Design, Synthesis, and Spectral Properties of Novel 2-Mercaptobenzothiazole Derivatives
Abstract
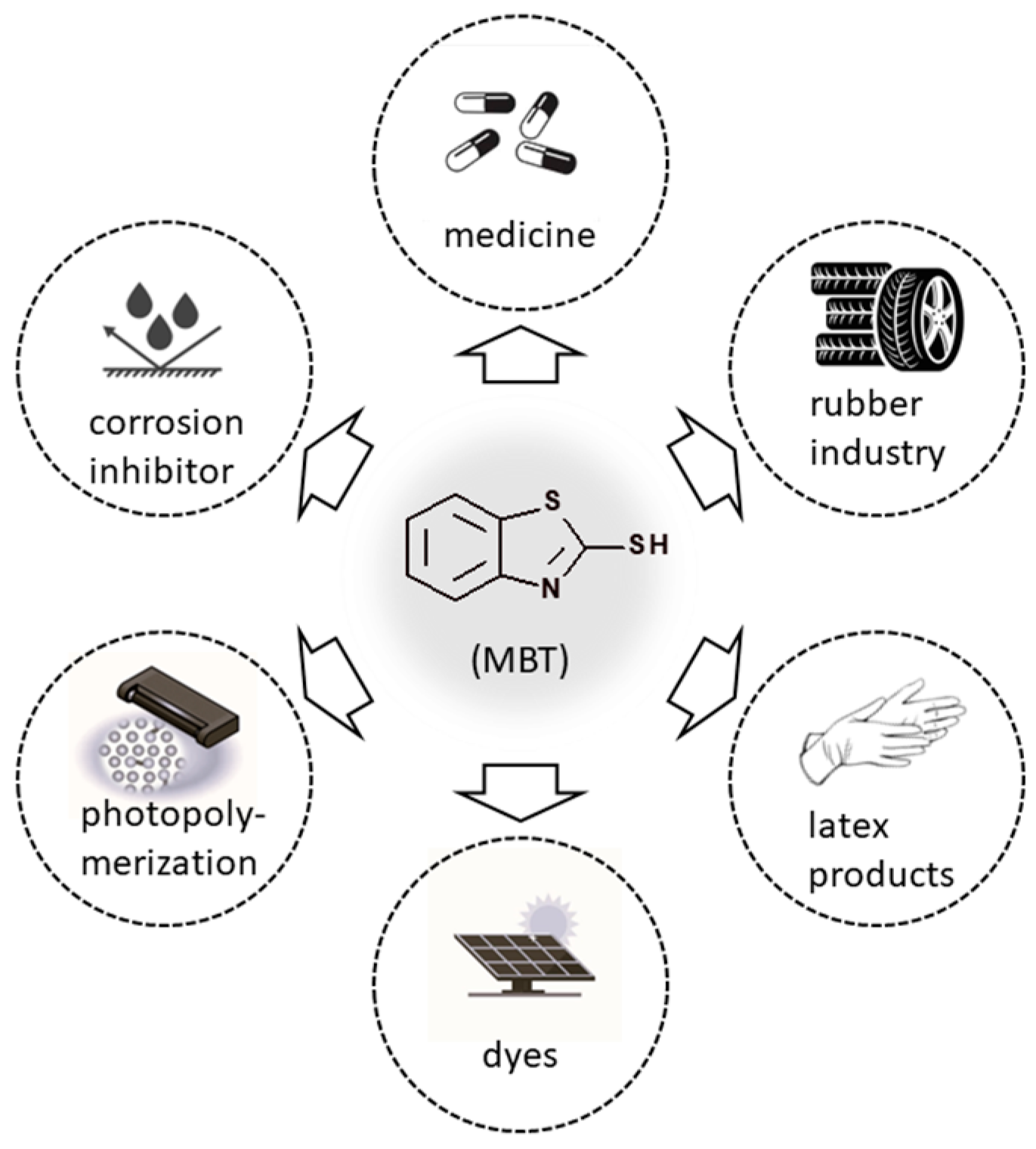
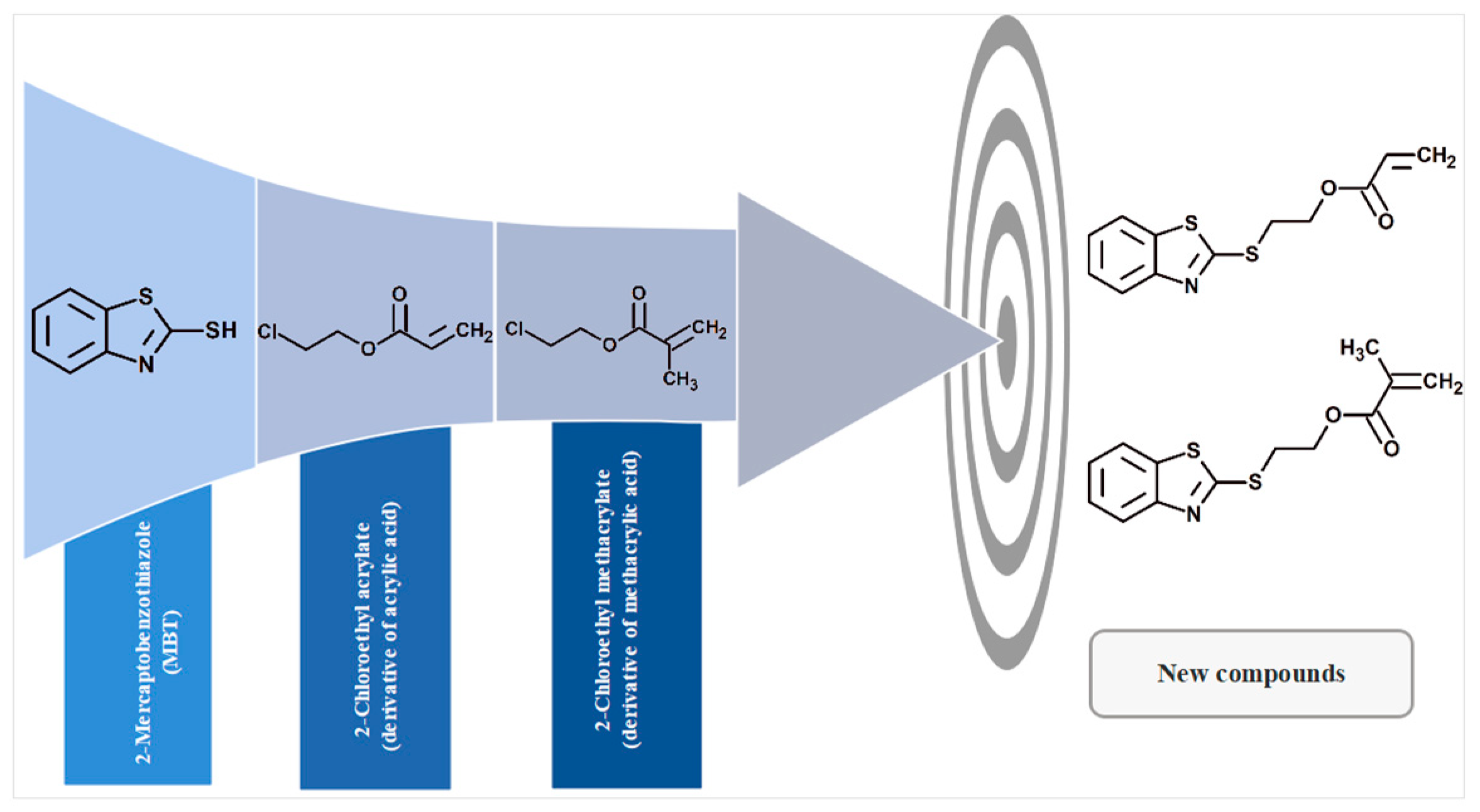
1. Introduction
2. Materials and Methods
2.1. Materials
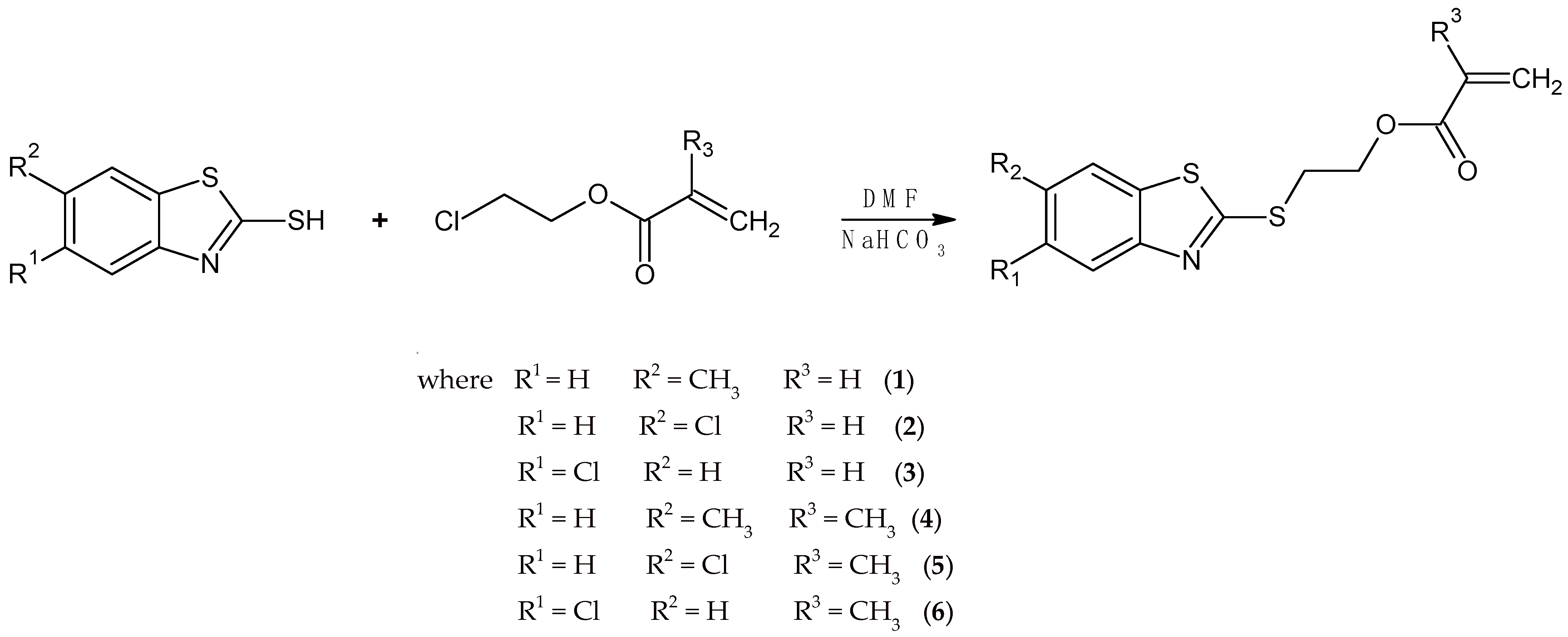
2.2. Synthesis
Elemental Analysis
- Elemental analysis was as follows:
2.3. Experimental Measurements
2.3.1. NMR Measurements
2.3.2. Elemental Analysis Measurements
2.3.3. UV-Vis Measurements
2.3.4. FT-IR Measurements
2.3.5. Boiling Point
2.3.6. GC-MS Measurements
2.3.7. Quantum Mechanical Calculations
3. Results and Discussion
3.1. Synthesis and NMR Data
3.2. Vibrational Analysis
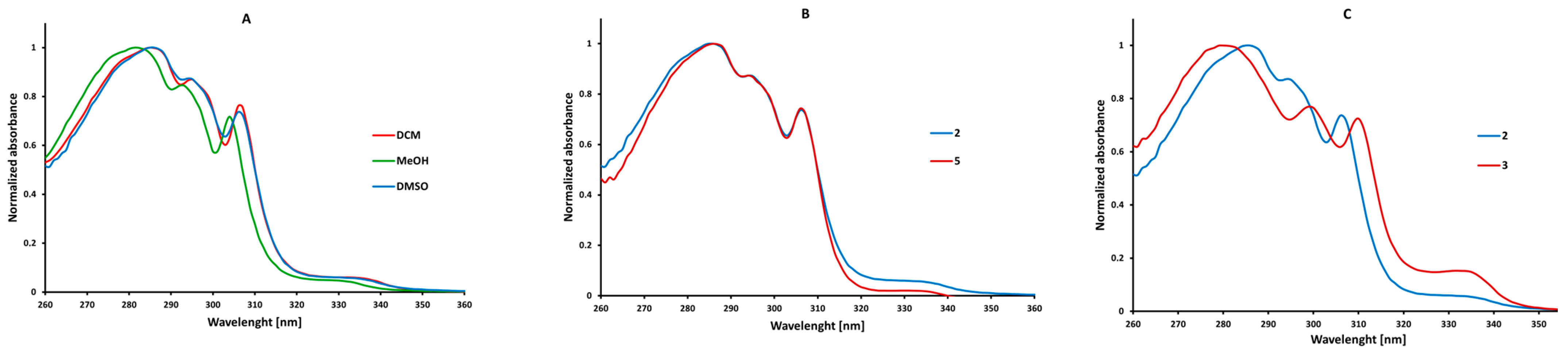
3.3. Spectroscopic Studies
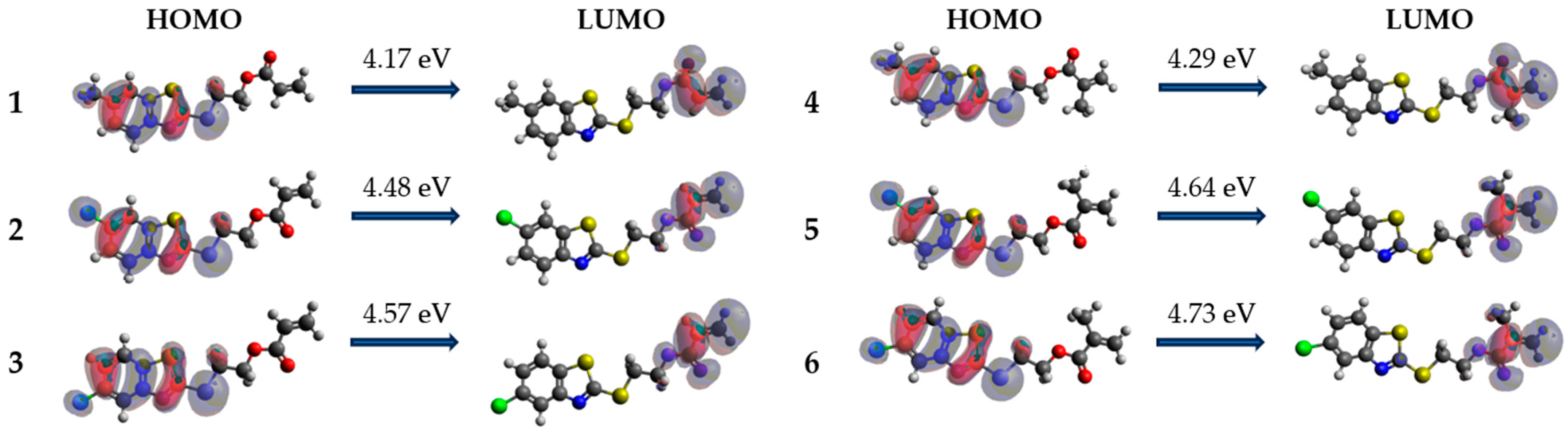
3.4. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azam, M.A.; Suresh, B. Biological activities of 2-mercaptobenzothiazole derivatives: A review. Sci. Pharm. 2012, 80, 789–823. [Google Scholar] [CrossRef] [PubMed]
- Shafi, S.; Mahboob Alam, M.; Mulakayala, N.; Mulakayala, C.; Vanaja, G.; Kalle, A.M.; Pallu, R.; Alam, M.S. Synthesis of novel 2-mercapto benzothiazole and 1,2,3-triazole based bis-heterocycles: Their anti-inflammatory and anti-nociceptive activities. Eur. J. Med. Chem. 2012, 49, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Gulab, H.; Shah, Z.; Mahmood, M.; Shah, S.R.; Ali, S.; Iqbal, M.; Khan, M.N.; Flörke, U.; Khan, S.A. Synthesis, characterization and antibacterial activity of a new calcium complex using sodium 2-mercaptobenzothiazole and 1,10-phenanthroline as ligands. J. Mol. Struct. 2018, 1154, 140–144. [Google Scholar] [CrossRef]
