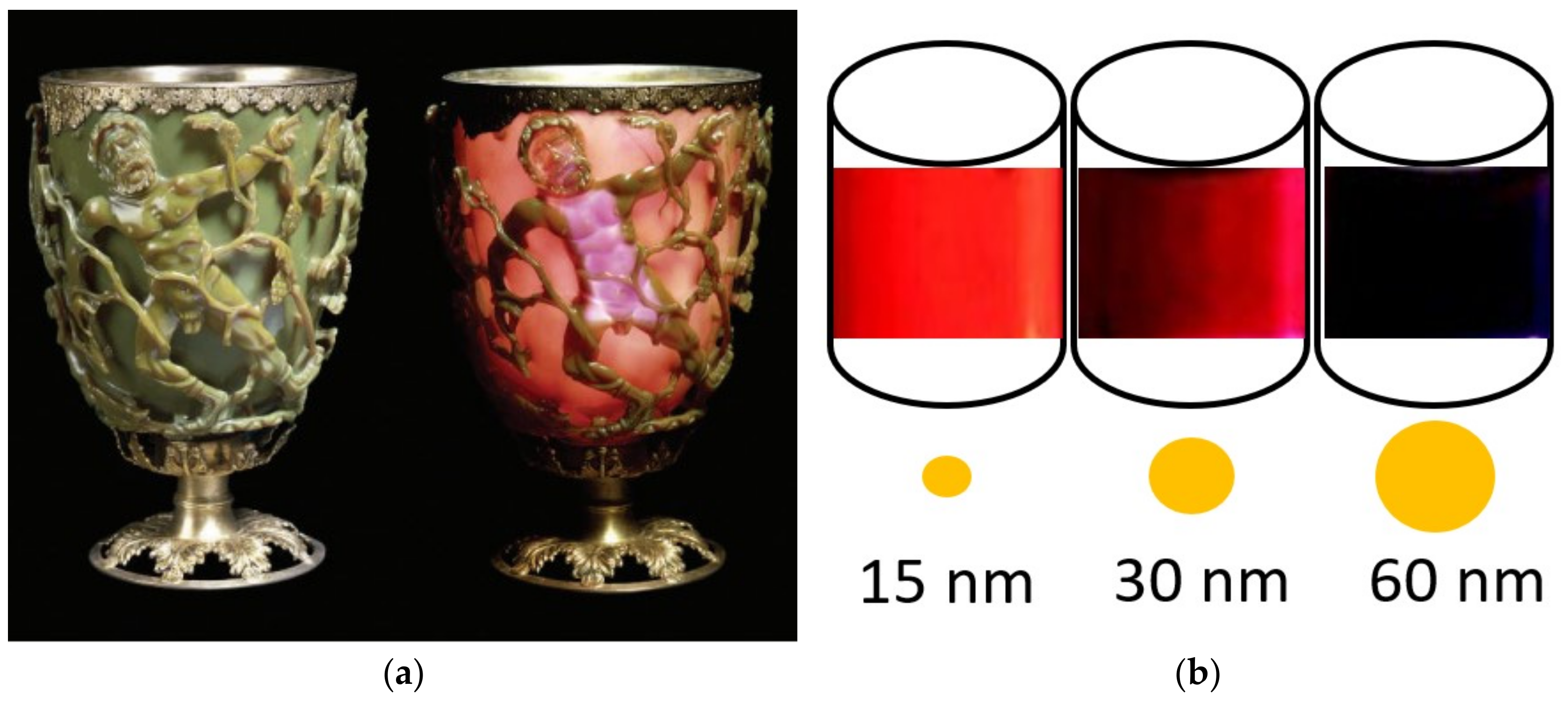
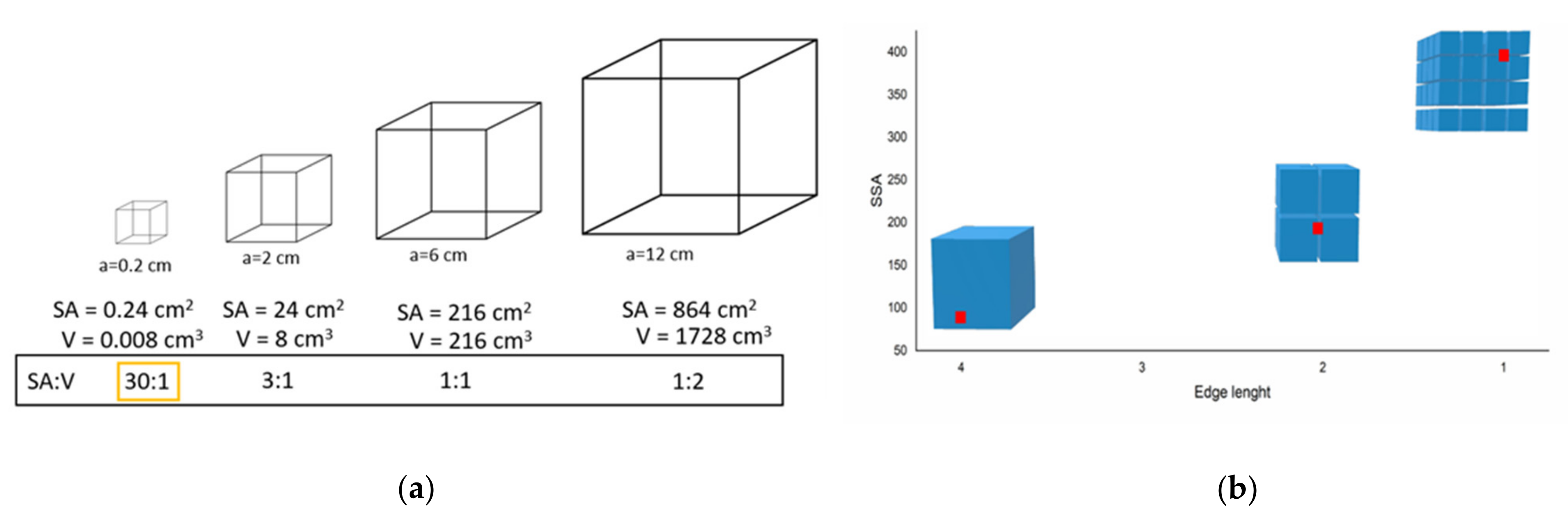
The Boom in Nanomaterials for Built Heritage Conservation: Why Does Size Matter?
Abstract
Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feynman, R.P. There’s plenty of room at the bottom, a talk given at a meeting of the American Physical Society, December 29, 1959. Eng. Sci. 1960, 23, 22–26. [Google Scholar]
- Bhushan, B.; Bhushan, B. Baumann Springer Handbook of Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2007; Volume 2. [Google Scholar]
- Lindsay, S. Introduction to Nanoscience; Oxford University Press: Oxford, UK; New York, NY, USA, 2010; ISBN 0199544212. [Google Scholar]
- Bailey, C. The Greek Atomists and Epicurus; Cambridge University Press: Oxford, UK, 1928. [Google Scholar]
- British Museum Webpage. Available online: https://www.britishmuseum.org/ (accessed on 18 November 2022).
- Wagner, F.; Haslbeck, S.; Stievano, L.; Caloger, S.; Pankhunrst, Q.A.; Martinek, K.P. Before striking gold in gold-ruby glass. Nature 2000, 407, 691–692. [Google Scholar] [CrossRef] [PubMed]
- Caiger-Smith, A. Lustre Pottery: Technique. In Tradition and Innovation in Islam and the Western World; Faber & Faber: London, UK, 1985. [Google Scholar]
- Pérez-Arantegui, J.; Molera, J.; Larrea, A.; Pradell, T.; Vendrell-Saz, M.; Borgia, I.; Brunetti, B.G.; Cariati, F.; Fermo, P.; Mellini, M.; et al. Luster Pottery from the Thirteenth Century to the Sixteenth Century: A Nanostructured Thin Metallic Film. J. Am. Ceram. Soc. 2001, 84, 442–446. [Google Scholar] [CrossRef]
- Pérez-Arantegui, J.; Larrea, A. The secret of early nanomaterials is revealed, thanks to transmission electron microscopy. Trends Anal. Chem. 2003, 22, 327–329. [Google Scholar] [CrossRef]
- Scott, D.A. The deterioration of gold alloys and some aspects of their conservation. Stud. Conserv. 1983, 28, 194–203. [Google Scholar]
- Hunt, L.B. The true story of Purple of Cassius. Gold Bull. 1976, 9, 134–139. [Google Scholar] [CrossRef]
- Cardell, C.; Guerra, I. Natural corrosion-induced gold nanoparticles yield purple color of Alhambra palaces decoration. Sci. Adv. 2022, 8, eabn2541. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, C.; Zeng, Q.; Xu, B.; Yin, S.; Wang, H.; Xu, S.; Bai, C. Alkane-assisted adsorption and assembly of phthalocyanines and porphyrins. J. Am. Chem. Soc. 2000, 122, 5550–5556. [Google Scholar] [CrossRef]
- Chamundeeswari, M.; Jeslin, J.; Verma, M.L. Nanocarriers for drug delivery applications. Environ. Chem. Lett. 2019, 17, 849–865. [Google Scholar] [CrossRef]
- Choi, O.; Deng, K.K.; Kim, N.-J.; Ross, L., Jr.; Surampalli, R.Y.; Hu, Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef]
