Study on Reversible Solubilization by Adjusting Surfactant Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Tension Measurement
2.3. pH Measurement
2.4. Solubilization Measurement
2.5. Controlled Release Measurement
3. Results and Discussion
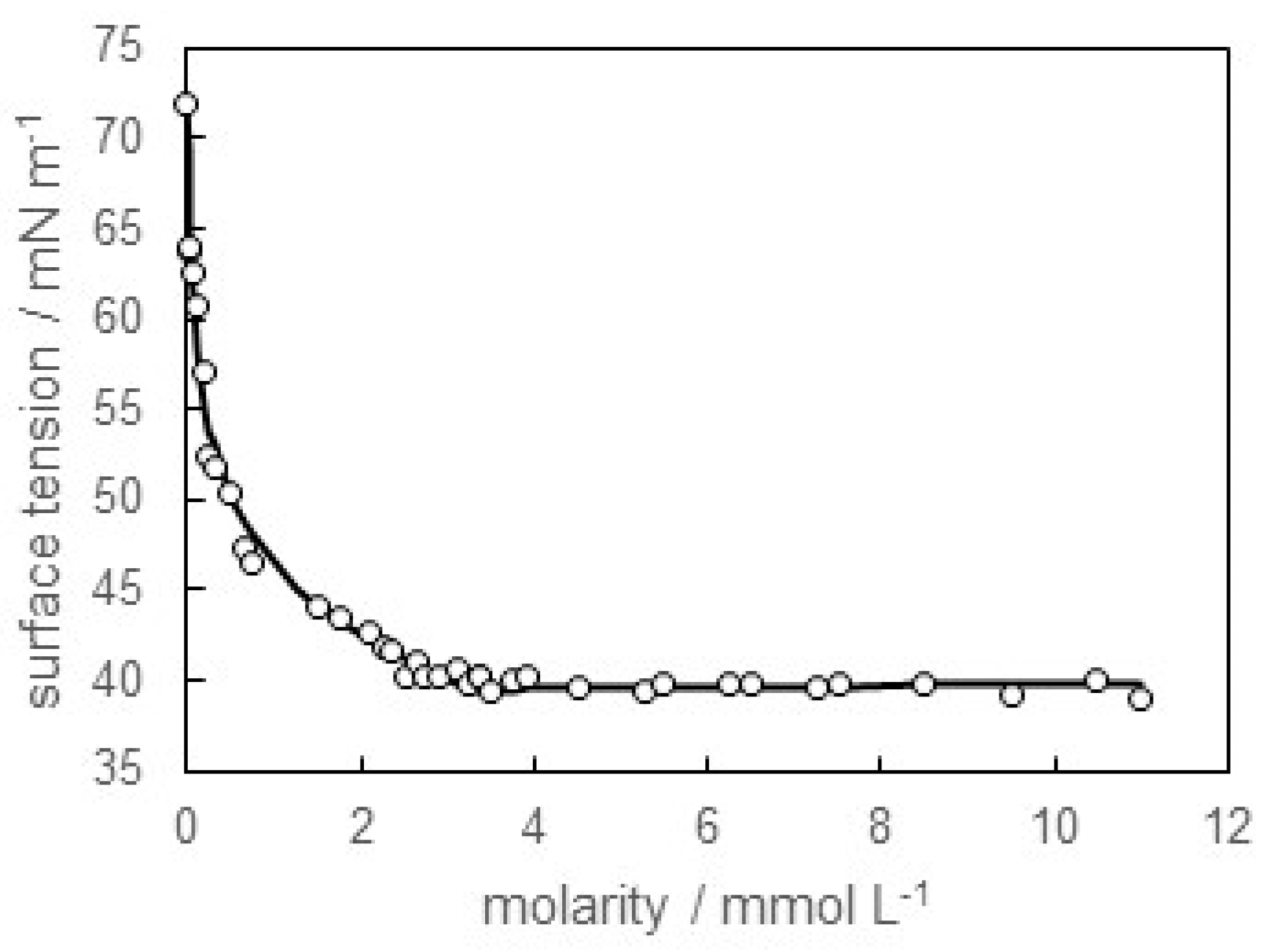
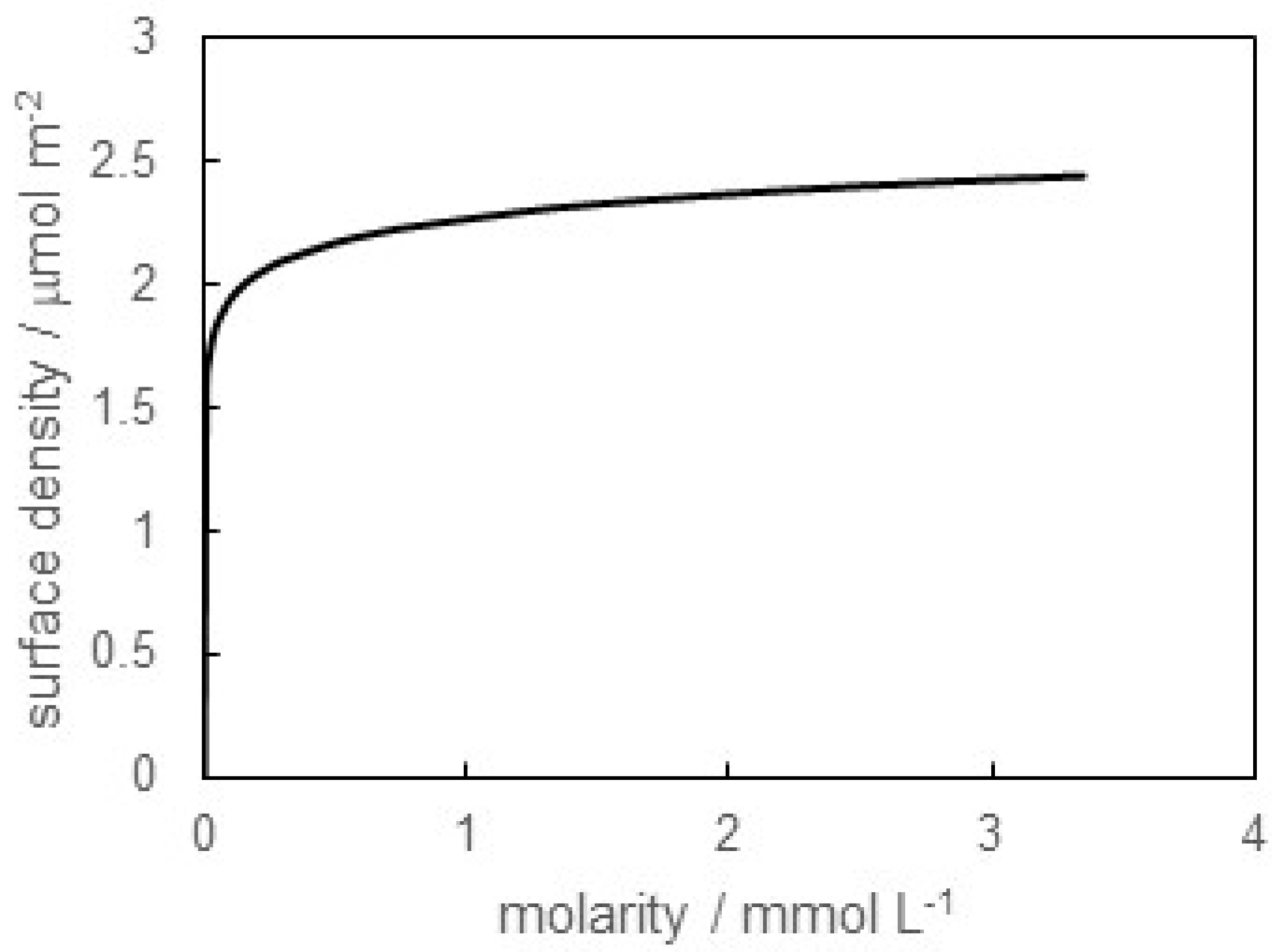
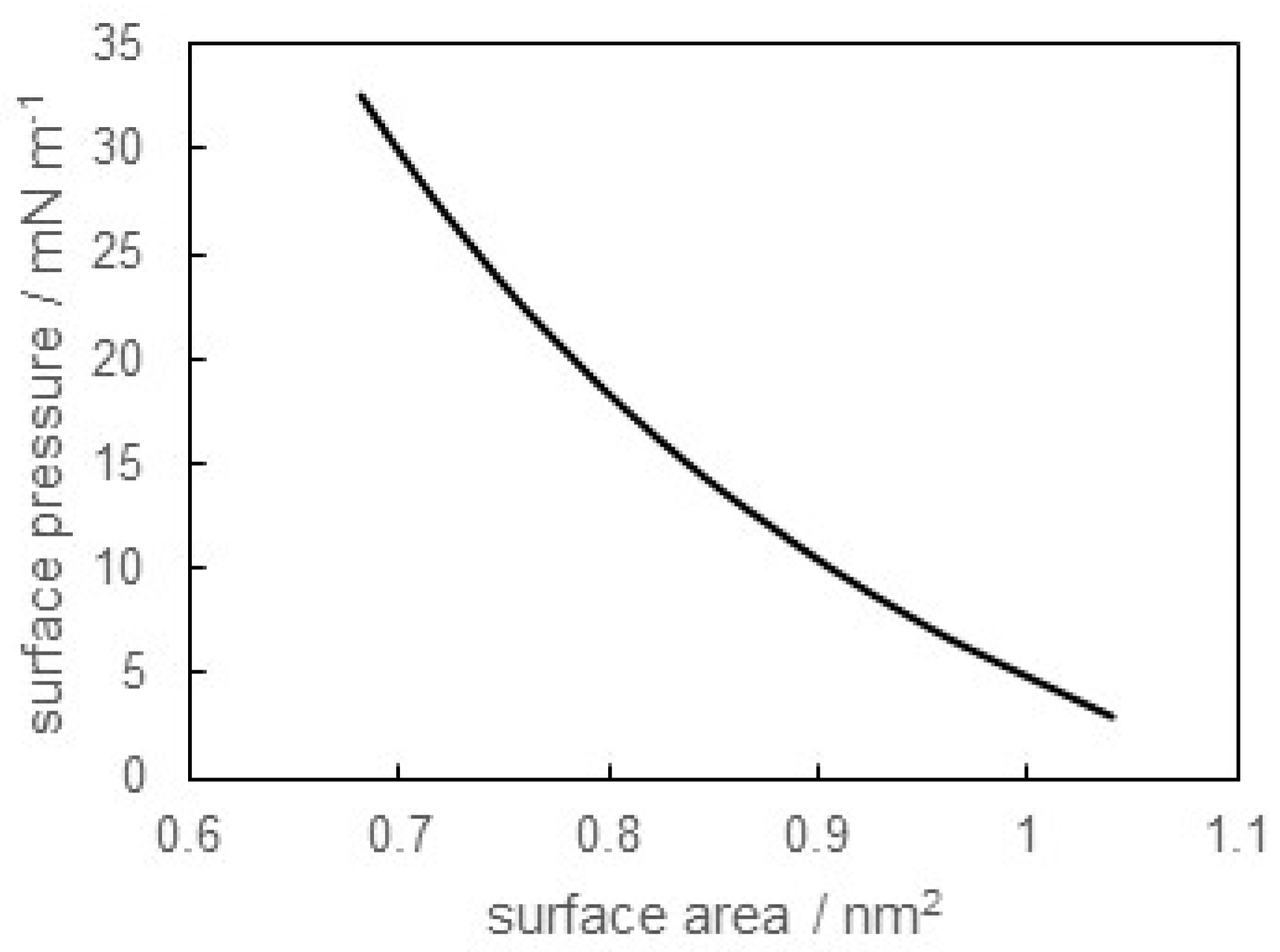
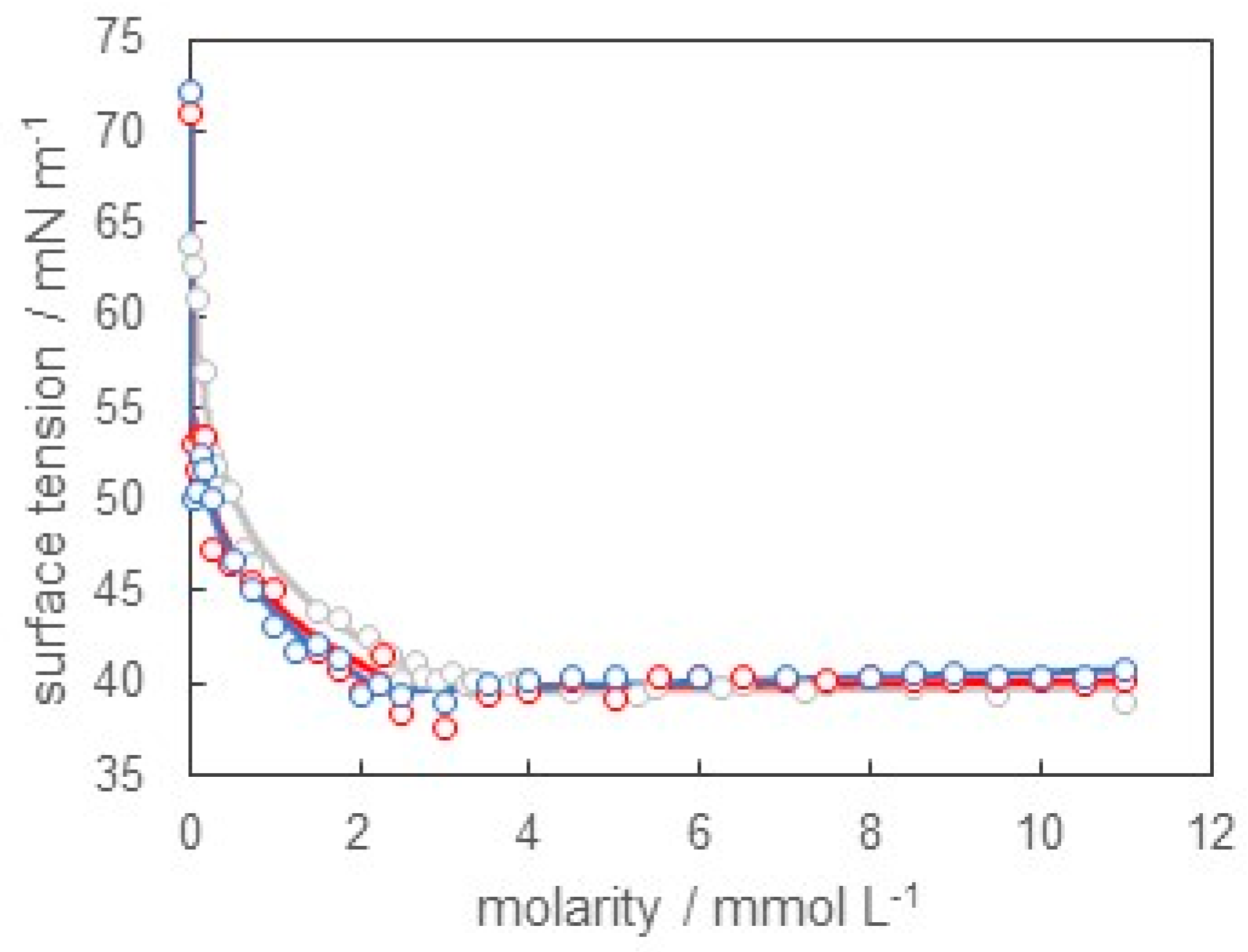
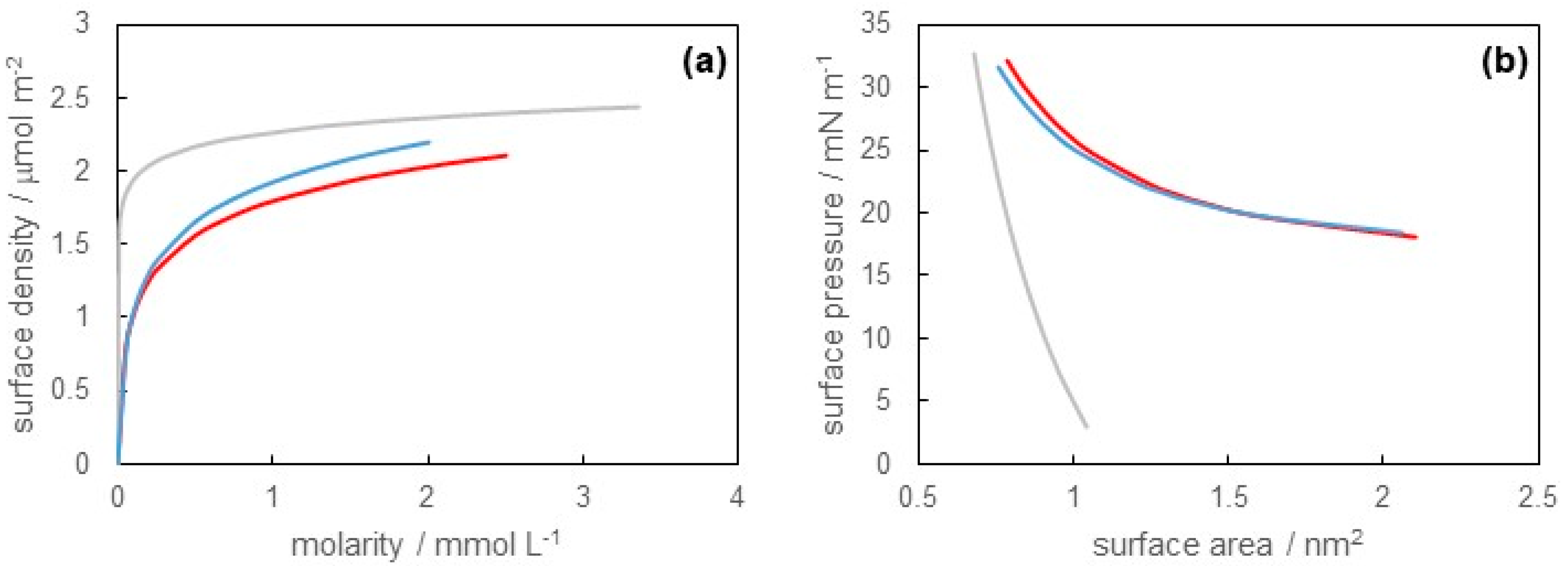
3.1. Surface Tension of SB-12 Solution
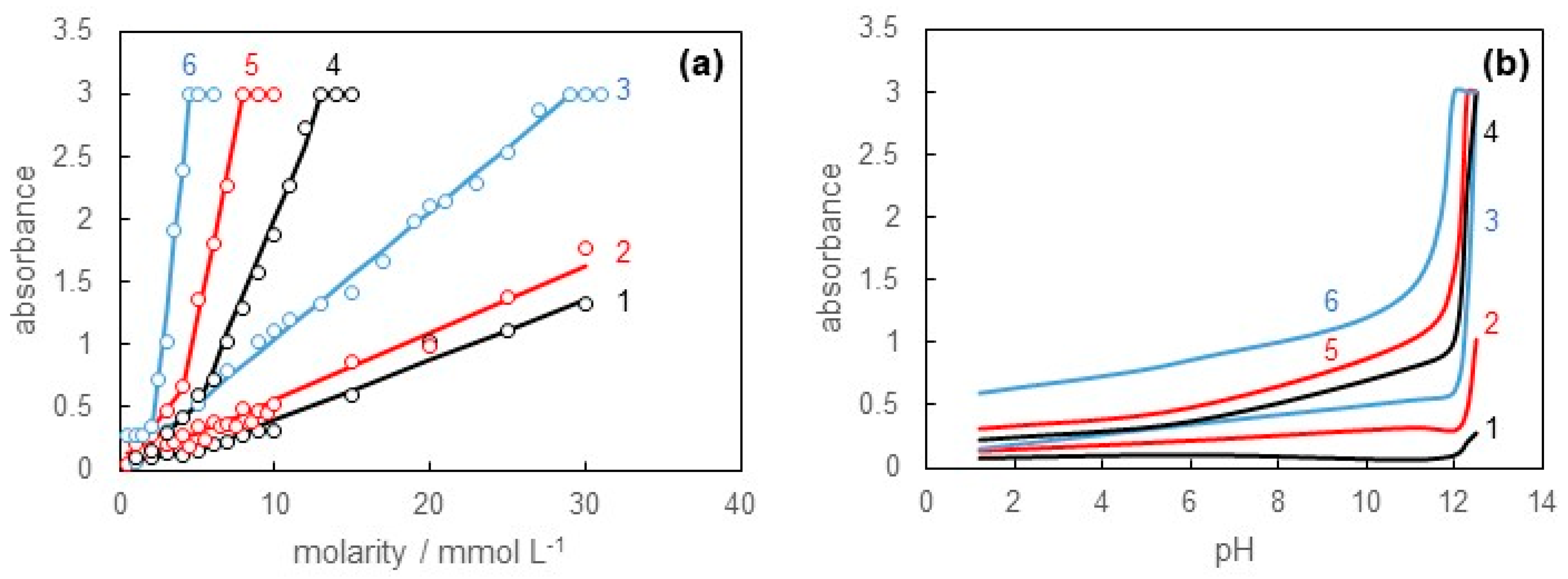
3.2. Solubilization of Sudan III into SB-12 Solution
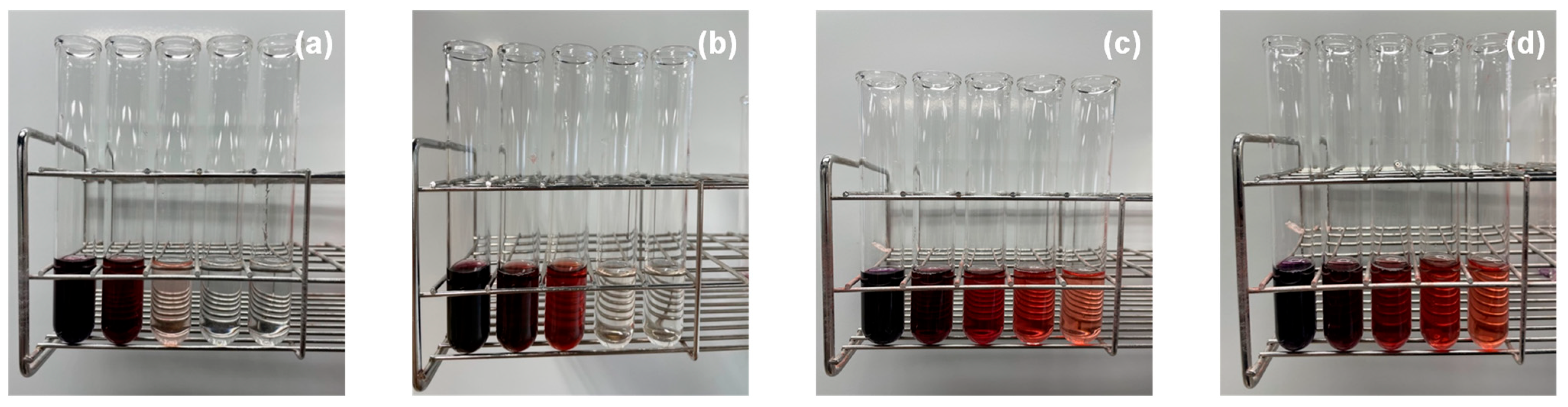
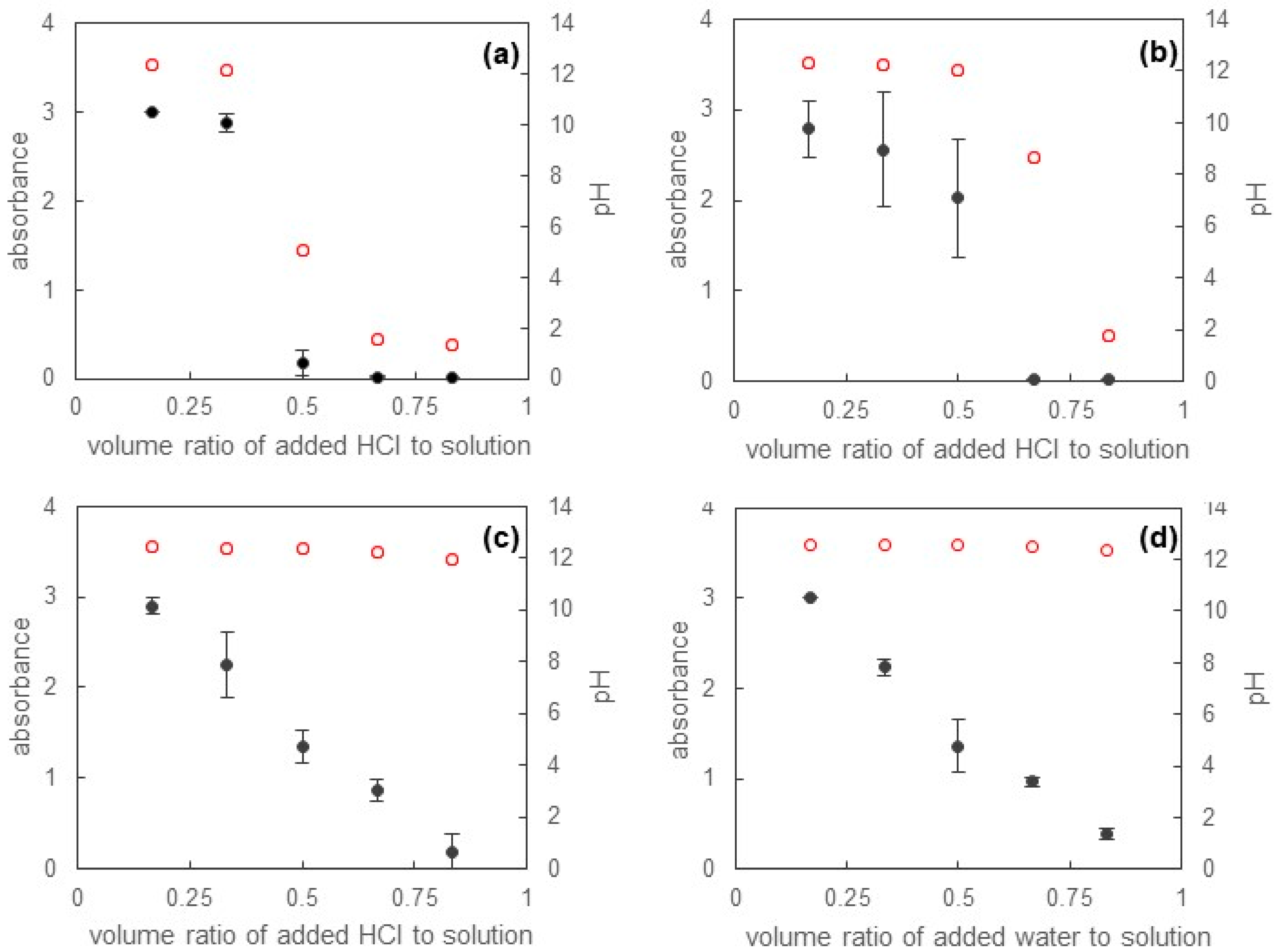
3.3. Release of Solubilized Sudan III from Micellar Solutions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thummar, A.D.; Sastry, N.V.; Verma, G.; Hassan, P.A. Aqueous block copolymer-surfactant mixtures—Surface tension, DLS and viscosity measurements and their utility in solubilization of hydrophobic drug and its controlled release. Colloids Surf. A 2011, 386, 54–64. [Google Scholar] [CrossRef]
