Recent Advances in Alkali-Activated Materials with Seawater and Sea Sand
Abstract
1. Introduction
2. Properties of Fresh and Hardened AAMs with Seawater and Sea Sand
2.1. Workability
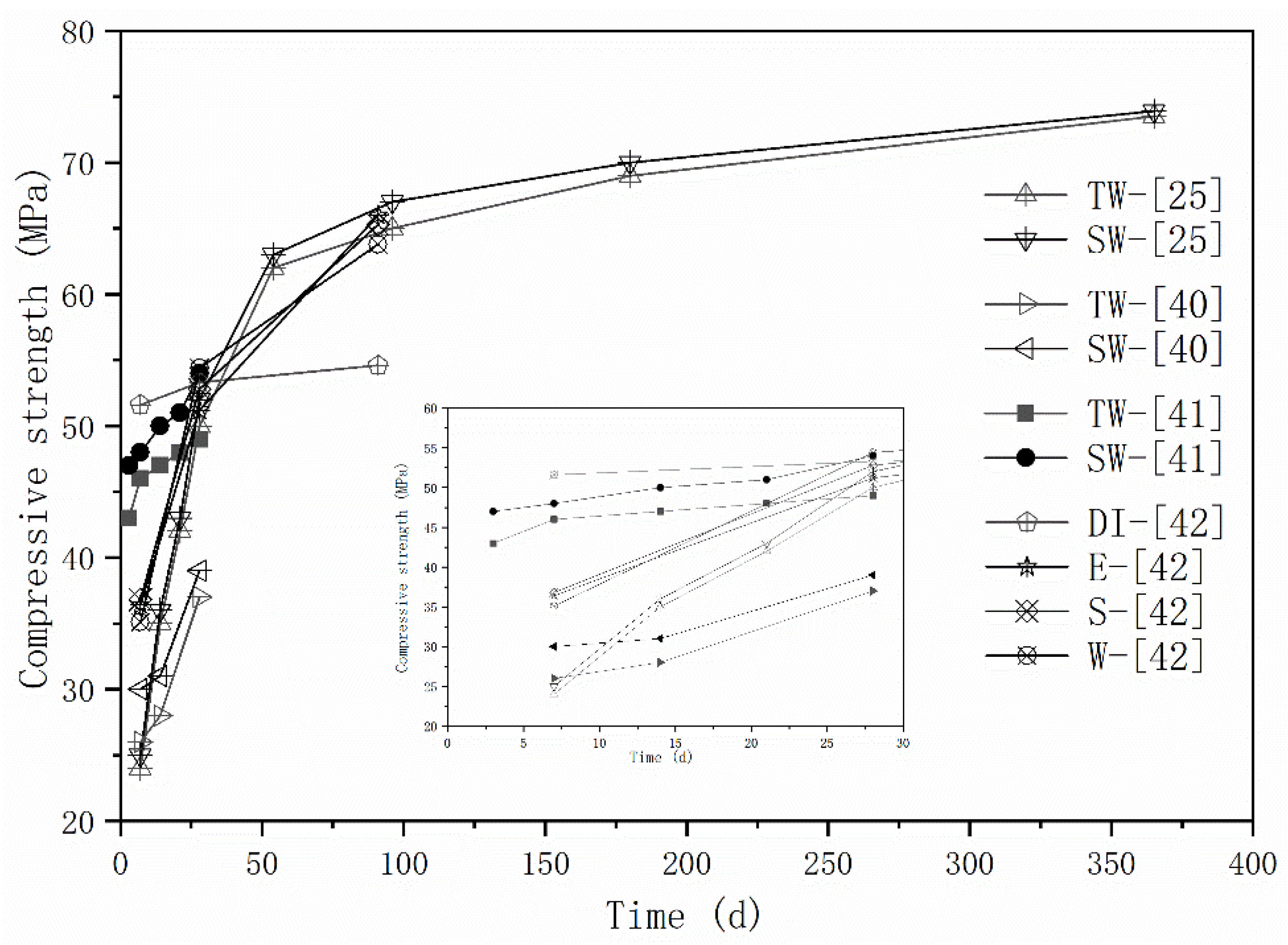
2.2. Mechanical Properties
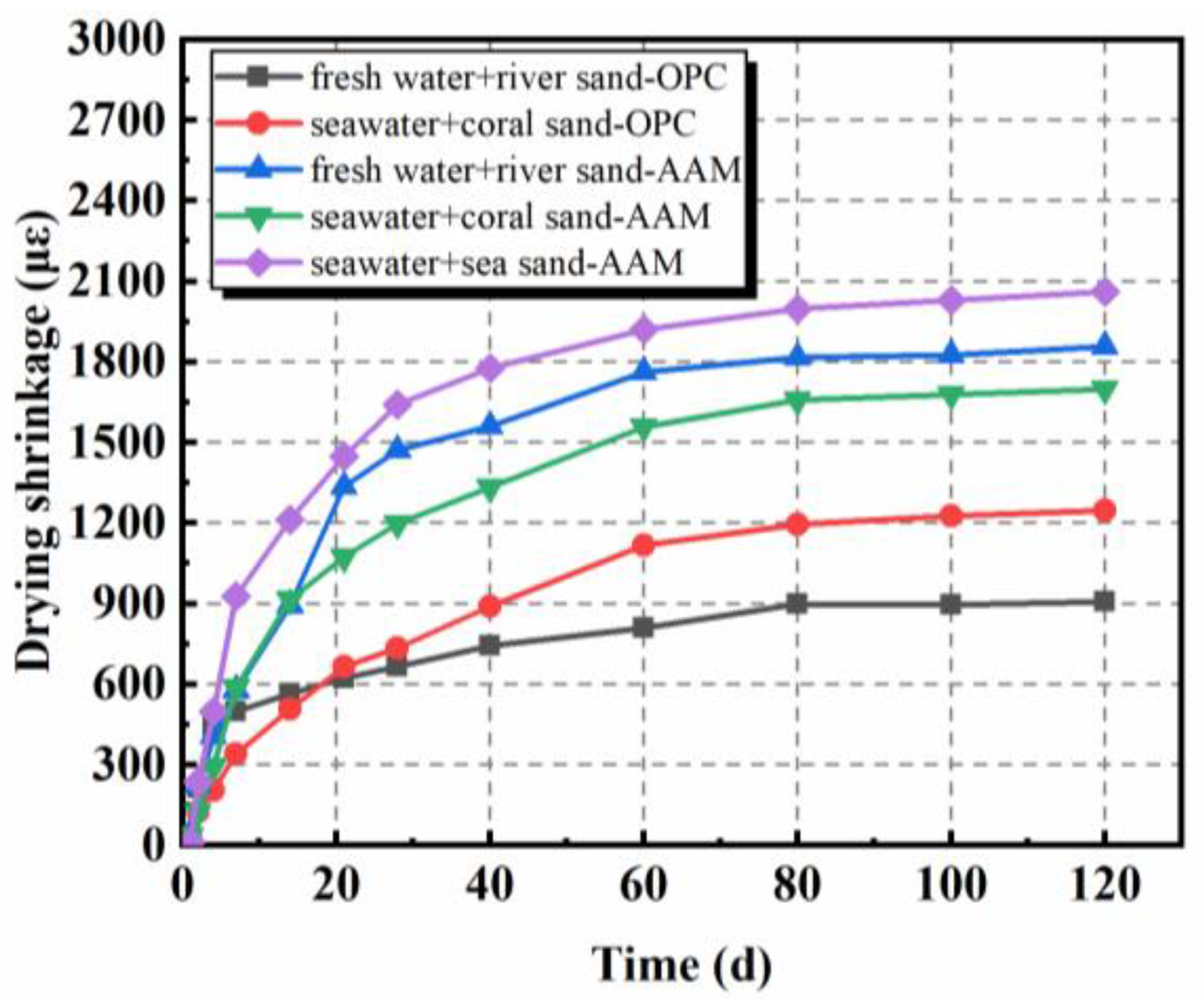
2.3. Drying Shrinkage
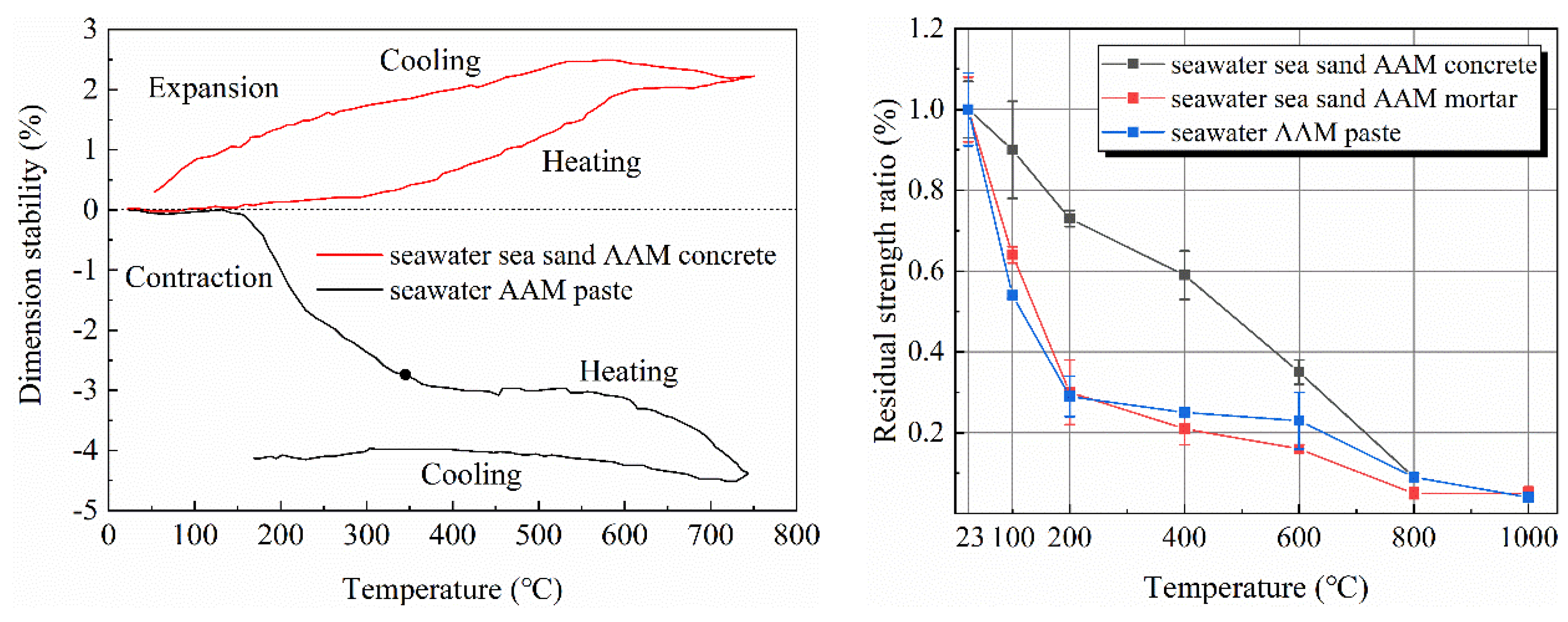
2.4. Thermal Properties
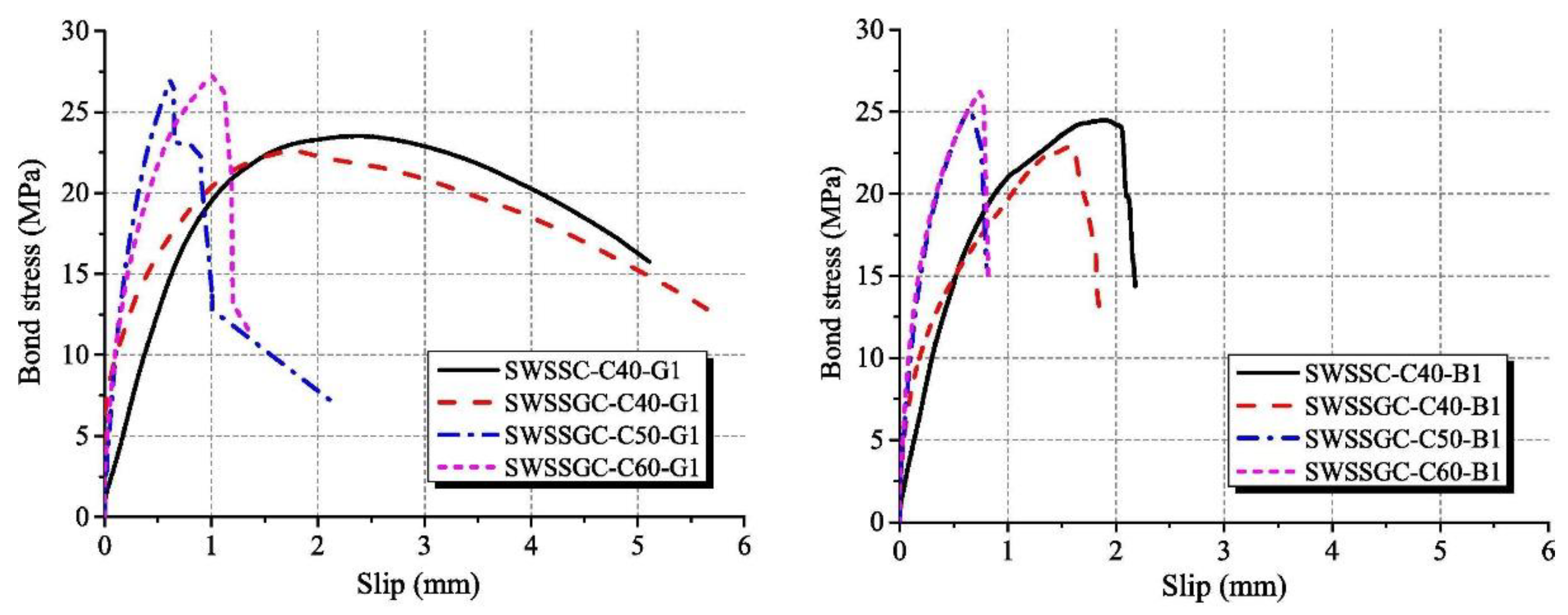
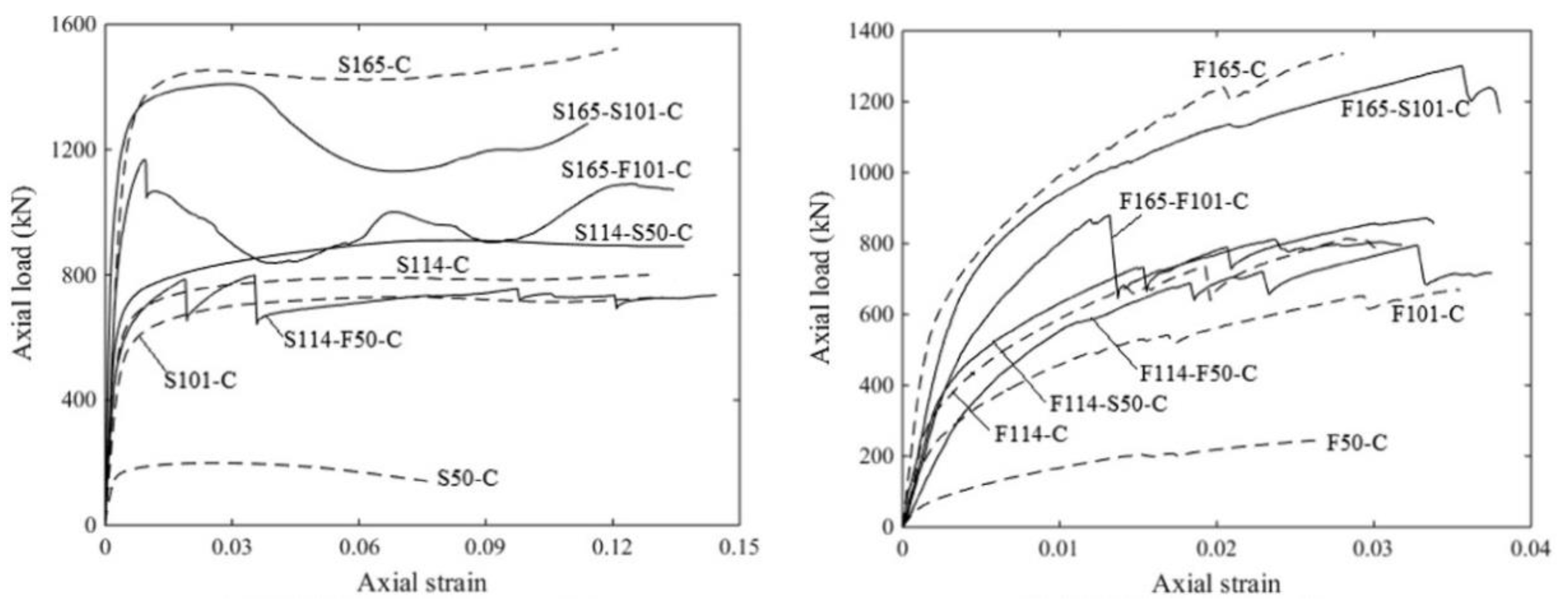
2.5. Reinforced and Confined AAMs
3. Reaction Products and Microstructure of AAMs with Seawater and Sea Sand
3.1. Reaction Products
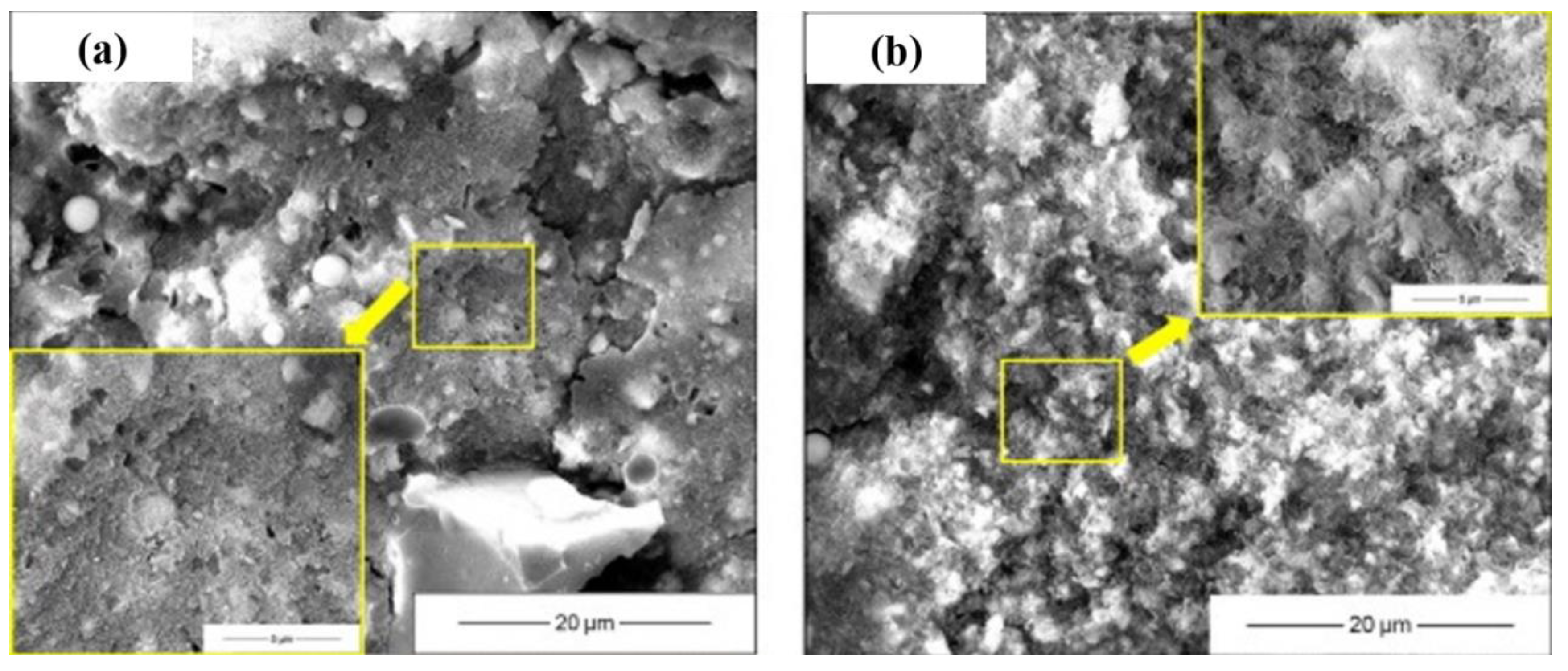
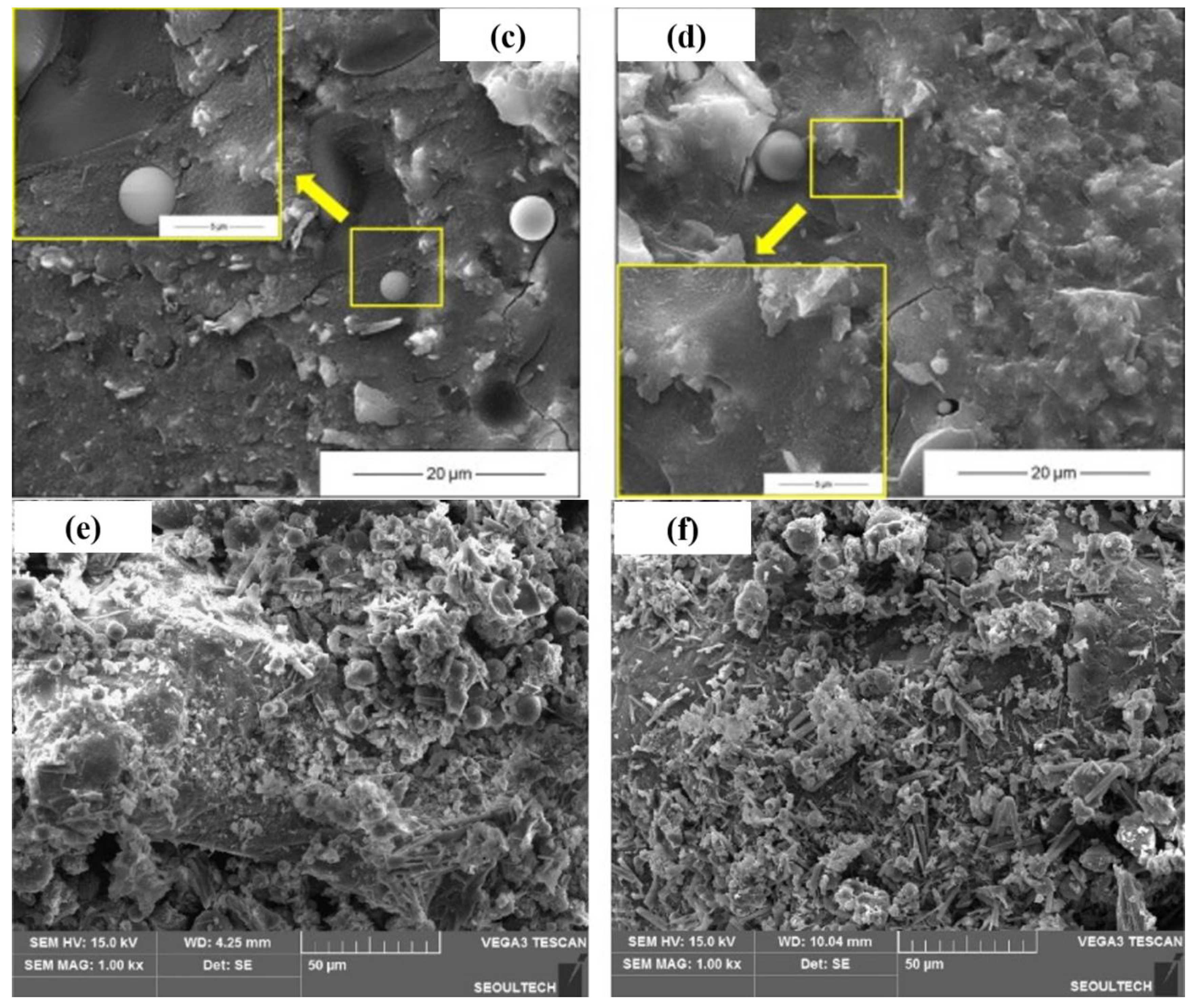
3.2. Microstructure
4. Durability of AAMs with Seawater and Sea Sand
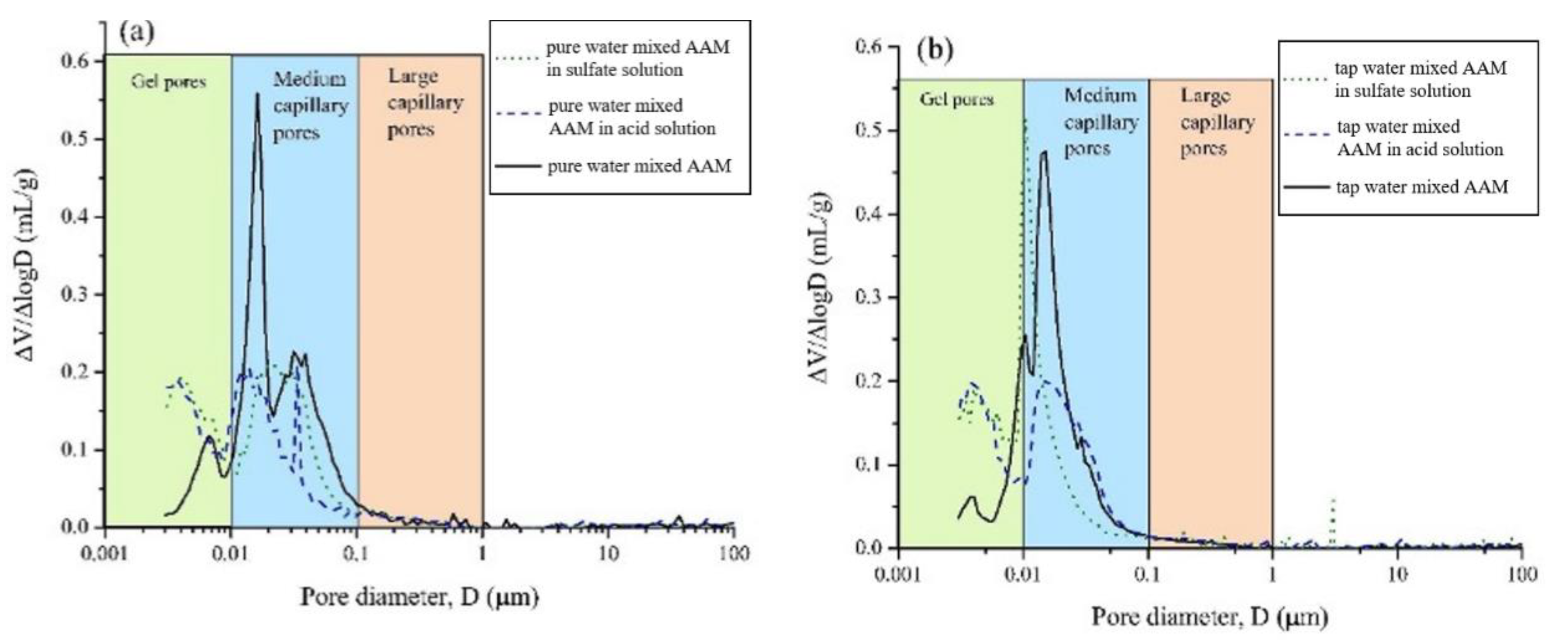
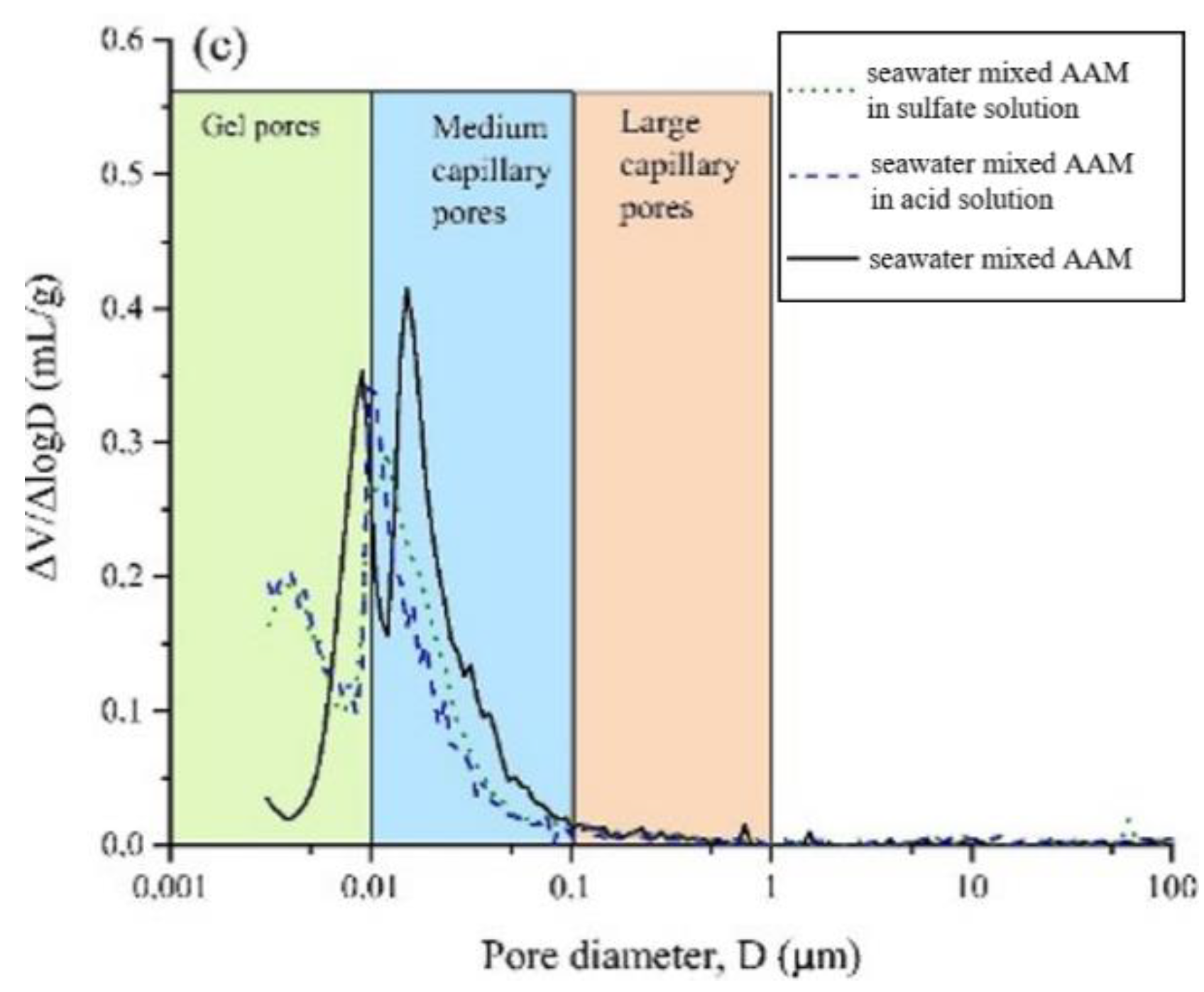
4.1. Acid and Sulfate Resistance
4.2. Reinforcement Corrosion
5. Challenges and Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jun, Y.; Yoon, S.; Oh, J. A Comparison Study for Chloride-Binding Capacity between Alkali-Activated Fly Ash and Slag in the Use of Seawater. Appl. Sci. 2017, 7, 971. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Geopolymers: Structures, Processing, Properties and Industrial Applications; Woodhead Publishing Limited: Cambridge, UK, 2009. [Google Scholar]
- Provis, J.L.; Bernal, S.A. Geopolymers and Related Alkali-Activated Materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A. One year geopolymerisation of sodium silicate activated fly ash and metakaolin geopolymers. Cem. Concr. Compos. 2019, 95, 98–110. [Google Scholar] [CrossRef]
- Provis, J.; van Deventer, J. Alkali Activated Materials; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Davidovits, J. Geopolymer Chemistry and Applications; Geopolymer Institute: Compiegne, France, 2011. [Google Scholar]
- Kuehl, H. Slag Cement and Process of Making the Same. U.S. Patent No. 900,939, 13 October 1908. [Google Scholar]
- Purdon, A.O. The Action of Alkalis on Blast Furnace Slag. J. Soc. Chem. Ind. 1940, 59, 191–202. [Google Scholar]
- Buchwald, A.; Vanooteghem, M.; Gruyaert, E.; Hilbig, H.; De Belie, N. Purdocement: Application of alkali-activated slag cement in Belgium in the 1950s. Mater. Struct. 2015, 48, 501–511. [Google Scholar] [CrossRef]
- Glukhovsky, V.D. Gruntosilikaty (Soil Silicates); Gosstroyizdat: Kiev, Ukraine, 1959. [Google Scholar]
- Davidovits, J. The Need to Create a New Technical Language for the Transfer of Basic Scientific Information. In Transfer and Exploitation of Scientific and Technical Information; Office for Official Publications of the European Communities: Luxembourg, 1982. [Google Scholar]
