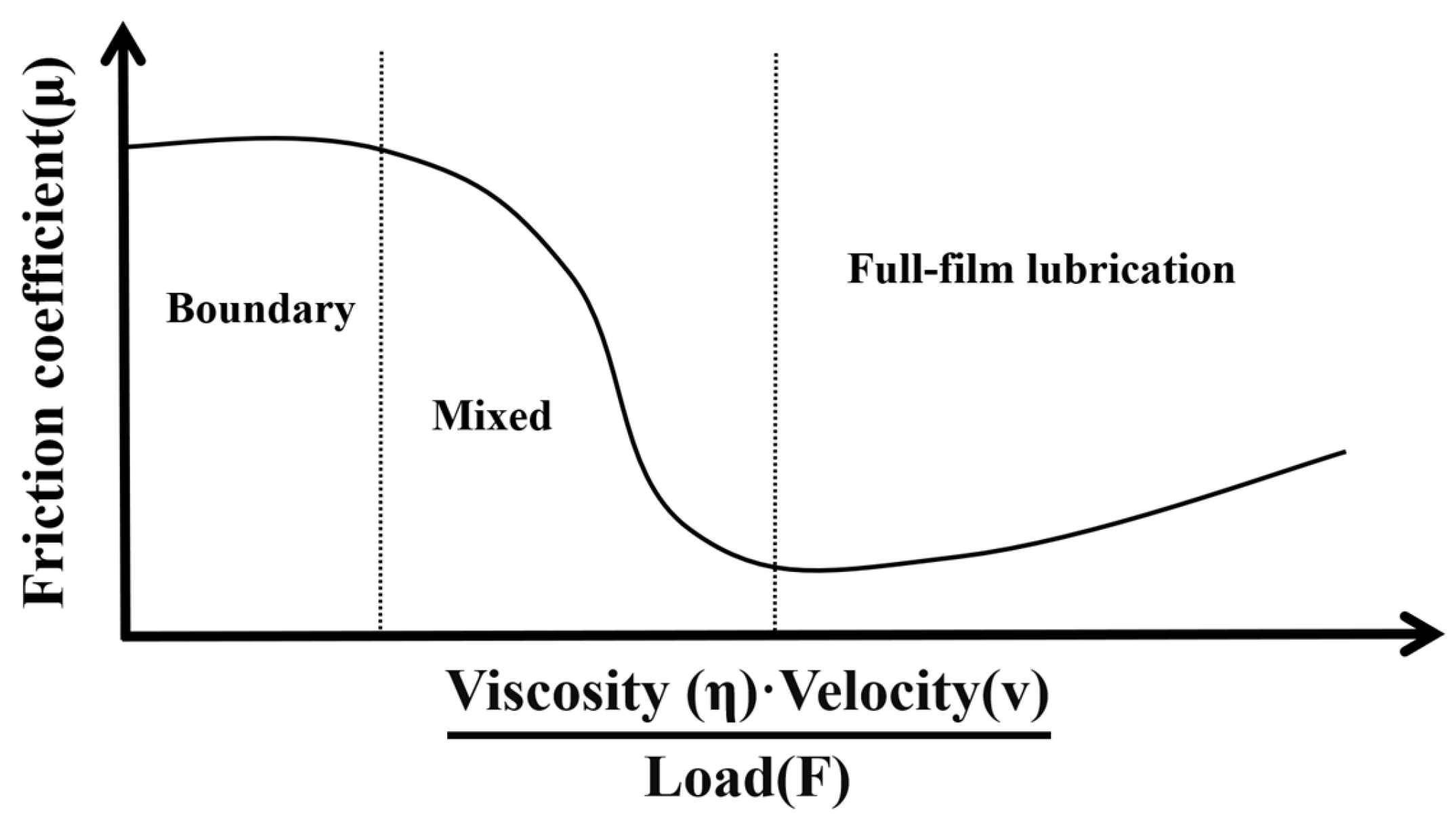
32]. Due to rapid loading, the stiffness of the agarose gel increases, making it more resistant to deformation. This phenomenon can be explained from two aspects. Firstly, the higher the speed, the smaller the puncture angle becomes, resulting in more localized damage to the agarose gel. Secondly, according to the theory of the Stribeck curve in the field of tribology, due to the high water content of the agarose gel created for this study, the friction between the electrode and the agarose gel exists in a state between boundary and mixed lubrication, as shown in
Figure 10. In such a state, the greater the speed, the lower the coefficient of friction between the surfaces in contact.
