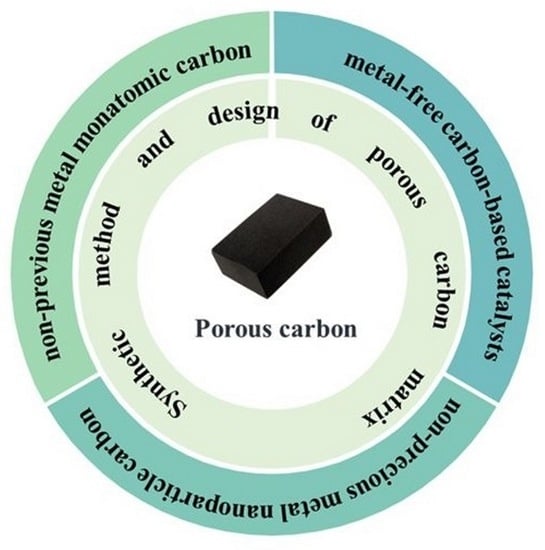
Research Progress on Porous Carbon-Based Non-Precious Metal Electrocatalysts
Abstract
1. Introduction
2. Preparation of Porous Carbon Materials
2.1. Template Method
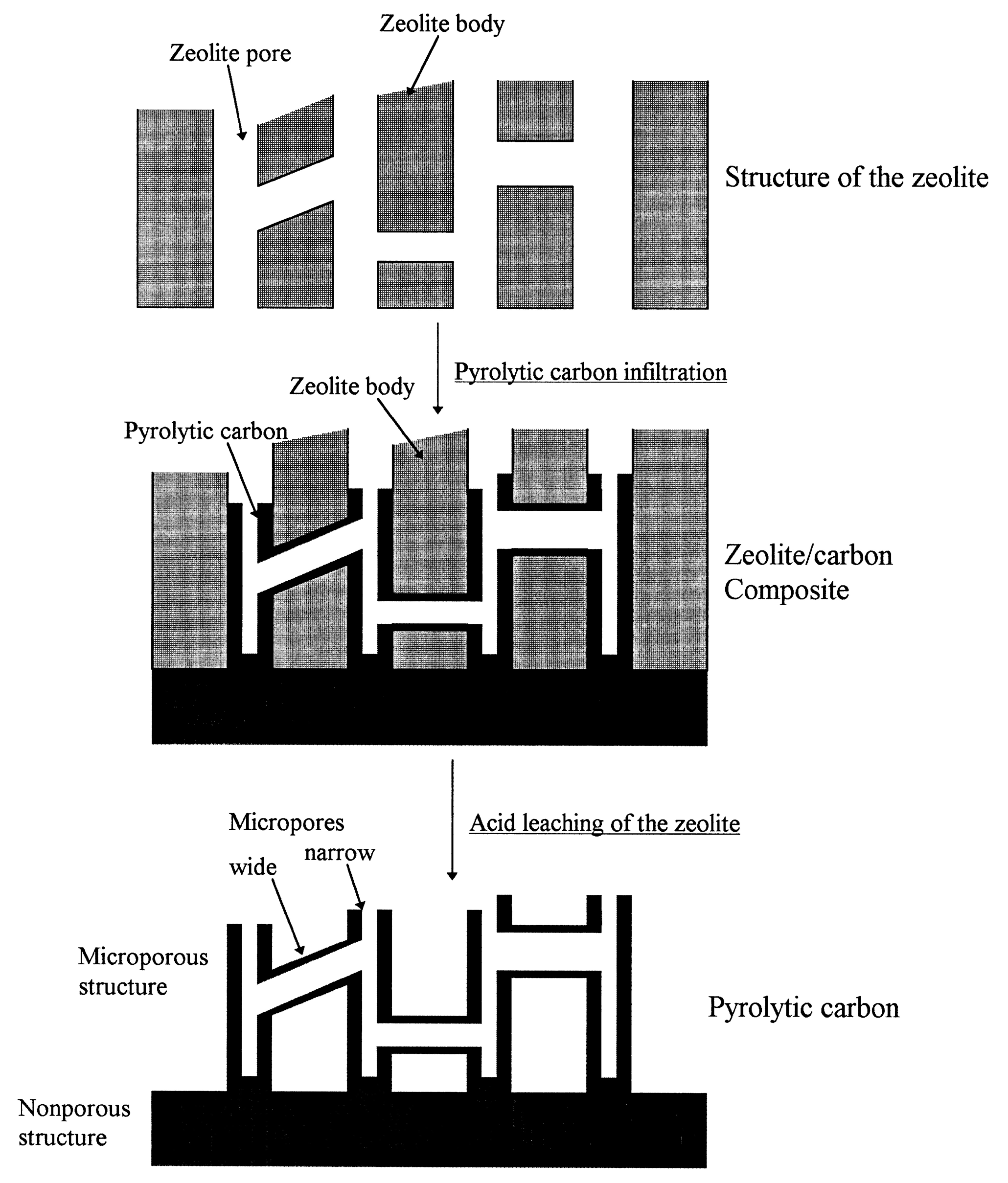
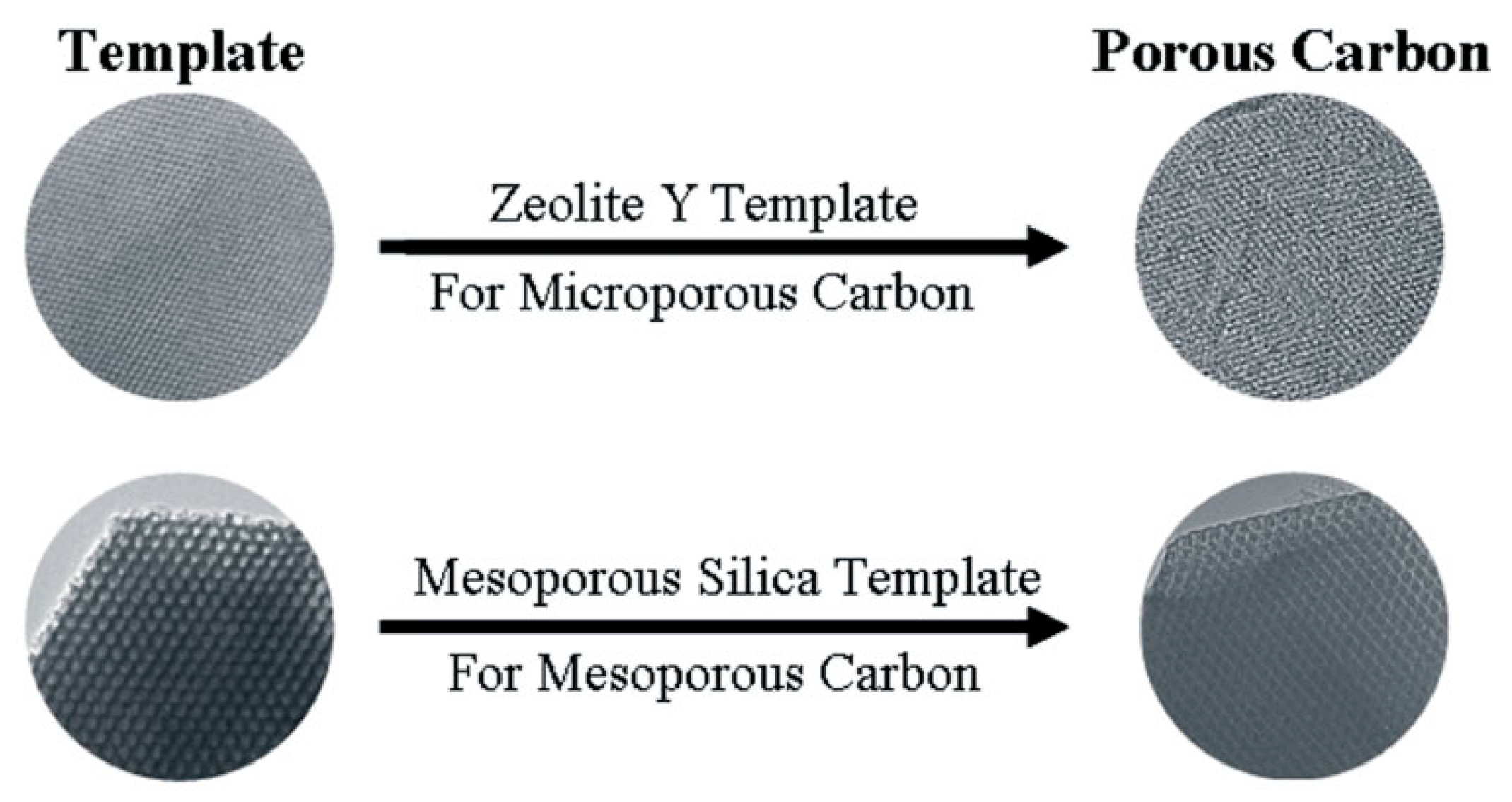
2.1.1. Hard Template Method
2.1.2. Soft Template Method
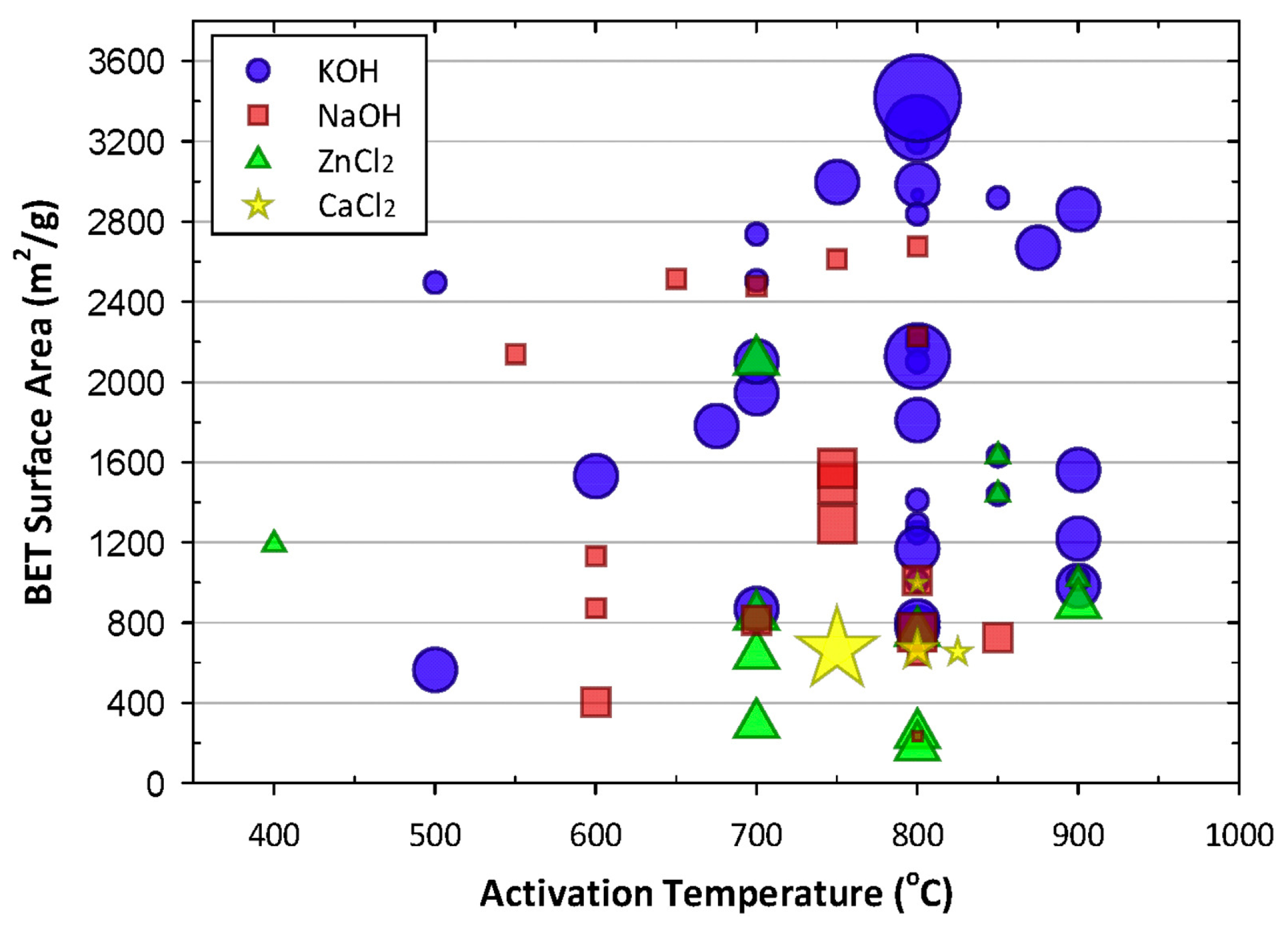
2.1.3. Activation Method
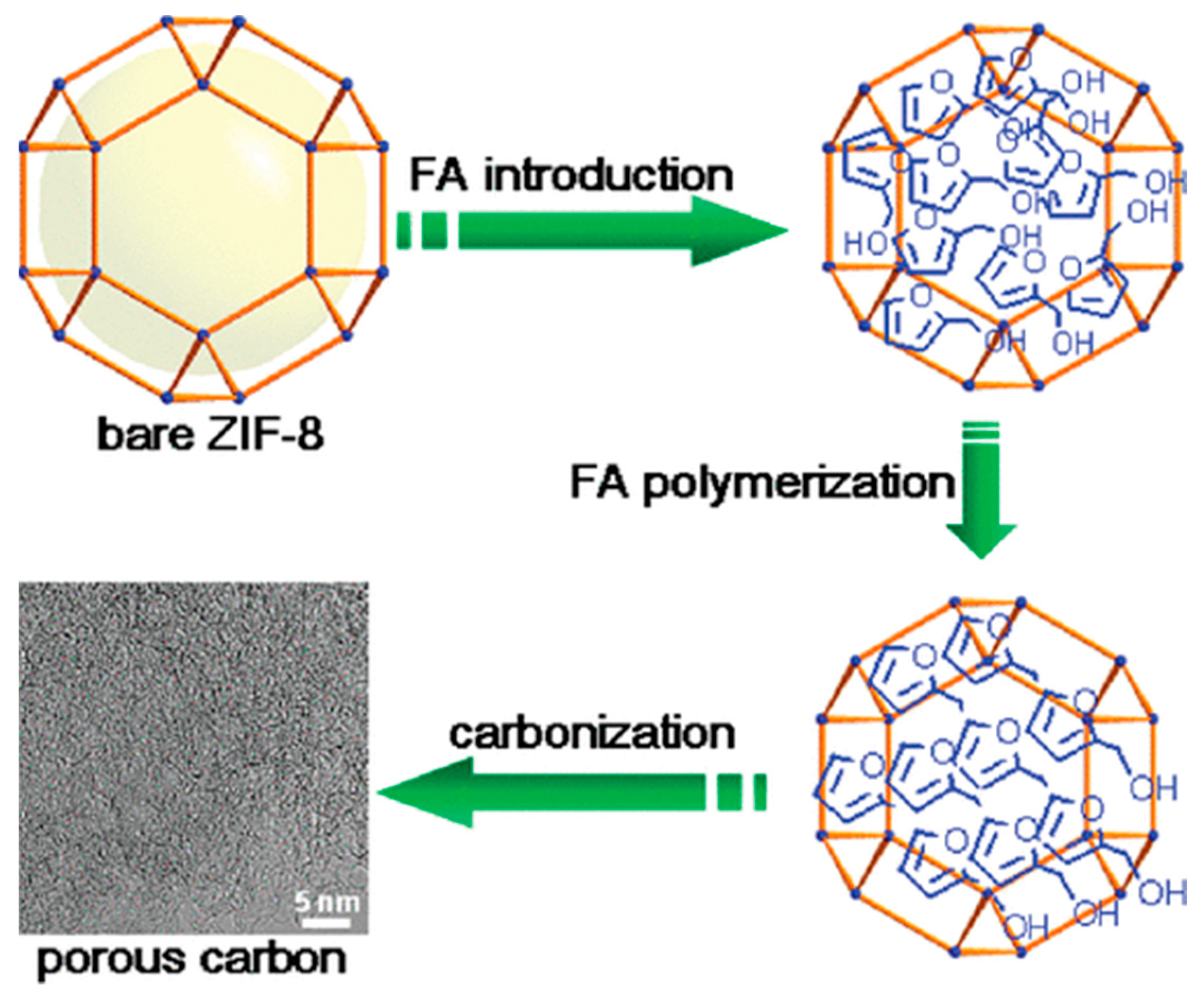
2.1.4. Direct Pyrolysis Method
3. Research Progress of Porous Carbon-Based Non-Precious Metal Electrocatalysts
3.1. Metal-Free Carbon-Based Catalysts
3.2. Non-Precious Metal Nanoparticle Carbon-Based Catalysts
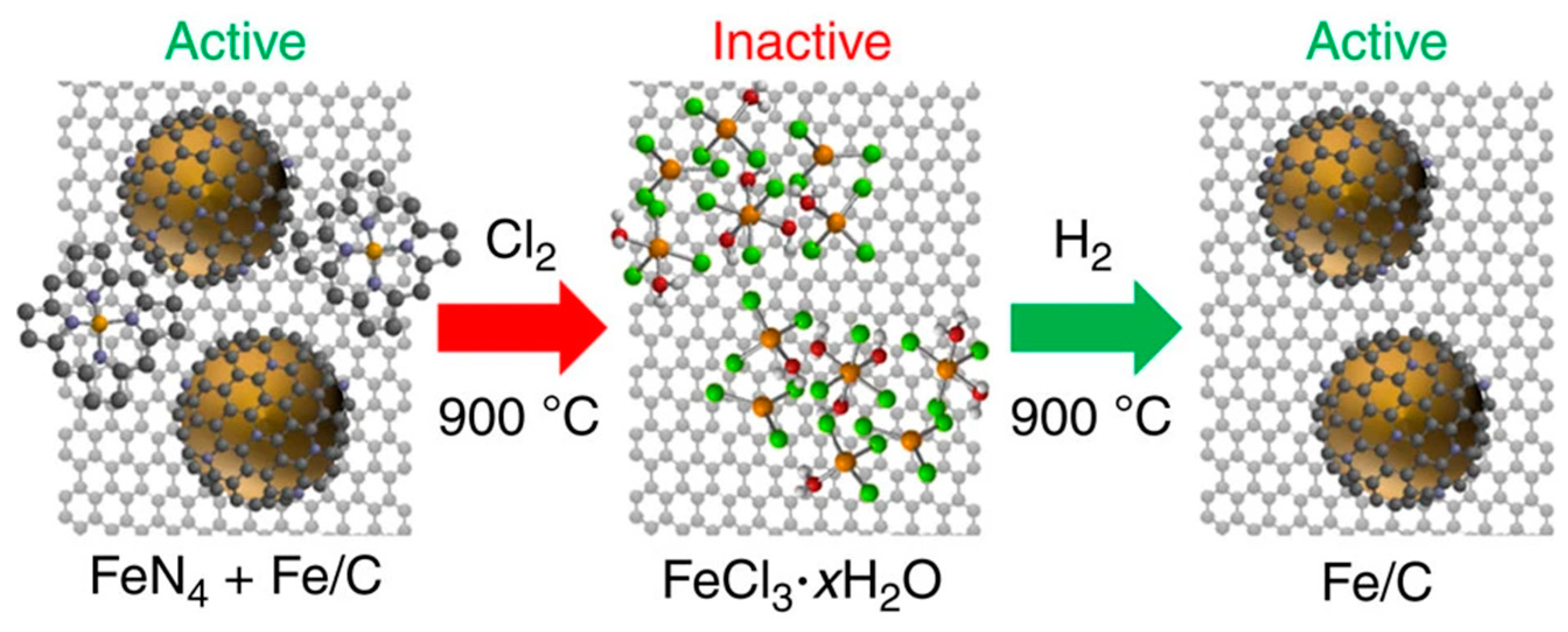
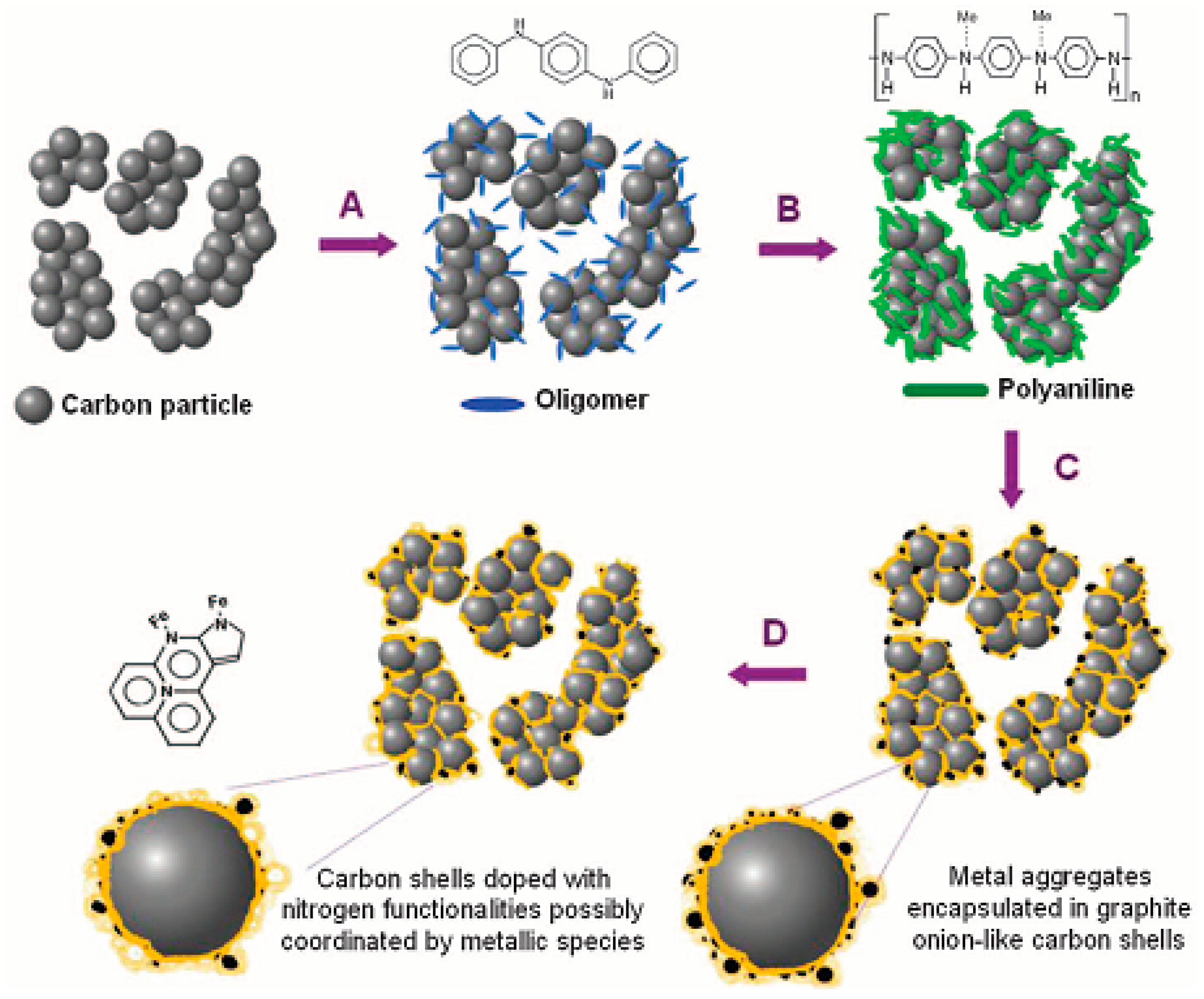
3.2.1. Iron Carbide Nanoparticle/Carbon Composite Electrocatalyst
3.2.2. Fe/Co Nanoparticles/Carbon Composite Electrocatalyst
