Comparison of Orthodontic Tooth Movement of Regenerated Bone Induced by Carbonated Hydroxyapatite or Deproteinized Bovine Bone Mineral in Beagle Dogs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Scaffold Implantation
2.2. Attaching Orthodontic Appliances and Moving Teeth
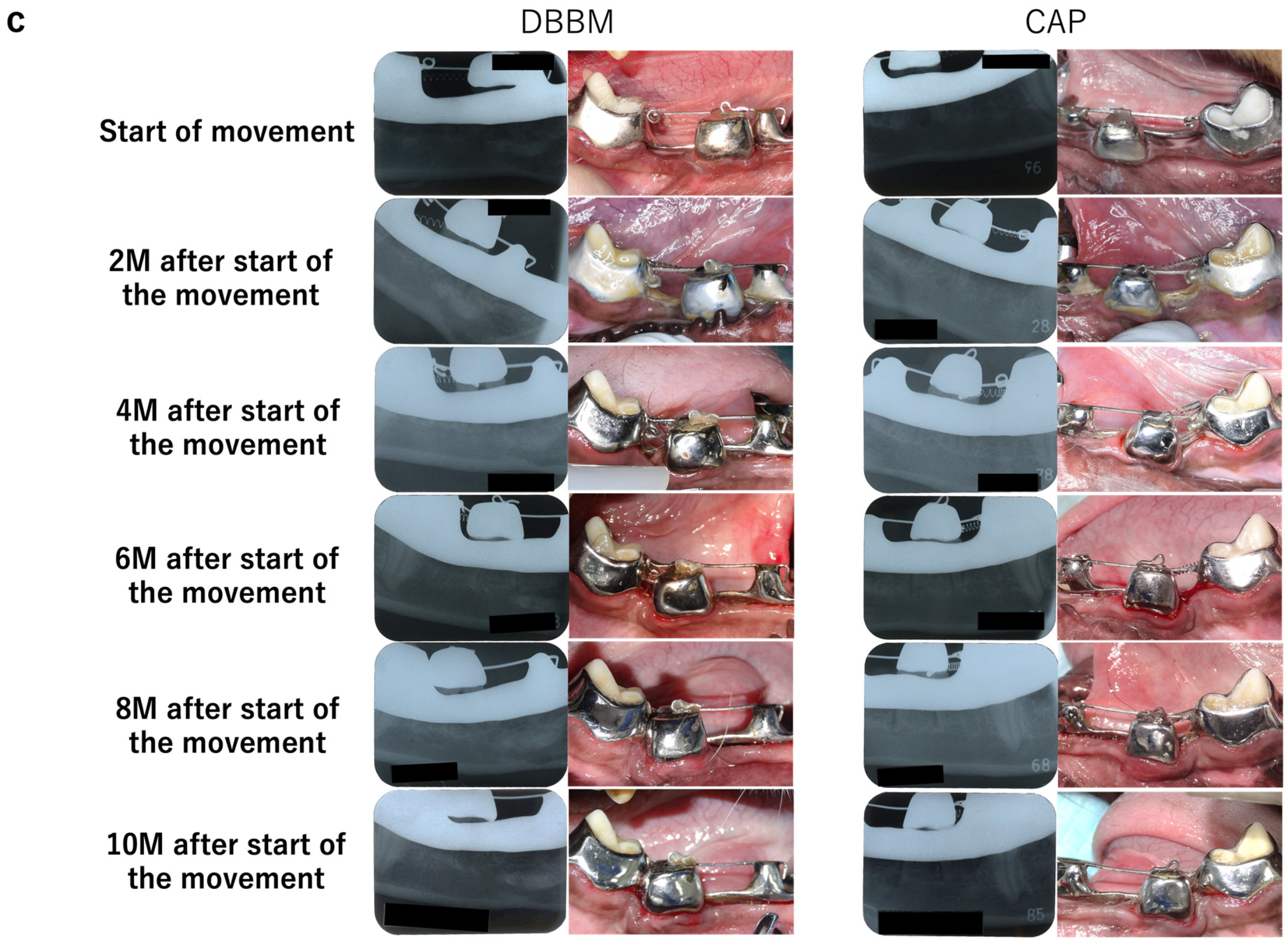
2.3. Intraoral and Radiographic Photographs
2.4. Evaluation of Tooth Movement by an Intraoral Scanner (IOS)
2.5. Histological Evaluation of Regenerated Bone
2.6. Statistical Analysis
3. Results
3.1. Intraoral and Radiographic Evaluation
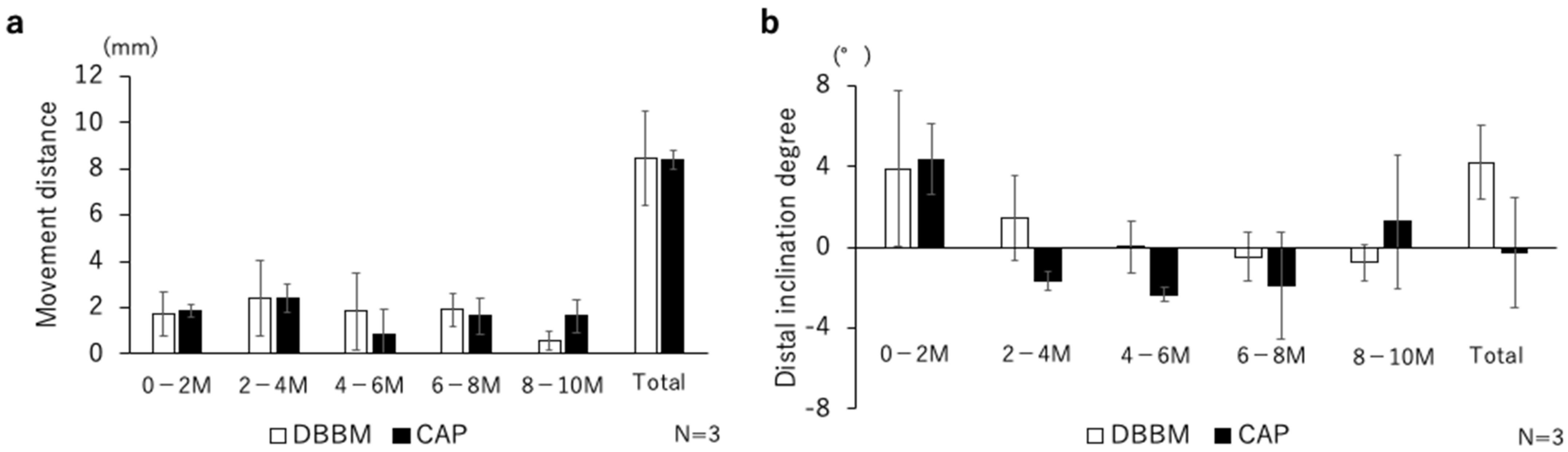
3.2. 3D Tooth Movement Evaluation
3.3. Histological Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hiraki, T.; Kunimatsu, R.; Nakajima, K.; Abe, T.; Yamada, S.; Rikitake, K.; Tanimoto, K. Stem cell-derived conditioned media from human exfoliated deciduous teeth promote bone regeneration. Oral Dis. 2020, 26, 381–390. [Google Scholar] [CrossRef]
- Jensen, T.; Schou, S.; Stavropoulos, A.; Terheyden, H.; Holmstrup, P. Maxillary sinus floor augmentation with Bio-Oss or Bio-Oss mixed with autogenous bone as graft: A systematic review. Clin. Oral Implant. Res. 2012, 23, 263–273. [Google Scholar] [CrossRef]
- Cosso, M.G.; De Brito, R.B.; Piattelli, A.; Shibli, J.A.; Zenóbio, E.G. Volumetric dimensional changes of autogenous bone and the mixture of hydroxyapatite and autogenous bone graft in humans maxillary sinus augmentation. A multislice tomographic study. Clin. Oral Implant. Res. 2014, 25, 1251–1256. [Google Scholar] [CrossRef]
- Guillaume, B. Filling bone defects with β-TCP in maxillofacial surgery: A review. Morphologie. 2017, 101, 113–119. [Google Scholar] [CrossRef]
- Sugimoto, A.; Ohno, K.; Michi, K.; Kanegae, H.; Aigase, S.; Tachikawa, T. Effect of calcium phosphate ceramic particle insertion on tooth eruption. Oral Surg. Oral Med. Oral Pathol. 1993, 76, 141–148. [Google Scholar] [CrossRef]
- Wiltfang, J.; Merten, H.A.; Schlegel, K.A.; Schultze-Mosgau, S.; Kloss, F.R.; Rupprecht, S.; Kessler, P. Degradation characteristics of α and β tri-calcium-phosphate (TCP) in minipigs. J. Biomed. Mater. Res. 2002, 63, 115–121. [Google Scholar] [CrossRef]
- Moreira, C.; Silva, J.R.; Samico, P.; Nishioka, G.N.d.M.; Nishioka, R.S. Application of bio-oss in tissue regenerative treatment prior to implant installation: Literature review. Braz. Dent. Sci. 2019, 22, 147–154. [Google Scholar] [CrossRef]
