The Study on Fatigue Crack Growth Rate of 4130X Material under Different Hydrogen Corrosion Conditions
Abstract
1. Introduction
2. Materials, Experimental Procedures, and Simulations
2.1. Preparation of Materials
2.2. Experimental Solutions
2.3. Experimental Procedure
- V crack opening displacement, ΔV = Vmax − Vmin, mm;
- P load, ΔP = Pmax − Pmin, kN;
- B specimen thickness, mm;
- E elastic modulus, GPa;
- ai crack length per cycle, mm;
- W specimen width, mm;
- C6 dimensionless flexibility, function of ai/W, see Equation (2).
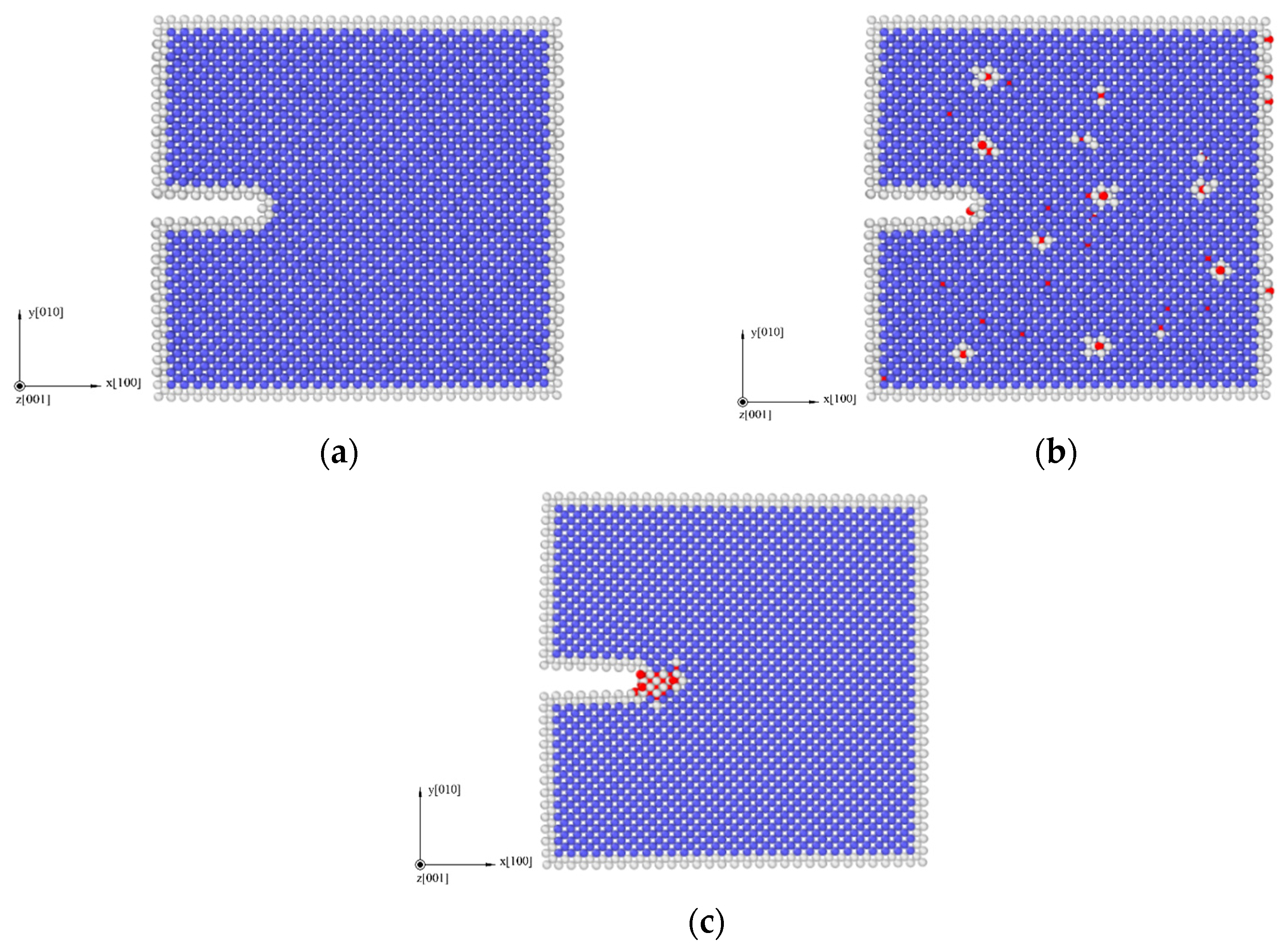
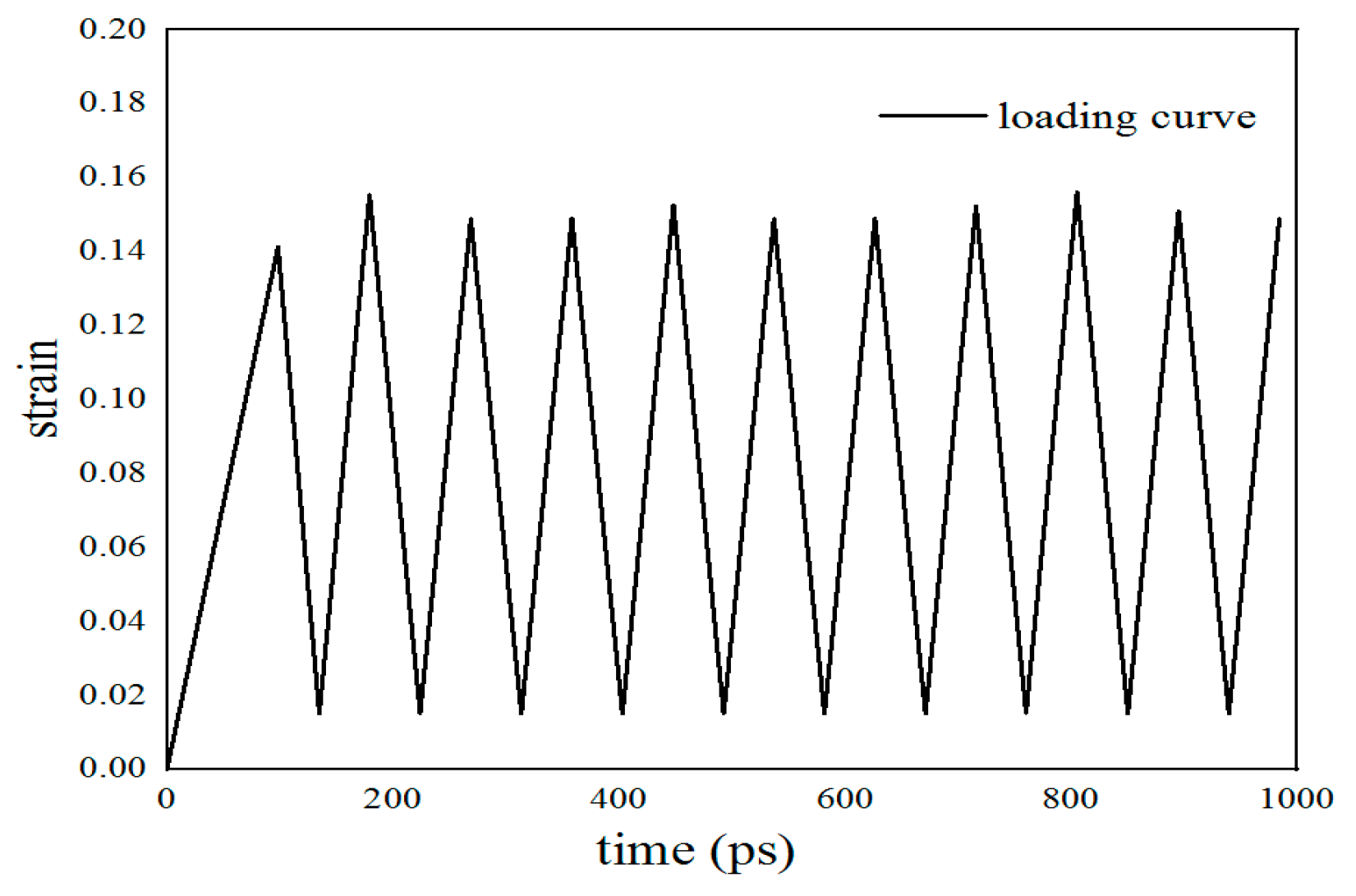
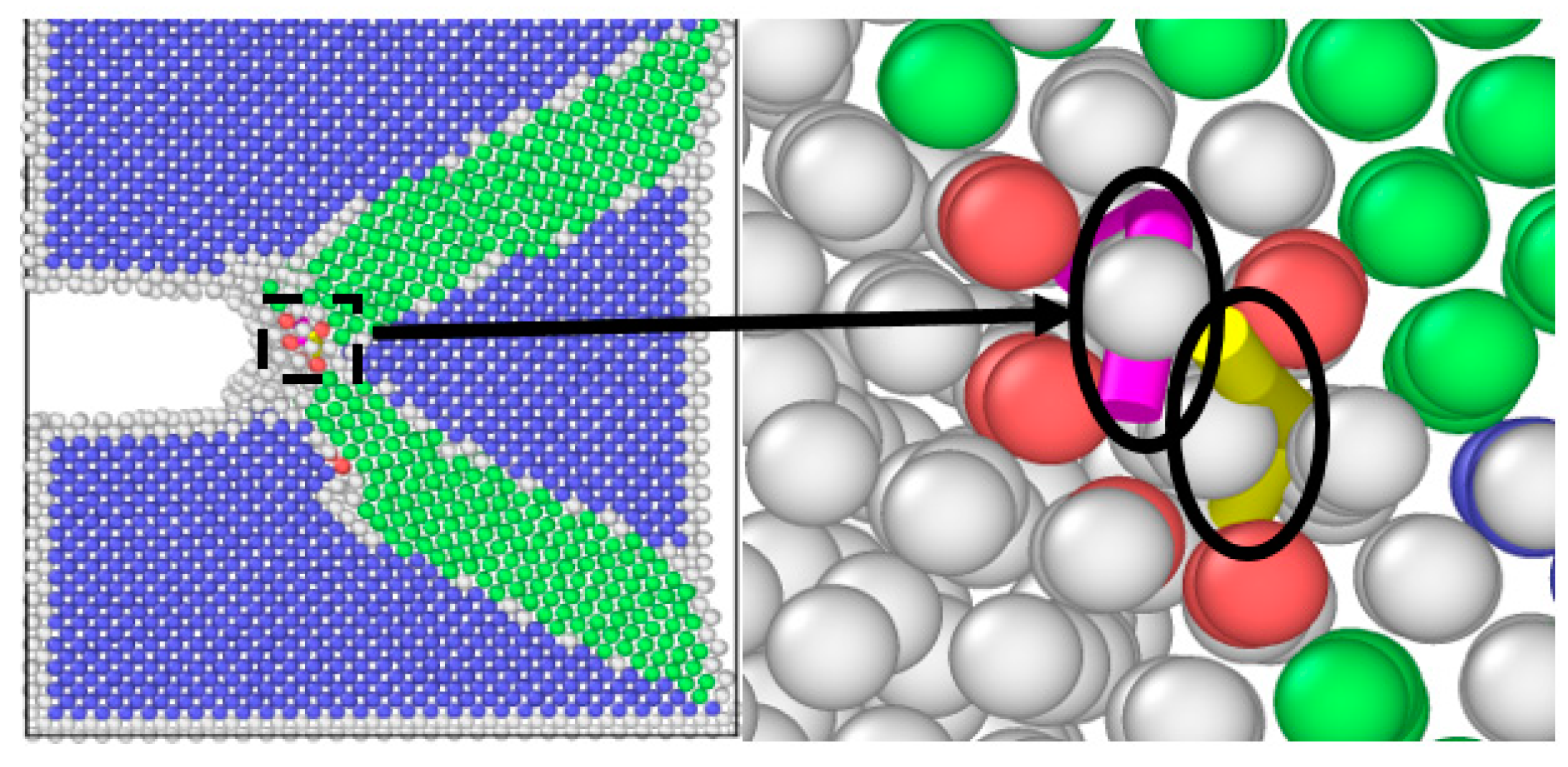
2.4. Molecular Dynamics Simulation
3. Experimental and Simulation Results
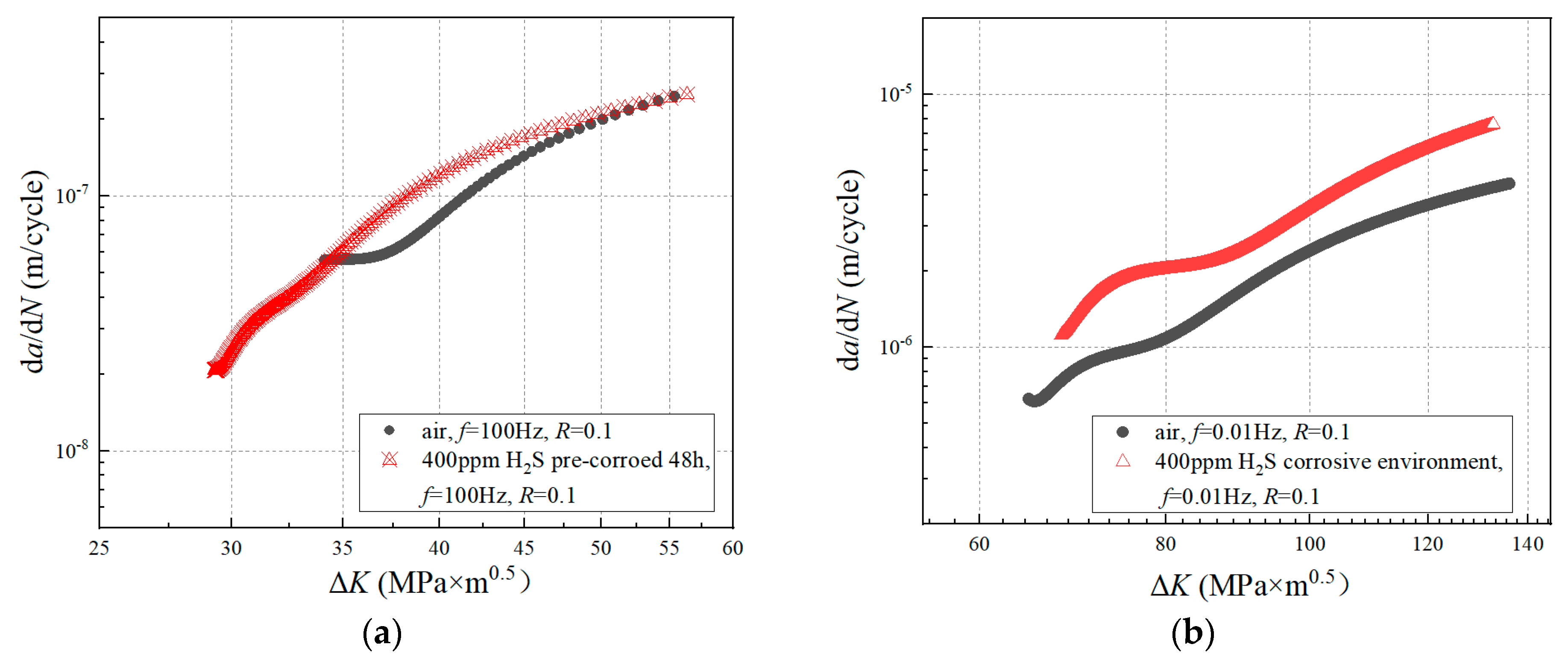
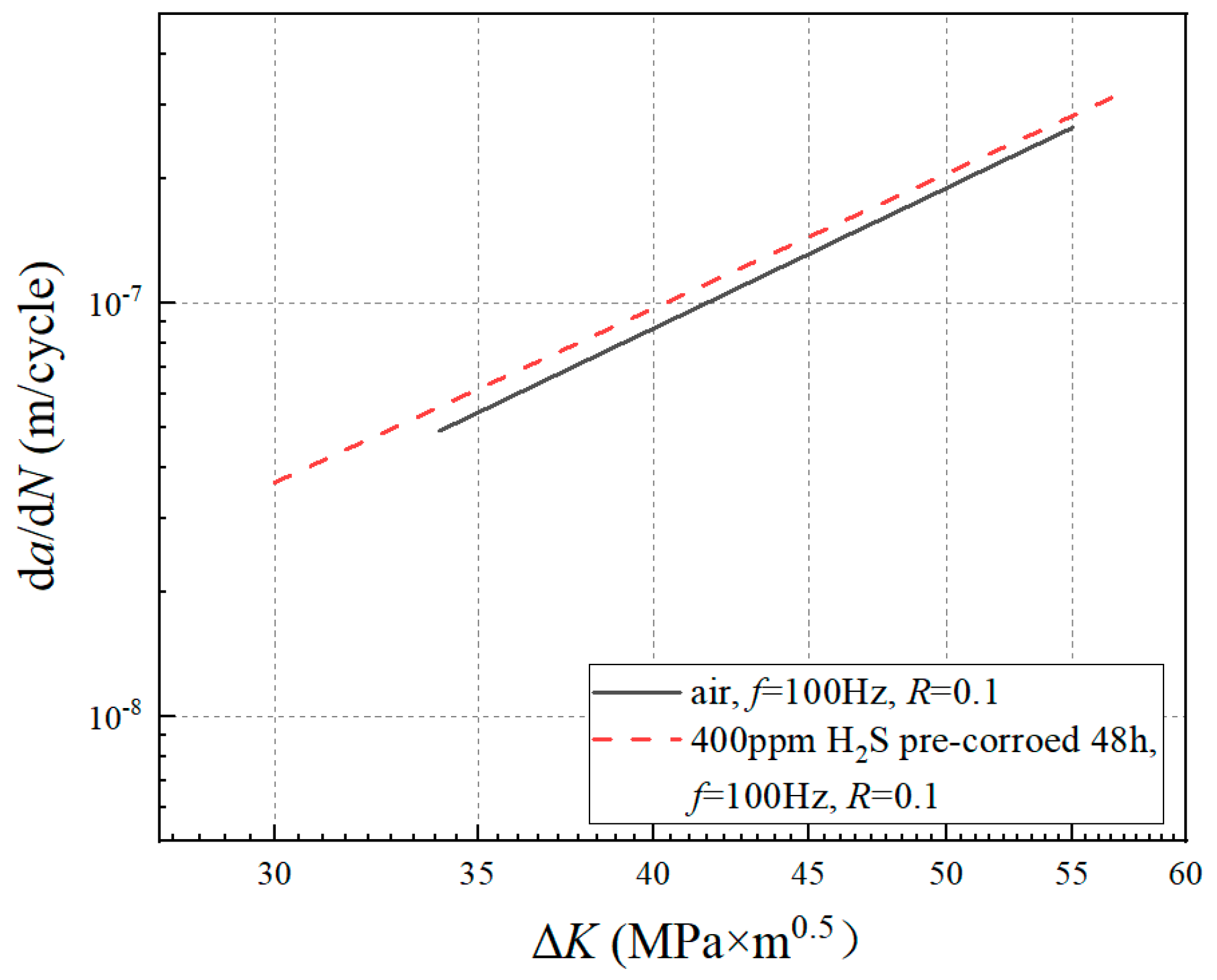
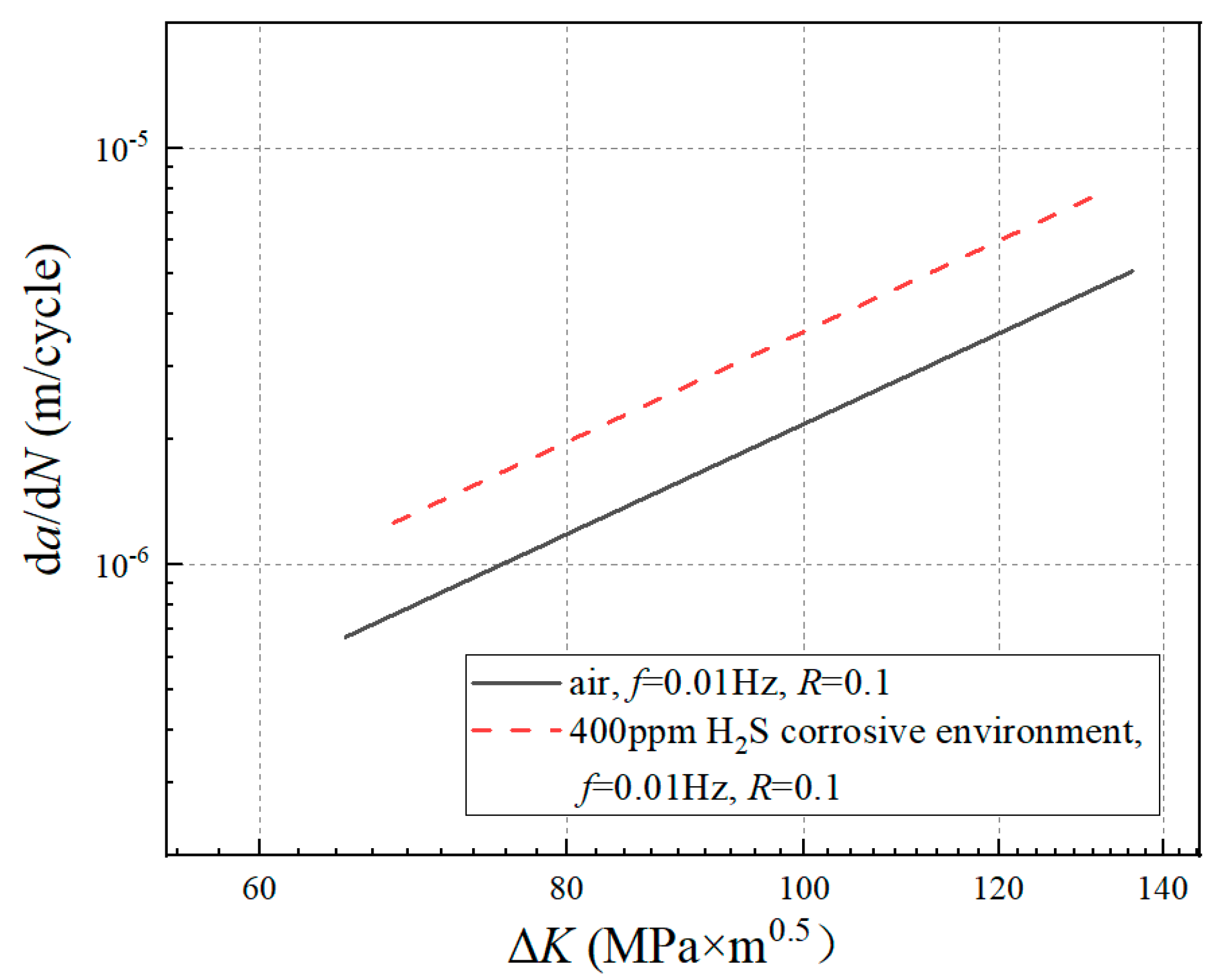
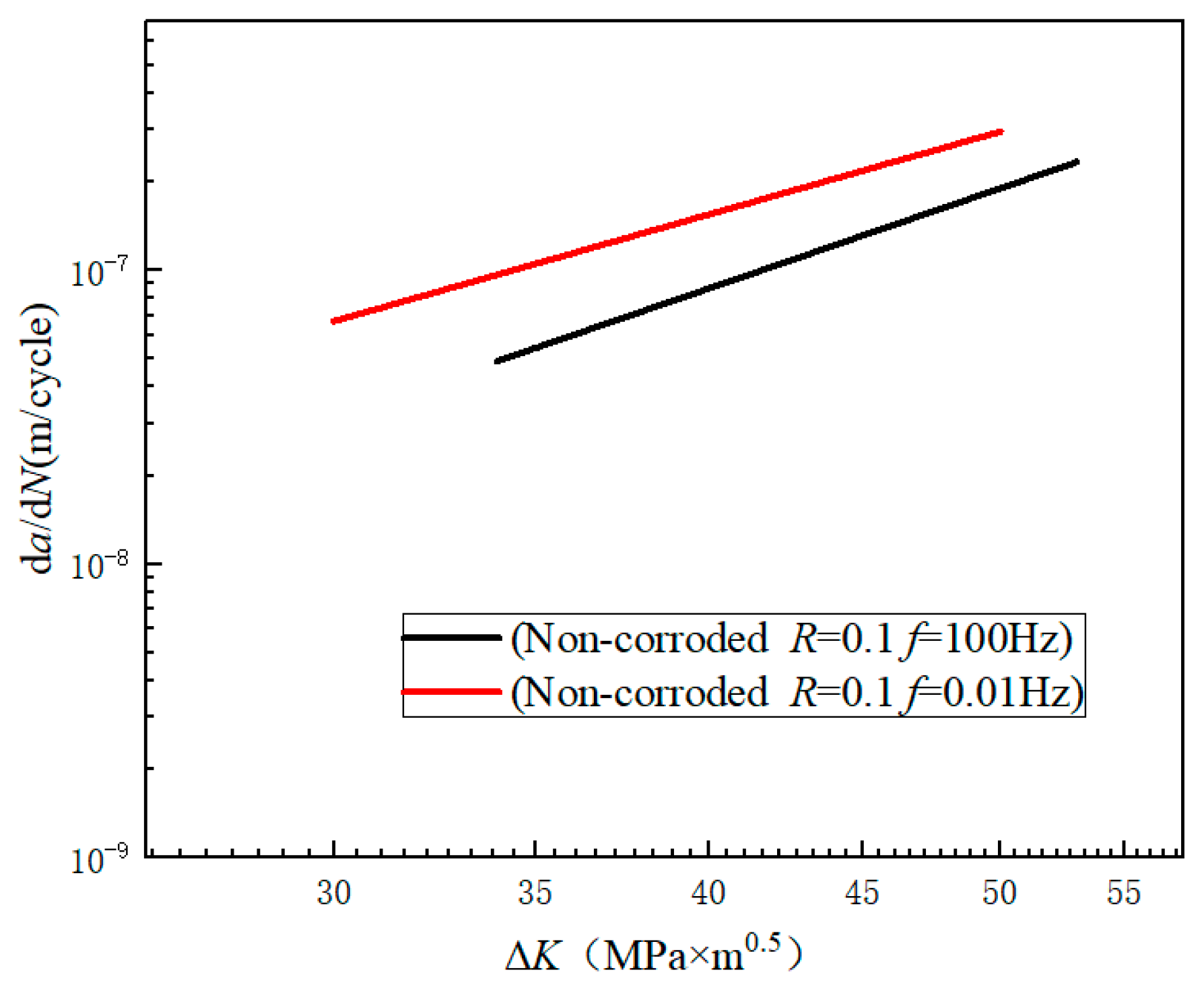
3.1. da/dN-ΔK Curves under Different Experimental Conditions
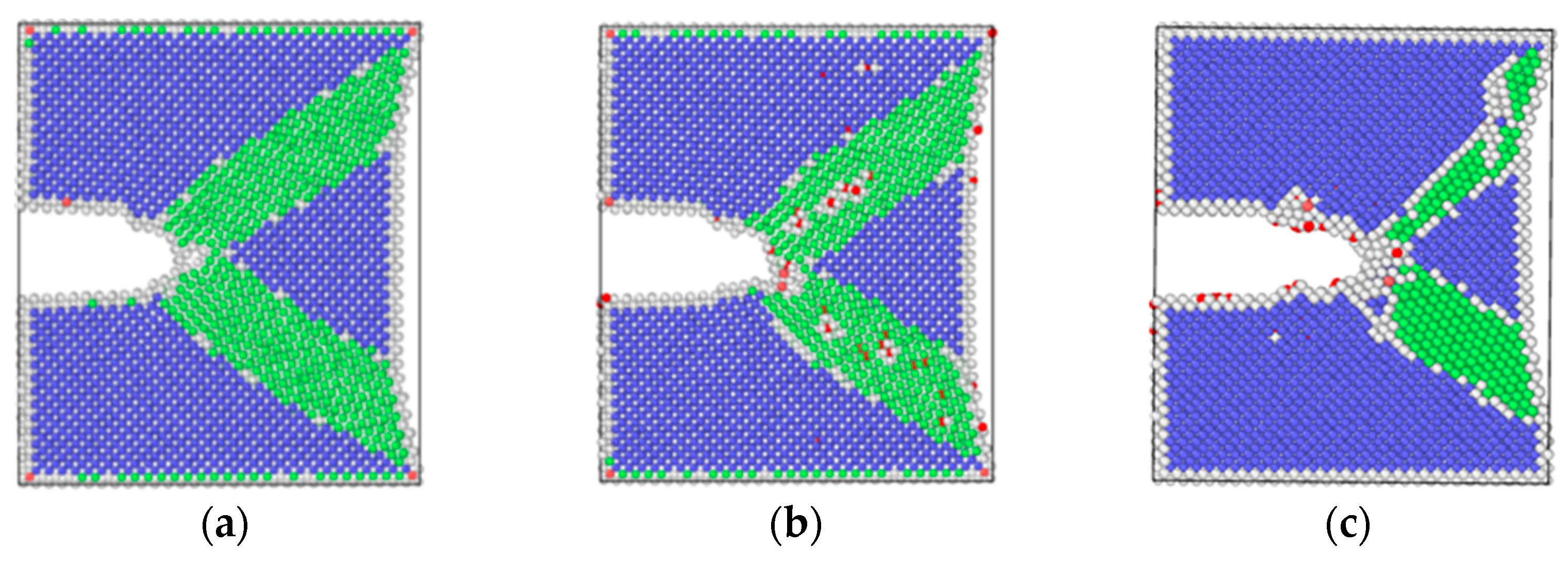
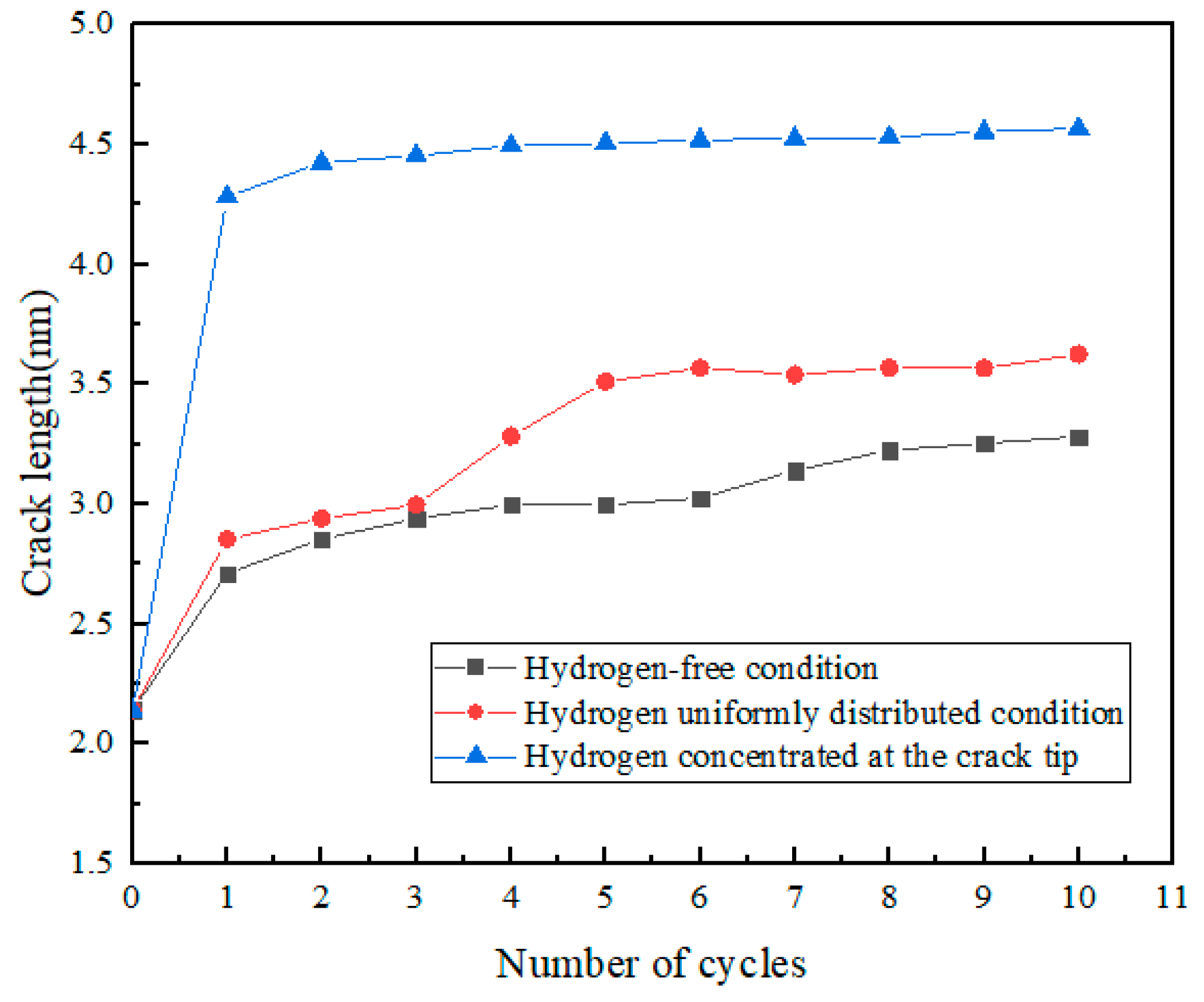
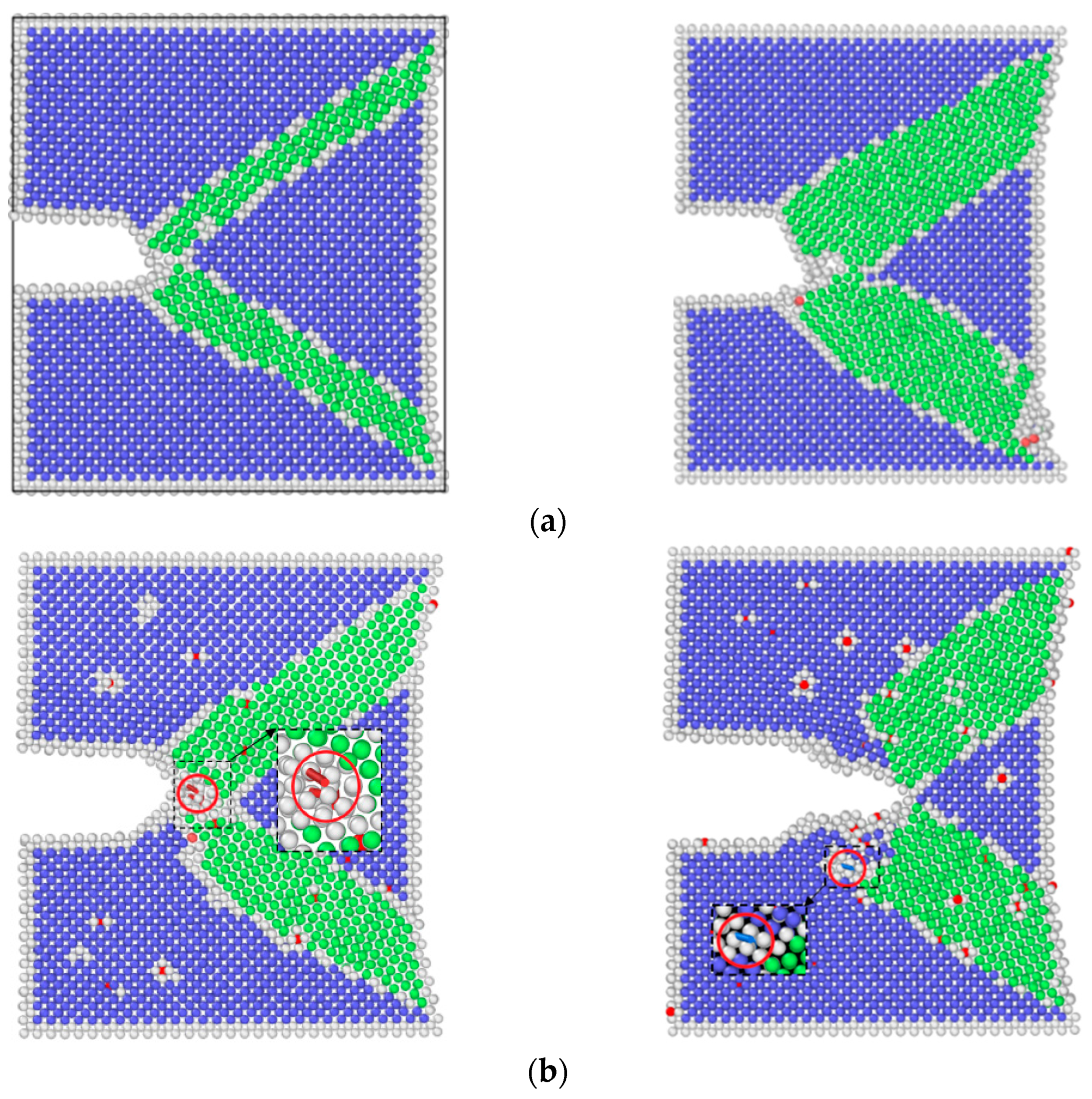
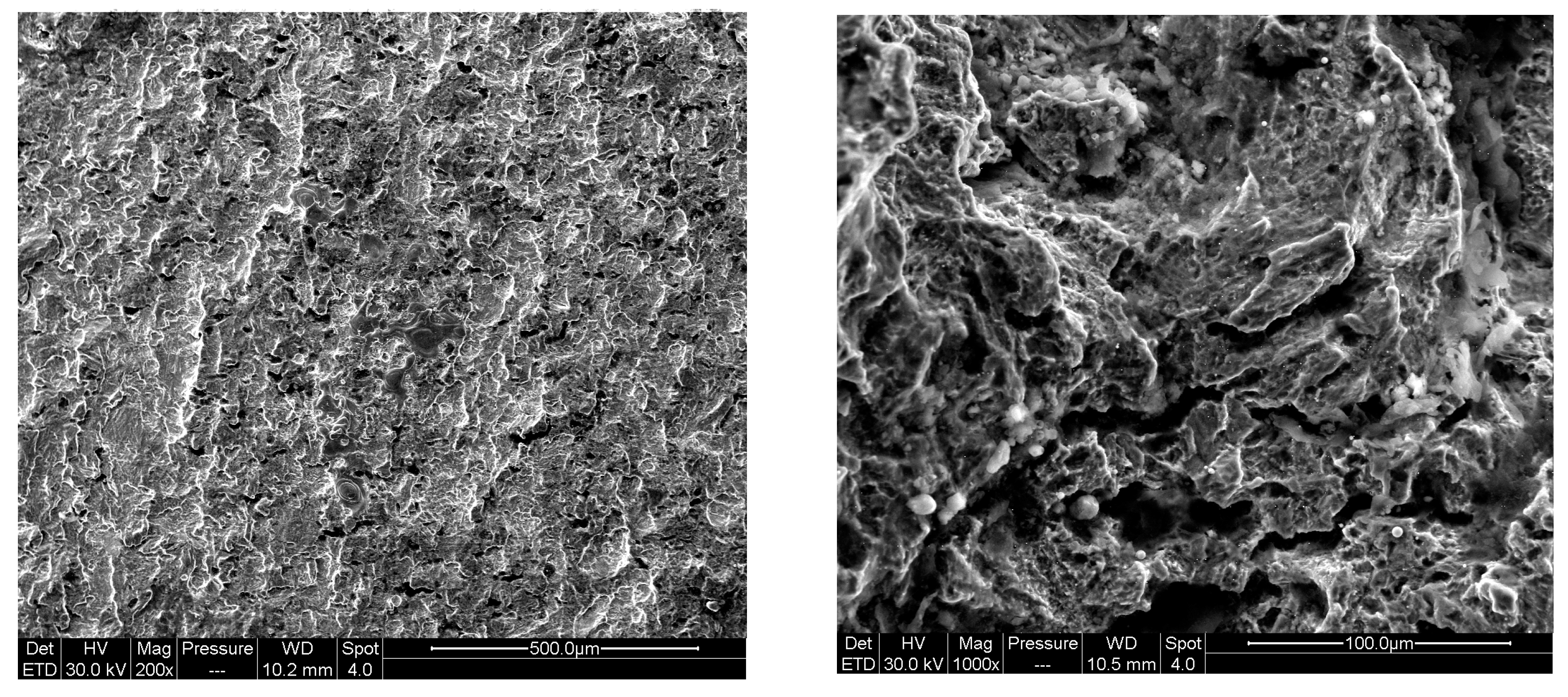
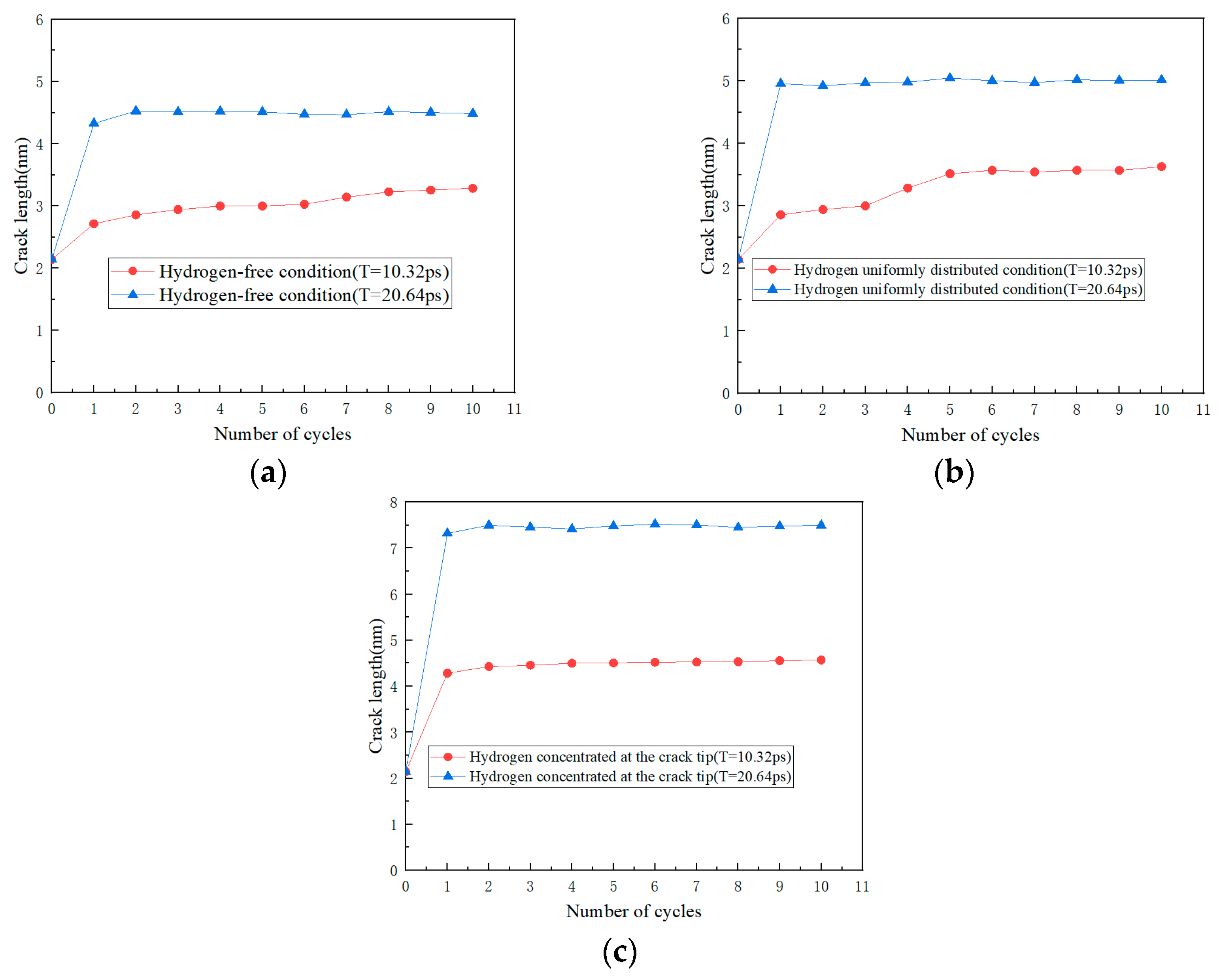
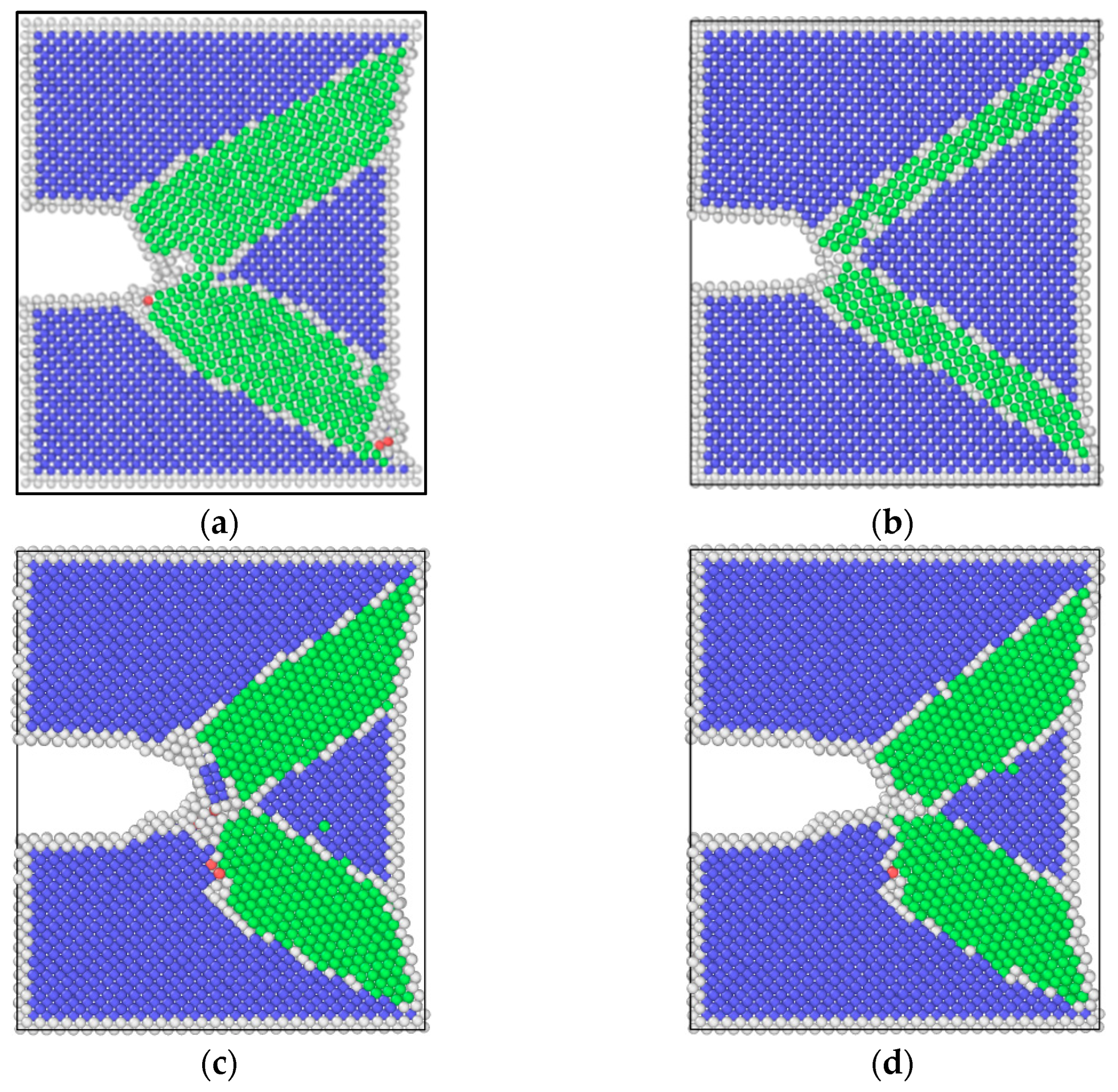
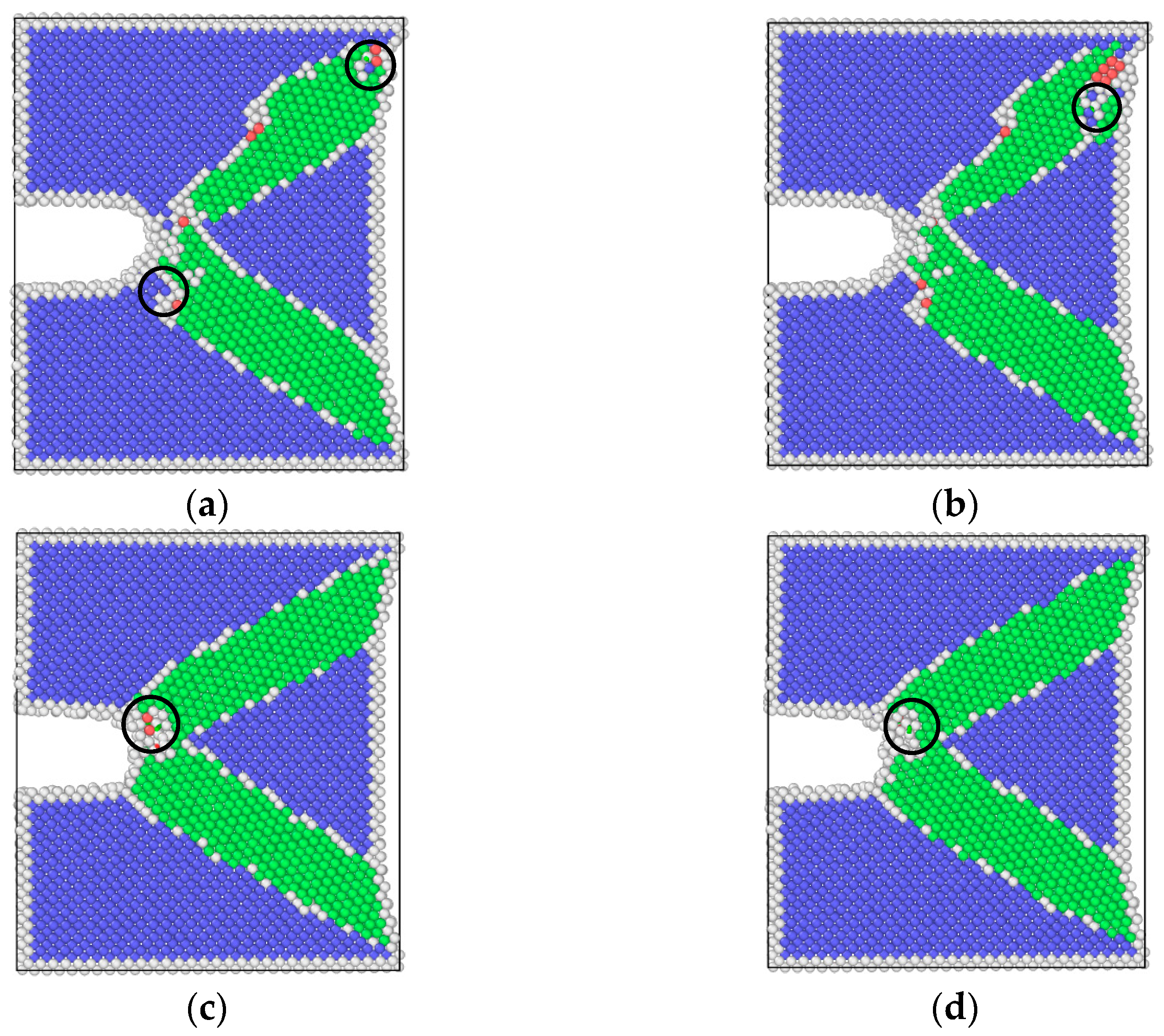