3.2.3. Transition Metal Phosphide Nanoparticles/Carbon Composite Electrocatalyst
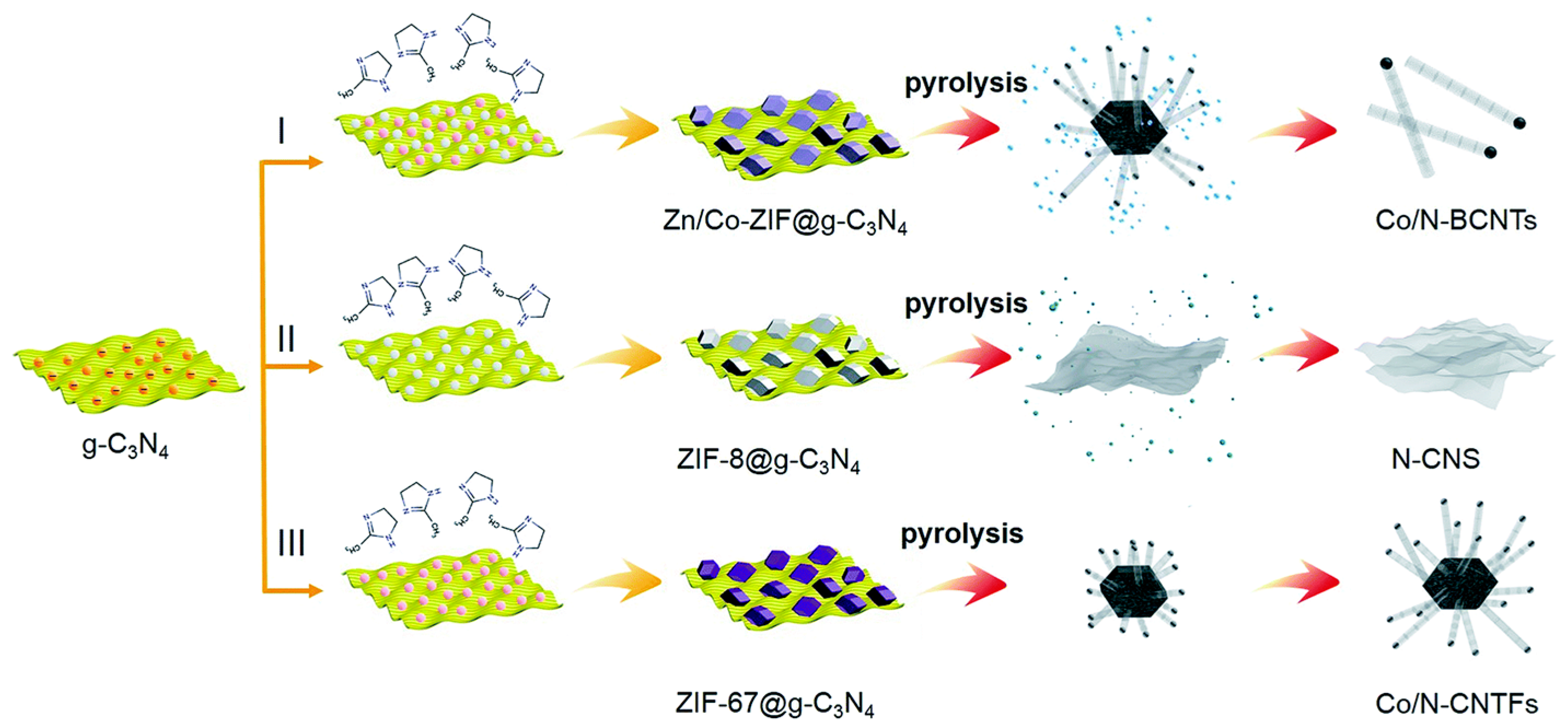
3.3. Non-Previous Metal Porous Carbon-Based Monatomic Catalyst
3.4. Performance of Carbon-Based Non-Precious Metal Electrocatalysts in Fuel Cells
4. Summary and Outlook
- Non-previous metal porous carbon-based monatomic catalyst has an atomic utilization ratio close to 100%, which is a worthy research direction. However, its current performance cannot be compared with that of commonly used Pt/C catalysts. It is still necessary to develop more advanced synthetic methods to improve the stability of non-noble metal carbon-based monatomic catalysts and conduct more in-depth research on their structure-activity relationship to guide the synthesis of catalysts and improve their catalytic activity.