- Davidovits, J. Geopolymers: Inorganic Polymeric New Materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Puertas, F.; Sobrados, I.; Sanz, J. Structure of Calcium Silicate Hydrates Formed in Alkaline-Activated Slag: Influence of the Type of Alkaline Activator. J. Am. Ceram. Soc. 2003, 86, 1389–1394. [Google Scholar] [CrossRef]
- Brough, A.R.; Atkinson, A. Sodium silicate-based, alkali-activated slag mortars: Part I. Strength, hydration and microstructure. Cem. Concr. Res. 2002, 32, 865–879. [Google Scholar] [CrossRef]
- Myers, R.J.; Bernal, S.A.; Nicolas, R.S.; Provis, J.L. Generalized Structural Description of Calcium–Sodium Aluminosilicate Hydrate Gels: The Cross-Linked Substituted Tobermorite Model. Langmuir 2013, 29, 5294–5306. [Google Scholar] [CrossRef]
- Richardson, I.G.; Brough, A.R.; Groves, G.W.; Dobson, C.M. The characterization of hardened alkali-activated blast-furnace slag pastes and the nature of the calcium silicate hydrate (C-S-H) phase. Cem. Concr. Res. 1994, 24, 813–829. [Google Scholar] [CrossRef]
- Puertas, F.; Palacios, M.; Manzano, H.; Dolado, J.S.; Rico, A.; Rodríguez, J. A model for the C-A-S-H gel formed in alkali-activated slag cements. J. Eur. Ceram. Soc. 2011, 31, 2043–2056. [Google Scholar] [CrossRef]
- Skinner, L.B.; Chae, S.R.; Benmore, C.J.; Wenk, H.R.; Monteiro, P.J. Nanostructure of calcium silicate hydrates in cements. Phys. Rev. Lett. 2010, 104, 195502. [Google Scholar] [CrossRef]
- Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. Do Geopolymers Actually Contain Nanocrystalline Zeolites? A Reexamination of Existing Results. Chem. Mater. 2005, 17, 3075–3085. [Google Scholar] [CrossRef]
- Glukhovsky, V.D. Ancient, Modern and Future Concretes. In Proceedings of the 2nd International Seminar, Gothenburg, Sweden; 1989. [Google Scholar]
- Sun, Z.; Vollpracht, A. Isothermal calorimetry and in-situ XRD study of the NaOH activated fly ash, metakaolin and slag. Cem. Concr. Res. 2018, 103, 110–122. [Google Scholar] [CrossRef]
- Miller, S.A.; Horvath, A.; Monteiro, P.J.M. Impacts of booming concrete production on water resources worldwide. Nat. Sustain. 2018, 1, 69–76. [Google Scholar] [CrossRef]
- UN-Water. 2017 UN World Water Development Report: Wastewater, the Untapped Resource; UN-Water: New York, NY, USA, 2017. [Google Scholar]
- Shi, D.; Yao, Y.; Ye, J.; Zhang, W. Effects of seawater on mechanical properties, mineralogy and microstructure of calcium silicate slag-based alkali-activated materials. Constr. Build. Mater. 2019, 212, 569–577. [Google Scholar] [CrossRef]
- Lv, W.; Sun, Z.; Su, Z. Study of seawater mixed one-part alkali activated GGBFS-fly ash. Cem. Concr. Compos. 2020, 106, 103484. [Google Scholar] [CrossRef]
- Luukkonen, T.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. Sustainable batching water options for one-part alkali-activated slag mortar: Sea water and reverse osmosis reject water. PLoS ONE 2020, 15, e0242462. [Google Scholar] [CrossRef]