3.2. Simulation Results of Molecular Dynamics
4. Analysis and Discussion
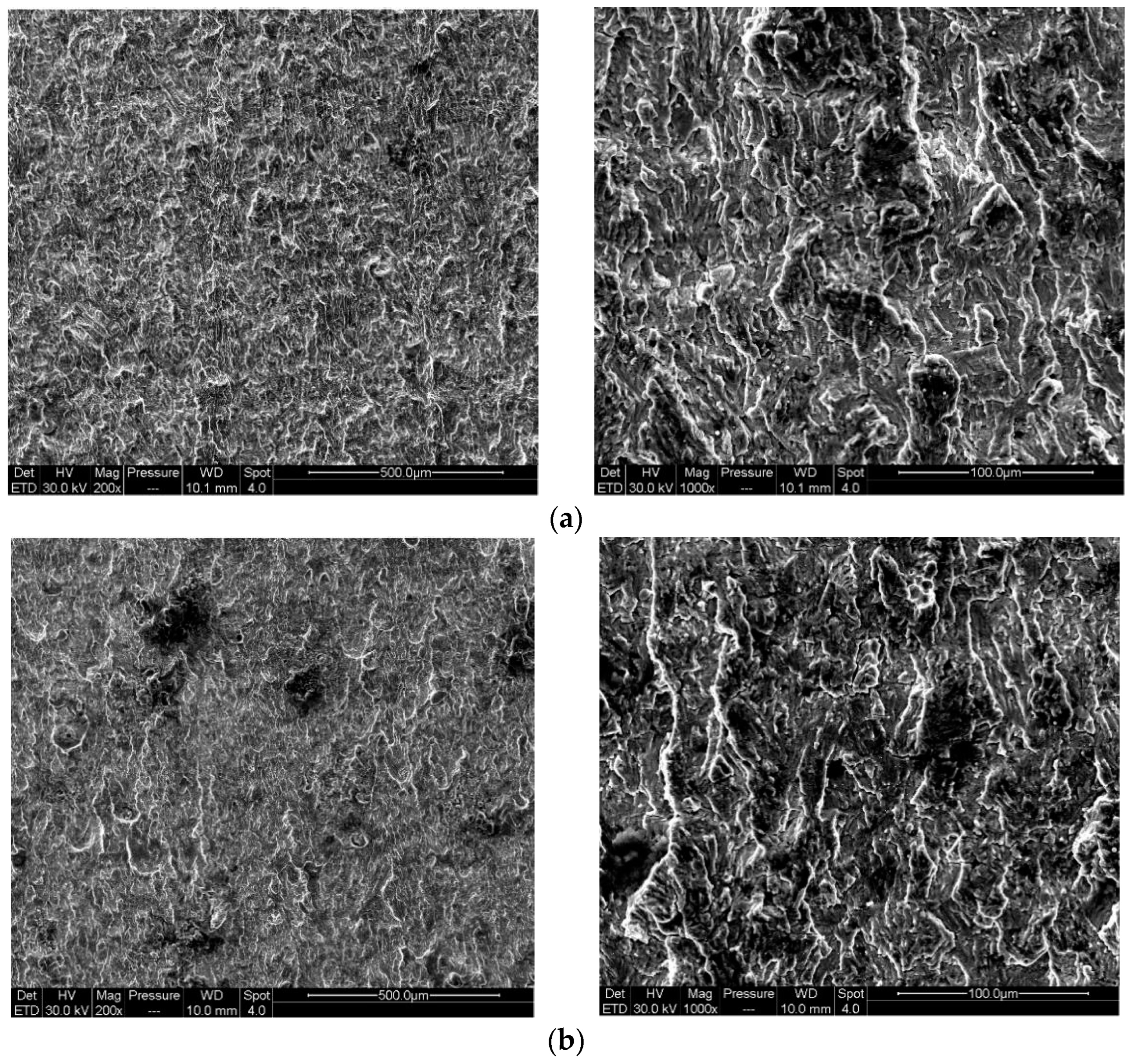
4.1. Effect of Pre-Corrosion on Crack Growth Rate
4.2. Effect of Hydrogen Corrosion Environment on Crack Growth Rate
4.3. Effect of Frequency on Crack Growth Rate
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, M.; Xuan, F.; Tu, S. Interpreting load ratio dependence of near-threshold fatigue crack growth by a new crack closure model. Int. J. Press. Vessel. Pip. 2013, 110, 9–13. [Google Scholar] [CrossRef]
- Olugbade, T.O.; Ojo, O.T.; Omiyale, B.O.; Olutomilola, E.O.; Olorunfemi, B.J. A review on the corrosion fatigue strength of surface-modified stainless steels. J. Braz. Soc. Mech. Sci. Eng. 2021, 43, 421. [Google Scholar] [CrossRef]
- Dian, L.; Lu, W.; Chen, B.; Gou, G.; Che, X.; Zhu, Z.; Gao, W. Corrosion fatigue crack propagation of 7XXX series aluminum alloys from key components of high-speed train. Int. J. Mod. Phys. B 2022, 36, 2240023. [Google Scholar] [CrossRef]
- Giarola, J.M.; Avila, J.A.; Cintho, O.M.; Pinto, H.C.; Oliveira, M.F.; Bose Filho, W.W. The effect of hydrogen on the fracture toughness of friction-stir welded API 5L X70 pipeline steels. Fatigue Fract. Eng. Mater. Struct. 2022, 45, 3009–3024. [Google Scholar] [CrossRef]
