Relevant Choices Affecting the Fatigue Analysis of Ni-Ti Endovascular Devices
Abstract
1. Introduction
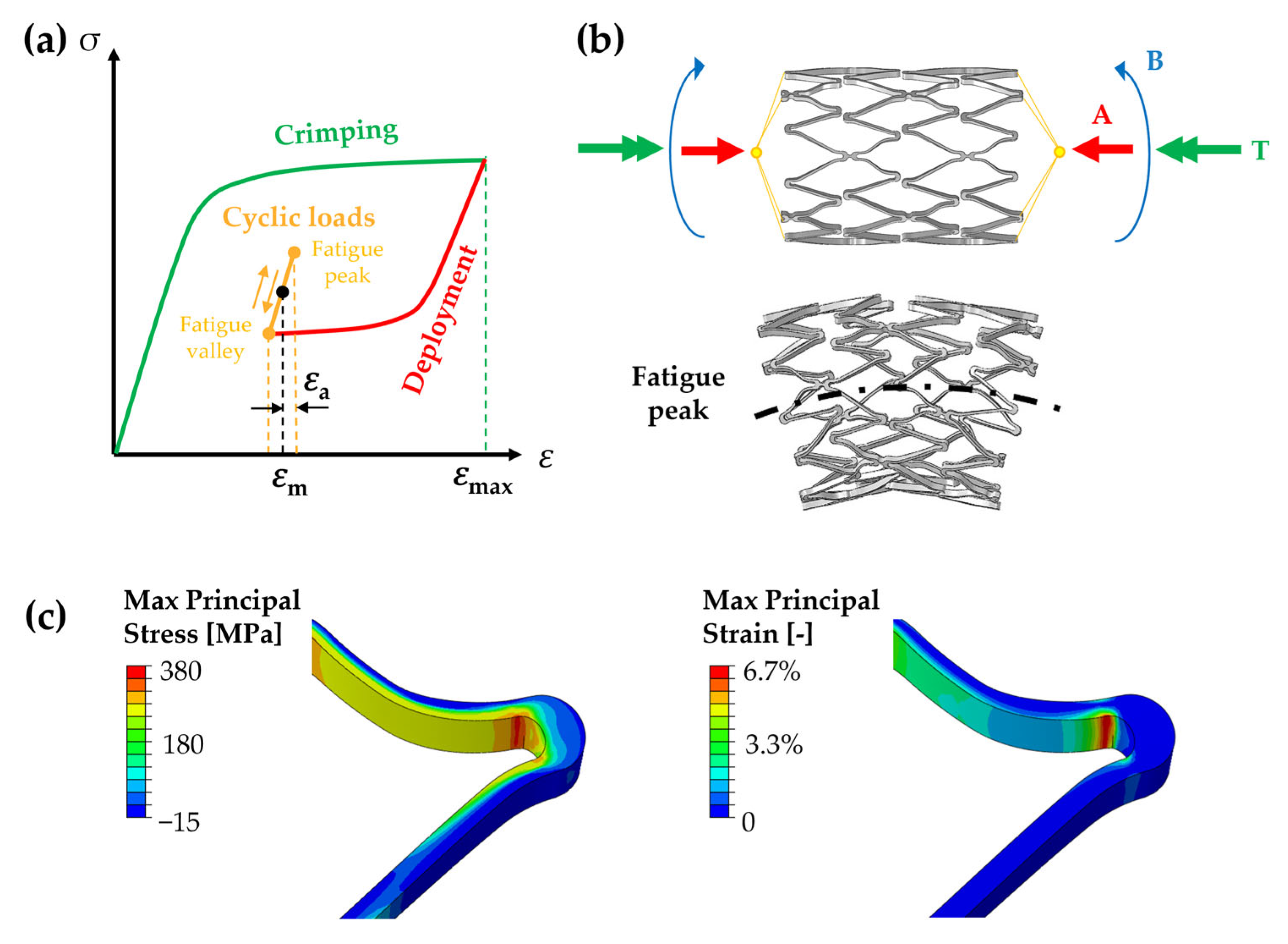
2. Experimental Aspects Affecting Fatigue Analysis
2.1. Type of Specimens
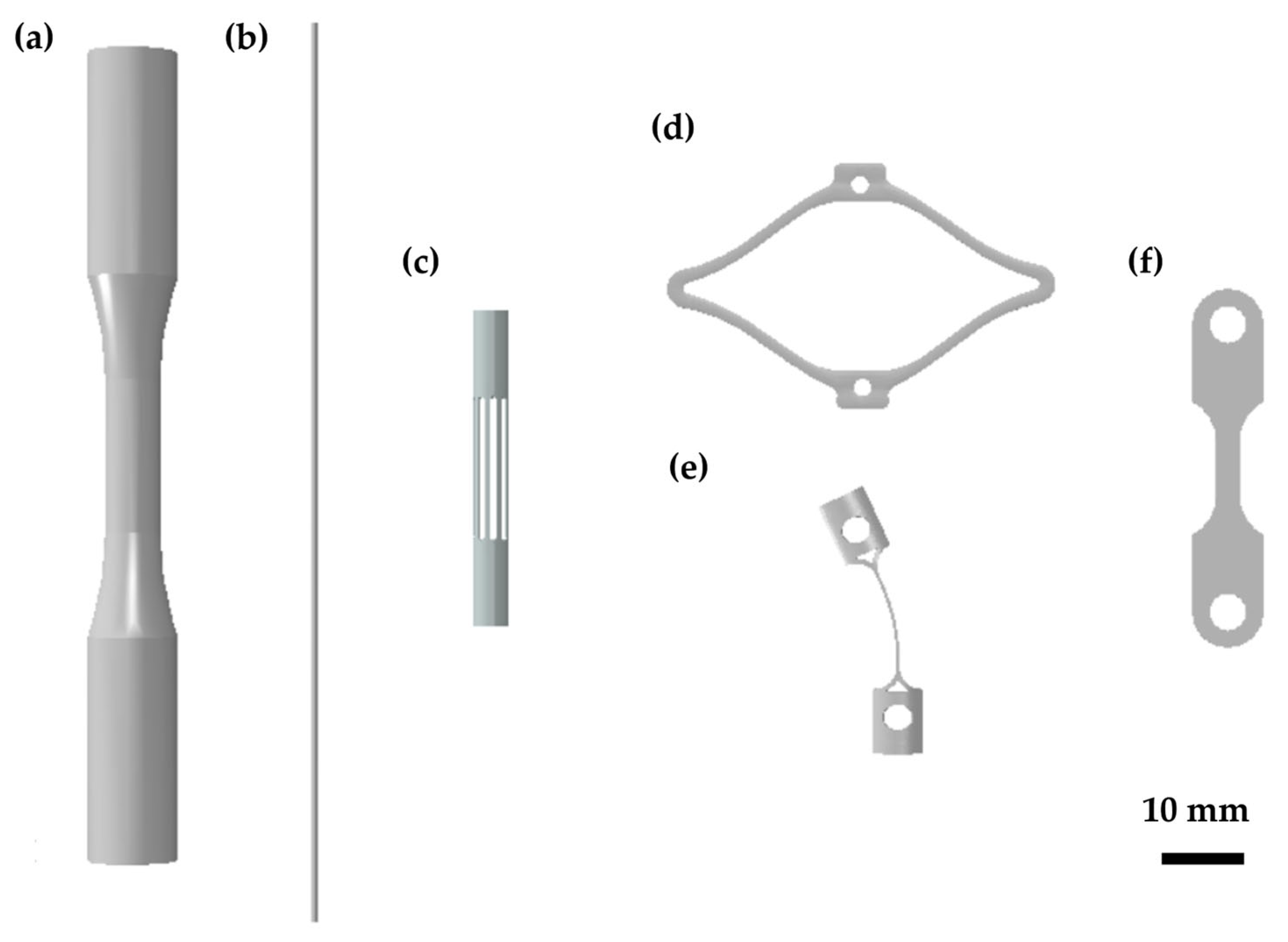
2.1.1. Bulk Samples
2.1.2. Single Wires
2.1.3. Laser-Cut Wires
2.1.4. Surrogate Specimens
2.1.5. Macroscopic Thin Samples
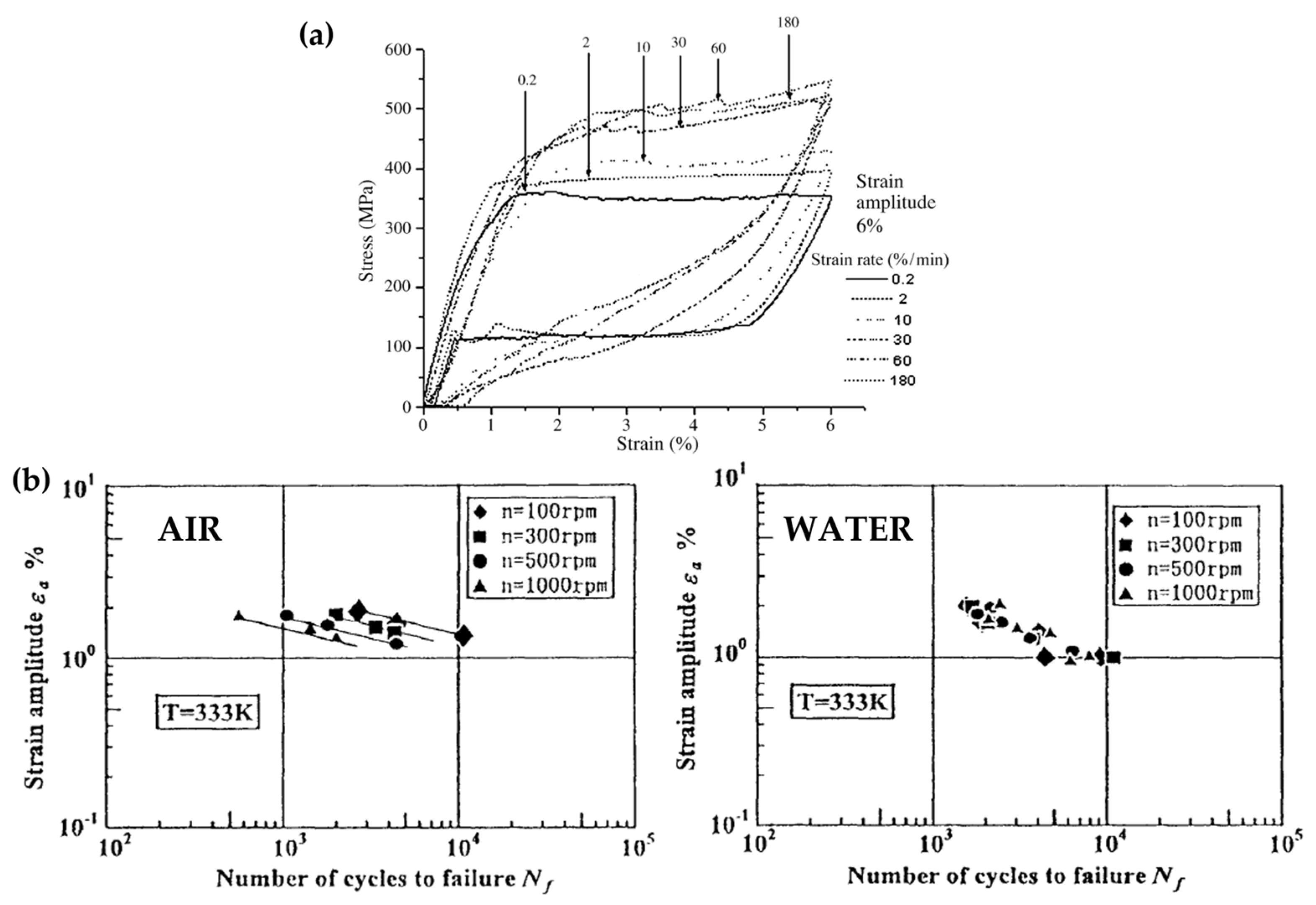
2.2. Testing Frequency and Environment
3. Numerical Aspects Affecting Fatigue Analysis
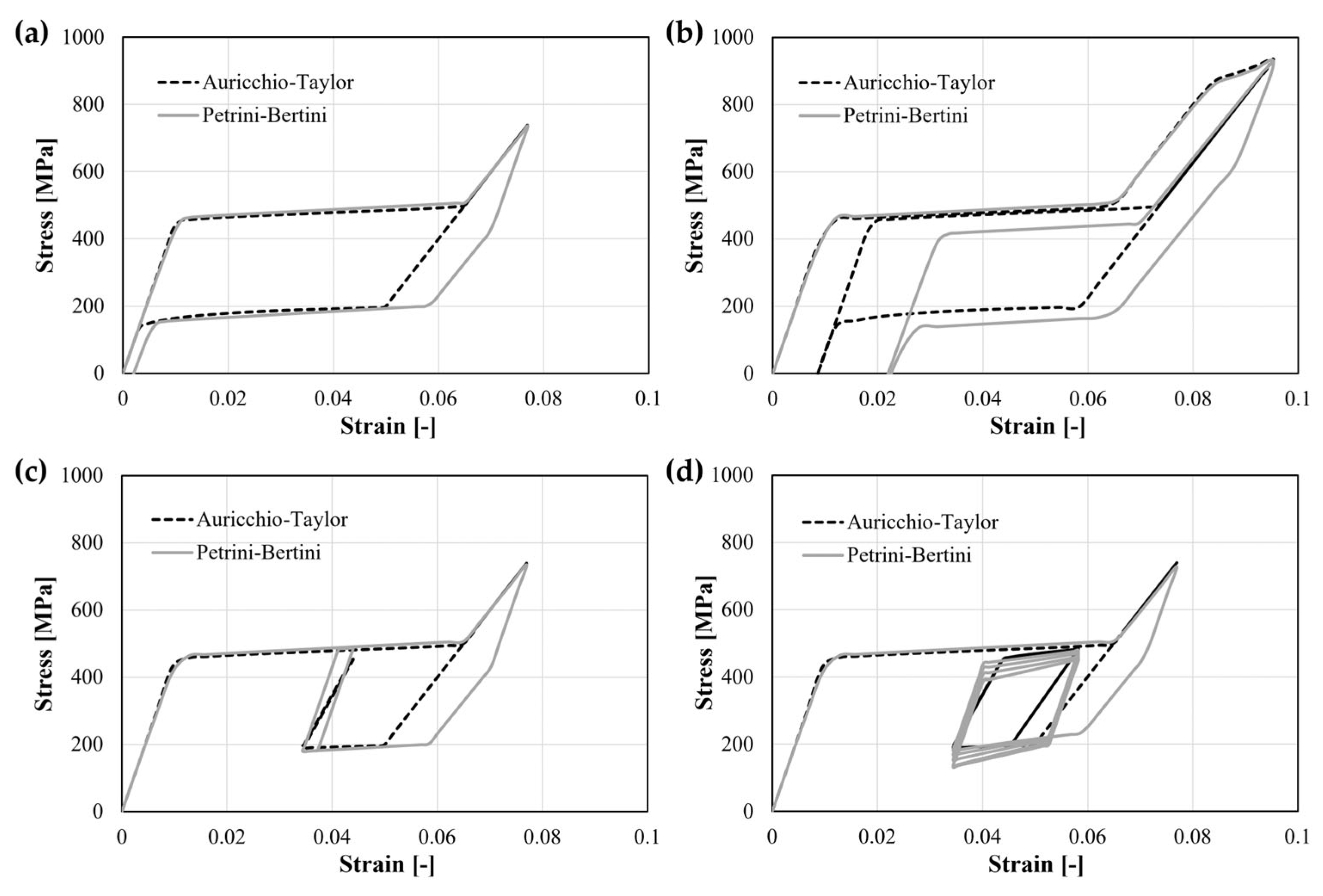
3.1. Model Reliability
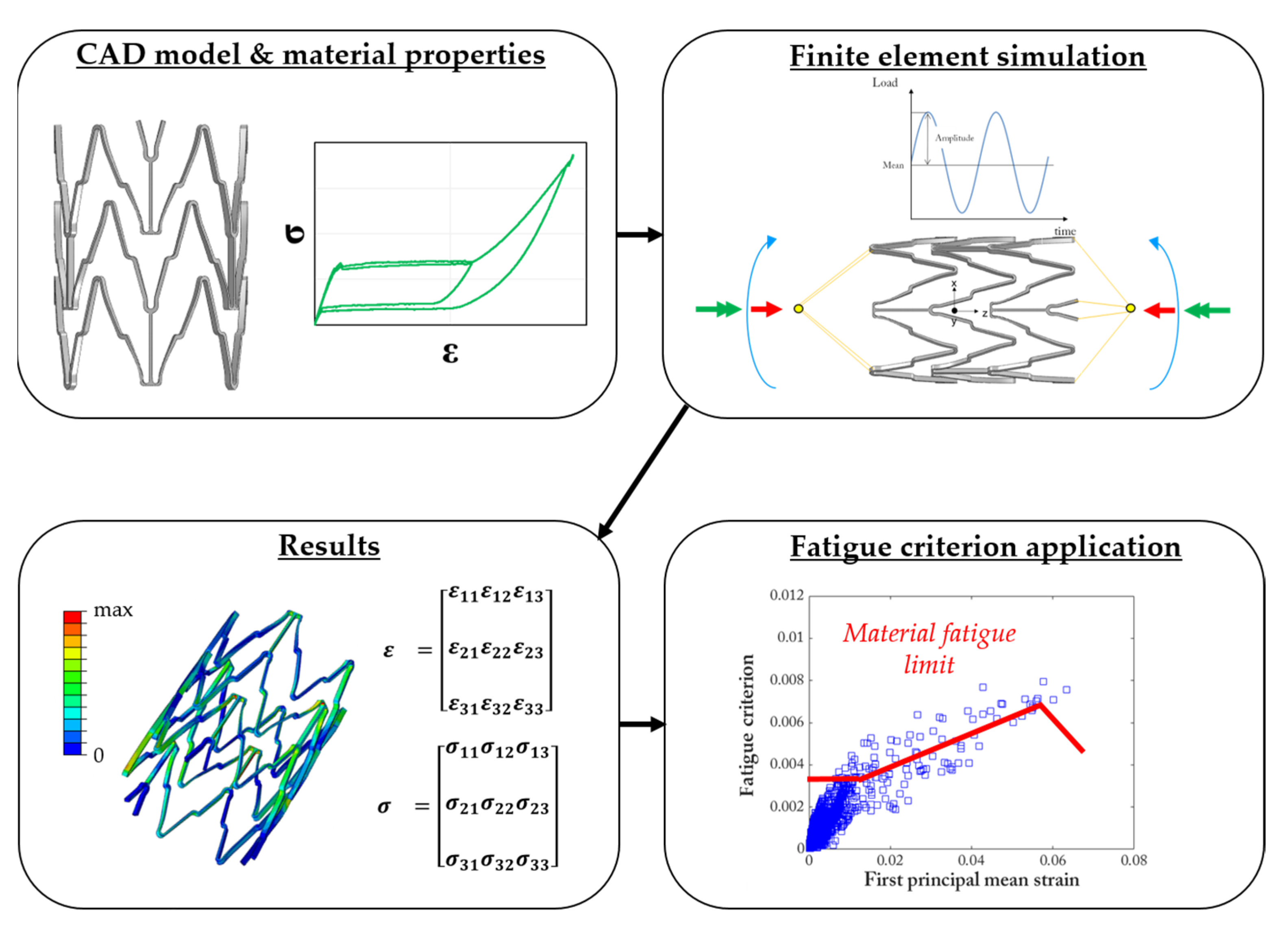
3.2. Simulation of Fatigue Tests
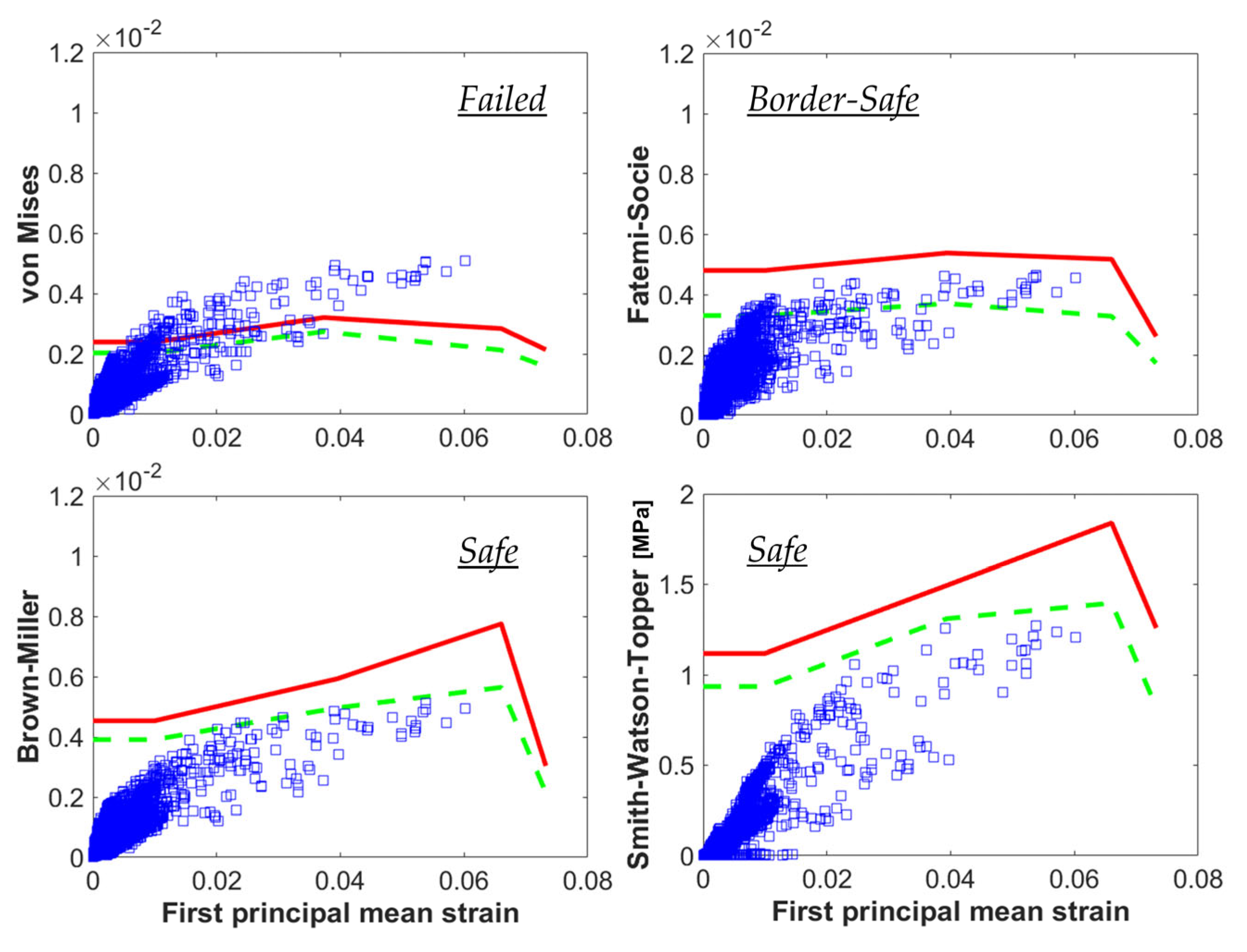
3.3. Fatigue Criteria
4. Conclusions
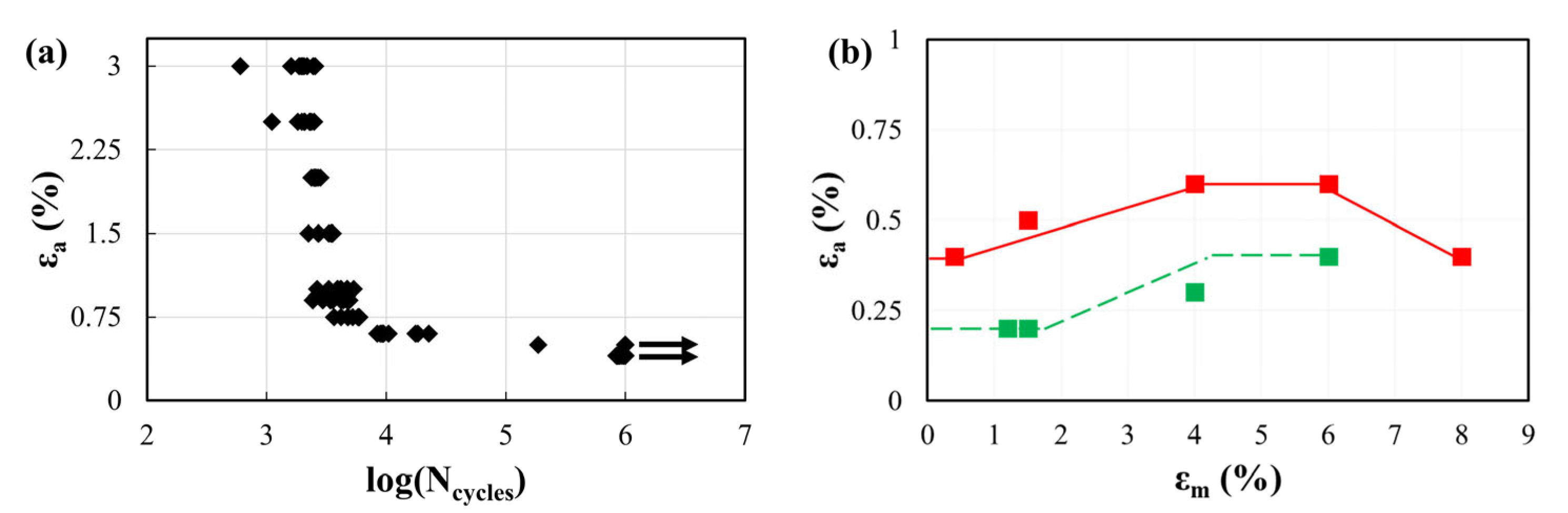
- Material fatigue characterization can be performed using different samples, whose design also inevitably affects the testing configuration (e.g., rotary bending, axial tension/compression, pure bending, etc.). This, in turn, leads to the generation of material fatigue limit data that should be discussed before use; for example, results from testing wire specimens overestimate the failure risk of a stent v-strut loaded under the same maximum strain. The samples that are closer to stents in terms of their geometry, strut dimension, manufacturing process, and loading conditions are the surrogate diamond-shaped samples, which, however, mandatorily require a finite element model to assess the local stress and strain;
- The need for accelerated durability tests opens questions regarding the material strain rate sensitivity, which alters the stress–strain behavior due to the intrinsic heat of the sample due to the fast and repeated lattice transformation. Standard regulators suggest performing tests in a liquid environment to allow a better heat exchange and to reduce such effects, which, however, cannot be discarded when working at values of tenths of a Hz;
- When preparing a device computational model, it is crucial to work with an accurate geometry associated with proper mechanical properties. However, due to the bending-dominated deformation of the v-struts, even minor discrepancies in the model might introduce sensible differences in the global behavior. Moreover, different constitutive formulations for shape-memory alloys are available in the literature that are able to differently account for the complex material phenomenology, thus, affecting the numerical outcomes;