When implanting electrodes at high speed, the impact on the tissue is instantaneous and concentrated, allowing the electrodes to quickly penetrate the agarose gel, reducing the movement of the surrounding tissue, and thus localizing the damage. However, this rapid penetration also results in a greater mechanical pressure being exerted on the local area, which may increase the severity of the damage. Therefore, although the area of damage is more focused, the extent of the damage may intensify due to the increased mechanical force. Therefore, when planning and conducting implantation experiments, it is crucial to comprehensively consider the dual effects of implantation speed on load increase and potential damage to find an optimal implantation speed that balances minimizing damage and ensuring effective implantation. In our experimental results, we observed that when the implantation speed was 2.63 mm/s, the maximum angular deflections of the sample in both the x and y directions were the smallest relative to other implantation speeds, indicating the least degree of damage. Additionally, the area of damage was predominantly localized around the needle tip and along the needle shaft, as depicted in
Figure 9. When the implantation speed was increased to 5 mm/s, it was evident that both the extent of damage and the maximum angular deflections had increased. Therefore, we consider 2.63 mm/s to be the appropriate implantation speed. This finding provides valuable reference for future research by other scholars in this field.
3.3. Array Electrode Implantation Experiments
A single electrode is capable of measuring only limited signals in the vicinity of its tip. Even with multiple recording points on the electrode, these points can only capture brain electrical activity along the electrode’s axis. This constrains the ability to thoroughly record brain electrical signals on the same horizontal level. Hence, to achieve a more comprehensive gathering of brain electrical signals for the purposes of signal decoding and analysis, it is common practice to implant several electrodes simultaneously during animal or human experiments.
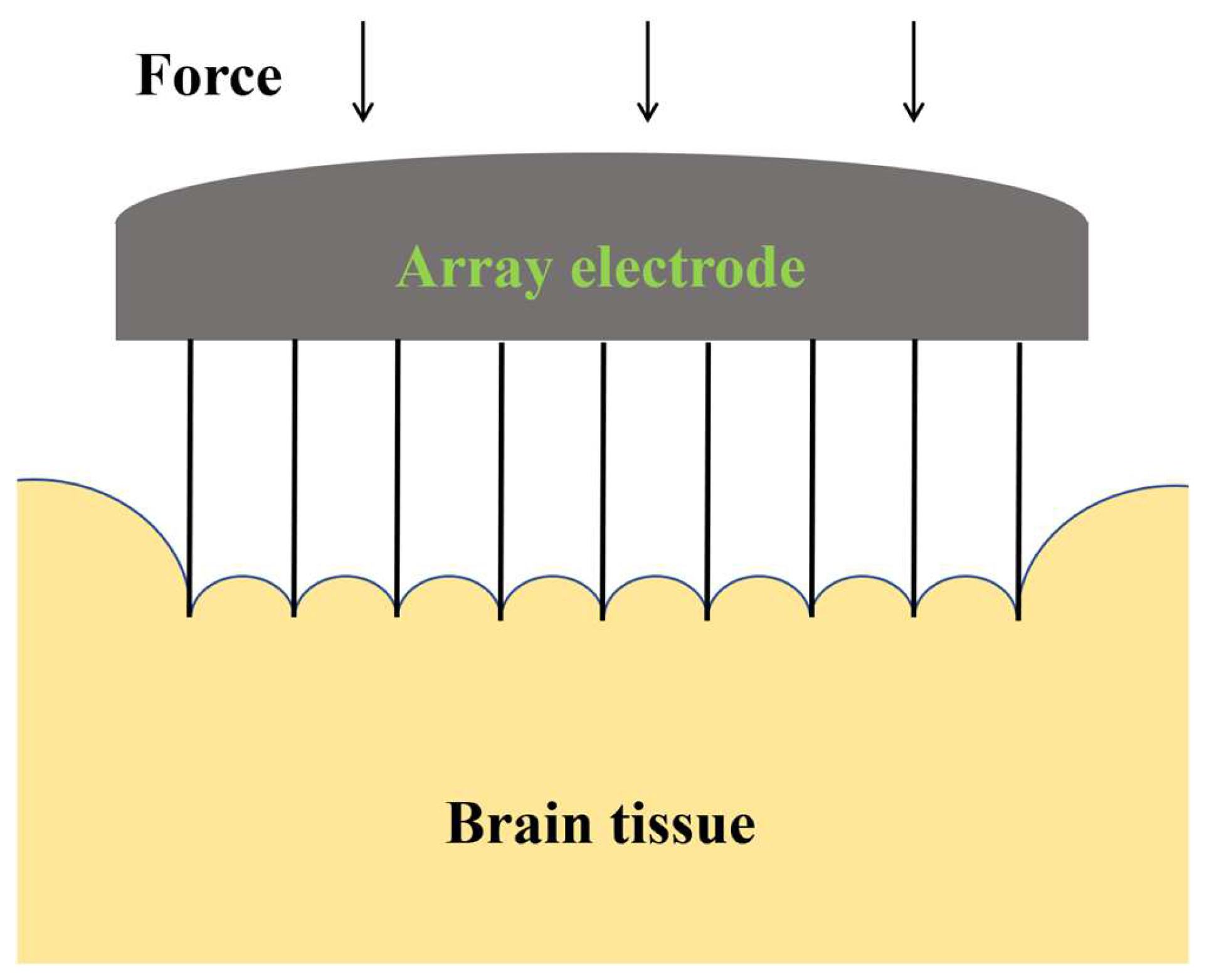
