β-Cyclodextrin Nanosponges Inclusion Compounds Associated with Silver Nanoparticles to Increase the Antimicrobial Activity of Quercetin
Abstract
1. Introduction
- The development of an NSs–QRC formulation to improve the stability of free QRC.
- The association of AgNPs to the NSs–QRC complex to generate the AgNPs–NSs–QRC ternary system and the further evaluation of its antimicrobial properties.
- Evaluating the biocompatibility and biotoxicity of the NS–QRC and AgNPs–NSs–QRC complexes.
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AgNPs
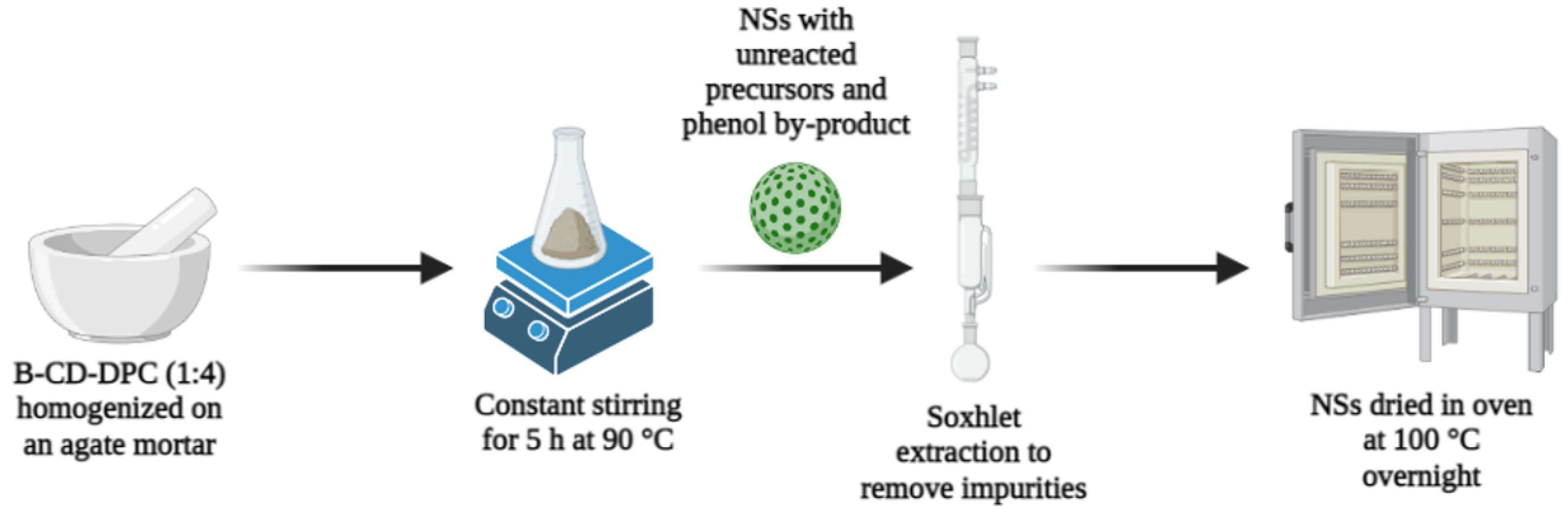
2.3. Synthesis and Purification of the NSs Matrix
2.4. Formation of the NSs-QRC Inclusion Compound
2.5. Formation of the ICs–AgNPs Ternary Complexes
2.6. Characterization Methods
2.7. Complexation Efficiencies of the NS–QRC Formulation
2.8. Antibacterial Activity
2.9. MTS Cell Metabolic Activity Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the ICs
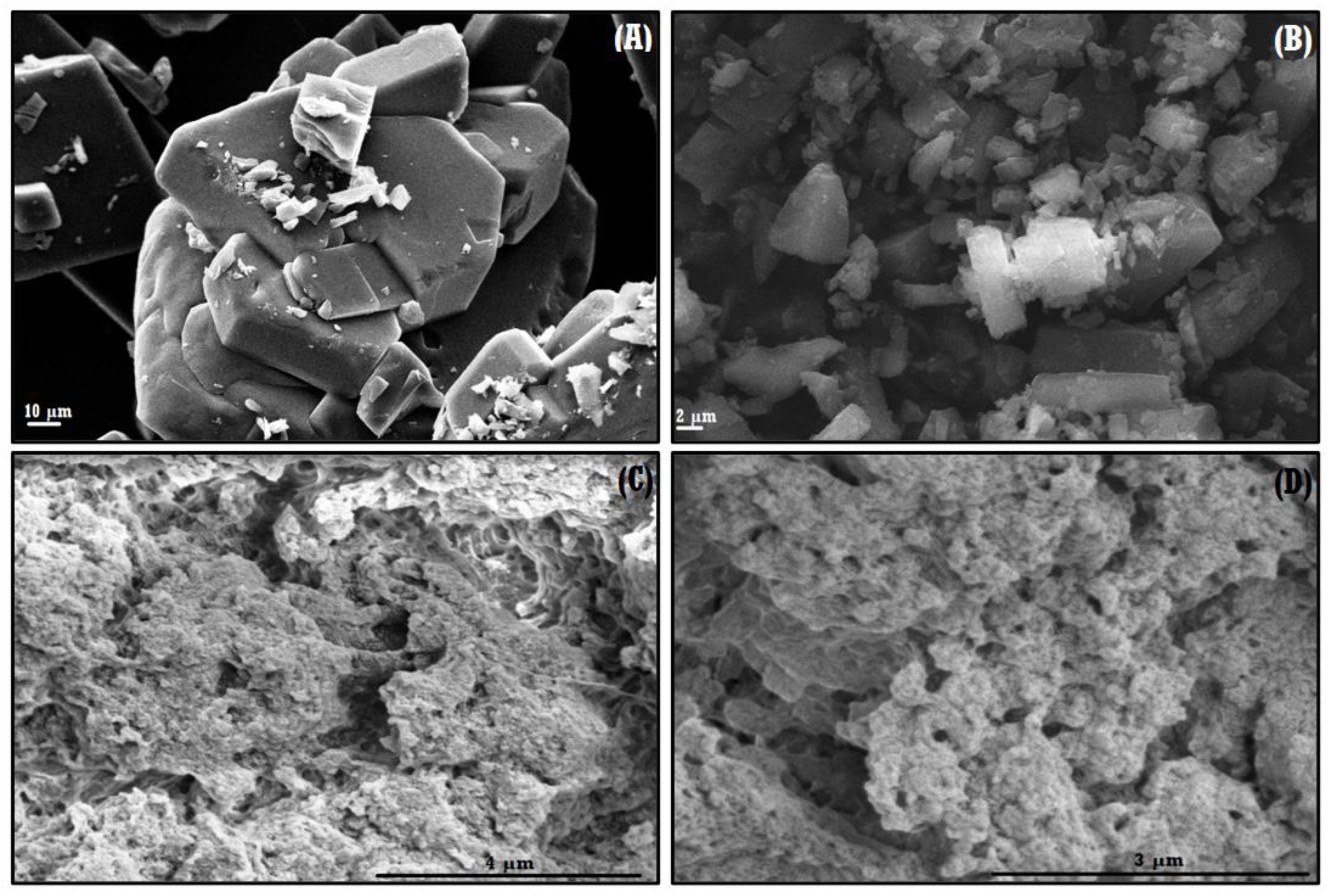
3.1.1. FE–SEM of the NS–QRC Complex
3.1.2. TGA of the NS–QRC Complexes
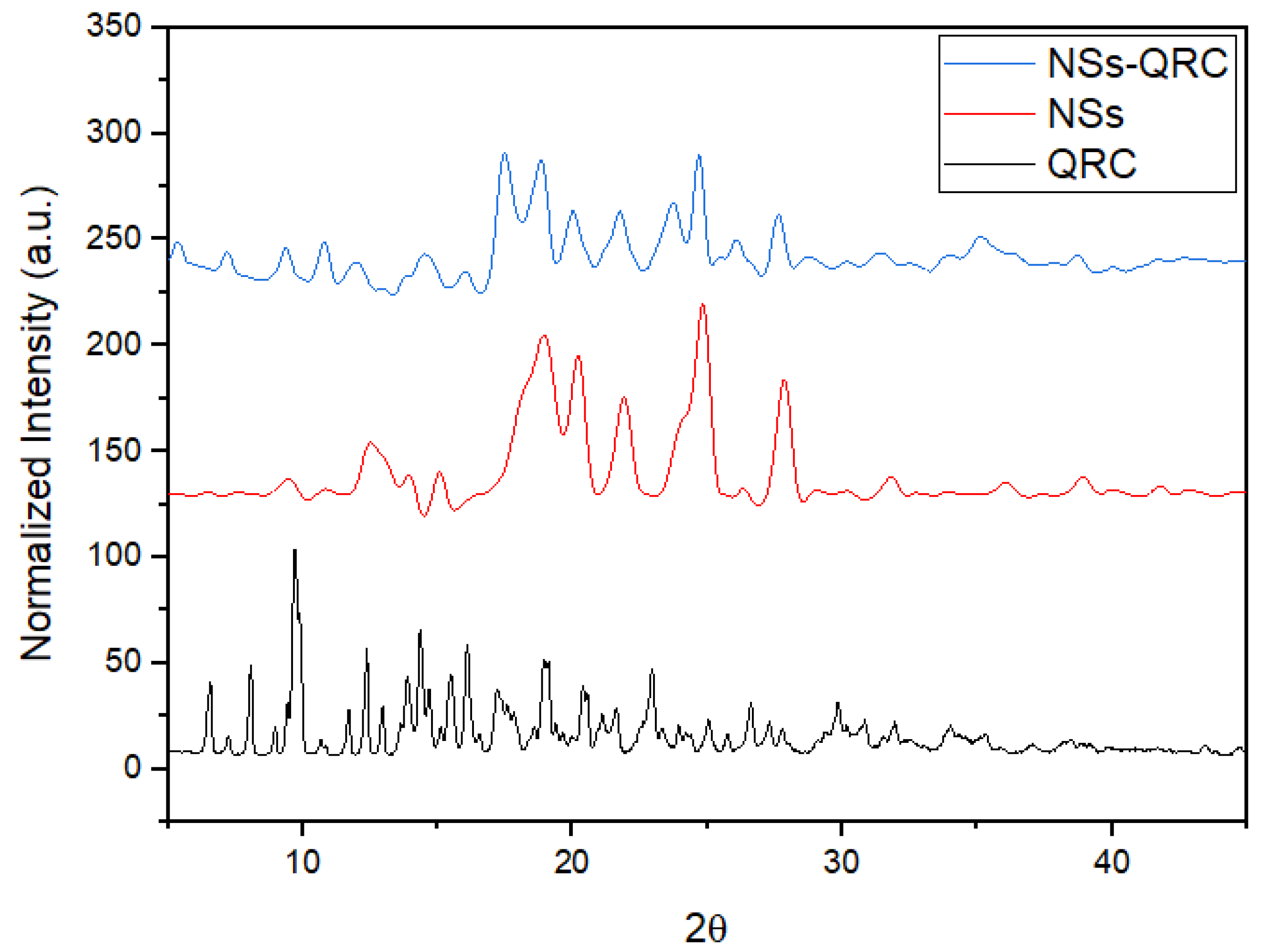
3.1.3. XRPD of the NS–QRC Complexes
3.1.4. Loading Capacity (LC%) and Encapsulation Efficiencies (EE%) of the NS–QRC Complex
3.1.5. 1H–NMR of the NS–QRC Complexes
3.2. Characterization of NS–QRC Associated with AgNPs
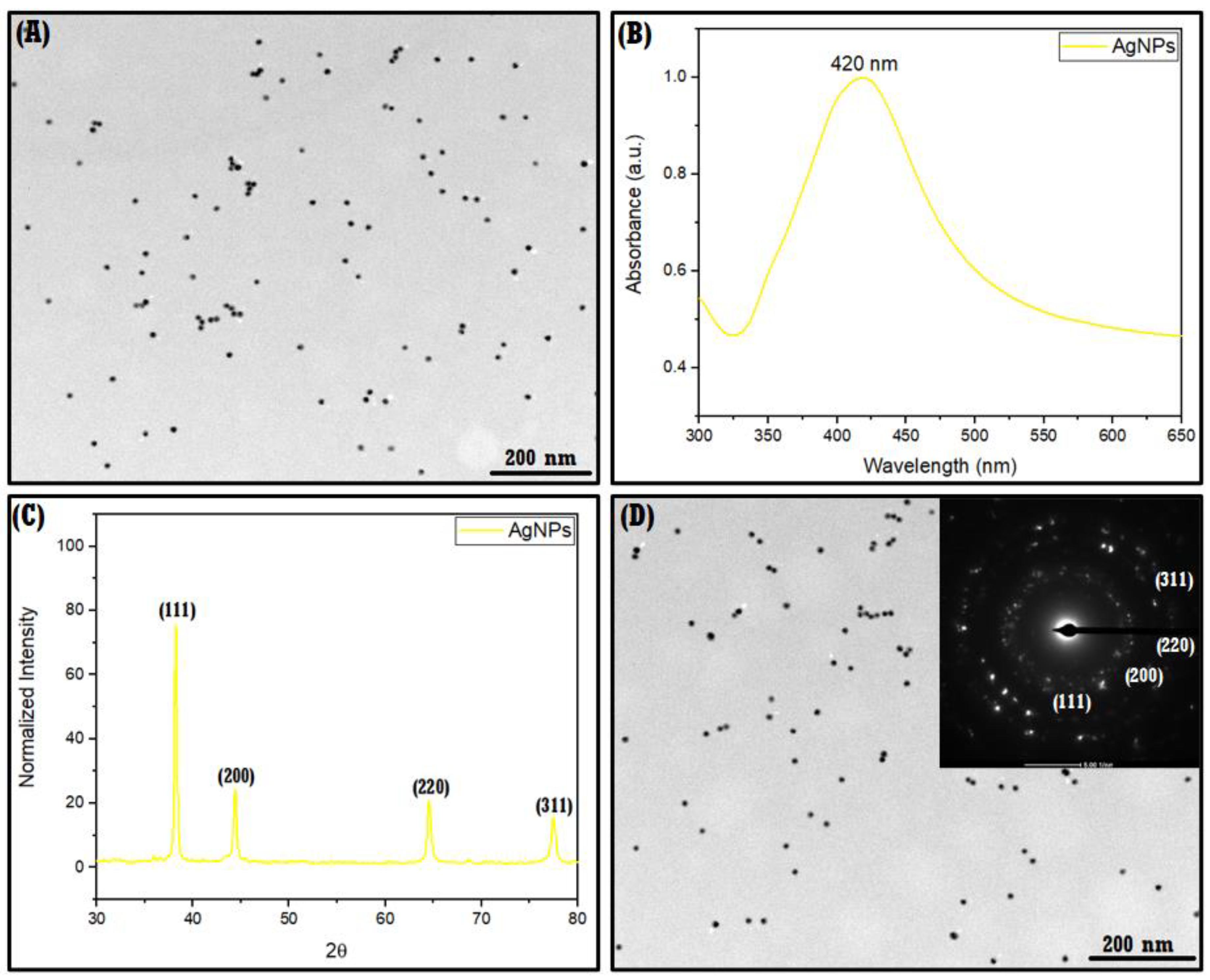
3.2.1. TEM, UV–Vis, XRPD, SAED, DLS, and ζ-Potential of AgNPs
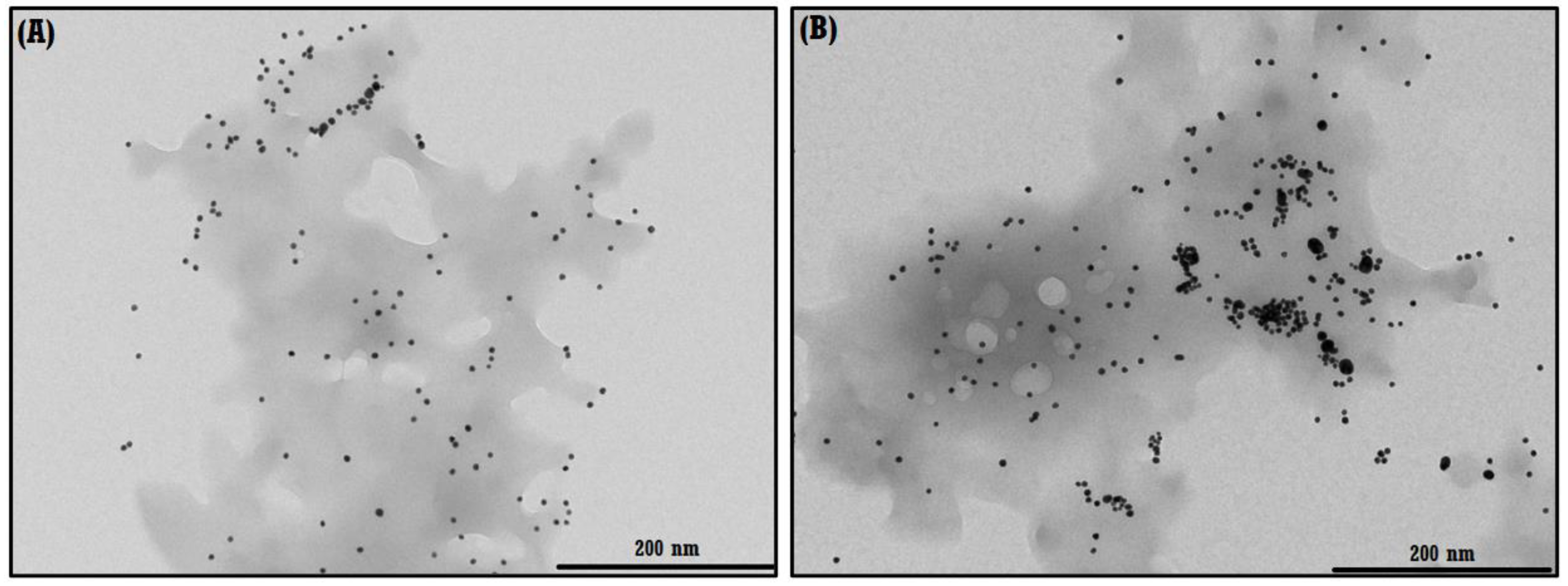
3.2.2. TEM of the NS–QRC Complex Associated to the AgNPs
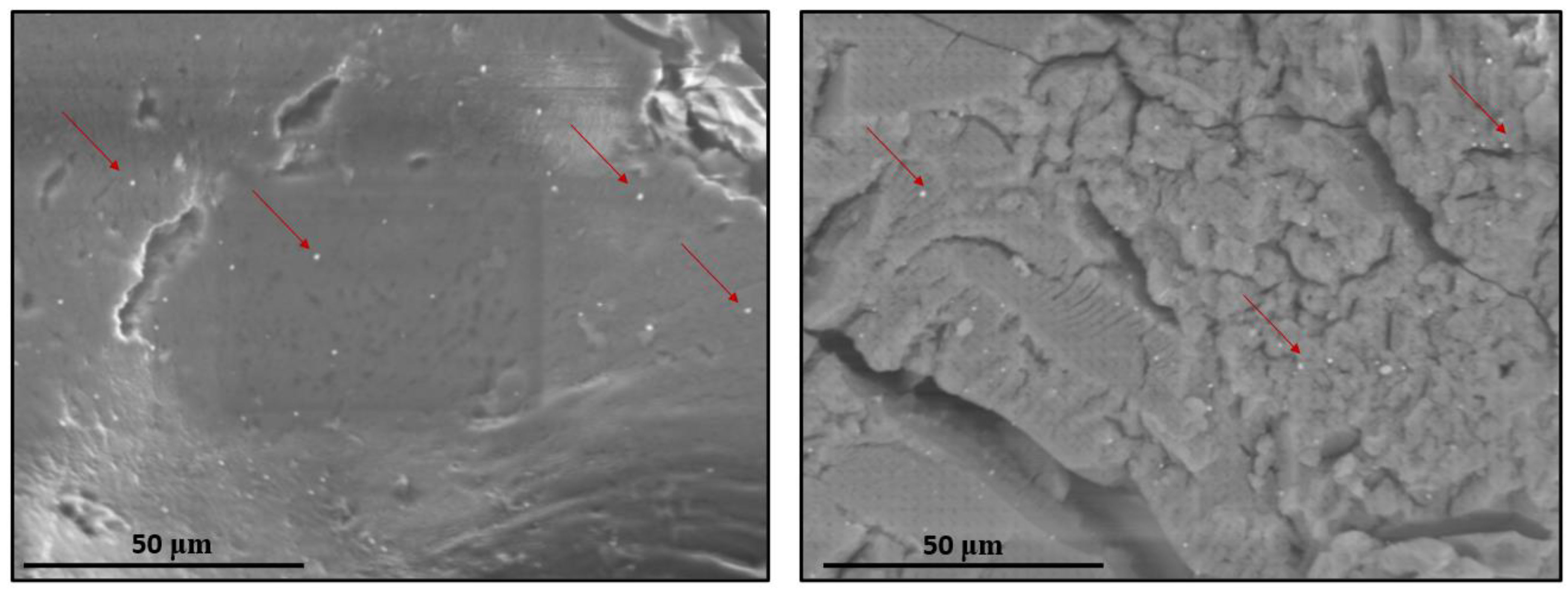
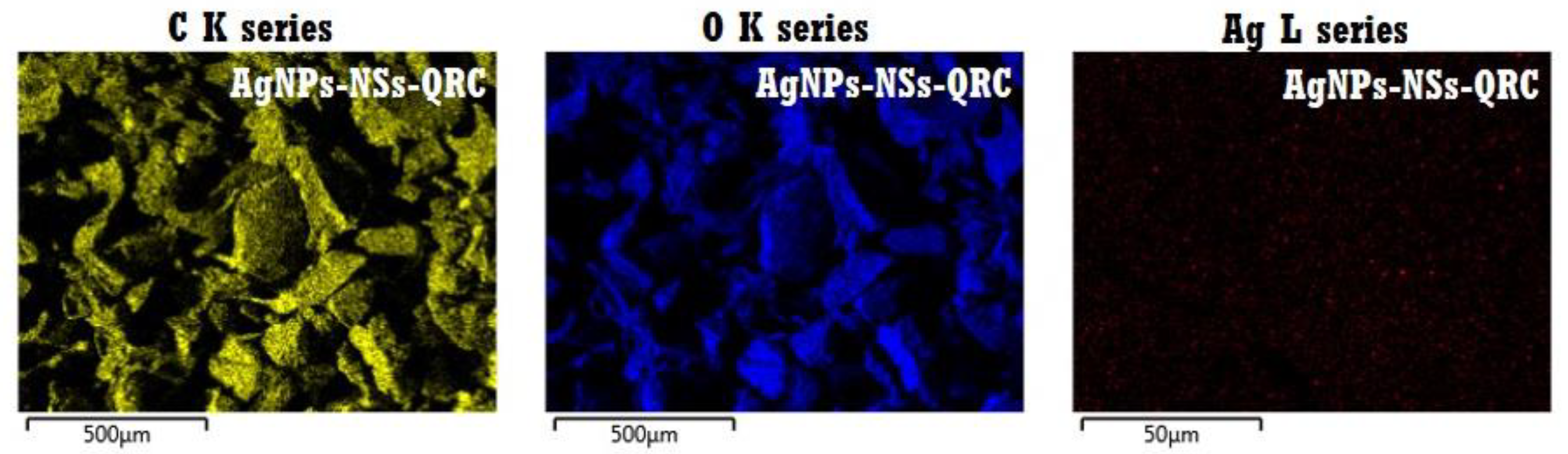
3.2.3. FE–SEM and EDS Analyses of NS–QRC Associated with AgNPs
3.2.4. UV–Visible Spectrum of NS–QRC Associated with AgNPs
3.2.5. DLS and ζ-Potential of NS–QRC Associated to the AgNPs
3.3. Antibacterial Assays of NS–QRC Associated with the AgNPs
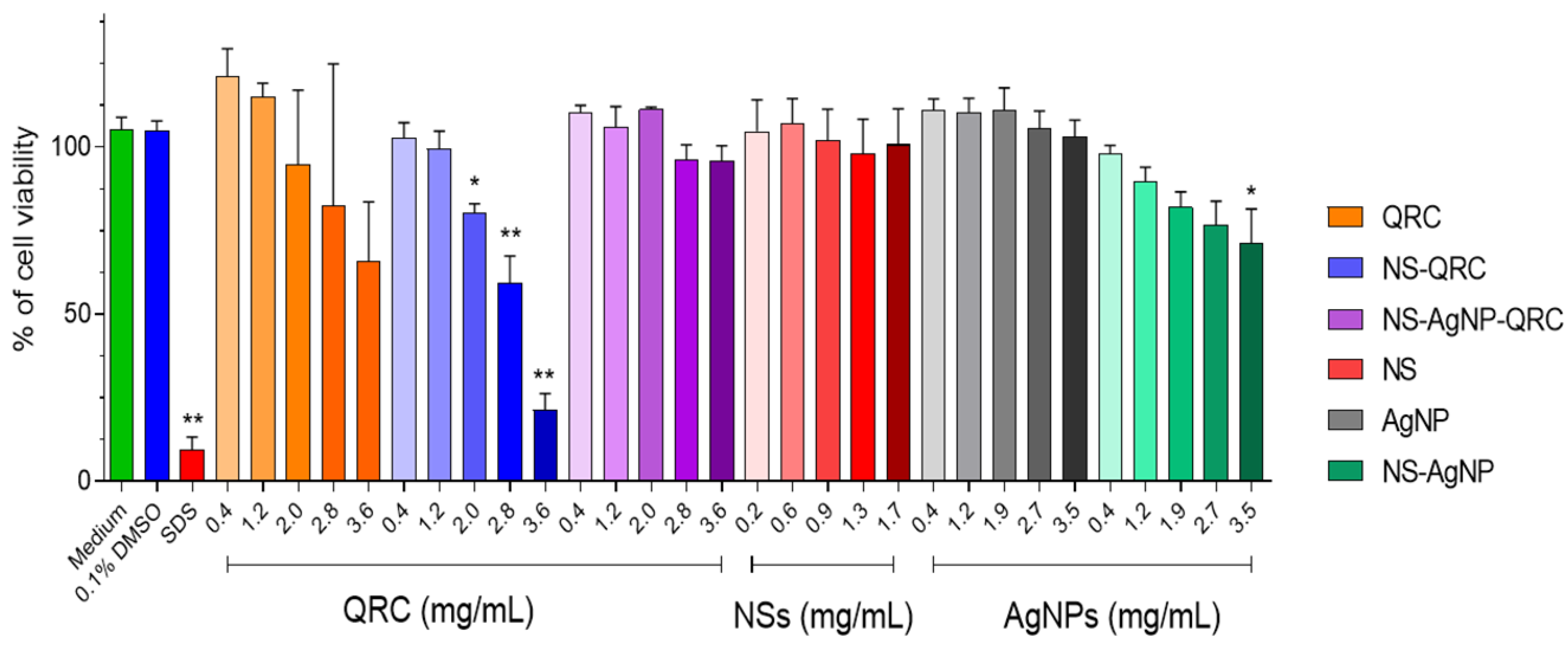
3.4. Cell Metabolic Activity Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
FE–SEM Images of Free NSs

Appendix B
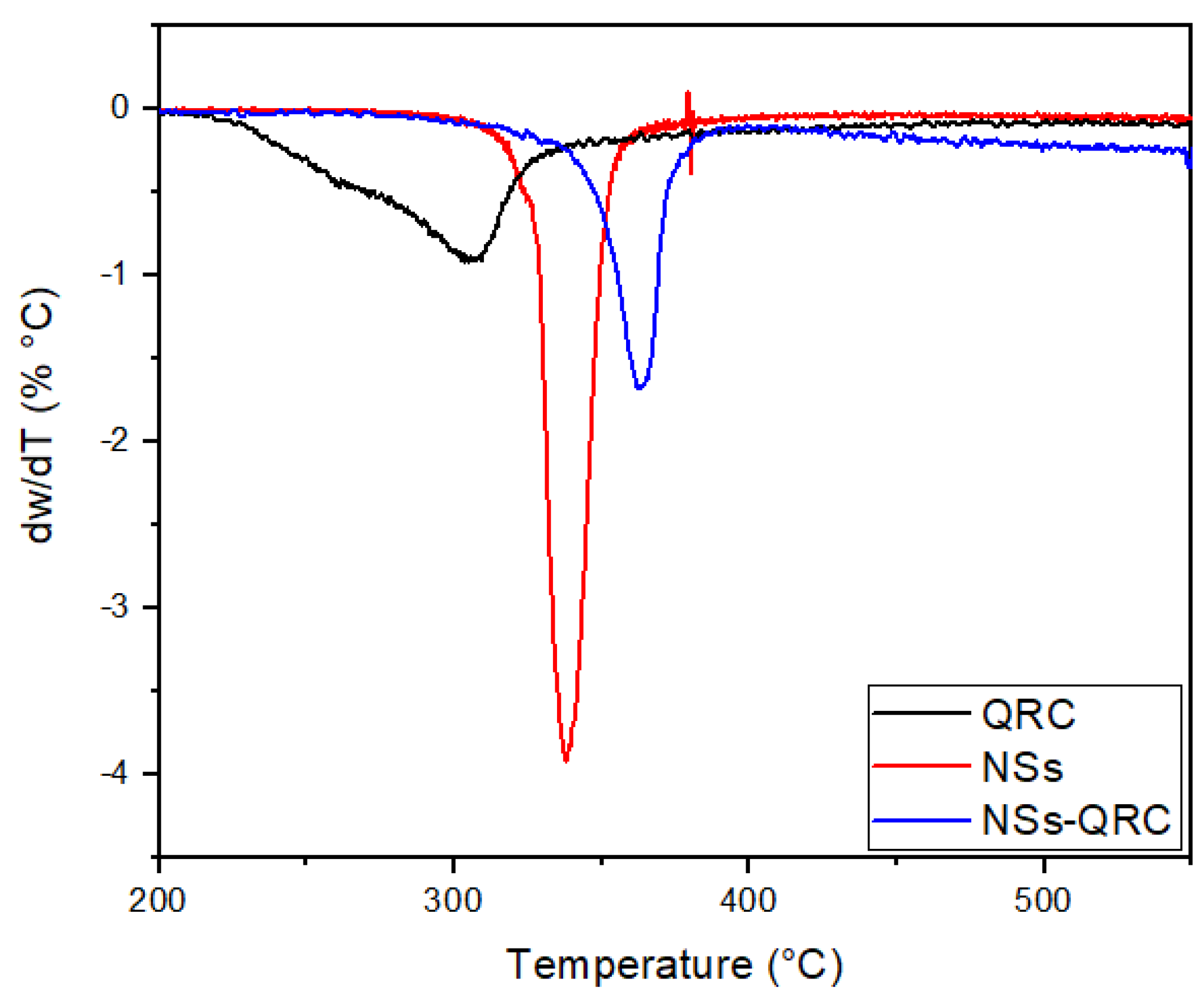
Determination of QRC Content Using Derivative Thermogravimetry (DTG)
Appendix C
UV–Visible Spectra, DLS, and ζ-Potentials of AgNP–NS–QRC after 20 Days of Storage
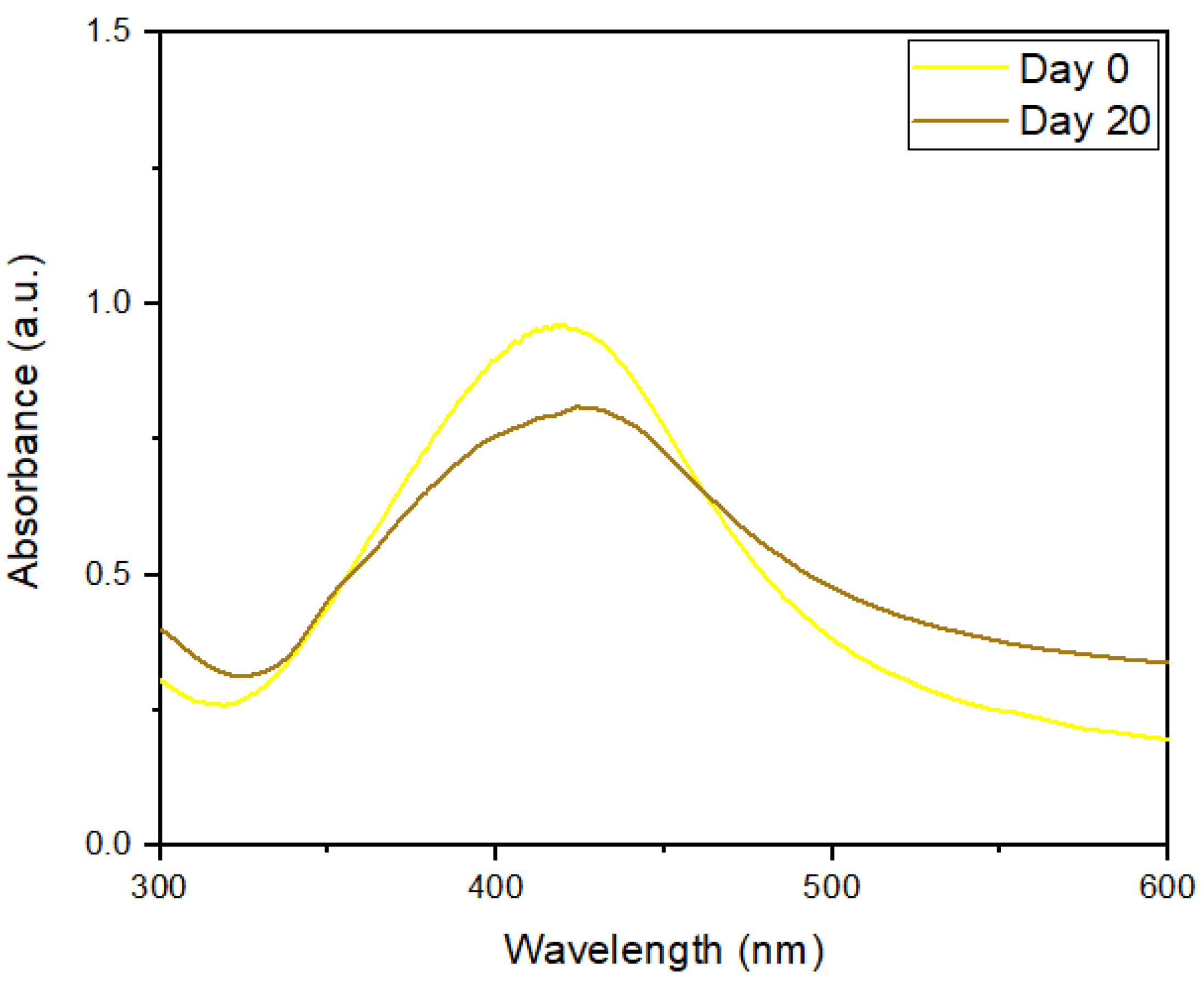
| Sample | Dh (nm) | ζ-Potentials (mV) | PDI |
|---|---|---|---|