- The choice of easily reproducible and controllable experiments is of paramount importance for comparing simulation results with the reality of interest;
- For conducting a computational fatigue analysis, a proper fatigue criterion should be selected for the interpretation of the local tensors of stress and strain. There are no available criteria specifically formulated for Ni-Ti and shape-memory alloys, and the choice of different approaches might lead to different outcomes. It is recommendable to prefer those criteria that are able to account for complex multiaxial loading conditions, which are known as critical plane-based criteria.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Humbeeck, J. Shape Memory Materials: State of the Art and Requirements for Future Applications. J. Phys. IV 1997, 7, C5-3–C5-12. [Google Scholar] [CrossRef]
- Duerig, T.; Pelton, A.; Stöckel, D. An Overview of Nitinol Medical Applications. Mater. Sci. Eng. 1999, 273–275, 149–160. [Google Scholar] [CrossRef]
- Auricchio, F.; Constantinescu, A.; Conti, M.; Scalet, G. Fatigue of Metallic Stents: From Clinical Evidence to Computational Analysis. Ann. Biomed. Eng. 2016, 44, 287–301. [Google Scholar] [CrossRef] [PubMed]
- MacTaggart, J.N.; Phillips, N.Y.; Lomneth, C.S.; Pipinos, I.I.; Bowen, R.; Timothy Baxter, B.; Johanning, J.; Matthew Longo, G.; Desyatova, A.S.; Moulton, M.J.; et al. Three-Dimensional Bending, Torsion and Axial Compression of the Femoropopliteal Artery during Limb Flexion. J. Biomech. 2014, 47, 2249–2256. [Google Scholar] [CrossRef]
- Duda, S.H.; Bosiers, M.; Lammer, J.; Scheinert, D.; Zeller, T.; Oliva, V.; Tielbeek, A.; Anderson, J.; Wiesinger, B.; Tepe, G.; et al. Drug-Eluting and Bare Nitinol Stents for the Treatment of Atherosclerotic Lesions in the Superficial Femoral Artery: Long-Term Results from the SIROCCO Trial. J. Endovasc. Ther. 2006, 13, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Babaev, A.; Hari, P.; Zavlunova, S.; Kurayev, A. Role of Nitinol Stent Fractures in the Development of In-Stent Restenosis in the Superficial Femoral Artery. JACC Cardiovasc. Interv. 2014, 7, S35. [Google Scholar] [CrossRef]