Unlike single electrodes, the spacing between electrodes in an array is a critical factor that affects both the health of brain tissue and the quality of signal acquisition. If the electrodes are too closely spaced, the coupling effect between adjacent electrodes may cause excessive damage to the brain tissue, as shown in
Figure 11. Conversely, if the spacing between electrodes is too wide, it may not capture all necessary signals within the region. Therefore, studying the optimal spacing of electrode arrays is crucial for achieving precise signal collection and minimizing brain tissue damage. Considering the Utah electrode’s well-established use in clinical settings, where the inter-electrode spacing is conventionally set at five times the diameter of the individual electrodes, such as those sold by Blackrock, we tailored our experimental design accordingly. Given that the diameter of the electrodes we are using in our study is 0.5 mm, we have established the distances between electrodes for our implantation experiments as 2, 3, and 4 mm. This decision was made to align with the clinical standards while accommodating the specific requirements of our research.
Figure 12 presents the load–displacement curves for array electrode implantation experiments at an insertion speed of 2.63 mm/s, with varying electrode spacings, from the initial contact of the electrode array with the agarose gel to the cessation of implantation. The load–displacement curves can be divided into four distinct phases. The first phase is akin to the single electrode implantation experiment, with the load continuously increasing from the moment the electrode first comes into contact with the agarose gel until just before the puncture. During the second phase, a noticeable reduction in the peak amplitude of the load–displacement curve is observed compared to that of a single electrode, which may be attributed to the distribution and interaction of the array electrodes. By analyzing images captured during the implantation process by a CCD camera, we observed that the vibration of the array electrodes during implantation is reduced compared to a single electrode.