| AgNP–NS–QRC (Day 0) | 261 ± 11 | −28 ± 5 | 0.48 |
| AgNP–NS–QRC (Day 20) | 273 ± 15 | −23 ± 8 | 0.56 |
Appendix D
Cumulative Release of QRC from the NS–QRC and AgNP–QRC–NS Complexes

Appendix E
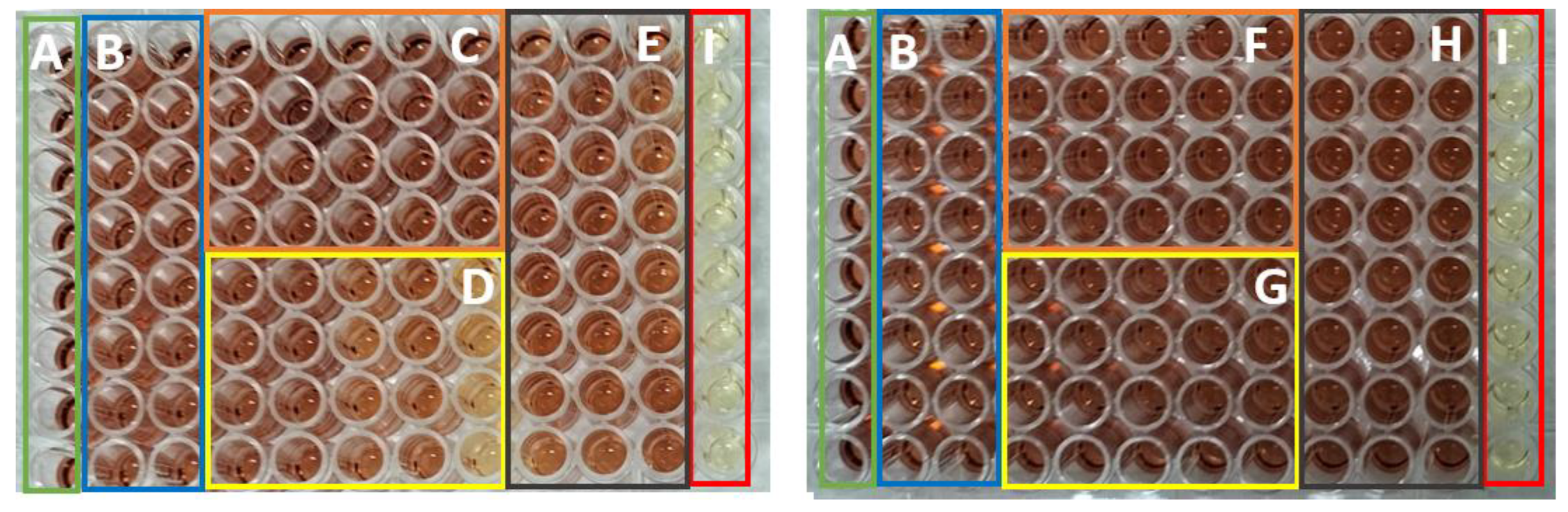
Selected Images for the Antimicrobial Activity Experiments

Appendix F
Selected Images from MTS Assay
References
- Munir, R.; Hadi, A.; Khan, S.-U.; Asghar, S.; Irfan, M.; Khan, I.U.; Hameed, M.; Inam, S.; Islam, N.; Hassan, S.F.; et al. Solubility and Dissolution Enhancement of Dexibuprofen with Hydroxypropylbetacyclodextrin (HPβCD) and Poloxamers (188/407) Inclusion Complexes: Preparation and In Vitro Characterization. Polymers 2022, 14, 579. [Google Scholar] [CrossRef]
- Bernad-Bernad, M.-J.; Gracia-Mora, J.; Díaz, D.; Castillo-Blum, S.E. Thermodynamic study of cyclodextrins complexation with benzimidazolic antihelmintics in different reaction media. Curr. Cancer Drug Targets 2008, 5, 146–153. [Google Scholar] [CrossRef]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from molecules to applications. Environ. Chem. Lett. 2018, 16, 1361–1375. [Google Scholar] [CrossRef]
- Loftsson, T. Cyclodextrins in Parenteral Formulations. J. Pharm. Sci. 2021, 110, 654–664. [Google Scholar] [CrossRef]
- Braga, S.S.; Barbosa, J.S.; Santos, N.E.; El-Saleh, F.; Paz, F.A.A. Cyclodextrins in Antiviral Therapeutics and Vaccines. Pharmaceutics 2021, 13, 409. [Google Scholar] [CrossRef]
- Adeoye, O.; Bártolo, I.; Conceição, J.; da Silva, A.B.; Duarte, N.; Francisco, A.P.; Taveira, N.; Cabral-Marques, H. Pyromellitic dianhydride crosslinked soluble cyclodextrin polymers: Synthesis, lopinavir release from sub-micron sized particles and anti-HIV-1 activity. Int. J. Pharm. 2020, 583, 119356. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; Li, W.; Liu, K.; Tang, T.; Liu, J.; Jiang, W. One-step synthesis of an environment-friendly cyclodextrin-based nanosponge and its applications for the removal of dyestuff from aqueous solutions. Res. Chem. Intermed. 2020, 46, 1715–1734. [Google Scholar] [CrossRef]
- Shoaib, Q.-U.; Abbas, N.; Irfan, M.; Hussain, A.; Arshad, M.S.; Hussain, S.Z.; Latif, S.; Bukhari, N.I. Development and evaluation of scaffold-based nanosponge formulation for controlled drug delivery of naproxen and ibuprofen. Trop. J. Pharm. Res. 2018, 17, 1465. [Google Scholar] [CrossRef]