- Ma, K.; Hua, Z.; Gu, C.; Zhang, Z.; Ye, S.; Yao, Y. Effects of crack position on fatigue life of large seamless storage vessels made of 4130X for hydrogen refueling station. Int. J. Hydrogen Energy 2019, 44, 22559–22568. [Google Scholar] [CrossRef]
- Xiao, H.; Zeng, Q.; Xia, L.; Fu, Z.; Zhu, S. Hydrogen-assisted fatigue crack propagation behavior of equiatomic Co–Cr–Fe–Mn–Ni high-entropy alloy. Mater. Corros. 2022, 73, 550–557. [Google Scholar] [CrossRef]
- Cao, B.; Cheng, S.; Li, A.; Deng, Y.; Zhao, F. Fatigue Crack Propagation Study of Bridge Steel Q345qD Based on XFEM Considering the Influence of the Stress Ratio. Appl. Sci. 2022, 12, 12782. [Google Scholar] [CrossRef]
- Iacoviello, F.; Di Cocco, V. Sintered stainless steels: Fatigue crack propagation resistance under hydrogen charging conditions. Corros. Sci. 2007, 49, 2099–2117. [Google Scholar] [CrossRef]
- Li, W.; Chao, B.; Ke, W.; Yong-Zheng, L. A simplified prediction model of dwell-fatigue crack growth behaviour for titanium alloy. Ships Offshore Struct. 2022, 17, 2408–2415. [Google Scholar] [CrossRef]
- Dey, S.; Sivaprasad, S.; Das, N.; Chattoraj, I. Influence of Hydrogen on Fatigue Crack Growth in 7075 Aluminum Alloy. J. Mater. Eng. Perform. 2023, 32, 782–792. [Google Scholar] [CrossRef]