- Tanaka, M. Takahashi, Study on the Salting-out Effect using Silica Species by FAB-MS. J. Solut. Chem. 2007, 36, 27–37. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, H.; Shah, K.W.; Dong, Z.; Wu, J. Performance evaluation and microstructure characterization of seawater and coral/sea sand alkali-activated mortars. Constr. Build. Mater. 2020, 259, 120403. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, H.; Shah, K.W.; Feng, P. Dong, Optimization of mix proportion of alkali-activated slag mortars prepared with seawater and coral sand. Constr. Build. Mater. 2021, 284, 122805. [Google Scholar] [CrossRef]
- Cwirzen, A.; Sztermen, P.; Habermehl-Cwirzen, K. Effect of Baltic Seawater and Binder Type on Frost Durability of Concrete. J. Mater. Civ. Eng. 2014, 26, 283–287. [Google Scholar] [CrossRef]
- Moukwa, M. Deterioration of concrete in cold sea waters. Cem. Concr. Res. 1990, 20, 439–446. [Google Scholar] [CrossRef]
- Kawamura, M.; Takeuchi, K. Alkali-silica reaction and pore solution composition in mortars in sea water. Cem. Concr. Res. 1996, 26, 1809–1819. [Google Scholar] [CrossRef]
- Ganjian, E.; Pouya, H.S. Effect of magnesium and sulfate ions on durability of silica fume blended mixes exposed to the seawater tidal zone. Cem. Concr. Res. 2005, 35, 1332–1343. [Google Scholar] [CrossRef]
- De Weerdt, K.; Justnes, H.; Geiker, M.R. Changes in the phase assemblage of concrete exposed to sea water. Cem. Concr. Compos. 2014, 47, 53–63. [Google Scholar] [CrossRef]
- Wang, J.; Liu, E.; Li, L. Multiscale investigations on hydration mechanisms in seawater OPC paste. Constr. Build. Mater. 2018, 191, 891–903. [Google Scholar] [CrossRef]
- Cotruvo, J.A. Water desalination processes and associated health and environmental issues. Water Cond. Purif. 2005, 47, 13–17. [Google Scholar]
- Siddique, S.; Jang, J.G. Acid and sulfate resistance of seawater based alkali activated fly ash: A sustainable and durable approach. Constr. Build. Mater. 2021, 281, 122601. [Google Scholar] [CrossRef]
- Li, Y.-L.; Zhao, X.-L.; Raman, R.K.S.; Al-Saadi, S. Thermal and mechanical properties of alkali-activated slag paste, mortar and concrete utilising seawater and sea sand. Constr. Build. Mater. 2018, 159, 704–724. [Google Scholar] [CrossRef]
- Kang, C.; Kim, T. Pore and strength characteristics of alkali-activated slag paste with seawater. Mag. Concr. Res. 2020, 72, 499–508. [Google Scholar] [CrossRef]
- Jun, Y.; Kim, T.; Kim, J.H. Chloride-bearing characteristics of alkali-activated slag mixed with seawater: Effect of different salinity levels. Cem. Concr. Compos. 2020, 112, 103680. [Google Scholar] [CrossRef]