- Berglundh, T.; Lindhe, J. Healing around implants placed in bone defects treated with Bio-Oss: An experimental study in the dog. Clin. Oral Implant. Res. 1997, 8, 117–124. [Google Scholar] [CrossRef]
- Vasilyev, A.V.; Kuznetsova, V.S.; Bukharova, T.B.; Grigoriev, T.E.; Zagoskin, Y.D.; Galitsina, E.V.; Fatkhudinova, N.L.; Babichenko, I.I.; Chvalun, S.N.; Goldstein, D.V.; et al. Osteoinductive potential of highly porous polylactide granules and Bio-Oss impregnated with low doses of BMP-2. IOP Conf. Ser. Earth Environ. Sci. 2020, 421, 052035. [Google Scholar] [CrossRef]
- Araújo, M.G.; Carmagnola, D.; Berglundh, T.; Thilander, B.; Lindhe, J. Orthodontic movement in bone defects augmented with Bio-Oss. An experimental study in dogs. J. Clin. Periodontol. 2001, 28, 73–80. [Google Scholar] [CrossRef]
- Ishikawa, K.; Hayashi, K. Carbonate apatite artificial bone. Sci. Technol. Adv. Mater. 2021, 22, 683–694. [Google Scholar] [CrossRef]
- Mano, T.; Akita, K.; Fukuda, N.; Kamada, K.; Kurio, N.; Ishikawa, K.; Miyamoto, Y. Histological comparison of three apatitic bone substitutes with different carbonate contents in alveolar bone defects in a beagle mandible with simultaneous implant installation. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1450–1459. [Google Scholar] [CrossRef]
- Fukuda, N.; Ishikawa, K.; Miyamoto, Y. Alveolar ridge preservation in beagle dogs using carbonate apatite bone substitute. Ceram. Int. 2022, 48, 1796–1804. [Google Scholar] [CrossRef]
- Ogino, Y.; Ayukawa, Y.; Tachikawa, N.; Shimogishi, M.; Miyamoto, Y.; Kudoh, K.; Fukuda, N.; Ishikawa, K.; Koyano, K. Staged sinus floor elevation using novel low-crystalline carbonate apatite granules: Prospective results after 3-year functional loading. Materials 2021, 14, 5760. [Google Scholar] [CrossRef]
- Kitamura, M.; Yamashita, M.; Miki, K.; Ikegami, K.; Takedachi, M.; Kashiwagi, Y.; Nozaki, T.; Yamanaka, K.; Masuda, H.; Ishihara, Y.; et al. An exploratory clinical trial to evaluate the safety and efficacy of combination therapy of REGROTH® and Cytrans® granules for severe periodontitis with intrabony defects. Regen. Ther. 2022, 21, 104–113. [Google Scholar] [CrossRef]
- Tanimoto, K.; Sumi, K.; Yoshioka, M.; Oki, N.; Tanne, Y.; Awada, T.; Kato, Y.; Sugiyama, M.; Tanne, K. Experimental tooth movement into new bone area regenerated by use of bone marrow-derived mesenchymal stem cells. Cleft Palate Craniofac. J. 2015, 52, 386–394. [Google Scholar] [CrossRef]