- Petrini, L.; Trotta, A.; Dordoni, E.; Migliavacca, F.; Dubini, G.; Lawford, P.V.; Gosai, J.N.; Ryan, D.M.; Testi, D.; Pennati, G. A Computational Approach for the Prediction of Fatigue Behaviour in Peripheral Stents: Application to a Clinical Case. Ann. Biomed. Eng. 2016, 44, 536–547. [Google Scholar] [CrossRef]
- Garcia-Toca, M.; Rodriguez, H.E.; Naughton, P.A.; Keeling, A.; Phade, S.V.; Morasch, M.D.; Kibbe, M.R.; Eskandari, M.K. Are Carotid Stent Fractures Clinically Significant? Cardiovasc. Interv. Radiol. 2012, 35, 263–267. [Google Scholar] [CrossRef]
- Scheinert, D.; Scheinert, S.; Sax, J.; Piorkowski, C.; Bräunlich, S.; Ulrich, M.; Biamino, G.; Schmidt, A. Prevalence and Clinical Impact of Stent Fractures after Femoropopliteal Stenting. J. Am. Coll. Cardiol. 2005, 45, 312–315. [Google Scholar] [CrossRef]
- Gökgöl, C.; Schumann, S.; Diehm, N.; Zheng, G.; Büchler, P. In-Vivo Quantification of Femoro-Popliteal Artery Deformations: Percutaneous Transluminal Angioplasty vs. Nitinol Stent Placement. J. Endovasc. Ther. 2016, 24, 27–34. [Google Scholar] [CrossRef]
- ASTM E1823-13; Standard Terminology Relating to Fatigue and Fracture Testing. ASTM International: West Conshohocken, PA, USA, 2013.
- Eggeler, G.; Hornbogen, E.; Yawny, A.; Heckmann, A.; Wagner, M. Structural and Functional Fatigue of NiTi Shape Memory Alloys. Mater. Sci. Eng. A 2004, 378, 24–33. [Google Scholar] [CrossRef]
- Berti, F.; Spagnoli, A.; Petrini, L. A Numerical Investigation on Multiaxial Fatigue Assessment of Nitinol Peripheral Endovascular Devices with Emphasis on Load Non-Proportionality Effects. Eng. Fract. Mech. 2019, 216, 106512. [Google Scholar] [CrossRef]
- Berti, F.; Wang, P.J.; Spagnoli, A.; Pennati, G.; Migliavacca, F.; Edelman, E.R.; Petrini, L. Nickel–Titanium Peripheral Stents: Which Is the Best Criterion for the Multi-Axial Fatigue Strength Assessment? J. Mech. Behav. Biomed. Mater. 2021, 113, 104142. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services; Food and Drug Administration; Centre for Devices and Radiological Health Guidance Document for Industry and FDA Staff. Non-Clinical Engineering Tests and Recommended Labeling for Intravascular Stents and Associated Delivery Systems; U.S. Food and Drug Administration: Rockwall, MD, USA, 2010; pp. 18–20. [Google Scholar]