- Rashad, A.M.; Ezzat, M. A Preliminary study on the use of magnetic, Zamzam, and sea water as mixing water for alkali-activated slag pastes. Constr. Build. Mater. 2019, 207, 672–678. [Google Scholar] [CrossRef]
- Siddique, S.; Jang, J.G. Mechanical Properties, Microstructure, and Chloride Content of Alkali-Activated Fly Ash Paste Made with Sea Water. Materials 2020, 13, 1467. [Google Scholar] [CrossRef] [PubMed]
- Shinde, B.H.; Kadam, K.N. Strength Properties of Fly Ash Based Geopolymer Concrete with Sea Sand. Am. J. Eng. Res. 2016, 5, 129–132. [Google Scholar]
- Anbarasan, I.; Soundarapandian, N. Investigation of mechanical and micro structural properties of geopolymer concrete blended by dredged marine sand and manufactured sand under ambient curing conditions. Struct. Concr. 2020, 21, 992–1003. [Google Scholar] [CrossRef]
- Rashad, A.M. A comprehensive overview about recycling rubber as fine aggregate replacement in traditional cementitious materials. Int. J. Sustain. Built Environ. 2016, 5, 46–82. [Google Scholar] [CrossRef]
- Xiao, J.; Qiang, C.; Nanni, A.; Zhang, K. Use of sea-sand and seawater in concrete construction: Current status and future opportunities. Constr. Build. Mater. 2017, 155, 1101–1111. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Le, T.A.; Lee, K. Evaluation of the mechanical properties of sea sand-based geopolymer concrete and the corrosion of embedded steel bar. Constr. Build. Mater. 2018, 169, 462–472. [Google Scholar] [CrossRef]
- Rashad, A.M.; Gharieb, M. An investigation on the effect of sea sand on the properties of fly ash geopolymer mortars. Innov. Infrastruct. Solut. 2021, 6, 53. [Google Scholar] [CrossRef]
- Wang, A.; Lyu, B.; Zhang, Z.; Liu, K.; Xu, H.; Sun, D. The development of coral concretes and their upgrading technologies: A critical review. Constr. Build. Mater. 2018, 187, 1004–1019. [Google Scholar] [CrossRef]
- Yang, S.; Xu, J.; Zang, C.; Li, R.; Yang, Q.; Sun, S. Mechanical properties of alkali-activated slag concrete mixed by seawater and sea sand. Constr. Build. Mater. 2019, 196, 395–410. [Google Scholar] [CrossRef]
- Matalkah, F.; Salem, T.; Shaafaey, M.; Soroushian, P. Drying shrinkage of alkali activated binders cured at room temperature. Constr. Build. Mater. 2019, 201, 563–570. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Monteiro, P.; Mehta, P.K. Concrete: Microstructure, Properties and Materials; McGraw-Hill Education: Berkshire, UK, 2006. [Google Scholar]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Physical evolution of Na-geopolymer derived from metakaolin up to 1000 °C. J. Mater. Sci. 2007, 42, 3044–3054. [Google Scholar] [CrossRef]
- Rahier, H.; Simons, W.; Van Mele, B.; Biesemans, M. Low-temperature synthesized aluminosilicate glasses: Part III Influence of the composition of the silicate solution on production, structure and properties. J. Mater. Sci. 1997, 32, 2237–2247. [Google Scholar] [CrossRef]