- Fong, J.; Yuan, M.; Jakobsen, T.H.; Mortensen, K.T.; Delos Santos, M.M.S.; Chua, S.L.; Yang, L.; Tan, C.H.; Nielsen, T.E.; Givskov, M. Disulfide Bond-Containing Ajoene Analogues As Novel Quorum Sensing Inhibitors of Pseudomonas aeruginosa. J. Med. Chem. 2017, 60, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Cano, N.H.; Ballari, M.S.; López, A.G.; Santiago, A.N. New Synthesis and Biological Evaluation of Benzothiazole Derivates as Antifungal Agents. J. Agric. Food Chem. 2015, 63, 3681–3686. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.P.; Rahman, M.A.; Nishad, S.; Maurya, S.K.; Anas, M.; Mujahid, M. Synthesis and biological activities of benzothiazole derivatives: A review. Intell. Pharm. 2023, 1, 122–132. [Google Scholar] [CrossRef]
- Sun, X.; Liu, S.; Hu, P.; Sun, S.; Xie, Z.; Hu, G.; Hu, D.; Zhang, M. Microscale Corrosion Inhibition Behavior of Four Corrosion Inhibitors (BTA, MBI, MBT, and MBO) on Archeological Silver Artifacts Based on Scanning Electrochemical Cell Microscopy. Anal. Chem. 2023, 95, 14686–14694. [Google Scholar] [CrossRef]
- Garg, V.; Sharma, S.B.; Zanna, S.; Seyeux, A.; Wiame, F.; Maurice, V.; Marcus, P. Enhanced corrosion inhibition of copper in acidic environment by cathodic control of interface formation with 2-mercaptobenzothiazole. Electrochim. Acta 2023, 447, 142162. [Google Scholar] [CrossRef]
- Vernack, E.; Zanna, S.; Seyeux, A.; Costa, D.; Chiter, F.; Tingaut, P.; Marcus, P. ToF-SIMS, XPS and DFT study of the adsorption of 2-mercaptobenzothiazole on copper in neutral aqueous solution and corrosion protection in chloride solution. Corros. Sci. 2023, 210, 110854. [Google Scholar] [CrossRef]
- Finšgar, M.; Čakara, D. Spectroscopic analysis and in situ adsorption of 2-mercaptobenzothiazole corrosion inhibitor on Zn from a chloride solution. Appl. Surf. Sci. 2022, 606, 154843. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J.; Khaled, M. CS2 mediated synthesis of corrosion-inhibiting mercaptobenzothiazole molecule for industrial zinc: Experimental studies and molecular dynamic simulations. J. Mol. Liq. 2021, 324, 115129. [Google Scholar] [CrossRef]