- As an alternative to previous metal-based electrocatalysts, the large-scale production of non-previous metal porous carbon-based catalysts must be investigated. However, as far as the current technology is concerned, the large-scale preparation of non-precious metal electrocatalysts cannot be realized. Finding industrial-scale scale-up synthesis methods while maintaining low cost is the key to the practical application of non-precious metal catalysts. In addition, the uniformity of products should be ensured while mass production, which is a very challenging research direction in the future.
- Porous carbon supports can improve the dispersion of active components and provide channels for material and charge transport. Therefore, carbon support is a key factor in the performance of the catalyst. However, the traditional carbon matrix will be corroded under the battery working conditions and lose the metal-support interaction. Therefore, the improvement of the carbon matrix is an important research direction. The design of porous carbon matrix channels can achieve a more uniform catalyst load, expose more active sites, and promote the mass transfer of reactants, thus, improving catalytic activity.
- Many aspects are still poorly understood, such as the molecular-level description of solvents, cations, and anions near the electrode–electrolyte interface. On the basis of experimental research, using theoretical simulation to optimize the synthesis conditions and deduce the structure-activity relationship will be conducive to a more in-depth understanding.
Funding
Data Availability Statement
Conflicts of Interest
References
- Florides, G.A.; Christodoulides, P. Global warming and carbon dioxide through sciences. Environ. Int. 2009, 35, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Markovic, N.M. Interfacing electrochemistry. Nat. Mater. 2013, 12, 101–102. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [PubMed]
- Badwal, S.P.; Giddey, S.S.; Munnings, C.; Bhatt, A.I.; Hollenkamp, A.F. Emerging electrochemical energy conversion and storage technologies. Front. Chem. 2014, 2, 79. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Shao-Horn, Y.; Sheng, W.; Chen, S.; Ferreira, P.J.; Holby, E.; Morgan, D. Instability of supported platinum nanoparticles in low-temperature fuel cells. Top. Catal. 2007, 46, 285–305. [Google Scholar] [CrossRef]
- Roth, C.; Shao-Horn, Y.; Myers, D.; Inukai, J. Batteries and Fuel Cells: Leading the Way to a Cleaner and Brighter Future; Wiley Online Library: Hoboken, NJ, USA, 2015; Volume 2, pp. 1408–1409. [Google Scholar]
- Su, H.; Chen, T.-H. Preparation of PtSn2–SnO2/C nanocatalyst and its high performance for methanol electro-oxidation. Chin. Chem. Lett. 2016, 27, 1083–1086. [Google Scholar] [CrossRef]
- Maillard, F.; Schreier, S.; Hanzlik, M.; Savinova, E.R.; Weinkauf, S.; Stimming, U. Influence of particle agglomeration on the catalytic activity of carbon-supported Pt nanoparticles in CO monolayer oxidation. Phys. Chem. Chem. Phys. 2005, 7, 385–393. [Google Scholar] [CrossRef]
- Shao, Y.; Yin, G.; Gao, Y. Understanding and approaches for the durability issues of Pt-based catalysts for PEM fuel cell. J. Power Sources 2007, 171, 558–566. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, B. Recent advances in porous Pt-based nanostructures: Synthesis and electrochemical applications. Chem. Soc. Rev. 2014, 43, 2439–2450. [Google Scholar] [CrossRef]
- Yamada, Y.; Miyamoto, K.; Hayashi, T.; Iijima, Y.; Todoroki, N.; Wadayama, T. Oxygen reduction reaction activities for Pt-enriched Co/Pt (111), Co/Pt (100), and Co/Pt (110) model catalyst surfaces prepared by molecular beam epitaxy. Surf. Sci. 2013, 607, 54–60. [Google Scholar] [CrossRef]
- Hussain, S.; Erikson, H.; Kongi, N.; Sarapuu, A.; Solla-Gullón, J.; Maia, G.; Kannan, A.M.; Alonso-Vante, N.; Tammeveski, K. Oxygen reduction reaction on nanostructured Pt-based electrocatalysts: A review. Int. J. Hydrog. Energ. 2020, 45, 31775–31797. [Google Scholar] [CrossRef]
- Guo, Y.G.; Hu, J.S.; Wan, L.J. Nanostructured materials for electrochemical energy conversion and storage devices. Adv. Mater. 2008, 20, 2878–2887. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Mun, B.S.; Mayrhofer, K.J.; Ross, P.N.; Markovic, N.M. Effect of surface composition on electronic structure, stability, and electrocatalytic properties of Pt-transition metal alloys: Pt-skin versus Pt-skeleton surfaces. J. Am. Chem. Soc. 2006, 128, 8813–8819. [Google Scholar] [CrossRef]
- Stephens, I.E.; Bondarenko, A.S.; Perez-Alonso, F.J.; Calle-Vallejo, F.; Bech, L.; Johansson, T.P.; Jepsen, A.K.; Frydendal, R.; Knudsen, B.P.; Rossmeisl, J. Tuning the activity of Pt (111) for oxygen electroreduction by subsurface alloying. J. Am. Chem. Soc. 2011, 133, 5485–5491. [Google Scholar] [CrossRef]
- Paul, S. Hydrogenation and Dehydrogenation by Catalysis. Ber. Dtsch. Chem. Ges. 1911, 44, 17. [Google Scholar]
- Jayasayee, K.; Van Veen, J.R.; Manivasagam, T.G.; Celebi, S.; Hensen, E.J.; De Bruijn, F.A. Oxygen reduction reaction (ORR) activity and durability of carbon supported PtM (Co, Ni, Cu) alloys: Influence of particle size and non-noble metals. Appl. Catal. B Environ. 2012, 111, 515–526. [Google Scholar] [CrossRef]
- Zhang, J.; Vukmirovic, M.B.; Xu, Y.; Mavrikakis, M.; Adzic, R.R. Controlling the catalytic activity of platinum-monolayer electrocatalysts for oxygen reduction with different substrates. Angew. Chem. 2005, 117, 2170–2173. [Google Scholar] [CrossRef]
- Jiang, W.-J.; Tang, T.; Zhang, Y.; Hu, J.-S. Synergistic modulation of non-precious-metal electrocatalysts for advanced water splitting. Acc. Chem. Res. 2020, 53, 1111–1123. [Google Scholar] [CrossRef]
- Zhao, D.; Shui, J.L.; Grabstanowicz, L.R.; Chen, C.; Commet, S.M.; Xu, T.; Lu, J.; Liu, D.J. Highly efficient non-precious metal electrocatalysts prepared from one-pot synthesized zeolitic imidazolate frameworks. Adv. Mater. 2014, 26, 1093–1097. [Google Scholar] [CrossRef]
- Chen, Z.; Higgins, D.; Yu, A.; Zhang, L.; Zhang, J. A review on non-precious metal electrocatalysts for PEM fuel cells. Energ. Environ. Sci. 2011, 4, 3167–3192. [Google Scholar] [CrossRef]
- Gao, J.; Tao, H.; Liu, B. Progress of nonprecious-metal-based electrocatalysts for oxygen evolution in acidic media. Adv. Mater. 2021, 33, 2003786. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, A.S.; Varghese, A.; Nidhin, M. Transition metal oxides in electrochemical and bio sensing: A state-of-art review. Appl. Surf. Sci. Adv. 2021, 4, 100072. [Google Scholar] [CrossRef]
- Liu, K.; Kattel, S.; Mao, V.; Wang, G. Electrochemical and computational study of oxygen reduction reaction on nonprecious transition metal/nitrogen doped carbon nanofibers in acid medium. J. Phys. Chem. C 2016, 120, 1586–1596. [Google Scholar] [CrossRef]
- Dai, L. Carbon-based catalysts for metal-free electrocatalysis. Curr. Opin. Electrochem. 2017, 4, 18–25. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, L.; Li, R.; Qu, K.; Wang, L.; Kang, W.; Li, H.; Xiong, S. Electrocatalytic oxygen reduction of COF-derived porous Fe-Nx nanoclusters/carbon catalyst and application for high performance Zn-air battery. Microporous Mesoporous Mater. 2022, 330, 111609. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Wang, X.; Zheng, B.; Zhang, W.; Chen, Y. Scalable synthesis of porous hollow CoSe2–MoSe2/carbon microspheres for highly efficient hydrogen evolution reaction in acidic and alkaline media. J. Mater. Chem. A 2018, 6, 12701–12707. [Google Scholar] [CrossRef]
- Yang, X.; Chen, C.; Zhou, Z.; Sun, S. Advances in active site structure of carbon-based non-precious metal catalysts for oxygen reduction reaction. Acta Phys. Chim. Sin. 2019, 35, 472–485. [Google Scholar] [CrossRef]
- Cheng, N.; Ren, L.; Xu, X.; Du, Y.; Dou, S.X. Recent development of zeolitic imidazolate frameworks (ZIFs) derived porous carbon based materials as electrocatalysts. Adv. Energy Mater. 2018, 8, 1801257. [Google Scholar] [CrossRef]
- Zhu, C.; Li, H.; Fu, S.; Du, D.; Lin, Y. Highly efficient nonprecious metal catalysts towards oxygen reduction reaction based on three-dimensional porous carbon nanostructures. Chem. Soc. Rev. 2016, 45, 517–531. [Google Scholar] [CrossRef]
- Lu, Q.; Eid, K.; Li, W. Heteroatom-Doped Porous Carbon-Based Nanostructures for Electrochemical CO2 Reduction. Nanomaterials 2022, 12, 2379. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, S.; Zhu, P.; Zhao, X.; Wang, G. Fe–N4 engineering of S and N co-doped hierarchical porous carbon-based electrocatalysts for enhanced oxygen reduction in Zn–air batteries. Dalton Trans. 2020, 49, 14847–14853. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Mahmood, A.; Liang, Z.; Zou, R.; Guo, S. Earth-abundant nanomaterials for oxygen reduction. Angew. Chem. Int. Ed. 2016, 55, 2650–2676. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.-F.; Li, W.; Bhardwaj, A.; Nuguri, S.; Ribbe, A.; Watkins, J.J. Ordered nanoporous carbons with broadly tunable pore size using bottlebrush block copolymer templates. J. Am. Chem. Soc. 2019, 141, 17006–17014. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Santiago, A.R.P.; Sanad, M.F.; Weller, J.M.; Fernandez-Delgado, O.; Barrera, L.A.; Maturano-Rojas, V.; Alvarado-Tenorio, B.; Chan, C.K.; Noveron, J.C. Tissue paper-derived porous carbon encapsulated transition metal nanoparticles as advanced non-precious catalysts: Carbon-shell influence on the electrocatalytic behaviour. J. Colloid. Interf. Sci. 2021, 581, 905–918. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, Y.; Jiang, C.; Lu, L. Structural effects of a carbon matrix in non-precious metal O2-reduction electrocatalysts. Chem. Soc. Rev. 2016, 45, 2396–2409. [Google Scholar] [CrossRef]