- Mashaqbeh, H.; Obaidat, R.; Al-Shar’i, N. Evaluation and Characterization of Curcumin-β-Cyclodextrin and Cyclodextrin-Based Nanosponge Inclusion Complexation. Polymers 2021, 13, 4073. [Google Scholar] [CrossRef]
- Argenziano, M.; Haimhoffer, A.; Bastiancich, C.; Jicsinszky, L.; Caldera, F.; Trotta, F.; Scutera, S.; Alotto, D.; Fumagalli, M.; Musso, T.; et al. In Vitro Enhanced Skin Permeation and Retention of Imiquimod Loaded in β-Cyclodextrin Nanosponge Hydrogel. Pharmaceutics 2019, 11, 138. [Google Scholar] [CrossRef]
- Salehi, O.; Sami, M.; Rezaei, A. Limonene loaded cyclodextrin nanosponge: Preparation, characterization, antibacterial activity and controlled release. Food Biosci. 2021, 42, 101193. [Google Scholar] [CrossRef]
- Hayiyana, Z.; Choonara, Y.; Makgotloe, A.; du Toit, L.; Kumar, P.; Pillay, V. Ester-Based Hydrophilic Cyclodextrin Nanosponges for Topical Ocular Drug Delivery. Curr. Pharm. Des. 2017, 22, 6988–6997. [Google Scholar] [CrossRef]
- Allahyari, S.; Zahednezhad, F.; Khatami, M.; Hashemzadeh, N.; Zakeri-Milani, P.; Trotta, F. Cyclodextrin nanosponges as potential anticancer drug delivery systems to be introduced into the market, compared with liposomes. J. Drug Deliv. Sci. Technol. 2022, 67, 102931. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, R. Enhancing efficacy and safety of azelaic acid via encapsulation in cyclodextrin nanosponges: Development, characterization and evaluation. Polym. Bull. 2020, 78, 5275–5302. [Google Scholar] [CrossRef]
- Jain, A.; Prajapati, S.K.; Kumari, A.; Mody, N.; Bajpai, M. Engineered nanosponges as versatile biodegradable carriers: An insight. J. Drug Deliv. Sci. Technol. 2020, 57, 101643. [Google Scholar] [CrossRef]
- Singireddy, A.; Subramanian, S. Cyclodextrin Nanosponges to Enhance the Dissolution Profile of Quercetin by Inclusion Complex Formation. Part. Sci. Technol. 2016, 34, 341–346. [Google Scholar] [CrossRef]
- Anandam, S.; Selvamuthukumar, S. Fabrication of cyclodextrin nanosponges for quercetin delivery: Physicochemical characterization, photostability, and antioxidant effects. J. Mater. Sci. 2014, 49, 8140–8153. [Google Scholar] [CrossRef]