- Rickard, W.D.A.; Temuujin, J.; van Riessen, A. Thermal analysis of geopolymer pastes synthesised from five fly ashes of variable composition. J. Non-Cryst. Solids 2012, 358, 1830–1839. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G. Effect of elevated temperatures on geopolymer paste, mortar and concrete. Cem. Concr. Res. 2010, 40, 334–339. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, H.; Cao, R.; Ding, J.; Chen, X. Feasibility of using geopolymers to investigate the bond behavior of FRP bars in seawater sea-sand concrete. Constr. Build. Mater. 2021, 282, 122636. [Google Scholar] [CrossRef]
- Li, Y.L.; Zhao, X.L.; Singh, R.K.R.; Al-Saadi, S. Experimental study on seawater and sea sand concrete filled GFRP and stainless steel tubular stub columns. Thin-Walled Struct. 2016, 106, 390–406. [Google Scholar] [CrossRef]
- Lam, D.; Gardner, L. Structural design of stainless steel concrete filled columns. J. Constr. Steel Res. 2008, 64, 1275–1282. [Google Scholar] [CrossRef]
- Uy, B.; Tao, Z.; Han, L.H. Behaviour of short and slender concrete-filled stainless steel tubular columns. J. Constr. Steel Res. 2011, 67, 360–378. [Google Scholar] [CrossRef]
- Haha, M.B.; Lothenbach, B.; Le Saout, G.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part I: Effect of MgO. Cem. Concr. Res. 2011, 41, 955–963. [Google Scholar] [CrossRef]
- Rousselot, I.; Taviot-Guého, C.; Leroux, F.; Léone, P.; Palvadeau, P.; Besse, J.-P. Insights on the Structural Chemistry of Hydrocalumite and Hydrotalcite-like Materials: Investigation of the Series Ca2M3+(OH)6Cl·2H2O (M3+: Al3+, Ga3+, Fe3+, and Sc3+) by X-ray Powder Diffraction. J. Solid State Chem. 2002, 167, 137–144. [Google Scholar] [CrossRef]
- Nishida, T.; Otsuki, N.; Ohara, H.; Garba-Say, Z.M.; Nagata, T. Some Considerations for Applicability of Seawater as Mixing Water in Concrete. J. Mater. Civ. Eng. 2015, 27, B4014004. [Google Scholar] [CrossRef]
- El-Didamony, H.; Amer, A.A.; Ela-ziz, H.A. Properties and durability of alkali-activated slag pastes immersed in sea water. Ceram. Int. 2012, 38, 3773–3780. [Google Scholar] [CrossRef]
- Otsuki, N.; Saito, T.; Tadokoro, Y. Possibility of Sea Water as Mixing Water in Concrete. J. Civ. Eng. Archit. 2012, 6, 1273–1279. [Google Scholar]
- Zhang, J.; Shi, C.; Zhang, Z. Chloride binding of alkali-activated slag/fly ash cements. Constr. Build. Mater. 2019, 226, 21–31. [Google Scholar] [CrossRef]
- Nawa, T. Adsorption of chloride ions on the C-S-H and C-A-S-H. In Proceedings of the 14th International Congress on the Chemistry of Cement, Beijing, China, 13–16 October 2015; p. 1. [Google Scholar]