- Ten Heggeler, J.M.A.G.; Slot, D.E.; Van Der Weijden, G.A. Effect of socket preservation therapies following tooth extraction in non-molar regions in humans: A systematic review. Clin. Oral Implant. Res. 2011, 22, 779–788. [Google Scholar] [CrossRef]
- Fukuba, S.; Okada, M.; Nohara, K.; Iwata, T. Alloplastic bone substitutes for periodontal and bone regeneration in dentistry: Current status and prospects. Materials 2021, 14, 1096. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Katagiri, H.; Kono, M.; Schaller, B.; Iizuka, T.; Safi, A.F. The impact of the size of bone substitute granules on macrophage and osteoblast behaviors in vitro. Clin. Oral Investig. 2021, 25, 4949–4958. [Google Scholar] [CrossRef]
- Maeno, S.; Niki, Y.; Matsumoto, H.; Morioka, H.; Yatabe, T.; Funayama, A.; Toyama, Y.; Taguchi, T.; Tanaka, J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 2005, 26, 4847–4855. [Google Scholar] [CrossRef]
- Sato, N.; Handa, K.; Venkataiah, V.S.; Hasegawa, T.; Njuguna, M.M.; Yahata, Y.; Saito, M. Comparison of the vertical bone defect healing abilities of carbonate apatite, β-tricalcium phosphate, hydroxyapatite and bovine-derived heterogeneous bone. Dent. Mater. J. 2020, 39, 309–318. [Google Scholar] [CrossRef]
- Canterelli, M.; Godoi, A.; Sinhoerti, M.; Neres, J.; Santos, E.; Sobrinho, L.; Costa, A. Effect of horizontal slot of maxillary canines’ brackets with varying wire angulations—An in vitro study. Braz. Dent. J. 2022, 33, 55–63. [Google Scholar] [CrossRef]
- Feller, L.; Khammissa, R.; Thomadakis, G.; Fourie, J.; Lemmer, J. Apical External Root Resorption and Repair in Orthodontic Tooth Movement: Biological Events. Biomed. Res. Int. 2016, 2016, 4864195. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, Z.; Zheng, J.; Chen, X.; Xu, W.; Zou, D.; Zhang, S.; Yang, C. Timing of force application on buccal tooth movement into bone-grafted alveolar defects: A pilot study in dogs. Am. J. Orthod. Dentofacial. Orthop. 2021, 159, e123–e134. [Google Scholar] [CrossRef]
- Alalola, B.; Asiri, A.; Binmoghaiseeb, I.; Baharoon, W.; Alrassi, Y.; Alanizy, B.; Alsayari, H. Impact of Bone-Grafting Materials on the Rate of Orthodontic Tooth Movement: A Systematic Review. Cureus 2023, 15, e44535. [Google Scholar] [CrossRef]
- Ru, N.; Liu, S.; Bai, Y.; Li, Y.; Liu, Y.; Wei, X. BoneCeramic graft regenerates alveolar defects but slows orthodontic tooth movement with less root resorption. Am. J. Orthod. Dentofacial. Orthop. 2016, 149, 523–532. [Google Scholar] [CrossRef]