- Sujitha, Y.S.; Muzib, Y.I. Formulation and optimization of quercetin loaded nanosponges topical gel: Ex vivo, pharmacodynamic and pharmacokinetic studies. Int. J. Appl. Pharm. 2019, 11, 156–165. [Google Scholar] [CrossRef]
- Gupta, B.; Dalal, P.; Rao, R. Cyclodextrin decorated nanosponges of sesamol: Antioxidant, anti-tyrosinase and photostability assessment. Food Biosci. 2021, 42, 101098. [Google Scholar] [CrossRef]
- Singh, P.; Ren, X.; Guo, T.; Wu, L.; Shakya, S.; He, Y.; Wang, C.; Maharjan, A.; Singh, V.; Zhang, J. Biofunctionalization of β-cyclodextrin nanosponges using cholesterol. Carbohydr. Polym. 2018, 190, 23–30. [Google Scholar] [CrossRef]
- Omar, S.M.; Ibrahim, F.; Ismail, A. Formulation and evaluation of cyclodextrin-based nanosponges of griseofulvin as pediatric oral liquid dosage form for enhancing bioavailability and masking bitter taste. Saudi Pharm. J. 2020, 28, 349–361. [Google Scholar] [CrossRef]
- Gholibegloo, E.; Mortezazadeh, T.; Salehian, F.; Forootanfar, H.; Firoozpour, L.; Foroumadi, A.; Ramazani, A.; Khoobi, M. Folic acid decorated magnetic nanosponge: An efficient nanosystem for targeted curcumin delivery and magnetic resonance imaging. J. Colloid Interface Sci. 2019, 556, 128–139. [Google Scholar] [CrossRef]
- Caldera, F.; Nisticò, R.; Magnacca, G.; Matencio, A.; Monfared, Y.K.; Trotta, F. Magnetic Composites of Dextrin-Based Carbonate Nanosponges and Iron Oxide Nanoparticles with Potential Application in Targeted Drug Delivery. Nanomaterials 2022, 12, 754. [Google Scholar] [CrossRef]
- Salazar, S.; Guerra, D.; Yutronic, N.; Jara, P. Removal of Aromatic Chlorinated Pesticides from Aqueous Solution Using β-Cyclodextrin Polymers Decorated with Fe3O4 Nanoparticles. Polymers 2018, 10, 1038. [Google Scholar] [CrossRef]
- Salazar, S.; Yutronic, N.; Jara, P. Magnetic β-Cyclodextrin Nanosponges for Potential Application in the Removal of the Neonicotinoid Dinotefuran from Wastewater. Int. J. Mol. Sci. 2020, 21, 4079. [Google Scholar] [CrossRef]
- Asela, I.; Donoso-González, O.; Yutronic, N.; Sierpe, R. β-Cyclodextrin-Based Nanosponges Functionalized with Drugs and Gold Nanoparticles. Pharmaceutics 2021, 13, 513. [Google Scholar] [CrossRef]
- Sandoval, S.S.; Cortés-Adasme, E.; Gallardo-Toledo, E.; Araya, I.; Celis, F.; Yutronic, N.; Jara, P.; Kogan, M.J. β-Cyclodextrin-Based Nanosponges Inclusion Compounds Associated with Gold Nanorods for Potential NIR-II Drug Delivery. Pharmaceutics 2022, 14, 2206. [Google Scholar] [CrossRef]