- Potirniche, G.; Horstemeyer, M.; Jelinek, B.; Wagner, G. Fatigue damage in nickel and copper single crystals at nanoscale. Int. J. Fatigue 2005, 27, 1179–1185. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, L.; Zhu, Q.; Chen, G.; Wang, C.; Fan, G.; Qin, H.; Tian, Q.; Gan, B. Evolution of fatigue mechanical properties and micro defects in nickel-based single crystal superalloys: A molecular dynamics research. Mater. Today Commun. 2023, 34, 105044. [Google Scholar] [CrossRef]
- Wu, W.; Ding, Z.; Chen, B.; Shen, H.; Li, Y. Effect of rhenium on low cycle fatigue behaviors of Ni-based single crystal superalloys: A molecular dynamics simulation. J. Mater. Res. Technol. 2022, 18, 5144–5160. [Google Scholar] [CrossRef]
- Dogan, O.; Kapci, M.F.; Esat, V.; Bal, B. Experimental and Molecular Dynamics Simulation-Based Investigations on Hydrogen Embrittlement Behavior of Chromium Electroplated 4340 Steel. J. Eng. Mater. Technol. 2021, 143, 041010. [Google Scholar] [CrossRef]
- Xing, X.; Zhang, Y.; Wang, S.; Li, Z.; Yang, C.; Cui, G.; Zhang, S.; Liu, J.; Gou, J.; Yu, H. Atomistic simulation of hydrogen-induced plastic zone compression during cyclic loading. Int. J. Hydrogen Energy 2020, 45, 15697–15709. [Google Scholar] [CrossRef]