- Chiter, F.; Costa, D.; Maurice, V.; Marcus, P. Chemical interaction, self-ordering and corrosion inhibition properties of 2-mercaptobenzothiazole monolayers: DFT atomistic modeling on metallic copper. Corros. Sci. 2022, 209, 110658. [Google Scholar] [CrossRef]
- Majidi, R.; Danaee, I.; Vrsalović, L.; Zarei, D. Development of a smart anticorrosion epoxy coating containing a pH-sensitive GO/MOF nanocarrier loaded with 2-mercaptobenzothiazole corrosion inhibitor. Mater. Chem. Phys. 2023, 308, 128291. [Google Scholar] [CrossRef]
- He, S.; Gao, Y.; Wu, C.; Chen, Z.; Cen, H. A self-healing and anticorrosion epoxy coating based on the novel polymer filler containing a side-linked grafting 2-mercaptobenzothiazole. J. Mater. Res. Technol. 2023, 26, 1107–1121. [Google Scholar] [CrossRef]
- Nawaz, M.; Kahraman, R.; Taryba, M.G.; Hassan, M.K.; Attaei, M.; Montemor, M.F.; Shakoor, R.A. Improved properties of polyolefin nanocomposite coatings modified with ceria nanoparticles loaded with 2-mercaptobenzothiazole. Prog. Org. Coat. 2022, 171, 107046. [Google Scholar] [CrossRef]
- Larsson, L.F.G.; Tractz, G.T.; Camargo Matheus, A.P.; Pinto Rodrigues, P.R. The use of 2-Mercaptobenzothiazole as a new co-adsorbent in dye-sensitized solar cells. Opt. Mater. 2022, 131, 112658. [Google Scholar] [CrossRef]
- Rojruthai, P.; Sakdapipanich, J.; Wiriyanantawong, J.; Ho, C.C.; Chaiear, N. Effect of Latex Purification and Accelerator Types on Rubber Allergens Prevalent in Sulphur Prevulcanized Natural Rubber Latex: Potential Application for Allergy-Free Natural Rubber Gloves. Polymers 2022, 14, 4679. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, X.; Wang, L.; Wang, J.; Song, C. Hydrogenation of tar residue derived from the synthesis of rubber vulcanization accelerator 2-mercaptobenzothiazole. Asia-Pac. J. Chem. Eng. 2017, 12, 400–405. [Google Scholar] [CrossRef]
- Morgan, B.; Mcgill, W.J. 2-Mercaptobenzothiazole as Accelerator for 2,3-Dimethyl-2-butene. J. Appl. Polym. Sci. 2000, 76, 1377–1385. [Google Scholar] [CrossRef]