- Salazar, S.; Yutronic, N.; Kogan, M.J.; Jara, P. Cyclodextrin Nanosponges Inclusion Compounds Associated with Gold Nanoparticles for Potential Application in the Photothermal Release of Melphalan and Cytoxan. Int. J. Mol. Sci. 2021, 22, 6446. [Google Scholar] [CrossRef]
- Silva, N.; Riveros, A.; Yutronic, N.; Lang, E.; Chornik, B.; Guerrero, S.; Samitier, J.; Jara, P.; Kogan, M.J. Photothermally Controlled Methotrexate Release System Using β-Cyclodextrin and Gold Nanoparticles. Nanomaterials 2018, 8, 985. [Google Scholar] [CrossRef]
- Vellaichamy, B.; Periakaruppan, P. Silver nanoparticle-embedded RGO-nanosponge for superior catalytic activity towards 4-nitrophenol reduction. RSC Adv. 2016, 6, 88837–88845. [Google Scholar] [CrossRef]
- Russo, M.; Spinella, A.; Di Vincenzo, A.; Lazzara, G.; Correro, M.R.; Shahgaldian, P.; Meo, P.L.; Caponetti, E. Synergistic Activity of Silver Nanoparticles and Polyaminocyclodextrins in Nanosponge Architectures. Chemistryselect 2019, 4, 873–879. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Rahman, A.; Kumar, S.; Bafana, A.; Dahoumane, S.A.; Jeffryes, C. Individual and Combined Effects of Extracellular Polymeric Substances and Whole Cell Components of Chlamydomonas reinhardtii on Silver Nanoparticle Synthesis and Stability. Molecules 2019, 24, 956. [Google Scholar] [CrossRef]
- Majoumouo, M.S.; Sibuyi, N.R.S.; Tincho, M.B.; Mbekou, M.; Boyom, F.F.; Meyer, M. Enhanced Anti-Bacterial Activity of Biogenic Silver Nanoparticles Synthesized from Terminalia mantaly Extracts. Int. J. Nanomed. 2019, 14, 9031–9046. [Google Scholar] [CrossRef]
- Kumar, S.; Pooja; Trotta, F.; Rao, R. Encapsulation of Babchi Oil in Cyclodextrin-Based Nanosponges: Physicochemical Characterization, Photodegradation, and In Vitro Cytotoxicity Studies. Pharmaceutics 2018, 10, 169. [Google Scholar] [CrossRef]
- Rezaei, A.; Khavari, S.; Sami, M. Incorporation of thyme essential oil into the β-cyclodextrin nanosponges: Preparation, characterization and antibacterial activity. J. Mol. Struct. 2021, 1241, 130610. [Google Scholar] [CrossRef]
- Moin, A.; Roohi, N.K.F.; Rizvi, S.M.D.; Ashraf, S.A.; Siddiqui, A.J.; Patel, M.; Ahmed, S.M.; Gowda, D.V.; Adnan, M. Design and formulation of polymeric nanosponge tablets with enhanced solubility for combination therapy. RSC Adv. 2020, 10, 34869–34884. [Google Scholar] [CrossRef]