- US Department of Health and Human Services; Food and Drug Administration; Centre for Devices and Radiological Health Guidance Document for Industry and FDA Staff. Technical Considerations for Non-Clinical Assessment of Medical Devices Containing Nitinol; U.S. Food and Drug Administration: Rockwall, MD, USA, 2021. [Google Scholar]
- ASME V&V 10; Guide for Verification and Validation in Computational Solid Mechanics. ASME: New York, NY, USA, 2006.
- ASME V&V 40; Assessing Credibility of Computational Modeling Through Verification and Validation: Application to Medical Devices. ASME: New York, NY, USA, 2018.
- Bonsignore, C. Present and Future Approaches to Lifetime Prediction of Superelastic Nitinol. Theor. Appl. Fract. Mech. 2017, 92, 298–305. [Google Scholar] [CrossRef]
- Pelton, A.R. Nitinol Fatigue: A Review of Microstructures and Mechanisms. J. Mater. Eng. Perform. 2011, 20, 613–617. [Google Scholar] [CrossRef]
- Mahtabi, M.J.; Shamsaei, N. A Modified Energy-Based Approach for Fatigue Life Prediction of Superelastic NiTi in Presence of Tensile Mean Strain and Stress. Int. J. Mech. Sci. 2016, 117, 321–333. [Google Scholar] [CrossRef]
- Mahtabi, M.J.; Shamsaei, N. Fatigue Modeling for Superelastic NiTi Considering Cyclic Deformation and Load Ratio Effects. Shape Mem. Superelasticity 2017, 3, 250–263. [Google Scholar] [CrossRef]
- Schaffer, J.E.; Plumley, D.L. Fatigue Performance of Nitinol Round Wire with Varying Cold Work Reductions. J. Mater. Eng. Perform. 2009, 18, 563–568. [Google Scholar] [CrossRef]
- Pelton, A.R.; Fino-decker, J.; Vien, L.; Bonsignore, C.; Saffari, P. Rotary-Bending Fatigue Characteristics of Medical-Grade Nitinol Wire. J. Mech. Behav. Biomed. Mater. 2013, 27, 19–32. [Google Scholar] [CrossRef]
- Senthilnathan, K.; Shamimi, A.; Bonsignore, C.; Paranjape, H.; Duerig, T. Effect of Prestrain on the Fatigue Life of Superelastic Nitinol. J. Mater. Eng. Perform. 2019, 28, 5946–5958. [Google Scholar] [CrossRef]