- GB/T 15970.6-2007; Corrosion of Metals and Alloys—Stress Corrosion Testing—Part 6: Preparation and Use of Pre-Cracked Specimens for Tests under Constant Load or Constant Displacement. National Steel Standardization Technical Committee: Beijing, China, 2007.
- GB/T 6398-2017; Metallic Materials—Fatigue Testing—Fatigue Crack Growth Method. National Steel Standardization Technical Committee: Beijing, China, 2017.
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in’t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS—A flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Song, J.; Curtin, W.A. Atomic mechanism and prediction of hydrogen embrittlement in iron. Nat. Mater. 2013, 12, 145–151. [Google Scholar] [CrossRef]
- Ackland, G.J.; Mendelev, M.I.; Srolovitz, D.J.; Han, S.; Barashev, A.V. Development of an interatomic potential for phosphorus impurities in α-iron. J. Phys.-Condens. Matter 2004, 16, S2629–S2642. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO-the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Robertson, I.M.; Sofronis, P.; Nagao, A.; Martin, M.L.; Wang, S.; Gross, D.W.; Nygren, K.E. Hydrogen Embrittlement Understood. Metall. Mater. Trans. A 2015, 46, 2323–2341. [Google Scholar] [CrossRef]
- Kumar, R.; Mahajan, D.K. Modelling of hydrogen-assisted damage at the deforming single crystal crack-tip. Mech. Mater. 2023, 178, 104557. [Google Scholar] [CrossRef]
- Djukic, M.B.; Bakic, G.M.; Sijacki Zeravcic, V.; Sedmak, A.; Rajicic, B. The synergistic action and interplay of hydrogen embrittlement mechanisms in steels and iron: Localized plasticity and decohesion. Eng. Fract. Mech. 2019, 216, 106528. [Google Scholar] [CrossRef]
- Liu, D.; Tian, G.; Li, Y.; Jin, G.; Zhang, W. Fatigue Crack Propagation Behavior of the 2195-T8 Aluminum-Lithium Alloy with a Precorroded Hole. Int. J. Aerosp. Eng. 2022, 2022, 941089. [Google Scholar] [CrossRef]
- Burns, J.T.; Jones, J.J.; Thompson, A.D.; Locke, J.S.W. Fatigue crack propagation of aerospace aluminum alloy 7075-T651 in high altitude environments. Int. J. Fatigue 2018, 106, 196–207. [Google Scholar] [CrossRef]
- He, Y. First-Principle Study on Diffusion and Aggregation Behavior of Hydrogen Atoms in Austenite Steel. Ph.D. Thesis, China University of Petroleum, Beijing, China, 2017. [Google Scholar]