- Pawar, S.; Shende, P. Dual drug delivery of cyclodextrin cross-linked artemether and lumefantrine nanosponges for synergistic action using 23 full factorial designs. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125049. [Google Scholar] [CrossRef]
- Garrido, B.; González, S.; Hermosilla, J.; Millao, S.; Quilaqueo, M.; Guineo, J.; Acevedo, F.; Pesenti, H.; Rolleri, A.; Shene, C.; et al. Carbonate-β-Cyclodextrin-Based Nanosponge as a Nanoencapsulation System for Piperine: Physicochemical Characterization. J. Soil Sci. Plant Nutr. 2019, 19, 620–630. [Google Scholar] [CrossRef]
- Salgın, S.; Salgın, U.; Vatansever, O. Synthesis and Characterization of β-Cyclodextrin Nanosponge and Its Application for the Removal of p-Nitrophenol from Water. CLEAN–Soil Air Water 2017, 45, 1500837. [Google Scholar] [CrossRef]
- Shende, P.K.; Gaud, R.; Bakal, R.; Patil, D. Effect of inclusion complexation of meloxicam with β-cyclodextrin- and β-cyclodextrin-based nanosponges on solubility, in vitro release and stability studies. Colloids Surf. B Biointerfaces 2015, 136, 105–110. [Google Scholar] [CrossRef]
- Rafati, N.; Zarrabi, A.; Caldera, F.; Trotta, F.; Ghias, N. Pyromellitic dianhydride crosslinked cyclodextrin nanosponges for curcumin controlled release; formulation, physicochemical characterization and cytotoxicity investigations. J. Microencapsul. 2019, 36, 715–727. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Suo, Z.; Zhai, Y.; Li, H. Cyclodextrin and its derivatives as effective excipients for amorphous ulipristal acetate systems. RSC Adv. 2022, 12, 9170–9178. [Google Scholar] [CrossRef]
- Singh, H.; Du, J.; Singh, P.; Yi, T.H. Ecofriendly synthesis of silver and gold nanoparticles by Euphrasia officinalis leaf extract and its biomedical applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1163–1170. [Google Scholar] [CrossRef]
- Nagar, N.; Devra, V. A kinetic study on the degradation and biodegradability of silver nanoparticles catalyzed Methyl Orange and textile effluents. Heliyon 2019, 5, e01356. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Jasim, I.K.; Alhammid, S.N.A.; Abdulrasool, A.A. Synthesis and evaluation of B-cyclodextrin Based Nanosponges of 5-Fluorouracil by Using Ultrasound Assisted Method. Iraqi J. Pharm. Sci. 2020, 29, 88–98. [Google Scholar] [CrossRef]