- Allegretti, D.; Berti, F.; Migliavacca, F.; Pennati, G.; Petrini, L. Fatigue Assessment of Nickel–Titanium Peripheral Stents: Comparison of Multi-Axial Fatigue Models. Shape Mem. Superelasticity 2018, 4, 186–196. [Google Scholar] [CrossRef]
- Berti, F.; Brambilla, A.; Porcellato, R.; Patriarca, L.; Petrini, L. Nickel-Titanium Peripheral Stents: Can Fracture Mechanics Shed Light on Their Fatigue Failure? Procedia Struct. Integr. 2022, 42, 722–729. [Google Scholar] [CrossRef]
- Pelton, A.R.; Schroeder, V.; Mitchell, M.R.; Gong, X.Y.; Barney, M.; Robertson, S.W. Fatigue and Durability of Nitinol Stents. J. Mech. Behav. Biomed. Mater. 2008, 1, 153–164. [Google Scholar] [CrossRef]
- Cao, H.; Wu, M.H.; Zhou, F.; McMeeking, R.M.; Ritchie, R.O. The Influence of Mean Strain on the High-Cycle Fatigue of Nitinol with Application to Medical Devices. J. Mech. Phys. Solids 2020, 143, 104057. [Google Scholar] [CrossRef]
- Runciman, A.; Xu, D.; Pelton, A.R.; Ritchie, R.O. An Equivalent Strain/Coffin-Manson Approach to Multiaxial Fatigue and Life Prediction in Superelastic Nitinol Medical Devices. Biomaterials 2011, 32, 4987–4993. [Google Scholar] [CrossRef]
- Catoor, D.; Ma, Z.; Kumar, S. Cyclic Response and Fatigue Failure of Nitinol under Tension–Tension Loading. J. Mater. Res. 2019, 34, 3504–3522. [Google Scholar] [CrossRef]
- ASTM E 606-04; Standard Practice for Strain-Controlled Fatigue Testing. ASTM International: West Conshohocken, PA, USA, 2004.
- Patriarca, L.; Foletti, S.; Beretta, S. A Comparison of DIC-Based Techniques to Measure Crack Closure in LCF. Theor. Appl. Fract. Mech. 2018, 98, 230–243. [Google Scholar] [CrossRef]
- Robertson, S.W.; Gong, X.Y.; Ritchie, R.O. Effect of Product Form and Heat Treatment on the Crystallographic Texture of Austenitic Nitinol. J. Mater. Sci. 2006, 41, 621–630. [Google Scholar] [CrossRef]
- Ritchie, R.O.; Lankford, J. Small Fatigue Cracks: A Statement of the Problem and Potential Solutions. Mater. Sci. Eng. 1986, 84, 11–16. [Google Scholar] [CrossRef]
- Saidane, K.; Polizu, S.; Yahia, L. Accelerated Fatigue Behavior and Mechano-Physical Characterizations of in Vitro Physiological Simulation of Nitinol Stents. J. Appl. Biomater. Biomech. 2007, 5, 117–124. [Google Scholar]