- Parmar, A.; Chavda, S.; Bahadur, P. Pluronic–cationic surfactant mixed micelles: Solubilization and release of the drug hydrochlorothiazide. Colloids Surf. A 2014, 441, 389–397. [Google Scholar] [CrossRef]
- Singla, P.; Chabba, S.; Mahajan, R.K. A systematic physicochemical investigation on solubilization and in vitro release of poorly water soluble oxcarbazepine drug in pluronic micelles. Colloids Surf. A 2016, 504, 479–488. [Google Scholar] [CrossRef]
- Pillai, S.A.; Sheth, U.; Bahadur, A.; Aswal, V.K.; Bahadur, P. Salt induced micellar growth in aqueous solutions of a star block copolymer Tetronic® 1304: Investigating the role in solubilizing, release and cytotoxicity of model drugs. J. Mol. Liq. 2016, 224, 303–310. [Google Scholar] [CrossRef]
- Sastry, N.V.; Singh, D.K.; Thummar, A.D.; Verma, G.; Hassan, P.A. Effect of hydrocarbon surfactants on dexamethasone solubilization into silicone surfactant micelles in aqueous media and its release from agar films as carriers. J. Mol. Liq. 2017, 225, 11–19. [Google Scholar] [CrossRef]
- Rapoport, N. Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery. Prog. Polym. Sci. 2007, 32, 962–990. [Google Scholar] [CrossRef]
- Liang, Y.; Su, Z.; Yao, Y.; Zhang, N. Preparation of pH Sensitive Pluronic-Docetaxel Conjugate Micelles to Balance the Stability and Controlled Release Issues. Materials 2015, 8, 379–391. [Google Scholar] [CrossRef]
- Liu, X.; Abbott, N.L. Spatial and temporal control of surfactant systems. J. Colloid Interface Sic. 2009, 339, 1–18. [Google Scholar] [CrossRef]
- Brown, P.; Butts, C.P.; Eastoe, J. Stimuli-responsive surfactants. Soft Matter 2013, 9, 2365–2374. [Google Scholar] [CrossRef]
- Scermino, L.; Fabozzi, A.; Tommaso, G.D.; Valente, A.J.M.; Iuliano, M.; Paduano, L.; D’Errico, G. pH-responsive micellization of an amine oxide surfactant with branched hydrophobic tail. J. Mol. Liq. 2020, 316, 113799. [Google Scholar] [CrossRef]