- Azócar, M.I.; Tamayo, L.; Vejar, N.; Gómez, G.; Zhou, X.; Thompsom, G.; Cerda, E.; Kogan, M.J.; Salas, E.; Paez, M.A. A systematic study of antibacterial silver nanoparticles: Efficiency, enhanced permeability, and cytotoxic effects. J. Nanoparticle Res. 2014, 16, 2465. [Google Scholar] [CrossRef]
- Lönnroth, E.-C. Toxicity of Medical Glove Materials: A Pilot Study. Int. J. Occup. Saf. Ergon. 2005, 11, 131–139. [Google Scholar] [CrossRef]
- Ramos, P. Application of Thermal Analysis to Evaluate Pharmaceutical Preparations Containing Theophylline. Pharmaceuticals 2022, 15, 1268. [Google Scholar] [CrossRef]
- Ahari, H.; Lahijani, L.K. Migration of Silver and Copper Nanoparticles from Food Coating. Coatings 2021, 11, 380. [Google Scholar] [CrossRef]
- Sambalova, O.; Thorwarth, K.; Heeb, N.V.; Bleiner, D.; Zhang, Y.; Borgschulte, A.; Kroll, A. Carboxylate Functional Groups Mediate Interaction with Silver Nanoparticles in Biofilm Matrix. ACS Omega 2017, 3, 724–733. [Google Scholar] [CrossRef]

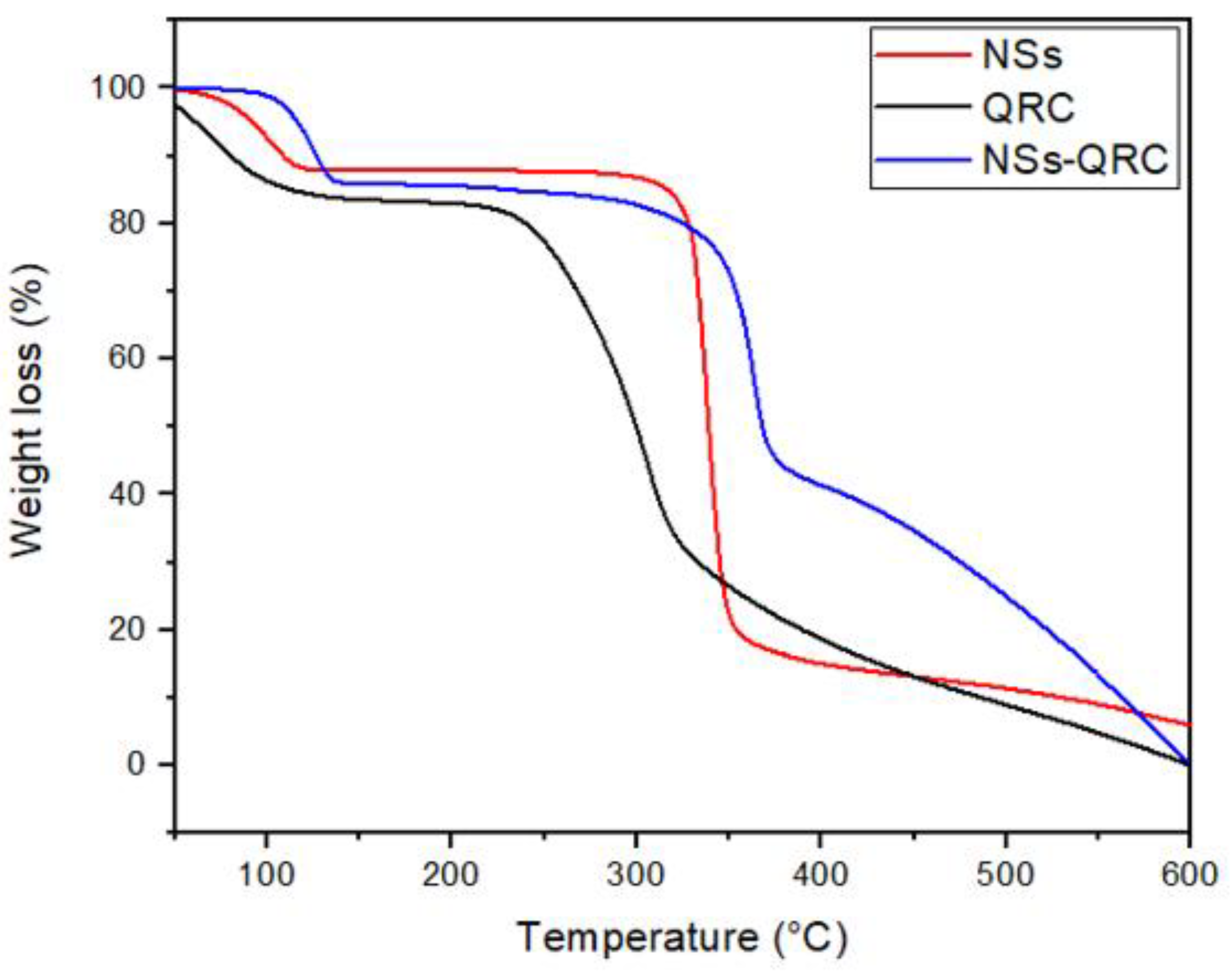


| Sample | Decomposition Temperature (°C) |
|---|---|
| QRC | 157.9–177.3 |
| QRC | 305.3–313.8 |
| NSs | 352.7–353.1 |
| NSs-QRC | 355.9–369.6 |
| Sample | RDC (%) |
|---|---|
| QRC | 83.3 ± 4.7 |
| NSs | 30.1 ± 2.3 |
| NS–QRC | 27.7 ± 5.9 |
| Sample | Encapsulation Efficiency (%) | Loading Capacity (%) |
|---|---|---|
| β–CD–QRC | 60.5 ± 2.2 | 5.77 ± 1.8 |
| NS–QRC | 88.1 ± 3.9 | 17.7 ± 3.5 |
| Sample | H1 | H2 | H3 | H4 | H5 | H6 | OH2 | OH3 | OH6 | |
|---|---|---|---|---|---|---|---|---|---|---|
| NSs | 4.823 | 3.298 | 3.627 | 3.360 | 3.577 | 3.653 | 5.703 | 5.677 | 4.447 | |
| NSs–QRC | 4.820 | 3.295 | 3.609 | 3.355 | 3.560 | 3.640 | 5.710 | 5.690 | 4.440 | |
| Δδ | 0.003 | 0.003 | 0.018 | 0.005 | 0.017 | 0.013 | −0.010 | −0.013 | −0.007 | |
| Sample | H′1 | H′2 | H′3 | H′4 | H′5 | H′6 | H’7 | H’8 | H’9 | H’10 |
| QRC | 9.637 | 9.323 | 7.455 | 6.837 | 7.635 | 9.111 | 6.355 | 12.57 | 6.055 | 10.88 |
| NSs–QRC | 9.622 | 9.310 | 7.445 | 6.822 | 7.622 | 9.101 | 6.340 | 12.44 | 6.044 | 10.75 |
| Δδ | 0.015 | 0.013 | 0.010 | 0.015 | 0.013 | 0.010 | 0.015 | 0.013 | 0.011 | 0.013 |