- Müller-Hülsbeck, S.; Schäfer, P.J.; Charalambous, N.; Yagi, H.; Heller, M.; Jahnke, T. Comparison of Second-Generation Stents for Application in the Superficial Femoral Artery: An In Vitro Evaluation Focusing on Stent Design. J. Endovasc. Ther. 2010, 17, 767–776. [Google Scholar] [CrossRef]
- Senol, K.; Cao, H.; Tripathy, S. Characterization and Validation of Fatigue Strains for Superelastic Nitinol Using Digital Image Correlation. J. Med. Device. 2021, 15, 041005. [Google Scholar] [CrossRef]
- Zhang, Y.; You, Y.; Moumni, Z.; Anlas, G.; Zhu, J.; Zhang, W. Experimental and Theoretical Investigation of the Frequency Effect on Low Cycle Fatigue of Shape Memory Alloys. Int. J. Plast. 2017, 90, 1–30. [Google Scholar] [CrossRef]
- Xiao, Y.; Zeng, P.; Lei, L.; Du, H. Experimental Investigation on Rate Dependence of Thermomechanical Response in Superelastic NiTi Shape Memory Alloy. J. Mater. Eng. Perform. 2015, 24, 3755–3760. [Google Scholar] [CrossRef]
- Elibol, C.; Wagner, M.F.X. Strain Rate Effects on the Localization of the Stress-Induced Martensitic Transformation in Pseudoelastic NiTi under Uniaxial Tension, Compression and Compression-Shear. Mater. Sci. Eng. A 2015, 643, 194–202. [Google Scholar] [CrossRef]
- Kan, Q.; Yu, C.; Kang, G.; Li, J.; Yan, W. Experimental Observations on Rate-Dependent Cyclic Deformation of Super-Elastic NiTi Shape Memory Alloy. Mech. Mater. 2016, 97, 48–58. [Google Scholar] [CrossRef]
- Dayananda, G.N.; Rao, M.S. Effect of Strain Rate on Properties of Superelastic NiTi Thin Wires. Mater. Sci. Eng. A 2008, 486, 96–103. [Google Scholar] [CrossRef]
- Sidharth, R.; Mohammed, A.S.K.; Abuzaid, W.; Sehitoglu, H. Unraveling Frequency Effects in Shape Memory Alloys: NiTi and FeMnAlNi. Shape Mem. Superelasticity 2021, 7, 235–249. [Google Scholar] [CrossRef]
- Pelton, A.R.; DiCello, J.; Miyazaki, S. Optimisation of Processing and Properties of Medical Grade Nitinol Wire. Minim. Invasive Ther. Allied Technol. 2000, 9, 107–118. [Google Scholar] [CrossRef]
- Tobushi, H.; Hachisuka, T.; Yamada, S.; Lin, P.H. Rotating-Bending Fatigue of a TiNi Shape-Memory Alloy Wire. Mech. Mater. 1997, 26, 35–42. [Google Scholar] [CrossRef]
- Shaw, J.A.; Kyriakides, S. Thermomechanical Aspects of NiTi. J. Mech. Phys. Solids 1995, 43, 1243–1281. [Google Scholar] [CrossRef]