- Yan, C.; Yang, L.; Mo, X.; Chen, K.; Niu, W.; Zhao, Z.; Li, G. Dual Thermo- and Photo-Responsive Micelles Based on Azobenzene-Containing Random Copolymer. Materials 2022, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhao, J.; Wang, B.; Jiang, R. Synthesis and self-assembly of new light-sensitive Gemini surfactants containing an azobenzene group. Colloids Surf. A 2009, 352, 24–30. [Google Scholar] [CrossRef]
- Fomina, N.; Sankaranarayanan, J.; Almutairi, A. Photochemical mechanisms of light-triggered release from nanocarriers. Adv. Drug Delivery Rev. 2012, 64, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Tian, S.; Niu, Y.; Li, G.; Ning, P. Reversible solubilization of typical polycyclic aromatic hydrocarbons by a photoresponsive surfactant. Colloids Surf. A 2014, 454, 172–179. [Google Scholar] [CrossRef]
- Esfandyari, H.; Rahimi, A.M.; Esmaeilzadeh, F.; Davarpanah, A.; Mohammadi, A.H. Amphoteric and cationic surfactants for enhancing oil recovery from carbonate oil reservoirs. J. Mol. Liq. 2021, 322, 114518. [Google Scholar] [CrossRef]
- Dai, C.; Han, Y.; Zhang, Y.; Duan, Y.; Tong, W.; Liu, S.; Tu, Y.; Hu, J.; Li, J. Cyclic solubilization and release of polycyclic aromatic hydrocarbons (PAHs) using gemini photosensitive surfactant combined with micro-nano bubbles: A promising enhancement technology for groundwater remediation. Sep. Purif. Technol. 2023, 309, 123042. [Google Scholar] [CrossRef]
- Takata, Y.; Ohtsuka, Y.; Ashida, T. Effect of Hydrophobic Chains on Solubilization of Hydrocarbon and Fluorocarbon Surfactant Mixtures in Aqueous Solution. J. Oleo Sci. 2019, 68, 855–861. [Google Scholar] [CrossRef]
- Matsuki, H.; Ikeda, N.; Aratono, M.; Kaneshina, S.; Motomura, K. Study on the Miscibility of Lithium Dodecyl Sulfate and Lithium Perfluorooctane Sulfonate in the Adsorbed Film and Micelle. J. Colloid Interface Sci. 1992, 150, 331–337. [Google Scholar] [CrossRef]
- Matsuki, H.; Ikeda, N.; Aratono, M.; Kaneshina, S.; Motomura, K. Study on the Miscibility of Lithium Tetradecyl Sulfate and Lithium Perfluorooctane Sulfonate in the Adsorbed Film and Micelle. J. Colloid Interface Sci. 1992, 154, 454–460. [Google Scholar] [CrossRef]