- Tao, L.; Qiao, M.; Jin, R.; Li, Y.; Xiao, Z.; Wang, Y.; Zhang, N.; Xie, C.; He, Q.; Jiang, D. Bridging the surface charge and catalytic activity of a defective carbon electrocatalyst. Angew. Chem. Int. Ed. 2019, 58, 1019–1024. [Google Scholar] [CrossRef]
- Oh, S.; Kim, K. Synthesis of a new mesoporous carbon and its application to electrochemical double-layer capacitors. Chem. Commun. 1999, 21, 2177–2178. [Google Scholar]
- Yamauchi, Y.; Kuroda, K. Rational design of mesoporous metals and related nanomaterials by a soft-template approach. Chem. Asian J. 2008, 3, 664–676. [Google Scholar] [CrossRef]
- Zhou, J.; Li, W.; Zhang, Z.; Xing, W.; Zhuo, S. Carbon dioxide adsorption performance of N-doped zeolite Y templated carbons. RSC Adv. 2012, 2, 161–167. [Google Scholar] [CrossRef]
- Xia, Y.; Mokaya, R.; Grant, D.M.; Walker, G.S. A simplified synthesis of N-doped zeolite-templated carbons, the control of the level of zeolite-like ordering and its effect on hydrogen storage properties. Carbon 2011, 49, 844–853. [Google Scholar] [CrossRef]
- Rodriguez-Mirasol, J.; Cordero, T.; Radovic, L.R.; Rodriguez, J.J. Structural and textural properties of pyrolytic carbon formed within a microporous zeolite template. Chem. Mater. 1998, 10, 550–558. [Google Scholar] [CrossRef]
- Nueangnoraj, K.; Tomai, T.; Nishihara, H.; Kyotani, T.; Honma, I. An organic proton battery employing two redox-active quinones trapped within the nanochannels of zeolite-templated carbon. Carbon 2016, 107, 831–836. [Google Scholar] [CrossRef]
- Lu, H.; Kim, K.; Kwon, Y.; Sun, X.; Ryoo, R.; Zhao, X. Zeolite-templated nanoporous carbon for high-performance supercapacitors. J. Mater. Chem. A 2018, 6, 10388–10394. [Google Scholar] [CrossRef]
- Kyotani, T.; Ma, Z.; Tomita, A. Template synthesis of novel porous carbons using various types of zeolites. Carbon 2003, 41, 1451–1459. [Google Scholar] [CrossRef]
- Lee, J.; Jin, S.; Hwang, Y.; Park, J.-G.; Park, H.M.; Hyeon, T. Simple synthesis of mesoporous carbon with magnetic nanoparticles embedded in carbon rods. Carbon 2005, 43, 2536–2543. [Google Scholar] [CrossRef]
- Liu, N.; Yin, L.; Wang, C.; Zhang, L.; Lun, N.; Xiang, D.; Qi, Y.; Gao, R. Adjusting the texture and nitrogen content of ordered mesoporous nitrogen-doped carbon materials prepared using SBA-15 silica as a template. Carbon 2010, 48, 3579–3591. [Google Scholar] [CrossRef]
- Janus, R.; Natkański, P.; Wądrzyk, M.; Lewandowski, M.; Michalik, M.; Kuśtrowski, P. Deposition of poly (furfuryl alcohol) in mesoporous silica template controlled by solvent polarity: A cornerstone of facile and versatile synthesis of high-quality CMK-type carbon replicas. Nanocasting of SBA-15, SBA-16, and KIT-6. Carbon 2022, 195, 292–307. [Google Scholar] [CrossRef]
- Gierszal, K.P.; Kim, T.-W.; Ryoo, R.; Jaroniec, M. Adsorption and Structural Properties of Ordered Mesoporous Carbons Synthesized by Using Various Carbon Precursors and Ordered Siliceous P6mm and Ia3d Mesostructures as Templates. J. Phys. Chem. B 2005, 109, 23263–23268. [Google Scholar] [CrossRef]
- Koyuncu, D.D.E.; Okur, M. Removal of AV 90 dye using ordered mesoporous carbon materials prepared via nanocasting of KIT-6: Adsorption isotherms, kinetics and thermodynamic analysis. Sep. Purif. Technol. 2021, 257, 117657. [Google Scholar] [CrossRef]
- Zakhidov, A.A.; Baughman, R.H.; Iqbal, Z.; Cui, C.; Khayrullin, I.; Dantas, S.O.; Marti, J.; Ralchenko, V.G. Carbon structures with three-dimensional periodicity at optical wavelengths. Science 1998, 282, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, R.; Joo, S.H.; Jun, S. Synthesis of highly ordered carbon molecular sieves via template-mediated structural transformation. J. Phys. Chem. B 1999, 103, 7743–7746. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Wu, D.; Nese, A.; Pietrasik, J.; Liang, Y.; He, H.; Kruk, M.; Huang, L.; Kowalewski, T.; Matyjaszewski, K. Preparation of polymeric nanoscale networks from cylindrical molecular bottlebrushes. ACS Nano 2012, 6, 6208–6214. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Kobayashi, S.; Kojin, F.; Tanaka, N.; Morishita, T.; Tryba, B. Pore structure of carbons coated on ceramic particles. Carbon 2004, 42, 3153–3158. [Google Scholar] [CrossRef]
- Yu, S.; Wang, H.; Hu, C.; Zhu, Q.; Qiao, N.; Xu, B. Facile synthesis of nitrogen-doped, hierarchical porous carbons with a high surface area: The activation effect of a nano-ZnO template. J. Mater. Chem. A 2016, 4, 16341–16348. [Google Scholar] [CrossRef]
- Przepiórski, J.; Karolczyk, J.; Tsumura, T.; Toyoda, M.; Inagaki, M.; Morawski, A.W. Effect of some thermally unstable magnesium compounds on the yield of char formed from poly (ethylene terephthalate). J. Therm. Anal. Calorim. 2012, 107, 1147–1154. [Google Scholar] [CrossRef]
- Li, X.-H.; Zhang, J.; Chen, X.; Fischer, A.; Thomas, A.; Antonietti, M.; Wang, X. Condensed graphitic carbon nitride nanorods by nanoconfinement: Promotion of crystallinity on photocatalytic conversion. Chem. Mater. 2011, 23, 4344–4348. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Hyeon, T. Recent progress in the synthesis of porous carbon materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Zhao, D. Mesoporous materials for energy conversion and storage devices. Nat. Rev. Mater. 2016, 1, 16023. [Google Scholar] [CrossRef]
- Chuenchom, L.; Kraehnert, R.; Smarsly, B.M. Recent progress in soft-templating of porous carbon materials. Soft Matter 2012, 8, 10801–10812. [Google Scholar] [CrossRef]
- Moriguchi, I.; Ozono, A.; Mikuriya, K.; Teraoka, Y.; Kagawa, S.; Kodama, M. Micelle-templated mesophases of phenol-formaldehyde polymer. Chem. Lett. 1999, 1999, 1171–1172. [Google Scholar] [CrossRef]
- Zheng, T.; Nishiyama, N.; Egashira, Y.; Ueyama, K. Ionic surfactant-mediated synthesis of Pt nanoparticles/nanoporous carbons composites. Colloids Surf. A Physicochem. Eng. Asp. 2005, 262, 52–56. [Google Scholar] [CrossRef]
- Wan, Y.; Shi, Y.; Zhao, D. Supramolecular aggregates as templates: Ordered mesoporous polymers and carbons. Chem. Mater. 2008, 20, 932–945. [Google Scholar] [CrossRef]
- Pérez-Mayoral, E.; Matos, I.; Bernardo, M.; Fonseca, I.M. New and advanced porous carbon materials in fine chemical synthesis. Emerging precursors of porous carbons. Catalysts 2019, 9, 133. [Google Scholar] [CrossRef]
- Meng, Y.; Gu, D.; Zhang, F.; Shi, Y.; Cheng, L.; Feng, D.; Wu, Z.; Chen, Z.; Wan, Y.; Stein, A. A family of highly ordered mesoporous polymer resin and carbon structures from organic−organic self-assembly. Chem. Mater. 2006, 18, 4447–4464. [Google Scholar] [CrossRef]
- Peng, L.; Hung, C.-T.; Wang, S.; Zhang, X.; Zhu, X.; Zhao, Z.; Wang, C.; Tang, Y.; Li, W.; Zhao, D. Versatile nanoemulsion assembly approach to synthesize functional mesoporous carbon nanospheres with tunable pore sizes and architectures. J. Am. Chem. Soc. 2019, 141, 7073–7080. [Google Scholar] [CrossRef]
- Lua, A.C.; Guo, J. Activated carbon prepared from oil palm stone by one-step CO2 activation for gaseous pollutant removal. Carbon 2000, 38, 1089–1097. [Google Scholar] [CrossRef]
- Kim, D.-W.; Kil, H.-S.; Nakabayashi, K.; Yoon, S.-H.; Miyawaki, J. Structural elucidation of physical and chemical activation mechanisms based on the microdomain structure model. Carbon 2017, 114, 98–105. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Amirkhiz, B.S.; Tan, X.; Xu, Z.; Wang, H.; Olsen, B.C.; Holt, C.M.; Mitlin, D. Carbonized chicken eggshell membranes with 3D architectures as high-performance electrode materials for supercapacitors. Adv. Energy Mater. 2012, 2, 431–437. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, H.M. High surface area microporous activated carbons prepared from Fox nut (Euryale ferox) shell by zinc chloride activation. Appl. Surf. Sci. 2015, 356, 753–761. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Suarez-Garcia, F.; Martinez-Alonso, A.; Tascón, J.M. Influence of porous texture and surface chemistry on the CO2 adsorption capacity of porous carbons: Acidic and basic site interactions. ACS Appl. Mater. Inter. 2014, 6, 21237–21247. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.L.; Li, G.C.; Fechler, N.; Yang, N.; Ma, Z.Y.; Wang, X.; Antonietti, M.; Yu, S.H. Polymerization under hypersaline conditions: A robust route to phenolic polymer-derived carbon aerogels. Angew. Chem. 2016, 128, 14843–14847. [Google Scholar] [CrossRef]
- Gao, M.; Pan, S.-Y.; Chen, W.-C.; Chiang, P.-C. A cross-disciplinary overview of naturally derived materials for electrochemical energy storage. Mater. Today Energy 2018, 7, 58–79. [Google Scholar] [CrossRef]