- Yu, C.; Kang, G.; Kan, Q.; Zhu, Y. Rate-Dependent Cyclic Deformation of Super-Elastic NiTi Shape Memory Alloy: Thermo-Mechanical Coupled and Physical Mechanism-Based Constitutive Model. Int. J. Plast. 2015, 72, 60–90. [Google Scholar] [CrossRef]
- Morrison, T.M.; Hariharan, P.; Funkhouser, C.M.; Afshari, P.; Goodin, M.; Horner, M. Assessing Computational Model Credibility Using a Risk-Based Framework: Application to Hemolysis in Centrifugal Blood Pumps. ASAIO J. 2019, 65, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Viceconti, M.; Juarez, M.; Curreli, C.; Pennisi, M.; Russo, G.; Pappalardo, F. POSITION PAPER: Credibility of In Silico Trial Technologies—A Theoretical Framing. IEEE J. Biomed. Health Inform. 2019, 24, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Azaouzi, M.; Makradi, A.; Belouettar, S. Deployment of a Self-Expanding Stent inside an Artery: A Finite Element Analysis. Mater. Des. 2012, 41, 410–420. [Google Scholar] [CrossRef]
- Conti, M.; Auricchio, F.; De Beule, M.; Verhegghe, B. Numerical Simulation of Nitinol Peripheral Stents: From Laser-Cutting to Deployment in a Patient Specific Anatomy. ESOMAT 2009, 2009, 6008. [Google Scholar] [CrossRef]
- Harvey, S.M. Nitinol Stent Fatigue in a Peripheral Human Artery Subjected to Pulsatile and Articulation Loading. J. Mater. Eng. Perform. 2011, 20, 697–705. [Google Scholar] [CrossRef]
- Rebelo, N.; Fu, R.; Lawrenchuk, M. Study of a Nitinol Stent Deployed into Anatomically Accurate Artery Geometry and Subjected to Realistic Service Loading. J. Mater. Eng. Perform. 2009, 18, 655–663. [Google Scholar] [CrossRef]
- Dordoni, E.; Meoli, A.; Wu, W.; Dubini, G.; Migliavacca, F.; Pennati, G.; Petrini, L. Fatigue Behaviour of Nitinol Peripheral Stents: The Role of Plaque Shape Studied with Computational Structural Analyses. Med. Eng. Phys. 2014, 36, 842–849. [Google Scholar] [CrossRef]
- Wu, W.; Qi, M.; Liu, X.P.; Yang, D.Z.; Wang, W.Q. Delivery and Release of Nitinol Stent in Carotid Artery and Their Interactions: A Finite Element Analysis. J. Biomech. 2007, 40, 3034–3040. [Google Scholar] [CrossRef]