- Van Der Horst, M.; Hendrikse, K.G.; Woolard, C.D. The role of thiol intermediates in 2-mercaptobenzothiazole accelerated sulfur vulcanization of rubber model compounds. J. Appl. Polym. Sci. 2003, 89, 47–54. [Google Scholar] [CrossRef]
- Andrzejewska, E.; Zych-Tomkowiak, D.; Andrzejewski, M.; Hug, G.L.; Marciniak, B. Heteroaromatic thiols as co-initiators for type II photoinitiating systems based on camphorquinone and isopropylthioxanthone. Macromolecules 2006, 39, 3777–3785. [Google Scholar] [CrossRef]
- Degirmenci, A.; Sanyal, R.; Arslan, M.; Sanyal, A. Benzothiazole-disulfide based redox-responsive polymers: Facile access to reversibly functionalizable polymeric coatings. Polym. Chem. 2022, 13, 2595–2607. [Google Scholar] [CrossRef]
- Pirman, T.; Ocepek, M.; Likozar, B. Radical Polymerization of Acrylates, Methacrylates, and Styrene: Biobased Approaches, Mechanism, Kinetics, Secondary Reactions, and Modeling. Ind. Eng. Chem. Res. 2021, 60, 9347–9367. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, Q.; Ding, Y.; Sato, S.I.; Xu, L.; Zang, C.; Shen, X.; Kakuchi, T. B(C6F5)3-Catalyzed Group Transfer Polymerization of Acrylates Using Hydrosilane: Polymerization Mechanism, Applicable Monomers, and Synthesis of Well-Defined Acrylate Polymers. Macromolecules 2019, 52, 844–856. [Google Scholar] [CrossRef]
- Brydson, J.A. Plastic Materials, 7th ed.; Butterworth-Heinemann: Oxford, UK, 1999; ISBN 9780750641326. [Google Scholar]
- Su, H.W.; Chen, W.C.; Lee, W.C.; King, J.S. New photocurable acrylic/silsesquioxane hybrid optical materials: Synthesis, properties, and patterning. Macromol. Mater. Eng. 2007, 292, 666–673. [Google Scholar] [CrossRef]
- Montheard, J.-P.; Chatzopoulos, M.; Chappard, D. 2-Hydroxyethyl Methacrylate (HEMA): Chemical Properties and Applications in Biomedical Fields. J. Macromol. Sci. Part C 1992, 32, 1–34. [Google Scholar] [CrossRef]