- Noman, M.T.; Ashraf, M.A.; Ali, A. Synthesis and applications of nano-TiO2: A review. Environ. Sci. Pollut. Res. 2019, 26, 3262–3291. [Google Scholar] [CrossRef]
- Kaneko, T.; Jupalli, T.T.; Moraru, D. Evaluation of Single-Electron Tunneling Operation in High-Concentration Codoped Si Nano-Transistors. In Proceedings of the 2022 IEEE Silicon Nanoelectronics Workshop (SNW), Honolulu, HI, USA, 11–12 June 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 1–2. [Google Scholar]
- Alivisatos, A.P. Perspectives on the physical chemistry of semiconductor nanocrystals. J. Phys. Chem. 1996, 100, 13226–13239. [Google Scholar] [CrossRef]
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef]
- Roduner, E. Nanoscopic materials: Size-dependent phenomena and growth principles. In Royal Society of Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 1782624945. [Google Scholar]
- Bai, C.; Liu, M. From chemistry to nanoscience: Not just a matter of size. Angew. Chem. Int. Ed. 2013, 52, 2678–2683. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef]
- Faraday, M. The Bakerian Lecture: Experimental Relations of Gold (and Other Metals) to Light. Philos. Trans. R. Soc. Lond. 1857, 147, 145–181. [Google Scholar]
- Arato, F.; Galilei, G. Discorsi e Dimostrazioni Matematiche Intorno a due Nuove Scienze Attinenti alla Mecanica ed i Movimenti Locali; Giusti, E., Ed.; Einaudi: Turin, Italy, 1992; Volume 169, p. 449. [Google Scholar]
- Planinšič, G.; Vollmer, M. The surface-to-volume ratio in thermal physics: From cheese cube physics to animal metabolism. Eur. J. Phys. 2008, 29, 369. [Google Scholar] [CrossRef]
- Tao, X.; Du, J.; Yang, Y.; Li, Y.; Xia, Y.; Gan, Y.; Huang, H.; Zhang, W.; Li, X. TiC nanorods derived from cotton fibers: Chloride-assisted VLS growth, structure, and mechanical properties. Cryst. Growth Des. 2011, 11, 4422–4426. [Google Scholar] [CrossRef]
- Edwards, P.P.; Johnston, R.L.; Rao, C.N.R. On the size-induced metal-insulator transition in clusters and small particles. In Metal Clusters in Chemistry; Wiley Press: Hoboken, NJ, USA, 1999; pp. 1454–1481. [Google Scholar]
- De Mello Donegá, C. Nanoparticles: Workhorses of Nanoscience; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 3662448238. [Google Scholar]
- Santos, C.; Gabriel, B.; Blancky, M.; Menes, O.; García, D.; Blanco, M.; Arconada, N.; Neto, V. Industrial Applications of Nanoparticles—A Prospective Overview. Mater. Today Proc. 2015, 2, 456–465. [Google Scholar] [CrossRef]
- Haruta, M. Chance and necessity: My encounter with gold catalysts. Angew. Chem. Int. Ed. 2014, 53, 52–56. [Google Scholar] [CrossRef]