- Yogarajah, E.; Nawa, T.; Kurumisawa, K. Influence of Surface Electrical Properties of C–S–H on Chloride Binding in Slag–Blended Cementitious Materials. J. Mater. Civ. Eng. 2018, 30, 04018064. [Google Scholar] [CrossRef]
- Rasheeduzzafar; Ehtesham Hussain, S.; Al-Saadoun, S.S. Effect of cement composition on chloride binding and corrosion of reinforcing steel in concrete. Cem. Concr. Res. 1991, 21, 777–794. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yogarajah, E.; Nawa, T. Impact of Ca/(Si+Al) Ratio of Calcium Aluminosilicate Hydrate (C-A-S-H) gel on Chloride Adsorption for Evaluating Durability of Reinforced Concrete; University of Moratuwa: Moratuwa, Sri Lanka, 2017. [Google Scholar]
- Ke, X.; Bernal, S.A.; Provis, J.L. Chloride binding capacity of synthetic C-(A)-S-H type gels in alkali-activated slag simulated pore solutions. In Proceedings of the International Conference of Construction Materials for Sustainable Future, Zadar, Croatia, 19 April 2017. [Google Scholar]
- Shehata, M.H.; Thomas, M.D.A. Alkali release characteristics of blended cements. Cem. Concr. Res. 2006, 36, 1166–1175. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, H.K. Influence of the slag content on the chloride and sulfuric acid resistances of alkali-activated fly ash/slag paste. Cem. Concr. Compos. 2016, 72, 168–179. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Zhang, Z. Effect of Na2O concentration and water/binder ratio on carbonation of alkali-activated slag/fly ash cements. Constr. Build. Mater. 2021, 269, 121258. [Google Scholar] [CrossRef]
- Sandberg, P. Studies of chloride binding in concrete exposed in a marine environment. Cem. Concr. Res. 1999, 29, 473–477. [Google Scholar] [CrossRef]
- Ma, Q.; Nanukuttan, S.V.; Basheer, P.A.M.; Bai, Y.; Yang, C. Chloride transport and the resulting corrosion of steel bars in alkali activated slag concretes. Mater. Struct./Mater. Et Constr. 2016, 49, 3663–3677. [Google Scholar] [CrossRef]
- Wang, J.; Xie, J.; Wang, Y.; Liu, Y.; Ding, Y. Rheological properties, compressive strength, hydration products and microstructure of seawater-mixed cement pastes. Cem. Concr. Compos. 2020, 114, 103770. [Google Scholar] [CrossRef]
- Edwards, G.C.; Angstadt, R.L. The effect of some soluble inorganic admixtures on the early hydration of portland cement. J. Appl. Chem. 1966, 16, 166–168. [Google Scholar] [CrossRef]
- Wilding, C.R.; Walter, A.; Double, D.D. A classification of inorganic and organic admixtures by conduction calorimetry. Cem. Concr. Res. 1984, 14, 185–194. [Google Scholar] [CrossRef]
- Suryavanshi, A.K.; Scantlebury, J.D.; Lyon, S.B. Pore size distribution of OPC & SRPC mortars in presence of chlorides. Cem. Concr. Res. 1995, 25, 980–988. [Google Scholar]