Supplementary Video S2 shows the complete implantation process for an array spacing of 4 mm. The third phase is identified where the electrode tips penetrate the agarose gel, starting from the initial puncture of the gel by the tips and continuing until all of the conical tips are fully embedded within the gel. The fourth phase represents the steady-state implantation process, where the diameter of the electrode remains constant. In this stage, as the electrode continues to be implanted, the agarose gel further depresses, and the load gradually increases, until the completion of implantation. The experimental results indicate that an increase in electrode spacing leads to a rise in implantation load at the same implantation depth. When the implantation depth is 8 mm, the implantation load increases from 61 mN to 115 mN, an increase of 88.5% when the electrode spacing is increased from 2 mm to 4 mm.
According to the research by Davidson et al. [
33], experiments with hollow microneedle arrays for transdermal drug delivery showed that increasing the spacing between arrays leads to an increase in the surface area of each microcirculation interface and lengthens the diffusion path from the center of the microneedle to the interface corners. Consequently, at the same depth of implantation, as spacing increases, the contact area between the agarose gel and the electrode array grows, resulting in a higher implantation load and making it more challenging for the electrode array to achieve the intended depth. Furthermore, we observed significant fluctuations in the load when the implantation displacement was between 6 and 8 mm. This is due to the fact that the length of the electrode is only 10 mm, and towards the end of the implantation, the electrode is susceptible to the influence of minor vibrations from the loading platform and other factors, resulting in greater fluctuations in the force experienced by the electrode compared to the earlier stages. However, the overall trend of the load is upward.
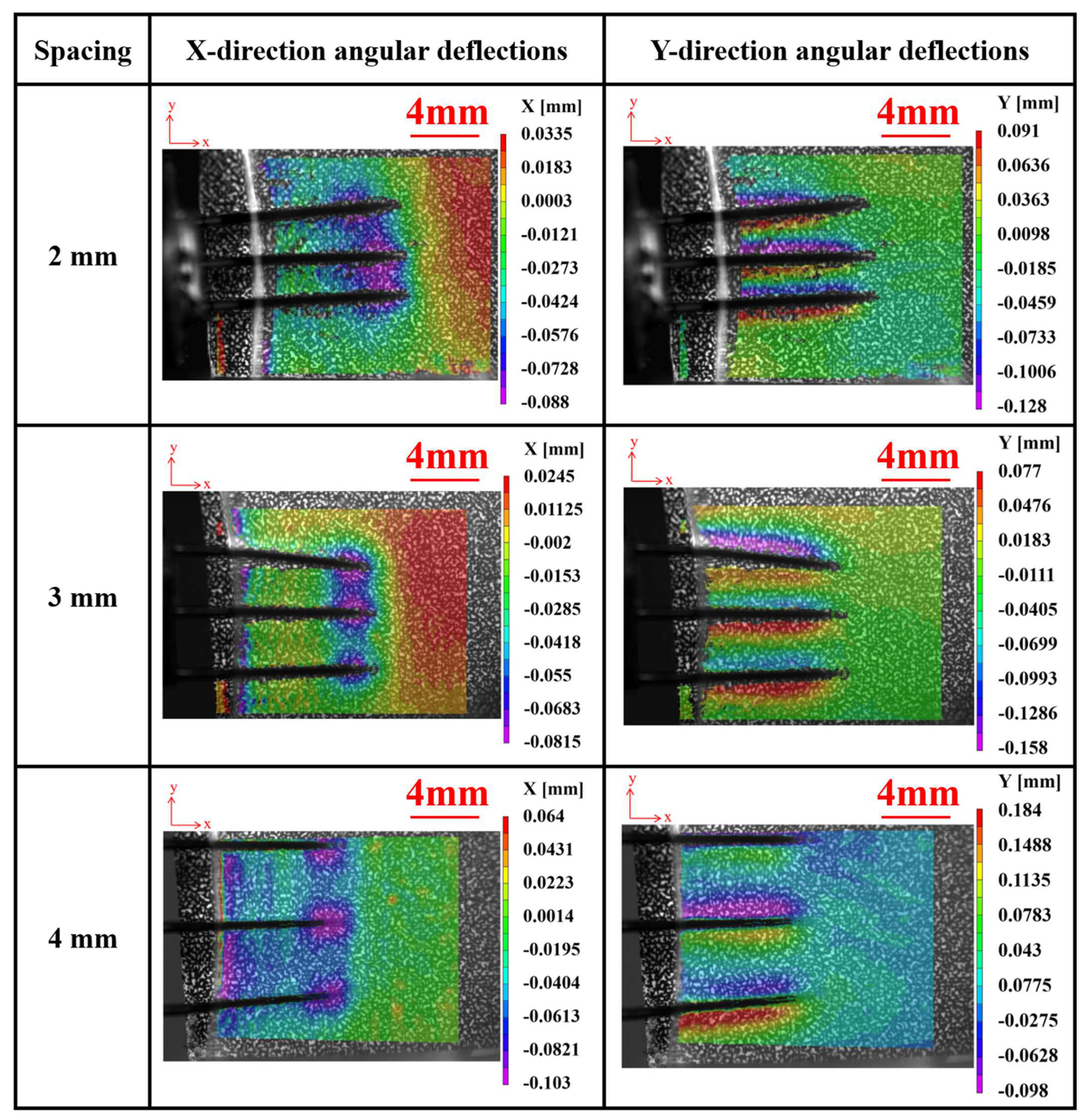
Figure 13 depicts the angular deflections of agarose gel in the X and Y directions during implantation experiments using electrodes with different array spacings at a velocity of 2.63 mm/s. It is observed that, similar to a single electrode, deformation primarily concentrates near the electrode tips. However, during the implantation process with array electrodes, tissues experience more pronounced indentations and exhibit an overall compressed state, contrasting with the implantation scenario of a single electrode. This phenomenon can be attributed to the coupling effect between adjacent electrodes, which gradually diminishes with increasing spacing. Further experimental observations indicate that when the spacing between electrodes increases to 4 mm, the coupling effect between them becomes weak and practically negligible.