- Jiang, W.; Guo, J.; Wen, W.; Jia, Y.-G.; Liu, S. Nano-Carriers Based on pH-Sensitive Star-Shaped Copolymers for Drug-Controlled Release. Materials 2019, 12, 1610. [Google Scholar] [CrossRef]
- Jafari, A.; Yan, L.; Mohamed, M.A.; Wu, Y.; Cheng, C. Well-Defined Diblock Poly(ethylene glycol)-b-Poly(ε-caprolactone)-Based Polymer-Drug Conjugate Micelles for pH-Responsive Delivery of Doxorubicin. Materials 2020, 13, 1510. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Holmberg, K.; Bordes, R. Micellization of true amphoteric surfactants. J. Colloid Interface Sci. 2013, 411, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Motomura, K.; Matubayasi, N.; Aratono, M.; Matuura, R. Thermodynamic Studies on Adsorption at Interfaces II. One Surface-Active Component System: Tetradecanol at Hexane/Water Interface. J. Colloid Interface Sci. 1978, 64, 356–361. [Google Scholar] [CrossRef]
- Motomura, K.; Iwanaga, S.; Hayami, Y.; Uryu, S.; Matuura, R. Thermodynamic Studies on Adsorption at Interfaces IV. Dodecylammonium Chloride at Water/Air Interface. J. Colloid Interface Sci. 1981, 80, 32–38. [Google Scholar] [CrossRef]