- Li, B.; Dai, F.; Xiao, Q.; Yang, L.; Shen, J.; Zhang, C.; Cai, M. Nitrogen-doped activated carbon for a high energy hybrid supercapacitor. Energ. Environ. Sci. 2016, 9, 102–106. [Google Scholar] [CrossRef]
- Estevez, L.; Dua, R.; Bhandari, N.; Ramanujapuram, A.; Wang, P.; Giannelis, E.P. A facile approach for the synthesis of monolithic hierarchical porous carbons–high performance materials for amine based CO2 capture and supercapacitor electrode. Energ. Environ. Sci. 2013, 6, 1785–1790. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Li, C.; Liang, H.W.; Chen, J.F.; Yu, S.H. Ultralight, flexible, and fire-resistant carbon nanofiber aerogels from bacterial cellulose. Angew. Chem. 2013, 125, 2997–3001. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Liu, B.; Lan, Y.-Q.; Kuratani, K.; Akita, T.; Shioyama, H.; Zong, F.; Xu, Q. From metal–organic framework to nanoporous carbon: Toward a very high surface area and hydrogen uptake. J. Am. Chem. Soc. 2011, 133, 11854–11857. [Google Scholar] [CrossRef]
- Lu, C.; Wang, D.; Zhao, J.; Han, S.; Chen, W. A continuous carbon nitride polyhedron assembly for high-performance flexible supercapacitors. Adv. Funct. Mater. 2017, 27, 1606219. [Google Scholar] [CrossRef]
- Ye, L.; Ying, Y.; Sun, D.; Zhang, Z.; Fei, L.; Wen, Z.; Qiao, J.; Huang, H. Highly efficient porous carbon electrocatalyst with controllable N-species content for selective CO2 reduction. Angew. Chem. Int. Ed. 2020, 59, 3244–3251. [Google Scholar] [CrossRef]
- Duan, W.; Li, G.; Lei, Z.; Zhu, T.; Xue, Y.; Wei, C.; Feng, C. Highly active and durable carbon electrocatalyst for nitrate reduction reaction. Water Res. 2019, 161, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dai, L. Carbon-based metal-free catalysts. Nat. Rev. Mater. 2016, 1, 16064. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, X.; Wu, M.; Li, Q.; Wang, Y.; Yao, J. Metal-free fluorine-doped carbon electrocatalyst for CO2 reduction outcompeting hydrogen evolution. Angew. Chem. Int. Ed. 2018, 57, 9640–9644. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Muthukrishnan, A.; Nagata, S.; Nabae, Y. Kinetic understanding of the reduction of oxygen to hydrogen peroxide over an N-doped carbon electrocatalyst. J. Phys. Chem. C 2019, 123, 4590–4596. [Google Scholar] [CrossRef]
- Kong, F.; Cui, X.; Huang, Y.; Yao, H.; Chen, Y.; Tian, H.; Meng, G.; Chen, C.; Chang, Z.; Shi, J. N-Doped Carbon Electrocatalyst: Marked ORR Activity in Acidic Media without the Contribution from Metal Sites? Angew. Chem. Int. Ed. 2022, 61, e202116290. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Parvez, K.; Yang, S.; Hernandez, Y.; Winter, A.; Turchanin, A.; Feng, X.; Müllen, K. Nitrogen-doped graphene and its iron-based composite as efficient electrocatalysts for oxygen reduction reaction. ACS Nano 2012, 6, 9541–9550. [Google Scholar] [CrossRef]
- Zhou, T.; Zhou, Y.; Ma, R.; Zhou, Z.; Liu, G.; Liu, Q.; Zhu, Y.; Wang, J. Nitrogen-doped hollow mesoporous carbon spheres as a highly active and stable metal-free electrocatalyst for oxygen reduction. Carbon 2017, 114, 177–186. [Google Scholar] [CrossRef]
- Zhao, Y.; Nakamura, R.; Kamiya, K.; Nakanishi, S.; Hashimoto, K. Nitrogen-doped carbon nanomaterials as non-metal electrocatalysts for water oxidation. Nat. Commun. 2013, 4, 2390. [Google Scholar] [CrossRef]
- Qiao, M.; Tang, C.; He, G.; Qiu, K.; Binions, R.; Parkin, I.; Zhang, Q.; Guo, Z.; Titirici, M. Graphene/nitrogen-doped porous carbon sandwiches for the metal-free oxygen reduction reaction: Conductivity versus active sites. J. Mater. Chem. A 2016, 4, 12658–12666. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, G.; Jin, S.; Zhou, Y.; Ji, Q.; Lan, H.; Liu, H.; Qu, J. Graphitic N in nitrogen-doped carbon promotes hydrogen peroxide synthesis from electrocatalytic oxygen reduction. Carbon 2020, 163, 154–161. [Google Scholar] [CrossRef]
- Zhou, X.; Bai, Z.; Wu, M.; Qiao, J.; Chen, Z. 3-Dimensional porous N-doped graphene foam as a non-precious catalyst for the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 3343–3350. [Google Scholar] [CrossRef]
- Chen, P.; Wang, L.-K.; Wang, G.; Gao, M.-R.; Ge, J.; Yuan, W.-J.; Shen, Y.-H.; Xie, A.-J.; Yu, S.-H. Nitrogen-doped nanoporous carbon nanosheets derived from plant biomass: An efficient catalyst for oxygen reduction reaction. Energ. Environ. Sci. 2014, 7, 4095–4103. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Preuß, K.; Qiao, M.; Titirici, M.-M.; Varcoe, J.; Cai, Q. Halloysite-derived nitrogen doped carbon electrocatalysts for anion exchange membrane fuel cells. J. Power Sources 2017, 372, 82–90. [Google Scholar] [CrossRef]
- Pampel, J.; Fellinger, T.P. Opening of bottleneck pores for the improvement of nitrogen doped carbon electrocatalysts. Adv. Energy Mater. 2016, 6, 1502389. [Google Scholar] [CrossRef]
- Liang, H.-W.; Wu, Z.-Y.; Chen, L.-F.; Li, C.; Yu, S.-H. Bacterial cellulose derived nitrogen-doped carbon nanofiber aerogel: An efficient metal-free oxygen reduction electrocatalyst for zinc-air battery. Nano Energy 2015, 11, 366–376. [Google Scholar] [CrossRef]
- Ito, Y.; Christodoulou, C.; Nardi, M.V.; Koch, N.; Sachdev, H.; Mullen, K. Chemical vapor deposition of N-doped graphene and carbon films: The role of precursors and gas phase. ACS Nano 2014, 8, 3337–3346. [Google Scholar] [CrossRef]
- Zabet-Khosousi, A.; Zhao, L.; Pálová, L.; Hybertsen, M.S.; Reichman, D.R.; Pasupathy, A.N.; Flynn, G.W. Segregation of sublattice domains in nitrogen-doped graphene. J. Am. Chem. Soc. 2014, 136, 1391–1397. [Google Scholar] [CrossRef]
- Deng, D.; Pan, X.; Yu, L.; Cui, Y.; Jiang, Y.; Qi, J.; Li, W.-X.; Fu, Q.; Ma, X.; Xue, Q. Toward N-doped graphene via solvothermal synthesis. Chem. Mater. 2011, 23, 1188–1193. [Google Scholar] [CrossRef]
- Pham, T.V.; Kim, J.G.; Jung, J.Y.; Kim, J.H.; Cho, H.; Seo, T.H.; Lee, H.; Kim, N.D.; Kim, M.J. High areal capacitance of N-doped graphene synthesized by arc discharge. Adv. Funct. Mater. 2019, 29, 1905511. [Google Scholar] [CrossRef]
- Geng, D.; Chen, Y.; Chen, Y.; Li, Y.; Li, R.; Sun, X.; Ye, S.; Knights, S. High oxygen-reduction activity and durability of nitrogen-doped graphene. Energ. Environ. Sci. 2011, 4, 760–764. [Google Scholar] [CrossRef]
- Rybin, M.; Pereyaslavtsev, A.; Vasilieva, T.; Myasnikov, V.; Sokolov, I.; Pavlova, A.; Obraztsova, E.; Khomich, A.; Ralchenko, V.; Obraztsova, E. Efficient nitrogen doping of graphene by plasma treatment. Carbon 2016, 96, 196–202. [Google Scholar] [CrossRef]
- Park, S.; Hu, Y.; Hwang, J.O.; Lee, E.-S.; Casabianca, L.B.; Cai, W.; Potts, J.R.; Ha, H.-W.; Chen, S.; Oh, J. Chemical structures of hydrazine-treated graphene oxide and generation of aromatic nitrogen doping. Nat. Commun. 2012, 3, 638. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef]
- Yang, H.B.; Miao, J.; Hung, S.-F.; Chen, J.; Tao, H.B.; Wang, X.; Zhang, L.; Chen, R.; Gao, J.; Chen, H.M. Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: Development of highly efficient metal-free bifunctional electrocatalyst. Sci. Adv. 2016, 2, e1501122. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Du, A.; Jaroniec, M.; Qiao, S.Z. Hydrogen evolution by a metal-free electrocatalyst. Nat. Commun. 2014, 5, 3783. [Google Scholar] [CrossRef]
- Sheng, Z.-H.; Shao, L.; Chen, J.-J.; Bao, W.-J.; Wang, F.-B.; Xia, X.-H. Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef]
- Jiang, H.; Gu, J.; Zheng, X.; Liu, M.; Qiu, X.; Wang, L.; Li, W.; Chen, Z.; Ji, X.; Li, J. Defect-rich and ultrathin N doped carbon nanosheets as advanced trifunctional metal-free electrocatalysts for the ORR, OER and HER. Energ. Environ. Sci. 2019, 12, 322–333. [Google Scholar] [CrossRef]
- Marinoiu, A.; Raceanu, M.; Carcadea, E.; Varlam, M.; Stefanescu, I. Iodinated carbon materials for oxygen reduction reaction in proton exchange membrane fuel cell. Scalable synthesis and electrochemical performances. Arab. J. Chem. 2019, 12, 868–880. [Google Scholar] [CrossRef]
- Marinoiu, A.; Ion-Ebrasu, D.; Soare, A.; Raceanu, M. Iodine-Doped Graphene Oxide: Fast Single-Stage Synthesis and Application as Electrocatalyst. Materials 2022, 15, 6174. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Xiong, D.; Hao, Y.; Kou, H.; Liu, W.; Li, D.; Niu, Z. Novel iodine-doped reduced graphene oxide anode for sodium ion batteries. RSC Adv. 2017, 7, 55060–55066. [Google Scholar] [CrossRef]
- Zhu, Y.; Ye, X.; Jiang, H.; Wang, L.; Zhao, P.; Yue, Z.; Wan, Z.; Jia, C. Iodine-steam doped graphene films for high-performance electrochemical capacitive energy storage. J. Power Sources 2018, 400, 605–612. [Google Scholar] [CrossRef]
- Kalita, G.; Wakita, K.; Takahashi, M.; Umeno, M. Iodine doping in solid precursor-based CVD growth graphene film. J. Mater. Chem. 2011, 21, 15209–15213. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhang, B.; Cao, L.; Wu, X.; Lin, Z.; Yu, X.; Zhang, X.; Zeng, D.; Xie, F.; Zhang, W. Iodine doped graphene as anode material for lithium ion battery. Carbon 2015, 94, 1–8. [Google Scholar] [CrossRef]