During the implantation process, the effect of the electrode on the surrounding tissue is primarily limited to its immediate surrounding area. Increasing the spacing means the overlap between the areas of influence of each electrode decreases, thereby reducing the coupling between electrodes. This is very important for reducing tissue damage and improving the accuracy of implantation. Furthermore, as the spacing between the arrays increases, the overlap in the range of signals captured by each electrode decreases, reducing signal redundancy and mutual interference, thereby enhancing the accuracy and stability of long-term signal acquisition.
It can also be observed from
Figure 13 that the array electrodes have exhibited an inclination to tilt inward or outward during the implantation process. Further observation reveals that this tilting of the electrodes predominantly occurs at the outermost electrodes. This is attributed to the fact that during the implantation of the array electrodes, as the depth of implantation increases, the brain tissue on both sides exerts a stronger boundary effect on the outermost electrodes. This leads to an imbalance in the forces acting on either side of the electrodes, resulting in the inward or outward tilting of the outermost electrodes. This suggests that during the implantation process of the array electrodes, the signals collected by the outermost electrodes may be affected by the boundary effect. This is particularly important for subsequent signal processing.
It should be emphasized that this study primarily focuses on providing a theoretical basis and experimental data support for the selection of electrode implantation speed and electrode array spacing during the process of electrode implantation into brain tissue. However, the research presented in this paper is limited to the application of the DGS system to transparent agarose gel models, which differs from the actual process of electrode implantation into non-transparent brain tissue. When applying the DGS system to observe the implantation of electrodes into non-transparent brain tissue, it is usually necessary to perform special surface treatments, such as coating, to enhance its observability within the DGS system [
34,
35]. Future research should fully consider the opacity of brain tissue and design new experiments based on the current experimental foundation. This includes selecting appropriate brain tissue models and further optimizing the DGS system, with the aim of achieving real-time observation of the electrode implantation process in biological brain tissue.