- Yu, Y.; Sun, W.; Tong, D. Effect of loading frequency on corrosion fatigue crack growth rate of 7N01 aluminum alloy. IOP Conf. Ser. Mater. Sci. Eng. 2020, 768, 22060. [Google Scholar] [CrossRef]
- Govender, K.; Zhang, Y.H. Fatigue crack growth rate parametric study on subsea X65 pipeline steel girth welds in H2S/CO2 environments. Int. J. Fatigue 2022, 163, 107078. [Google Scholar] [CrossRef]
- Igwemezie, V.; Mehmanparast, A. Waveform and frequency effects on corrosion-fatigue crack growth behaviour in modern marine steels. Int. J. Fatigue 2020, 134, 105484. [Google Scholar] [CrossRef]
- Ren, X.; Lu, Q.; Zhang, P.; Bai, Y.; Jin, J.; Ou, Y. Effect of Vibration Frequency on Laser Filler Wire Welded Joints. Steel Res. Int. 2020, 91, 1900550. [Google Scholar] [CrossRef]
- Wang, J.; Li, X. A Phenomenological Model for Fatigue Crack Growth Rate of X70 Pipeline Steel in H2S Corrosive Environment. J. Press. Vessel. Technol. 2014, 136, 041703. [Google Scholar] [CrossRef]


| Material | C | Si | Mn | Cr | Mo | P, S | Cu |
|---|---|---|---|---|---|---|---|
| 4130X | 0.26–0.34 | 0.17–0.37 | 0.40–0.70 | 0.80–1.10 | 0.15–0.25 | ≤0.035 | ≤0.30 |
| Material | Rm/MPa | RP0.2/MPa | E/GPa | A/% | Z/% |
|---|---|---|---|---|---|
| 4130X | 777 | 624 | 209 | 14.23 | 52.38 |