- Feng, M.; Sefton, M. V Hydroxyethyl methacrylate–methyl methacrylate (HEMA–MMA) copolymers for cell microencapsulation: Effect of HEMA purity. J. Biomater. Sci. Polym. Ed. 2000, 11, 537–545. [Google Scholar] [CrossRef]
- Huang, J.; Lean, J. Advances in acrylic structural adhesives. In Advances in Structural Adhesive Bonding, 2nd ed.; Woodhead Publishing: Sawston, UK, 2023; pp. 69–101. ISBN 9780323912143. [Google Scholar]
- Mori, H.; Müller, A.H.E. New polymeric architectures with (meth)acrylic acid segments. Prog. Polym. Sci. 2003, 28, 1403–1439. [Google Scholar] [CrossRef]
- Corsaro, C.; Neri, G.; Santoro, A.; Fazio, E. Acrylate and Methacrylate Polymers’ Applications: Second Life with Inexpensive and Sustainable Recycling Approaches. Materials 2022, 15, 282. [Google Scholar] [CrossRef]
- Parra, F.; Vázquez, B.; Benito, L.; Barcenilla, J.; San Román, J. Foldable antibacterial acrylic intraocular lenses of high refractive index. Biomacromolecules 2009, 10, 3055–3061. [Google Scholar] [CrossRef]
- Cracium, L.; Polishchuk, O.; Schriver, G.W.; Hainz, R. High Refractive Index Monomers, Compositions and Uses Thereof. U.S. Patent 2008/0200582 A1, 21 August 2008. [Google Scholar]
- Chisholm, B.J.; Pickett, J.E. Method of Making a High Refractive Index Optical Management Coating and the Coating. U.S. Patent 7,045,558 B2, 15 May 2006. [Google Scholar]
- Ong, B.K.; Woon, K.L.; Ariffin, A. Evaluation of various density functionals for predicting the electrophosphorescent host HOMO, LUMO and triplet energies. Synth. Met. 2014, 195, 54–60. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys 1993, 98, 5648. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Petersson, G.A.; Al-Laham, M.A. A complete basis set model chemistry. II. Open-shell systems and the total energies of the first-row atoms. J. Chem. Phys. 1991, 94, 6081–6090. [Google Scholar] [CrossRef]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, Inc., Wallingford, CT, USA. 2016. Available online: https://gaussian.com/g09citation/ (accessed on 25 December 2023).