- Aratono, M.; Uryu, S.; Hayami, Y.; Motomura, K.; Matuura, R. Mixed Adsorbed Film of Dodecylammonium Chloride with Decylammonium Chloride at Water/Air Interface. J. Colloid Interface Sci. 1983, 93, 162–168. [Google Scholar] [CrossRef]
- Gerola, A.P.; Costa, P.F.A.; Nome, F.; Quina, F. Micellization and adsorption of zwitterionic surfactants at the air/water interface. Curr. Opin. Colloid Interface Sci. 2017, 32, 48–56. [Google Scholar] [CrossRef]
- Silva, G.T.M.; Quina, F.H. Ion-micelle interactions and the modeling of reactivity in micellar solutions of simple zwitterionic sulfobetaine surfactants. Curr. Opin. Colloid Interface Sci. 2019, 44, 168–176. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Singh, R.G.; Holmberg, K. Solubilization of two organic dyes by cationic ester-containing gemini surfactants. J. Colloid Interface Sci. 2012, 376, 112–118. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Singh, R.G.; Holmberg, K. Solubilization of two organic dyes by anionic, cationic and nonionic surfactants. J. Colloid Interface Sci. 2013, 417, 133–139. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Holmberg, K. Solubilization of Hydrophobic Dyes in Surfactant Solutions. Materials 2013, 6, 580–608. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takata, Y.; Uchikura, A. Study on Reversible Solubilization by Adjusting Surfactant Properties. Materials 2023, 16, 3550. https://doi.org/10.3390/ma16093550
Takata Y, Uchikura A. Study on Reversible Solubilization by Adjusting Surfactant Properties. Materials. 2023; 16(9):3550. https://doi.org/10.3390/ma16093550
Chicago/Turabian StyleTakata, Youichi, and Amu Uchikura. 2023. "Study on Reversible Solubilization by Adjusting Surfactant Properties" Materials 16, no. 9: 3550. https://doi.org/10.3390/ma16093550
APA StyleTakata, Y., & Uchikura, A. (2023). Study on Reversible Solubilization by Adjusting Surfactant Properties. Materials, 16(9), 3550. https://doi.org/10.3390/ma16093550