- Marinoiu, A.; Raceanu, M.; Carcadea, E.; Varlam, M. Iodine-doped graphene–Catalyst layer in PEM fuel cells. Appl. Surf. Sci. 2018, 456, 238–245. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Huang, S.; Hou, Y.; Li, Y.; Zhao, Y.; Mu, S.; Zhang, J.; Zhao, Y. N, B-codoped defect-rich graphitic carbon nanocages as high performance multifunctional electrocatalysts. Nano Energy 2017, 42, 334–340. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444–452. [Google Scholar] [CrossRef]
- Yang, C.; Jin, H.; Cui, C.; Li, J.; Wang, J.; Amine, K.; Lu, J.; Wang, S. Nitrogen and sulfur co-doped porous carbon sheets for energy storage and pH-universal oxygen reduction reaction. Nano Energy 2018, 54, 192–199. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, M.; Zhang, L.; Dai, L.; Xia, Z. Design principles for heteroatom-doped carbon nanomaterials as highly efficient catalysts for fuel cells and metal-air batteries. Adv. Mater. 2015, 27, 6834–6840. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, Y.; Sun, L.; Li, J.; Liu, C.; Ren, W.; Li, F.; Gao, L.; Chen, J.; Liu, F. Elemental superdoping of graphene and carbon nanotubes. Nat. Commun. 2016, 7, 10921. [Google Scholar] [CrossRef]
- Ni, B.; Wu, L.; Chen, R.; Shi, C.; Chen, T. Fe/Co-based nanoparticles encapsulated in heteroatom-doped carbon electrocatalysts for oxygen reduction reaction. Sci. China Mater. 2019, 62, 1626–1641. [Google Scholar] [CrossRef]
- Xu, X.; Shi, C.; Li, Q.; Chen, R.; Chen, T. Fe–N-doped carbon foam nanosheets with embedded Fe2O3 nanoparticles for highly efficient oxygen reduction in both alkaline and acidic media. RSC Adv. 2017, 7, 14382–14388. [Google Scholar] [CrossRef]
- Hu, Y.; Jensen, J.O.; Zhang, W.; Cleemann, L.N.; Xing, W.; Bjerrum, N.J.; Li, Q. Hollow spheres of iron carbide nanoparticles encased in graphitic layers as oxygen reduction catalysts. Angew. Chem. Int. Ed. 2014, 53, 3675–3679. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Shi, C.-X.; Xu, X.-Y.; Sun, P.-C.; Chen, T.-H. Nitrogen-doped hierarchically porous carbon spheres as efficient metal-free electrocatalysts for an oxygen reduction reaction. J. Power Sources 2015, 283, 389–396. [Google Scholar] [CrossRef]
- Kramm, U.I.; Herrmann-Geppert, I.; Behrends, J.; Lips, K.; Fiechter, S.; Bogdanoff, P. On an easy way to prepare metal–nitrogen doped carbon with exclusive presence of MeN4-type sites active for the ORR. J. Am. Chem. Soc. 2016, 138, 635–640. [Google Scholar] [CrossRef]
- Sun, T.; Jiang, Y.; Wu, Q.; Du, L.; Zhang, Z.; Yang, L.; Wang, X.; Hu, Z. Is iron nitride or carbide highly active for oxygen reduction reaction in acidic medium? Catal. Sci. Technol. 2017, 7, 51–55. [Google Scholar] [CrossRef]
- Ren, G.; Lu, X.; Li, Y.; Zhu, Y.; Dai, L.; Jiang, L. Porous core–shell Fe3C embedded N-doped carbon nanofibers as an effective electrocatalysts for oxygen reduction reaction. ACS Appl. Mater. Inter. 2016, 8, 4118–4125. [Google Scholar] [CrossRef]
- Li, M.; Liu, T.; Bo, X.; Zhou, M.; Guo, L.; Guo, S. Hybrid carbon nanowire networks with Fe–P bond active site for efficient oxygen/hydrogen-based electrocatalysis. Nano Energy 2017, 33, 221–228. [Google Scholar] [CrossRef]
- Zhong, R.; Wu, Y.; Liang, Z.; Guo, W.; Zhi, C.; Qu, C.; Gao, S.; Zhu, B.; Zhang, H.; Zou, R. Fabricating hierarchically porous and Fe3C-embeded nitrogen-rich carbon nanofibers as exceptional electocatalysts for oxygen reduction. Carbon 2019, 142, 115–122. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Xu, X.X.; Hu, B.C.; Liang, H.W.; Lin, Y.; Chen, L.F.; Yu, S.H. Iron carbide nanoparticles encapsulated in mesoporous Fe-N-doped carbon nanofibers for efficient electrocatalysis. Angew. Chem. Int. Ed. 2015, 54, 8179–8183. [Google Scholar] [CrossRef]
- Wei, J.; Liang, Y.; Hu, Y.; Kong, B.; Simon, G.P.; Zhang, J.; Jiang, S.P.; Wang, H. A versatile iron-tannin-framework ink coating strategy to fabricate biomass-derived iron carbide/Fe-N-carbon catalysts for efficient oxygen reduction. Angew. Chem. Int. Ed. 2016, 55, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yao, Y.; Zhu, Y.; Liu, Y.; Su, Y.; Yang, X.; Li, C. Iron carbide nanoparticles encapsulated in mesoporous Fe–N-doped graphene-like carbon hybrids as efficient bifunctional oxygen electrocatalysts. ACS Appl. Mater. Inter. 2015, 7, 21511–21520. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mao, S.; Hou, Y.; Lei, L.; Yuan, C. 3D Edge-Enriched Fe3C@ C Nanocrystals with a Core–Shell Structure Grown on Reduced Graphene Oxide Networks for Efficient Oxygen Reduction Reaction. Chemsuschem 2018, 11, 3292–3298. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Xu, X.-Y.; Sun, P.-C.; Chen, T.-H. N-doped porous carbon nanosheets with embedded iron carbide nanoparticles for oxygen reduction reaction in acidic media. Int. J. Hydrogen Energ. 2015, 40, 4531–4539. [Google Scholar] [CrossRef]
- Guan, B.Y.; Yu, L.; Lou, X.W.D. A dual-metal–organic-framework derived electrocatalyst for oxygen reduction. Energ. Environ. Sci. 2016, 9, 3092–3096. [Google Scholar] [CrossRef]
- Kong, A.; Zhang, Y.; Chen, Z.; Chen, A.; Li, C.; Wang, H.; Shan, Y. One-pot synthesized covalent porphyrin polymer-derived core-shell Fe3C@ carbon for efficient oxygen electroreduction. Carbon 2017, 116, 606–614. [Google Scholar] [CrossRef]
- Yang, W.; Liu, X.; Yue, X.; Jia, J.; Guo, S. Bamboo-like carbon nanotube/Fe3C nanoparticle hybrids and their highly efficient catalysis for oxygen reduction. J. Am. Chem. Soc. 2015, 137, 1436–1439. [Google Scholar] [CrossRef]
- Liu, B.; Yao, H.; Daniels, R.A.; Song, W.; Zheng, H.; Jin, L.; Suib, S.L.; He, J. A facile synthesis of Fe3C@ mesoporous carbon nitride nanospheres with superior electrocatalytic activity. Nanoscale 2016, 8, 5441–5445. [Google Scholar] [CrossRef]
- Tan, H.; Li, Y.; Kim, J.; Takei, T.; Wang, Z.; Xu, X.; Wang, J.; Bando, Y.; Kang, Y.M.; Tang, J. Sub-50 nm Iron-Nitrogen-Doped Hollow Carbon Sphere-Encapsulated Iron Carbide Nanoparticles as Efficient Oxygen Reduction Catalysts. Adv. Sci. 2018, 5, 1800120. [Google Scholar] [CrossRef]
- Varnell, J.A.; Tse, E.C.; Schulz, C.E.; Fister, T.T.; Haasch, R.T.; Timoshenko, J.; Frenkel, A.I.; Gewirth, A.A. Identification of carbon-encapsulated iron nanoparticles as active species in non-precious metal oxygen reduction catalysts. Nat. Commun. 2016, 7, 12582. [Google Scholar] [CrossRef]
- Strickland, K.; Miner, E.; Jia, Q.; Tylus, U.; Ramaswamy, N.; Liang, W.; Sougrati, M.-T.; Jaouen, F.; Mukerjee, S. Highly active oxygen reduction non-platinum group metal electrocatalyst without direct metal–nitrogen coordination. Nat. Commun. 2015, 6, 7343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, J.; Chen, S.; Yang, J.; Song, L.; Wu, X.; Xu, H. Co nanoparticles encapsulated in N-doped carbon nanosheets: Enhancing oxygen reduction catalysis without metal–nitrogen bonding. ACS Appl. Mater. Inter. 2017, 9, 38499–38506. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Liu, Y.; Zhao, F.; Nie, K.; Han, N.; Wang, X.; Huang, W.; Song, X.; Zhong, J.; Li, Y. Metallic cobalt nanoparticles encapsulated in nitrogen-enriched graphene shells: Its bifunctional electrocatalysis and application in zinc-air batteries. Adv. Funct. Mater. 2016, 26, 4397–4404. [Google Scholar] [CrossRef]
- Li, C.; Wu, M.; Liu, R. High-performance bifunctional oxygen electrocatalysts for zinc-air batteries over mesoporous Fe/Co-NC nanofibers with embedding FeCo alloy nanoparticles. Appl. Catal. B Environ. 2019, 244, 150–158. [Google Scholar] [CrossRef]
- Wu, N.; Lei, Y.; Wang, Q.; Wang, B.; Han, C.; Wang, Y. Facile synthesis of FeCo@ NC core–shell nanospheres supported on graphene as an efficient bifunctional oxygen electrocatalyst. Nano Res. 2017, 10, 2332–2343. [Google Scholar] [CrossRef]
- Park, H.-S.; Han, S.-B.; Kwak, D.-H.; Han, J.-H.; Park, K.-W. Fe nanoparticles encapsulated in doped graphitic shells as high-performance and stable catalysts for oxygen reduction reaction in an acid medium. J. Catal. 2019, 370, 130–137. [Google Scholar] [CrossRef]
- Mahmood, J.; Li, F.; Kim, C.; Choi, H.-J.; Gwon, O.; Jung, S.-M.; Seo, J.-M.; Cho, S.-J.; Ju, Y.-W.; Jeong, H.Y. Fe@C2N: A highly-efficient indirect-contact oxygen reduction catalyst. Nano Energy 2018, 44, 304–310. [Google Scholar] [CrossRef]
- Mao, C.; Kong, A.; Wang, Y.; Bu, X.; Feng, P. MIL-100 derived nitrogen-embodied carbon shells embedded with iron nanoparticles. Nanoscale 2015, 7, 10817–10822. [Google Scholar] [CrossRef]
- Dou, S.; Li, X.; Tao, L.; Huo, J.; Wang, S. Cobalt nanoparticle-embedded carbon nanotube/porous carbon hybrid derived from MOF-encapsulated Co3O4 for oxygen electrocatalysis. Chem. Commun. 2016, 52, 9727–9730. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Dong, Y.; Pan, X.; Liu, X.; Zhao, Z.; Qiu, J. Nitrogen-doped carbon nanotubes decorated with cobalt nanoparticles derived from zeolitic imidazolate framework-67 for highly efficient oxygen reduction reaction electrocatalysis. Carbon 2018, 132, 580–588. [Google Scholar] [CrossRef]
- Wang, R.; Yan, T.; Han, L.; Chen, G.; Li, H.; Zhang, J.; Shi, L.; Zhang, D. Tuning the dimensions and structures of nitrogen-doped carbon nanomaterials derived from sacrificial gC3N4/metal–organic frameworks for enhanced electrocatalytic oxygen reduction. J. Mater. Chem. A 2018, 6, 5752–5761. [Google Scholar] [CrossRef]