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeerschd, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Kabatc-Borcz, J.; Czeleń, P.; Skotnicka, A. Synthesis and Spectroscopic Properties of Selected Acrylic and Methacrylic Derivatives of 2-Mercaptobenzothiazole. Symmetry 2023, 15, 370. [Google Scholar] [CrossRef]
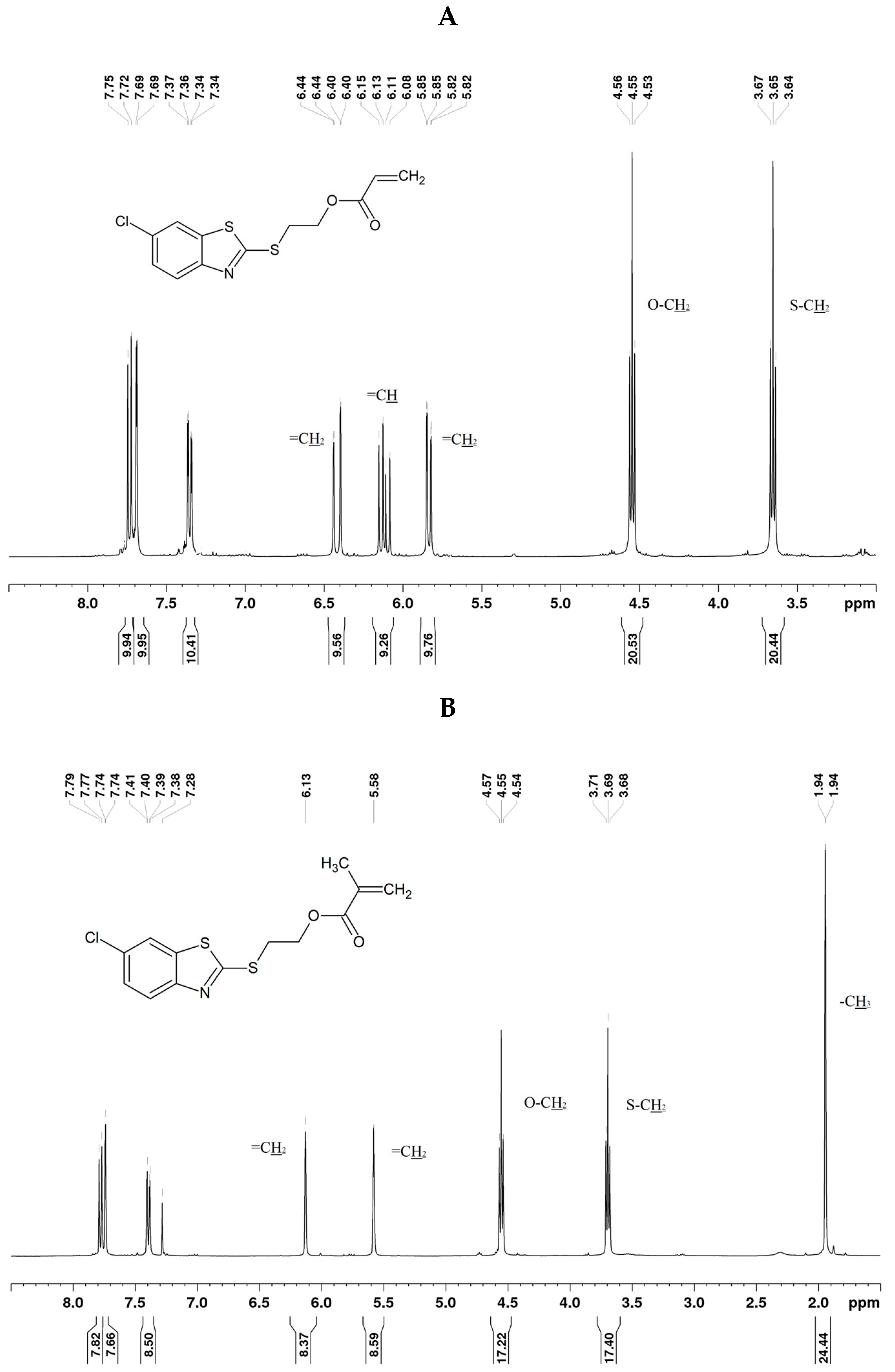
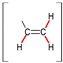
| Compound | Solvent | CH=CH2/C(CH3)=CH2 | CH=CH2 | C(CH3)=CH2 | CH2–O | S–CH2 |
|---|---|---|---|---|---|---|
| 1 | CHCl3 | 6.41; 5.82 | 6.11 | - | 4.53 | 3.63 |
| CHCl3 | 7.24; 6.48 | 6.58 | - | 4.42 | 3.47 | |
| DMSO-d6 | 7.23; 6.56 | 6.68 | - | 4.52 | 3.51 | |
| 2 | CHCl3 | 6.34; 5.76 | 6.04 | - | 4.46 | 3.58 |
| CHCl3 | 7.12; 6.29 | 6.47 | - | 4.38 | 3.46 | |
| DMSO-d6 | 7.02; 6.37 | 6.59 | - | 4.44 | 3.53 | |
| 3 | CHCl3 | 6.35; 5.75 | 6.04 | - | 4.47 | 3.60 |
| CHCl3 | 7.12; 6.41 | 6.44 | - | 4.40 | 3.48 | |
| DMSO-d6 | 7.01; 6.38 | 6.58 | - | 4.42 | 3.53 | |
| 4 | CHCl3 | 6.03; 5.47 | - | 1.83 | 4.44 | 3.56 |
| CHCl3 | 7.38; 6.17 | - | 2.27 | 4.84 | 3.46 | |
| DMSO-d6 | 7.36; 6.24 | - | 2.30 | 4.93 | 3.49 | |
| 5 | CHCl3 | 6.13; 5.58 | - | 1.94 | 4.55 | 3.69 |
| CHCl3 | 6.81; 6.12 | - | 2.22 | 4.34 | 3.61 | |
| DMSO-d6 | 6.77; 6.08 | - | 2.15 | 4.29 | 3.56 | |
| 6 | CHCl3 | 6.04; 5.49 | - | 1.85 | 4.46 | 3.60 |
| CHCl3 | 6.56; 5.84 | - | 2.24 | 4.47 | 3.63 | |
| DMSO-d6 | 6.70; 5.93 | - | 2.14 | 4.42 | 3.57 |
| Compound | Solvent | CH=CH2/C(CH3)=CH2 | CH=CH2/C(CH3)=CH2 | C=O | CH2–O | S–CH2 |
|---|---|---|---|---|---|---|
| 1 | CHCl3 | 131.23 | 128.02 | 165.77 | 62.75 | 31.86 |
| CHCl3 | 147.15 | 128.61 | 172.28 | 68.79 | 39.49 | |
| DMSO-d6 | 147.79 | 128.92 | 173.92 | 69.25 | 39.60 | |