- De Mello Donegá, C. Synthesis, and properties of colloidal heteronanocrystals. Chem. Soc. Rev. 2011, 40, 1512–1546. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.A.; Peng, S.; Cheng, K.; Sun, S. Magnetic nanoparticles: Synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev. 2009, 38, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Navarro, C.; Ruiz-Agudo, E. Nanolimes: From synthesis to application. Pure Appl. Chem. 2018, 90, 523–550. [Google Scholar] [CrossRef]
- Baglioni, P.; Chelazzi, D.; Giorgi, R. Nanotechnologies in the Conservation of Cultural Heritage: A Compendium of Materials and Techniques; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 9401793034. [Google Scholar]
- Ziegenbalg, G.; Drdácký, M.; Dietze, C.; Schuch, D. Nanomaterials in Architecture and Art Conservation; Pan Stanford Publishing: Singapore, 2018; ISBN 978-0-429-42875-3. [Google Scholar]
- Winkler, E. Stone in Architecture: Properties, Durability; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1997; ISBN 3540576266. [Google Scholar]
- Price, C.A.; Doehne, E. Stone conservation: An overview of current research. In Research in Conservaiton; The Getty Conservation Institute: Los Angeles, CA, USA, 2010. [Google Scholar]
- Sledge, J.; Elena, C.A.; DePriest Paula, T.; Koestler Robert, J. Conservation of the Exterior of the National Museum of the American Indian Building. In Smithsonian Contributions to Museum Conservation; SSN (ONLINE); Smithsonian Scholarly Press: Washington, DC, USA, 2017; Volume 6, pp. 1949–2367. [Google Scholar]
- Scherer, G.W.; Wheeler, G.S. Silicate consolidants for stone. In Proceedings of the Key Engineering Materials; Trans Tech Publications: Durnten-Zurich, Switzerland, 2009; Volume 391, pp. 1–25. [Google Scholar]
- Ashurst, J.; Ashurst, N. Practical Building Conservation-Mortars, Plasters and Renders; Ashgate Publishing: Farnham, UK, 1988; ISBN 0291397476. [Google Scholar]
- Baglioni, P.; Dei, L.; Giorgi, R.C.V. Basic Suspension, Its Preparation and Process for Paper Deacidification. U.S. Patent US2005042380A1, 15 January 2002. [Google Scholar]
- Matijević, E.; Cimaš, Š. Formation of Uniform Colloidal Iron (III) Oxides in Ethylene Glycol-Water Solutions. Colloid Polym. Sci. 1987, 265, 155–163. [Google Scholar] [CrossRef]
- Baglioni, P.; Chelazzi, D.; Giorgi, R.; Carretti, E.; Toccafondi, N.; Jaidar, Y. Commercial Ca (OH)2 nanoparticles for the consolidation of immovable works of art. Appl. Phys. A 2014, 114, 723–732. [Google Scholar] [CrossRef]
- Chelazzi, D.; Poggi, G.; Jaidar, Y.; Toccafondi, N.; Giorgi, R.; Baglioni, P. Hydroxide nanoparticles for cultural heritage: Consolidation and protection of wall paintings and carbonate materials. J. Colloid Interface Sci. 2013, 392, 42–49. [Google Scholar] [CrossRef]