- Aiken, T.A.; Kwasny, J.; Sha, W.; Soutsos, M.N. Effect of slag content and activator dosage on the resistance of fly ash geopolymer binders to sulfuric acid attack. Cem. Concr. Res. 2018, 111, 23–40. [Google Scholar] [CrossRef]
- Sturm, P.; Gluth, G.J.G.; Jäger, C.; Brouwers, H.J.H.; Kühne, H.C. Sulfuric acid resistance of one-part alkali-activated mortars. Cem. Concr. Res. 2018, 109, 54–63. [Google Scholar] [CrossRef]
- Aliques-Granero, J.; Tognonvi, M.T.; Tagnit-Hamou, A. Durability study of AAMs: Sulfate attack resistance. Constr. Build. Mater. 2019, 229, 117100. [Google Scholar] [CrossRef]
- Wilińska, I.; Pacewska, B. Comparative investigation of reactivity of different kinds of fly ash in alkaline media. J. Therm. Anal. Calorim. 2019, 138, 3857–3872. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; De Silva, P.; Sagoe-Crentsil, K.; Hanjitsuwan, S. Effect of SiO2 and Al2O3 on the setting and hardening of high calcium fly ash-based geopolymer systems. J. Mater. Sci. 2012, 47, 4876–4883. [Google Scholar] [CrossRef]
- Bonilla, A.; Baudouin, D.; Pérez-Ramírez, J. Desilication of ferrierite zeolite for porosity generation and improved effectiveness in polyethylene pyrolysis. J. Catal. 2009, 265, 170–180. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Moulijn, J.A.; Pérez-Ramírez, J. Mechanism of Hierarchical Porosity Development in MFI Zeolites by Desilication: The Role of Aluminium as a Pore-Directing Agent. Chem.—Eur. J. 2005, 11, 4983–4994. [Google Scholar] [CrossRef]
- Batis, G.; Pantazopoulou, P.; Tsivilis, S.; Badogiannis, E. The effect of metakaolin on the corrosion behavior of cement mortars. Cem. Concr. Compos. 2005, 27, 125–130. [Google Scholar] [CrossRef]
- Yeau, K.Y.; Kim, E.K. An experimental study on corrosion resistance of concrete with ground granulate blast-furnace slag. Cem. Concr. Res. 2005, 35, 1391–1399. [Google Scholar] [CrossRef]
- Badar, M.S.; Kupwade-Patil, K.; Bernal, S.A.; Provis, J.L.; Allouche, E.N. Corrosion of steel bars induced by accelerated carbonation in low and high calcium fly ash geopolymer concretes. Constr. Build. Mater. 2014, 61, 79–89. [Google Scholar] [CrossRef]
- Mundra, S.; Bernal, S.A.; Criado, M.; Hlaváček, P.; Ebell, G.; Reinemann, S.; Gluth, G.J.G.; Provis, J. Steel corrosion in reinforced alkali-activated materials. RILEM Tech. Lett. 2017, 2, 33–39. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Chen, Z.; Lee, H.; Lim, S. Chloride-Binding Capacity of Portland Cement Paste Blended with Synthesized CA2(CaO·2Al2O3). Adv. Mater. Sci. Eng. 2018, 2018, 5418930. [Google Scholar] [CrossRef]
- Effect of Sulfides in the Passive Layer of Steel Reinforcement in Alkali-activated Slags. In Proceedings of the International Conference on Alkali Activated Materials and Geopolymers: Versatile Materials Offering High Performance and Low Emissions, Tomar, Portugal, 27 May–1 June 2018.