| Loading Condition | Corrosion Condition | Crack Propagation Rate Expression |
|---|---|---|
| f = 100 Hz, R = 0.1 | Un-corroded | da/dN = 2.043 × 10−13(∆K)3.513 |
| Pre-corroded | da/dN = 3.900 × 10−13(∆K)3.368 | |
| f = 0.01 Hz, R = 0.1 | Un-corroded | da/dN = 3.360 × 10−12(∆K)2.911 |
| Corrosion fatigue | da/dN = 1.885 × 10−11(∆K)2.640 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Wang, J.; Zhao, B.; Zhang, E. The Study on Fatigue Crack Growth Rate of 4130X Material under Different Hydrogen Corrosion Conditions. Materials 2024, 17, 257. https://doi.org/10.3390/ma17010257
Jiang S, Wang J, Zhao B, Zhang E. The Study on Fatigue Crack Growth Rate of 4130X Material under Different Hydrogen Corrosion Conditions. Materials. 2024; 17(1):257. https://doi.org/10.3390/ma17010257
Chicago/Turabian StyleJiang, Shaolei, Jing Wang, Bo Zhao, and Enfeng Zhang. 2024. "The Study on Fatigue Crack Growth Rate of 4130X Material under Different Hydrogen Corrosion Conditions" Materials 17, no. 1: 257. https://doi.org/10.3390/ma17010257
APA StyleJiang, S., Wang, J., Zhao, B., & Zhang, E. (2024). The Study on Fatigue Crack Growth Rate of 4130X Material under Different Hydrogen Corrosion Conditions. Materials, 17(1), 257. https://doi.org/10.3390/ma17010257