- Hosseini, M.; Karapanagiotis, I. Advanced Materials for the Conservation of Stone; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 331972259X. [Google Scholar]
- Baglioni, P.; Giorgi, R. Soft and hard nanomaterials for restoration and conservation of cultural heritage. Soft Matter 2006, 2, 293–303. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Suzuki, A.; Ruiz-Agudo, E. Alcohol dispersions of calcium hydroxide nanoparticles for stone conservation. Langmuir 2013, 29, 11457–11470. [Google Scholar] [CrossRef]
- Otero, J.; Charola, A.E.; Grissom, C.A.; Starinieri, V. An overview of nanolime as a consolidation method for calcareous substrates. Ge-Conservación 2017, 1, 71–77. [Google Scholar] [CrossRef]
- Sierra-Fernandez, A.; Gomez-Villalba, L.S.; Fort, R. New nanomaterials for applications in conservation and restoration of stony materials: A review. Mater. Construcción 2017, 67, e107. [Google Scholar] [CrossRef]
- Franco-Castillo, I.; Hierro, L.; De La Fuente, J.; Michell, S.G. Perspectives for antimicrobial nanomaterials in cultural heritage conservation. Chem 2021, 7, 629–669. [Google Scholar] [CrossRef]
- Schifano, E.; Cavallini, D.; De Bellis, G.; Bracciale, M.P.; Felici, A.C.; Santarelli, M.L.; Sarto, M.S.; Uccelletti, D. Antibacterial Effect of Zinc Oxide-Based Nanomaterials on Environmental Biodeteriogens Affecting Historical Buildings. Nanomaterials 2020, 10, 335. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Shi, L.; Zhang, F.; Li, S.; Zeng, R. Corrosion resistance of a self-healing multilayer film based on SiO2 and CeO2 nanoparticles layer-by-layer assembly on Mg alloys. Mater. Lett. 2019, 237, 14–18. [Google Scholar] [CrossRef]
- Kolahalam, L.A.; Viswanath, I.K.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y. Review on nanomaterials: Synthesis and applications. Mater. Today Proc. 2019, 18, 2182–2190. [Google Scholar] [CrossRef]
- Das, S.K.; Choi, S.U.S.; Yu, W.; Pradeep, T. Nanofluids, Science and Technology; Wiley Intersicence Publication: Hoboken, NJ, USA, 2007; ISBN 978-0-470-07473-2. [Google Scholar]
- Yin, Y.; Talapin, D. The chemistry of functional nanomaterials. Chem. Soc. Rev. 2013, 42, 2484–2487. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H. A review on nanofluids: Preparation, stability mechanisms, and applications. J. Nanomater. 2012, 2012, 1–17. [Google Scholar] [CrossRef]
- Wong, K.; De Leon, O. Applications of Nanofluids: Current and Future. Corp. Adv. Mech. Eng. 2010, 2010, 519659. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q. Heat transfer enhancement of nanofluids. Int. J. Heat Fluid Flow 2000, 21, 58–64. [Google Scholar] [CrossRef]