- Hu, E.; Ning, J.; He, B.; Li, Z.; Zheng, C.; Zhong, Y.; Zhang, Z.; Hu, Y. Unusual formation of tetragonal microstructures from nitrogen-doped carbon nanocapsules with cobalt nanocores as a bi-functional oxygen electrocatalyst. J. Mater. Chem. A 2017, 5, 2271–2279. [Google Scholar] [CrossRef]
- Jafari, M.; Gharibi, H.; Parnian, M.J.; Nasrollahpour, M.; Vafaee, M. Iron-nanoparticle-loaded nitrogen-doped carbon nanotube/carbon sheet composites derived from MOF as electrocatalysts for an oxygen reduction reaction. ACS Appl. Nano Mater. 2021, 4, 459–477. [Google Scholar] [CrossRef]
- Zhao, R.; Ni, B.; Wu, L.; Sun, P.; Chen, T. Carbon-based iron-cobalt phosphate FeCoP/C as an effective ORR/OER/HER trifunctional electrocatalyst. Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 128118. [Google Scholar] [CrossRef]
- Zhao, R.; Wu, L.; Chen, R.; Sun, P.; Chen, T. In-situ growth of cobalt manganate spinel nanodots on carbon black toward high-performance zinc-air battery: Dual functions of 3-aminopropyltriethoxysilane. J. Colloid. Interf. Sci. 2022, 608, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, Y.; Liu, C. Nanostructured nickel phosphide supported on carbon nanospheres: Synthesis and application as an efficient electrocatalyst for hydrogen evolution. J. Power Sources 2015, 285, 169–177. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, N.; Chen, Y.; Lin, Y.; Li, Y.; Liu, Y.; Liu, C. Nickel phosphide nanoparticles-nitrogen-doped graphene hybrid as an efficient catalyst for enhanced hydrogen evolution activity. J. Power Sources 2015, 297, 45–52. [Google Scholar] [CrossRef]
- Hou, C.-C.; Cao, S.; Fu, W.-F.; Chen, Y. Ultrafine CoP nanoparticles supported on carbon nanotubes as highly active electrocatalyst for both oxygen and hydrogen evolution in basic media. ACS Appl. Mater. Inter. 2015, 7, 28412–28419. [Google Scholar] [CrossRef]
- Jiao, L.; Zhou, Y.-X.; Jiang, H.-L. Metal–organic framework-based CoP/reduced graphene oxide: High-performance bifunctional electrocatalyst for overall water splitting. Chem. Sci. 2016, 7, 1690–1695. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, J.; Cui, W.; Jiang, P.; Cheng, N.; Asiri, A.M.; Sun, X. Carbon nanotubes decorated with CoP nanocrystals: A highly active non-noble-metal nanohybrid electrocatalyst for hydrogen evolution. Angew. Chem. Int. Ed. 2014, 53, 6710–6714. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, J.; Zhou, X.; Wang, W.; Zuo, J.; Yang, Q. Alternative synthesis of cobalt monophosphide@ C core–shell nanocables for electrochemical hydrogen production. J. Power Sources 2015, 286, 464–469. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Xiong, Y.; Bo, X.; Zhang, Y.; Han, C.; Guo, L. Facile synthesis of various highly dispersive CoP nanocrystal embedded carbon matrices as efficient electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 4255–4265. [Google Scholar] [CrossRef]
- Pan, Y.; Lin, Y.; Chen, Y.; Liu, Y.; Liu, C. Cobalt phosphide-based electrocatalysts: Synthesis and phase catalytic activity comparison for hydrogen evolution. J. Mater. Chem. A 2016, 4, 4745–4754. [Google Scholar] [CrossRef]
- Chen, K.; Huang, X.; Wan, C.; Liu, H. Efficient oxygen reduction catalysts formed of cobalt phosphide nanoparticle decorated heteroatom-doped mesoporous carbon nanotubes. Chem. Commun. 2015, 51, 7891–7894. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Hao, Y.-N.; Zheng, L.; Cao, M. Organophosphoric acid-derived CoP quantum dots@ S, N-codoped graphite carbon as a trifunctional electrocatalyst for overall water splitting and Zn–air batteries. Nanoscale 2018, 10, 14613–14626. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Xu, Y.Y.; Asif, M.; Liu, H.; Xia, B.Y. Ball-milling synthesis of Co2P nanoparticles encapsulated in nitrogen doped hollow carbon rods as efficient electrocatalysts. J. Mater. Chem. A 2017, 5, 17563–17569. [Google Scholar] [CrossRef]
- Jiang, H.; Li, C.; Shen, H.; Liu, Y.; Li, W.; Li, J. Supramolecular gel-assisted synthesis Co2P particles anchored in multielement co-doped graphene as efficient bifunctional electrocatalysts for oxygen reduction and evolution. Electrochim. Acta 2017, 231, 344–353. [Google Scholar] [CrossRef]
- Ni, B.; Chen, R.; Wu, L.; Sun, P.; Chen, T. Encapsulated FeP nanoparticles with in-situ formed P-doped graphene layers: Boosting activity in oxygen reduction reaction. Sci. China-Mater. 2021, 64, 1159–1172. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, C.; Chen, W. FeP embedded in N, P dual-doped porous carbon nanosheets: An efficient and durable bifunctional catalyst for oxygen reduction and evolution reactions. J. Mater. Chem. A 2016, 4, 18723–18729. [Google Scholar] [CrossRef]
- Xu, X.; Shi, C.; Chen, R.; Chen, T. Iron phosphide nanocrystals decorated in situ on heteroatom-doped mesoporous carbon nanosheets used for an efficient oxygen reduction reaction in both alkaline and acidic media. RSC Adv. 2017, 7, 22263–22269. [Google Scholar] [CrossRef]
- Hu, K.; Xiao, Z.; Cheng, Y.; Yan, D.; Chen, R.; Huo, J.; Wang, S. Iron phosphide/N, P-doped carbon nanosheets as highly efficient electrocatalysts for oxygen reduction reaction over the whole pH range. Electrochim. Acta 2017, 254, 280–286. [Google Scholar] [CrossRef]
- Chen, M.; Goodman, D. The structure of catalytically active gold on titania. Science 2004, 306, 252–255. [Google Scholar] [CrossRef]
- Liu, C.; Pan, G.; Liang, N.; Hong, S.; Ma, J.; Liu, Y. Ir single atom catalyst loaded on amorphous carbon materials with high HER activity. Adv. Sci. 2022, 9, 2105392. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, S.; Chen, W. Metal single-atom catalysts for selective hydrogenation of unsaturated bonds. J. Mater. Chem. A 2021, 9, 5296–5319. [Google Scholar] [CrossRef]
- Yang, X.-F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Catalysis by supported metal single atoms. ACS Catal. 2017, 7, 34–59. [Google Scholar] [CrossRef]
- Jasinski, R. A new fuel cell cathode catalyst. Nature 1964, 201, 1212–1213. [Google Scholar] [CrossRef]
- Jahnke, H.; Schönborn, M.; Zimmermann, G. Organic dyestuffs as catalysts for fuel cells. Phys. Chem. Appl. Dyest. 1976, 61, 133–181. [Google Scholar]
- Gupta, S.; Tryk, D.; Bae, I.; Aldred, W.; Yeager, E. Heat-treated polyacrylonitrile-based catalysts for oxygen electroreduction. J. Appl. Electrochem. 1989, 19, 19–27. [Google Scholar] [CrossRef]
- Sharma, R.; Kar, K.K. Effects of structural disorder and nitrogen content on the oxygen reduction activity of polyvinylpyrrolidone-derived multi-doped carbon. J. Mater. Chem. A 2015, 3, 11948–11959. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Wang, H.; Liu, X.-P.; Su, L.; Yu, L.; Chen, P. Bulk production of N, Fe-doped carbon with high surface area and big pore volume and its excellent activity for oxygen reduction in acidic media. J. Electrochem. Soc. 2016, 163, H738. [Google Scholar] [CrossRef]
- Wang, W.; Luo, J.; Chen, W.; Li, J.; Xing, W.; Chen, S. Synthesis of mesoporous Fe/N/C oxygen reduction catalysts through NaCl crystallite-confined pyrolysis of polyvinylpyrrolidone. J. Mater. Chem. A 2016, 4, 12768–12773. [Google Scholar] [CrossRef]
- Wu, L.; Ni, B.; Chen, R.; Sun, P.; Chen, T. A general approach for hierarchically porous metal/N/C nanosphere electrocatalysts: Nano-confined pyrolysis of in situ-formed amorphous metal–ligand complexes. J. Mater. Chem. A 2020, 8, 21026–21035. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, H.; Zhang, X.; Liu, J.; Du, G.; Chen, T. Hierarchically Porous Three-Dimensional (3D) Carbon Nanorod Networks with a High Content of FeNx Sites for Efficient Oxygen Reduction Reaction. Langmuir 2022, 38, 11372–11381. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, M.; Proietti, E.; Jaouen, F.; Dodelet, J.-P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 2009, 324, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Proietti, E.; Jaouen, F.; Lefèvre, M.; Larouche, N.; Tian, J.; Herranz, J.; Dodelet, J.-P. Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells. Nat. Commun. 2011, 2, 416. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef]
- Shui, J.; Chen, C.; Grabstanowicz, L.; Zhao, D.; Liu, D.-J. Highly efficient nonprecious metal catalyst prepared with metal–organic framework in a continuous carbon nanofibrous network. Proc. Natl. Acad. Sci. USA 2015, 112, 10629–10634. [Google Scholar] [CrossRef]
- Chung, H.T.; Cullen, D.A.; Higgins, D.; Sneed, B.T.; Holby, E.F.; More, K.L.; Zelenay, P. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 2017, 357, 479–484. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Xu, D.; Zhong, H.-X.; Wang, J.; Meng, F.-L.; Zhang, X.-B. Gelatin-derived sustainable carbon-based functional materials for energy conversion and storage with controllability of structure and component. Sci. Adv. 2015, 1, e1400035. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Gui, M.; Asif, M.; Wang, Z.; Yu, Y.; Xiao, J.; Liu, H. Uniform Fe3O4/nitrogen-doped mesoporous carbon spheres derived from ferric citrate-bonded melamine resin as an efficient synergistic catalyst for oxygen reduction. ACS Appl. Mater. Inter. 2017, 9, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; He, S.; Lu, S.; Veder, J.P.; Johannessen, B.; Thomsen, L.; Saunders, M.; Becker, T.; De Marco, R.; Li, Q. Iron single atoms on graphene as nonprecious metal catalysts for high-temperature polymer electrolyte membrane fuel cells. Adv. Sci. 2019, 6, 1802066. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Chen, S.; Lin, Y.; Yang, L.; Chen, S.; Zheng, X.; Qi, Z.; Wang, C.; Long, R.; Chen, M. Precisely tuning the number of Fe atoms in clusters on N-doped carbon toward acidic oxygen reduction reaction. Chem 2019, 5, 2865–2878. [Google Scholar] [CrossRef]