| 2 | CHCl3 | 131.23 | 127.98 | 165.71 | 62.58 | 31.86 |
| CHCl3 | 139.15 | 134.17 | 170.93 | 64.66 | 38.02 | |
| DMSO-d6 | 142.74 | 135.31 | 175.04 | 66.37 | 39.31 | |
| 3 | CHCl3 | 131.25 | 127.90 | 165.57 | 62.46 | 31.75 |
| CHCl3 | 139.45 | 133.77 | 173.98 | 65.01 | 37.26 | |
| DMSO-d6 | 142.79 | 135.30 | 175.07 | 66.44 | 39.34 | |
| 4 | CHCl3 | 125.97 | 135.98 | 167.01 | 62.92 | 31.93 |
| CHCl3 | 148.30 | 143.64 | 171.72 | 72.90 | 40.91 | |
| DMSO-d6 | 148.57 | 144.15 | 173.44 | 73.27 | 40.98 | |
| 5 | CHCl3 | 126.05 | 122.18 | 167.01 | 62.73 | 31.98 |
| CHCl3 | 138.00 | 147.64 | 177.07 | 67.76 | 37.13 | |
| DMSO-d6 | 137.10 | 146.94 | 175.34 | 66.24 | 35.53 | |
| 6 | CHCl3 | 126.05 | 135.92 | 166.95 | 62.69 | 31.93 |
| CHCl3 | 138.15 | 148.23 | 176.98 | 68.08 | 40.00 | |
| DMSO-d6 | 136.40 | 146.98 | 175.24 | 66.38 | 39.53 |
| Compounds | νs and νas (C–H) | νs and νas (C–H) | ν (C=O) | ν (C=C) | ||
|---|---|---|---|---|---|---|
 | or | 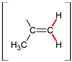 | 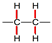 | |||
| 1 | 3031–3425 | 2860–2951 | 1720 | 1632 | ||
| 2 | 3036–3435 | 2690–2946 | 1720 | 1633 | ||
| 3 | 3035–3435 | 2658–2947 | 1721 | 1634 | ||
| 4 | 2950–3021 | 2734–2922 | 1715 | 1636 | ||
| 5 | 2951–3021 | 2734–2922 | 1715 | 1636 | ||
| 6 | 3066–3085 | 2836–2978 | 1714 | 1636 | ||
| Compound | Solvent | λab (nm) (Measured) | λab (nm) (Calculated) | ε (×104, M−1 · cm−1) |
|---|---|---|---|---|
| 1 | DCM | 283 | 278 | 1.21 |
| MeOH | 282 | 278 | 1.14 | |
| DMSO | 285 | 279 | 1.21 | |
| 2 | DCM | 285 | 282 | 1.21 |
| MeOH | 283 | 281 | 1.32 | |
| DMSO | 285 | 282 | 1.15 | |
| 3 | DCM | 279 | 283 | 1.16 |
| MeOH | 278 | 282 | 0.88 | |
| DMSO | 280 | 283 | 1.45 | |
| 4 | DCM | 285 | 279 | 1.42 |
| MeOH | 282 | 278 | 1.30 | |
| DMSO | 285 | 279 | 1.17 | |
| 5 | DCM | 285 | 282 | 1.62 |
| MeOH | 283 | 281 | 1.48 | |
| DMSO | 286 | 282 | 1.56 | |