- Carrillo-González, R.; Martínez-Gómez, M.A.; González-Chávez, M.d.C.A.; Hernández, J.C.M. Inhibition of microorganisms involved the in deterioration of an archaeological site by silver nanoparticles produced by a green synthesis method. Sci. Total Environ. 2016, 565, 872–881. [Google Scholar] [CrossRef]
- Otero, J.; Starinieri, V.; Charola, A.E. Influence of substrate pore structure and nanolime particle size on the effectiveness of nanolime treatments. Constr. Build. Mater. 2019, 209, 701–708. [Google Scholar] [CrossRef]
- Borsoi, G.; Lubelli, B.; van Hees, R.; Veiga, R.; Silva, A.S. Understanding the transport of nanolime consolidants within Maastricht limestone. J. Cult. Herit. 2016, 18, 242–249. [Google Scholar] [CrossRef]
- Barberena-Fernandez, A.M.; Blanco-Varela, M.T.; Carmona-Quiroga, P.M. Use of nanosilica-or nanolime-additioned TEOS to consolidate cementitious materials in heritage structures: Physical and mechanical properties of mortars. Cem. Concr. Compos. 2019, 95, 271–276. [Google Scholar] [CrossRef]
- Barbhuiya, G.; Danish Hasan, S. Effect of nano-silica on physio-mechanical properties and microstructure of soil: A comprehensive review. Mater. Today Proc. 2021, 44, 217–221. [Google Scholar] [CrossRef]
- Konsta-Gdoutos, M.S. Nanomaterials in self-consolidating concrete: A state-of-the-art review. J. Sustain. Cem. Based Mater 2014, 3, 167–180. [Google Scholar] [CrossRef]
- Van der Werf, I.; Ditaranto, N.; Sportelli, M.C.; Sabbatini, L. Development of a novel conservation treatment of stone monuments with bioactive nanocomposites. Herit. Sci. 2015, 3, 29. [Google Scholar] [CrossRef]
- Li, Q.; Hu, Y.; Zhang, B. Hydrophilic ZnO Nanoparticle-Based Antimicrobial Coatings for Sandstone Heritage Conservation. CS Appl. Nano Mater 2021, 4, 13908–13918. [Google Scholar] [CrossRef]
- Cappitelli, F.; Villa, F.; Sanmartin, P. Interactions of microorganisms and synthetic polymers in cultural heritage conservation. Int. Biodeterior. Biodegrad. 2021, 163, 105282. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otero, J.; Borsoi, G.; Monasterio-Guillot, L. The Boom in Nanomaterials for Built Heritage Conservation: Why Does Size Matter? Materials 2023, 16, 3277. https://doi.org/10.3390/ma16083277
Otero J, Borsoi G, Monasterio-Guillot L. The Boom in Nanomaterials for Built Heritage Conservation: Why Does Size Matter? Materials. 2023; 16(8):3277. https://doi.org/10.3390/ma16083277
Chicago/Turabian StyleOtero, Jorge, Giovanni Borsoi, and Luis Monasterio-Guillot. 2023. "The Boom in Nanomaterials for Built Heritage Conservation: Why Does Size Matter?" Materials 16, no. 8: 3277. https://doi.org/10.3390/ma16083277
APA StyleOtero, J., Borsoi, G., & Monasterio-Guillot, L. (2023). The Boom in Nanomaterials for Built Heritage Conservation: Why Does Size Matter? Materials, 16(8), 3277. https://doi.org/10.3390/ma16083277