| System | Dh (nm) | ζ-Potential (mV) | PDI |
|---|---|---|---|
| AgNPs | 31 ± 3 | −33 ± 5 | 0.21 |
| Sample | Dh (nm) | ζ-Potentials (mV) | PDI |
|---|---|---|---|
| NSs | 175 ± 18 | −35 ± 2 | 0.22 |
| NS–QRC | 233 ± 5 | −22 ± 3 | 0.33 |
| AgNP–NS–QRC | 261 ± 11 | −28 ± 5 | 0.48 |
| Concentration (mg/mL) | % Inhibition ± Standard Deviation | ||||
|---|---|---|---|---|---|
| Sample | |||||
| QRC | AgNPs | NS–AgNPs | NS–QRC | AgNP–NS–QRC | |
| 0.4 | 9.5 ± 2.7 | 10.5 ± 3.2 | 4.7 ± 3.1 | 3.9 ± 0.6 | 9.7 ± 1.7 |
| 1.2 | 16.2 ± 1.3 | 14.8 ± 1.8 | 8.5 ± 0.9 | 6.7 ± 2.9 | 15.7 ± 0.9 |
| 2.0 | 20.3 ± 1.2 | 17.4 ± 1.5 | 9.8 ± 0.6 | 39.1 ± 2.2 | 19.0 ± 2.7 |
| 2.8 | 27.5 ± 0.7 | 21.7 ± 3.2 | 15.2 ± 0.5 | 74.2 ± 0.8 | 26.0 ± 1.8 |
| 3.6 | 30.8 ± 0.7 | 26.3 ± 1.6 | 19.1 ± 0.8 | 85.0 ± 0.4 | 36.3 ± 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar Sandoval, S.; Bruna, T.; Maldonado-Bravo, F.; Bolaños, K.; Adasme-Reyes, S.; Riveros, A.; Caro, N.; Yutronic, N.; Silva, N.; Kogan, M.J.; et al. β-Cyclodextrin Nanosponges Inclusion Compounds Associated with Silver Nanoparticles to Increase the Antimicrobial Activity of Quercetin. Materials 2023, 16, 3538. https://doi.org/10.3390/ma16093538
Salazar Sandoval S, Bruna T, Maldonado-Bravo F, Bolaños K, Adasme-Reyes S, Riveros A, Caro N, Yutronic N, Silva N, Kogan MJ, et al. β-Cyclodextrin Nanosponges Inclusion Compounds Associated with Silver Nanoparticles to Increase the Antimicrobial Activity of Quercetin. Materials. 2023; 16(9):3538. https://doi.org/10.3390/ma16093538
Chicago/Turabian StyleSalazar Sandoval, Sebastián, Tamara Bruna, Francisca Maldonado-Bravo, Karen Bolaños, Sofía Adasme-Reyes, Ana Riveros, Nelson Caro, Nicolás Yutronic, Nataly Silva, Marcelo J. Kogan, and et al. 2023. "β-Cyclodextrin Nanosponges Inclusion Compounds Associated with Silver Nanoparticles to Increase the Antimicrobial Activity of Quercetin" Materials 16, no. 9: 3538. https://doi.org/10.3390/ma16093538
APA StyleSalazar Sandoval, S., Bruna, T., Maldonado-Bravo, F., Bolaños, K., Adasme-Reyes, S., Riveros, A., Caro, N., Yutronic, N., Silva, N., Kogan, M. J., & Jara, P. (2023). β-Cyclodextrin Nanosponges Inclusion Compounds Associated with Silver Nanoparticles to Increase the Antimicrobial Activity of Quercetin. Materials, 16(9), 3538. https://doi.org/10.3390/ma16093538