- Abe, T.; Sumi, K.; Kunimatsu, R.; Oki, N.; Tsuka, Y.; Awada, T.; Nakajima, K.; Sugiyama, M.; Tanimoto, K. Bone regeneration in a canine model of artificial jaw cleft using bone marrow–derived mesenchymal stem cells and carbonate hydroxyapatite Carrier. Cleft Palate Craniofac. J. 2020, 57, 208–217. [Google Scholar] [CrossRef]
- Suzuki, S.; Venkataiah, V.S.; Yahata, Y.; Kitagawa, A.; Inagaki, M.; Njuguna, M.M.; Nozawa, R.; Kakiuchi, Y.; Nakano, M.; Handa, K.; et al. Correction of large jawbone defect in the mouse using immature osteoblast–like cells and a 3D polylactic acid scaffold. PNAS Nexus. 2022, 1, pgac151. [Google Scholar] [CrossRef]
- Nakajima, K.; Kunimatsu, R.; Ando, K.; Ando, T.; Hayashi, Y.; Kihara, T.; Hiraki, T.; Tsuka, Y.; Abe, T.; Kaku, M.; et al. Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 497, 876–882. [Google Scholar] [CrossRef]
- Putranti, N.A.R.; Kunimatsu, R.; Rikitake, K.; Hiraki, T.; Nakajima, K.; Abe, T.; Tsuka, Y.; Sakata, S.; Nakatani, A.; Nikawa, H.; et al. Combination of carbonate hydroxyapatite and stem cells from human deciduous teeth promotes bone regeneration by enhancing BMP-2, VEGF and CD31 expression in immunodeficient mice. Cells 2022, 11, 1914. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, T.; Kunimatsu, R.; Tanimoto, K. Comparison of Orthodontic Tooth Movement of Regenerated Bone Induced by Carbonated Hydroxyapatite or Deproteinized Bovine Bone Mineral in Beagle Dogs. Materials 2024, 17, 112. https://doi.org/10.3390/ma17010112
Abe T, Kunimatsu R, Tanimoto K. Comparison of Orthodontic Tooth Movement of Regenerated Bone Induced by Carbonated Hydroxyapatite or Deproteinized Bovine Bone Mineral in Beagle Dogs. Materials. 2024; 17(1):112. https://doi.org/10.3390/ma17010112
Chicago/Turabian StyleAbe, Takaharu, Ryo Kunimatsu, and Kotaro Tanimoto. 2024. "Comparison of Orthodontic Tooth Movement of Regenerated Bone Induced by Carbonated Hydroxyapatite or Deproteinized Bovine Bone Mineral in Beagle Dogs" Materials 17, no. 1: 112. https://doi.org/10.3390/ma17010112
APA StyleAbe, T., Kunimatsu, R., & Tanimoto, K. (2024). Comparison of Orthodontic Tooth Movement of Regenerated Bone Induced by Carbonated Hydroxyapatite or Deproteinized Bovine Bone Mineral in Beagle Dogs. Materials, 17(1), 112. https://doi.org/10.3390/ma17010112