| 6 | DCM | 279 | 281 | 1.32 |
| MeOH | 277 | 281 | 1.10 | |
| DMSO | 279 | 282 | 1.23 |
| Compound | Solvent | HOMO (eV) | LUMO (eV) | Energy Gap (eV) | η (eV) |
|---|---|---|---|---|---|
| 1 | DCM | −6.1358 | −1.9627 | 4.1731 | 2.0865 |
| MeOH | −6.1415 | −1.9508 | 4.1908 | 2.0954 | |
| DMSO | −6.1423 | −1.9497 | 4.1927 | 2.0963 | |
| 2 | DCM | −6.2863 | −1.8019 | 4.4844 | 2.2422 |
| MeOH | −6.2822 | −1.7916 | 4.4906 | 2.2453 | |
| DMSO | −6.2819 | −1.7905 | 4.4914 | 2.2457 | |
| 3 | DCM | −6.3704 | −1.8044 | 4.5660 | 2.2830 |
| MeOH | −6.3628 | −1.7927 | 4.5701 | 2.2850 | |
| DMSO | −6.3622 | −1.7913 | 4.5709 | 2.2855 | |
| 4 | DCM | −6.1312 | −1.8419 | 4.2893 | 2.1446 |
| MeOH | −6.1383 | −1.8422 | 4.2961 | 2.1480 | |
| DMSO | −6.1394 | −1.8422 | 4.2972 | 2.1486 | |
| 5 | DCM | −6.2836 | −1.6389 | 4.6446 | 2.3223 |
| MeOH | −6.2808 | −1.6357 | 4.6452 | 2.3226 | |
| DMSO | −6.2806 | −1.6354 | 4.6452 | 2.3226 | |
| 6 | DCM | −6.3696 | −1.6416 | 4.7279 | 2.3640 |
| MeOH | −6.3606 | −1.6365 | 4.7241 | 2.3621 | |
| DMSO | −6.3598 | −1.6362 | 4.7236 | 2.3618 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skotnicka, A.; Kabatc-Borcz, J. Design, Synthesis, and Spectral Properties of Novel 2-Mercaptobenzothiazole Derivatives. Materials 2024, 17, 246. https://doi.org/10.3390/ma17010246
Skotnicka A, Kabatc-Borcz J. Design, Synthesis, and Spectral Properties of Novel 2-Mercaptobenzothiazole Derivatives. Materials. 2024; 17(1):246. https://doi.org/10.3390/ma17010246
Chicago/Turabian StyleSkotnicka, Agnieszka, and Janina Kabatc-Borcz. 2024. "Design, Synthesis, and Spectral Properties of Novel 2-Mercaptobenzothiazole Derivatives" Materials 17, no. 1: 246. https://doi.org/10.3390/ma17010246
APA StyleSkotnicka, A., & Kabatc-Borcz, J. (2024). Design, Synthesis, and Spectral Properties of Novel 2-Mercaptobenzothiazole Derivatives. Materials, 17(1), 246. https://doi.org/10.3390/ma17010246