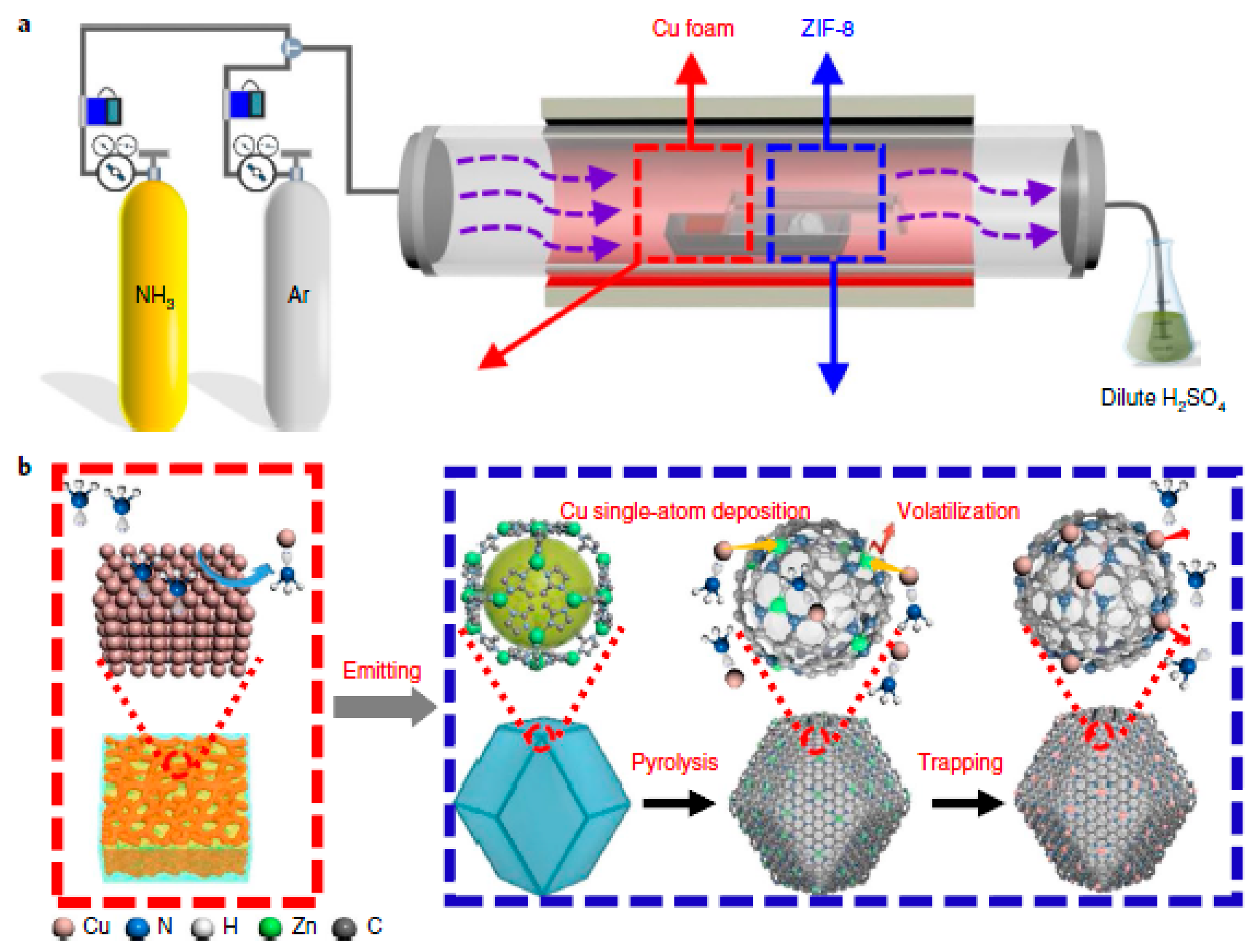
- Qu, Y.; Li, Z.; Chen, W.; Lin, Y.; Yuan, T.; Yang, Z.; Zhao, C.; Wang, J.; Zhao, C.; Wang, X. Direct transformation of bulk copper into copper single sites via emitting and trapping of atoms. Nat. Catal. 2018, 1, 781–786. [Google Scholar] [CrossRef]
- Wu, M.; Hu, X.; Li, C.; Li, J.; Zhou, H.; Zhang, X.; Liu, R. Encapsulation of metal precursor within ZIFs for bimetallic N-doped carbon electrocatalyst with enhanced oxygen reduction. Int. J. Hydrogen Energ. 2018, 43, 14701–14709. [Google Scholar] [CrossRef]
- Li, Z.; Shao, M.; Zhou, L.; Yang, Q.; Zhang, C.; Wei, M.; Evans, D.G.; Duan, X. Carbon-based electrocatalyst derived from bimetallic metal-organic framework arrays for high performance oxygen reduction. Nano Energy 2016, 25, 100–109. [Google Scholar] [CrossRef]
- Yuan, S.; Cui, L.L.; Dou, Z.; Ge, X.; He, X.; Zhang, W.; Asefa, T. Nonprecious Bimetallic Sites Coordinated on N-Doped Carbons with Efficient and Durable Catalytic Activity for Oxygen Reduction. Small 2020, 16, 2000742. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Luo, G.; Li, Z.; Zhao, C.; Zhang, H.; Zhu, M.; Xu, Q.; Wang, X.; Zhao, C. Synergistic effect of well-defined dual sites boosting the oxygen reduction reaction. Energ. Environ. Sci. 2018, 11, 3375–3379. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Fan, C.; Meng, Q.; Tang, Y.; Qiu, X.; Fu, G.; Ma, T. Dual single-atomic Ni-N4 and Fe-N4 sites constructing Janus hollow graphene for selective oxygen electrocatalysis. Adv. Mater. 2020, 32, 2003134. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, R.; Du, G.; Wang, H.; Hou, M.; Zhang, W.; Sun, P.; Chen, T. Hierarchically porous Fe/N/S/C nanospheres with high-content of Fe-Nx for enhanced ORR and Zn-air battery performance. Green Energy Environ. 2022. [Google Scholar] [CrossRef]
- Ni, B.; Chen, R.; Wu, L.; Xu, X.; Shi, C.; Sun, P.; Chen, T. Optimized enhancement effect of sulfur in Fe–N–S codoped carbon nanosheets for efficient oxygen reduction reaction. ACS Appl. Mater. Inter. 2020, 12, 23995–24006. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xiang, Q.; Peng, T.; Song, C.; Shang, W.; Deng, T.; Wu, J. Reconsidering the benchmarking evaluation of catalytic activity in oxygen reduction reaction. IScience 2020, 23, 101532. [Google Scholar] [CrossRef] [PubMed]
- Borup, R.L.; Kusoglu, A.; Neyerlin, K.C.; Mukundan, R.; Ahluwalia, R.K.; Cullen, D.A.; More, K.L.; Weber, A.Z.; Myers, D.J. Recent developments in catalyst-related PEM fuel cell durability. Curr. Opin. Electrochem. 2020, 21, 192–200. [Google Scholar] [CrossRef]
- Palanivelu, K.; Prabhakaran, V.; Ramani, V.K.; Ramanujam, K. Controlling the nitrogen content of metal-nitrogen-carbon based non-precious-metal electrocatalysts via selenium addition. J. Electrochem. Soc. 2015, 162, F475. [Google Scholar] [CrossRef]
- Ma, Y.-Y.; Wu, C.-X.; Feng, X.-J.; Tan, H.-Q.; Yan, L.-K.; Liu, Y.; Kang, Z.-H.; Wang, E.-B.; Li, Y.-G. Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoP@C electrocatalyst superior to Pt/C. Energ. Environ. Sci. 2017, 10, 788–798. [Google Scholar] [CrossRef]
- Jo, W.-K.; Moru, S.; Lee, D.-E.; Tonda, S. Cobalt-and iron-coordinated graphitic carbon nitride on reduced graphene oxide: A nonprecious bimetallic M–Nx–C analogue electrocatalyst for efficient oxygen reduction reaction in acidic media. Appl. Surf. Sci. 2020, 531, 147367. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, P.; Zhang, C.; Cui, X.; Xu, Y.; Qu, H.; Shi, J. Towards understanding ORR activity and electron-transfer pathway of M-Nx/C electro-catalyst in acidic media. J. Catal. 2017, 356, 229–236. [Google Scholar] [CrossRef]
- Yang, H.; Chen, X.; Chen, W.-T.; Wang, Q.; Cuello, N.C.; Nafady, A.; Al-Enizi, A.M.; Waterhouse, G.I.; Goenaga, G.A.; Zawodzinski, T.A. Tunable synthesis of hollow metal–nitrogen–carbon capsules for efficient oxygen reduction catalysis in proton exchange membrane fuel cells. ACS Nano 2019, 13, 8087–8098. [Google Scholar] [CrossRef]
- Lim, S.Y.; Martin, S.; Gao, G.; Dou, Y.; Simonsen, S.B.; Jensen, J.O.; Li, Q.; Norrman, K.; Jing, S.; Zhang, W. Self-standing nanofiber electrodes with Pt-Co derived from electrospun zeolitic imidazolate framework for high temperature PEM fuel cells. Adv. Funct. Mater. 2021, 31, 2006771. [Google Scholar] [CrossRef]
- Lilloja, J.; Kibena-Põldsepp, E.; Sarapuu, A.; Käärik, M.; Kozlova, J.; Paiste, P.; Kikas, A.; Treshchalov, A.; Leis, J.; Tamm, A. Transition metal and nitrogen-doped mesoporous carbons as cathode catalysts for anion-exchange membrane fuel cells. Appl. Catal. B Environ. 2022, 306, 121113. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Zhang, L.-C.; Wu, Y.; Chen, H.; Li, T.; Xu, M.; Bao, S.-J. A gel-limiting strategy for large-scale fabrication of Fe–N–C single-atom ORR catalysts. J. Mater. Chem. A 2021, 9, 7137–7142. [Google Scholar] [CrossRef]
- Yang, W.; Fellinger, T.-P.; Antonietti, M. Efficient metal-free oxygen reduction in alkaline medium on high-surface-area mesoporous nitrogen-doped carbons made from ionic liquids and nucleobases. J. Am. Chem. Soc. 2011, 133, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Xiaoyan, S.; Rui, W.; Dangsheng, S. Research progress in metal-free carbon-based catalysts. Chin. J. Catal. 2013, 34, 508–523. [Google Scholar]
- Zhang, Y.; Ge, J.; Wang, L.; Wang, D.; Ding, F.; Tao, X.; Chen, W. Manageable N-doped graphene for high performance oxygen reduction reaction. Sci. Rep. 2013, 3, 2771. [Google Scholar] [CrossRef] [PubMed]
- Schiros, T.; Nordlund, D.; Pálová, L.; Prezzi, D.; Zhao, L.; Kim, K.S.; Wurstbauer, U.; Gutiérrez, C.; Delongchamp, D.; Jaye, C. Connecting dopant bond type with electronic structure in N-doped graphene. Nano Lett. 2012, 12, 4025–4031. [Google Scholar] [CrossRef] [PubMed]
- Shui, J.; Wang, M.; Du, F.; Dai, L. N-doped carbon nanomaterials are durable catalysts for oxygen reduction reaction in acidic fuel cells. Sci. Adv. 2015, 1, e1400129. [Google Scholar] [CrossRef]
- Dou, Y.; Liao, T.; Ma, Z.; Tian, D.; Liu, Q.; Xiao, F.; Sun, Z.; Kim, J.H.; Dou, S.X. Graphene-like holey Co3O4 nanosheets as a highly efficient catalyst for oxygen evolution reaction. Nano Energy 2016, 30, 267–275. [Google Scholar] [CrossRef]














| Sample | Template | BET Surface Area/m2 g−1 | Pore Size/nm | Carbonization Conditions | Ref. |
|---|---|---|---|---|---|
| YTC8 | Zeolite Y | 1544 | 1.9 | 5 °C/min, 800 °C, 4 h | [41] |
| CEMC900 | Zeolite EMC-2 | 3360 | 1.5 | 900 °C, 3 h | [42] |
| M-OMC | SBA-15 | 643 | 2.9 | 1.58 °C/min, 700 °C, 3 h | [47] |
| N-MC | SBA-15 | 535.2 | 4.15 | 3 °C/min, 600 °C, 5 h | [48] |
| CMK-5 | SBA-15 | 2 84 | 4.1 | 4 °C/min, 850 °C, 1 h | [49] |
| CMK8-SC | KIT-6 | 740 | 5.0 | 1.4 °C/min, 900 °C, 2 h | [50] |
| OMC | KIT-6 | 905 | 4.0 | 900 °C, 3 h | [51] |
| Carbon | MgO | 1290 | 0.58 | 10 °C/min, 900 °C, 1 h | [56] |
| GZnC | ZnO | 2412 | 2–10 | 10 °C/min, 900 °C, 2 h | [57] |
| Sample | Electrolyte | Onset Potential | Electron Transfer Number | Ref. |
|---|---|---|---|---|
| N-doped hollow mesoporous carbon spheres | 0.1 M KOH | 0.92 V | 3.99 @ 0.4 V | [89] |
| Nitrogen-doped graphite nanomaterial | 0.1 M KOH | 1.52 ± 0.02 V | - | [90] |
| Graphene/nitrogen-doped porous carbon sandwich | 0.1 M KOH | 0.99 V | - | [91] |
| nitrogen-doped carbon | 0.1 M KOH | 0.74 V | 2.5–2.6 @ 0.1–0.5 V | [92] |
| 3D porous N-doped graphene foam | 0.1 M KOH | 1.02 V | 3.9 @ 0–0.85 V | [93] |
| N-doped nanoporous carbon nanosheet | 0.1 M KOH | 0.73 V | 3.85–3.96 @ 0.06–0.9 V | [94] |
| Sample | Electrolyte | Half-Wave Potential | Electron Transfer Number | Ref. |
| Flaky N-doped carbonaceous material | 0.1 M KOH | 0.75 V | 3.47 @ 1.2 V | [95] |
| Rod-like N-doped carbonaceous material | 0.1 M KOH | 0.76 V | 3.74 @ 1.2 V | [95] |
| Nitrogen-doped carbon | 0.1 M KOH | 0.88 V | - | [96] |
| Nitrogen-doped carbon nanofiber aerogel | 0.1 M KOH | 0.80 V | 3.96 @ 0.8 V | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Wu, L.; Ni, B.; Chen, T. Research Progress on Porous Carbon-Based Non-Precious Metal Electrocatalysts. Materials 2023, 16, 3283. https://doi.org/10.3390/ma16083283
Yu H, Wu L, Ni B, Chen T. Research Progress on Porous Carbon-Based Non-Precious Metal Electrocatalysts. Materials. 2023; 16(8):3283. https://doi.org/10.3390/ma16083283
Chicago/Turabian StyleYu, Hongda, Luming Wu, Baoxia Ni, and Tiehong Chen. 2023. "Research Progress on Porous Carbon-Based Non-Precious Metal Electrocatalysts" Materials 16, no. 8: 3283. https://doi.org/10.3390/ma16083283
APA StyleYu, H., Wu, L., Ni, B., & Chen, T. (2023). Research Progress on Porous Carbon-Based Non-Precious Metal Electrocatalysts. Materials, 16(8), 3283. https://doi.org/10.3390/ma16083283