- Holloway, M.; Sykes, J.M. Studies of the corrosion of mild steel in alkali-activated slag cement mortars with sodium chloride admixtures by a galvanostatic pulse method. Corros. Sci. 2005, 47, 3097–3110. [Google Scholar] [CrossRef]
- Criado, M.; Bernal, S.A.; Garcia-Triñanes, P.; Provis, J.L. Influence of slag composition on the stability of steel in alkali-activated cementitious materials. J. Mater. Sci. 2018, 53, 5016–5035. [Google Scholar] [CrossRef] [PubMed]
- Vollpracht, A.; Lothenbach, B.; Snellings, R.; Haufe, J. The pore solution of blended cements: A review. Mater. Struct. 2016, 49, 3341–3367. [Google Scholar] [CrossRef]
- Scott, A.; Alexander, M.G. Effect of supplementary cementitious materials (binder type) on the pore solution chemistry and the corrosion of steel in alkaline environments. Cem. Concr. Res. 2016, 89, 45–55. [Google Scholar] [CrossRef]
- Lothenbach, B.; Le Saout, G.; Haha, M.B.; Figi, R. Wieland, Hydration of a low-alkali CEM III/B–SiO2 cement (LAC). Cem. Concr. Res. 2012, 42, 410–423. [Google Scholar] [CrossRef]
- Tittarelli, F.; Mobili, A.; Giosuè, C.; Belli, A.; Bellezze, T. Corrosion behaviour of bare and galvanized steel in geopolymer and Ordinary Portland Cement based mortars with the same strength class exposed to chlorides. Corros. Sci. 2018, 134, 64–77. [Google Scholar] [CrossRef]
- Khatibmasjedi, S.; Basalo, F.; Nanni, A. SEACON: Redefining Sustainable Concrete. In Proceedings of the 4th international conference in Sustainable Construction Materials and Technologies (SCMT4), Las Vegas, NV, USA, 7–11 August 2016. [Google Scholar]
- Van Den Einde, L.; Zhao, L.; Seible, F. Use of FRP composites in civil structural applications. Constr. Build. Mater. 2003, 17, 389–403. [Google Scholar] [CrossRef]


| ID in Literature | Initial Setting Time (min) | Final Setting Time (min) | Slump (mm) | Mixture | Reference |
|---|---|---|---|---|---|
| Tw system | 65 | 125 | 275 | Precursor was a blend of calcium silicate slag, ground granulated blast furnace slag GGBS and fly ash (FA); tap water (Tw) or seawater (Sw) was mixed with sodium silicate to obtain Na2O/H2O mass ratio of 0.1. Water-to-blend ratio was 0.5. | [24] |
| Sw system | 60 | 125 | 235 | ||
| Tap water-mixed | 55 | 125 | - | GGBS and FA as precursor; solid sodium hydroxide, silicate and carbonate were used as activator, water-(tap water or seawater)-to-solid ratio was 0.45. | [25] |
| Seawater-mixed | 50 | 110 | - | ||
| Tap water | 23 | 29 | - | GGBS with anhydrous sodium metasilicate (SiO2/Na2O molar ratio 0.9) with mass ratio of 9:1 was used as solid source, and water-to-solid ratio was 1:2.9. | [26] |
| Sea water | 31 | 53 | - | ||
| FR-OPC | 186 | 336 | 183 | Precursor is a mixture of GGBS, FA and SF, while seawater was mixed with sodium hydroxide and silicate as activator to obtain modulus of 1.2. Paste was of Na2O to binder mass ratio 4%, and mortar was of sand (river sand, sea sand, coral sand) to binder ratio 2. | [28] |
| SC-OPC | 142 | 274 | 148 | ||
| FR-AAM | 133 | 239 | 180 | ||
| SC-AAM | 110 | 177 | 150 | ||
| SS-AAM | 177 | 279 | 196 | ||
| TM1 | 122 | 223 | 168 | Paste proportion was the same as above, but mixture of sea sand and coral sand (varying ratios) was used as aggregate. | [29] |
| TM2 | 113 | 213 | 168 | ||
| TM3 | 82 | 158 | 167 | ||
| TM4 | 72 | 106 | 170 | ||
| TM5 | 126 | 219 | 180 | ||
| TM6 | 133 | 210 | 196 | ||
| TM7 | 51 | 73 | 138 | ||
| TM8 | 53 | 74 | 144 | ||
| TM9 | 109 | 173 | 178 | ||
| TM10 | 85 | 133 | 156 | ||
| TM11 | 98 | 160 | 183 | ||
| TM12 | 53 | 74 | 140 | ||
| TM13 | 53 | 73 | 140 | ||
| TM14 | 40 | 60 | 143 | ||
| TM15 | 40 | 56 | 180 | ||
| TM16 | 32 | 48 | 178 |
| Cl− | Na+ | SO42− | Mg2+ | Ca2+ | K+ | Region | Reference |
|---|---|---|---|---|---|---|---|
| 3000 | 1800 | 410 | 240 | 98 | 67 | Baltic | [30] |
| 17,035 | 10,231 | 2800 | 1006 | 327 | - | Nanisivik, Baffin Island | [31] |
| 19,130 | 10,750 | 18,900 | 1370 | 320 | 380 | - | [32] |
| 22,330 | 11,400 | 3070 | 1328 | 422 | 399 | Kish Island, Iran | [33] |
| 19,400 | 9500 | 258 | 1100 | 350 | 350 | Trondheim fjord | [34] |
| 26,000 | 15,000 | 3700 | 2300 | 500 | 520 | Arabian Gulf near UAE | [35] |
| 18,980 | 10,556 | 2649 | 1262 | 400 | 380 | - | [36] |
| 41,942 | 7359 | 6802 | 1129 | 381.4 | 316.2 | West Sea in Republic of Korea | [37] |
| 20,700 | 11,940 | 3420 | 1430 | 439 | 622 | Melbourne | [38] |
| 22,100 | 8500 | 2600 | 1340 | 410 | 430 | Songjeong, Republic of Korea | [39] |
| 16,600 | 8300 | 2600 | 920 | 310 | 450 | Samcheok, Republic of Korea | [40] |
| 19,000 | 7500 | 3300 | 880 | 400 | 490 | Geoje, Republic of Korea | [40] |
| 16,000 | 7400 | 3000 | 890 | 390 | 470 | Seochen, Republic of Korea | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Li, X.; Liu, Q.; Tang, Q.; Lin, X.; Fan, X.; Huang, X.; Gan, M.; Chen, X.; Ji, Z. Recent Advances in Alkali-Activated Materials with Seawater and Sea Sand. Materials 2023, 16, 3571. https://doi.org/10.3390/ma16093571
Sun Z, Li X, Liu Q, Tang Q, Lin X, Fan X, Huang X, Gan M, Chen X, Ji Z. Recent Advances in Alkali-Activated Materials with Seawater and Sea Sand. Materials. 2023; 16(9):3571. https://doi.org/10.3390/ma16093571
Chicago/Turabian StyleSun, Zengqing, Xiaoyu Li, Qingsong Liu, Qingyu Tang, Xiaochen Lin, Xiaohui Fan, Xiaoxian Huang, Min Gan, Xuling Chen, and Zhiyun Ji. 2023. "Recent Advances in Alkali-Activated Materials with Seawater and Sea Sand" Materials 16, no. 9: 3571. https://doi.org/10.3390/ma16093571
APA StyleSun, Z., Li, X., Liu, Q., Tang, Q., Lin, X., Fan, X., Huang, X., Gan, M., Chen, X., & Ji, Z. (2023). Recent Advances in Alkali-Activated Materials with Seawater and Sea Sand. Materials, 16(9), 3571. https://doi.org/10.3390/ma16093571