- Arrigoni, M.; Auricchio, F.; Cacciafesta, V.; Petrini, L.; Pietrabissa, R. Mechanical Characterisation of Orthodontic Superelastic Ni-Ti Wires. Strain 2001, 11, 577–582. [Google Scholar] [CrossRef]
- Mckelvey, A.L.; Ritchie, R.O. Fatigue-Crack Propagation in Nitinol: A Shape-Memory and Superelastic Endovascular Stent Material Fatigue-Crack Propagation in Nitinol, a Shape-Memory and Superelastic Endovascular Stent Material. J. Biomed. Mater. Res. 1999, 47, 301–308. [Google Scholar] [CrossRef]
- Drexel, M.J.; Selvaduray, G.S.; Pelton, A.R. The Effects of Cold Work and Heat Treatment on the Properties of Nitinol Wire. Proc. Int. Conf. Shape Mem. Superelastic Technol. 2006, 447–454, 89–90. [Google Scholar]
- Lagoudas, D.; Hartl, D.; Chemisky, Y.; Machado, L.; Popov, P. Constitutive Model for the Numerical Analysis of Phase Transformation in Polycrystalline Shape Memory Alloys. Int. J. Plast. 2012, 32–33, 155–183. [Google Scholar] [CrossRef]
- Petrini, L.; Bertini, A. A Three-Dimensional Phenomenological Model Describing Cyclic Behavior of Shape Memory Alloys. Int. J. Plast. 2020, 125, 348–373. [Google Scholar] [CrossRef]
- Woodworth, L.A.; Wang, X.; Lin, G.; Kaliske, M. A Multi-Featured Shape Memory Alloy Constitutive Model Incorporating Tension–Compression Asymmetric Interpolation. Mech. Mater. 2022, 172, 104392. [Google Scholar] [CrossRef]
- Auricchio, F.; Taylor, R.L. Shape-Memory Alloys: Modelling and Numerical Simulations of the Finite-Strain Superelastic Behavior. Comput. Methods Appl. Mech. Eng. 1997, 143, 175–194. [Google Scholar] [CrossRef]
- BSI Standards BS EN ISO 25539-2; Cardiovascular Implants—Endovascular Devices. BSI Group: London, UK, 2020.
- Nikanorov, A.; Smouse, H.B.; Osman, K.; Bialas, M.; Shrivastava, S.; Schwartz, L.B. Fracture of Self-Expanding Nitinol Stents Stressed in Vitro under Simulated Intravascular Conditions. J. Vasc. Surg. 2008, 48, 435–440. [Google Scholar] [CrossRef]
- Berti, F.; Antonini, L.; Poletti, G.; Fiuza, C.; Vaughan, T.J.; Migliavacca, F.; Petrini, L.; Pennati, G. How to Validate in Silico Deployment of Coronary Stents: Strategies and Limitations in the Choice of Comparator. Front. Med. Technol. 2021, 3, 702656. [Google Scholar] [CrossRef]
- Meoli, A.; Dordoni, E.; Petrini, L.; Migliavacca, F.; Dubini, G.; Pennati, G. Computational Study of Axial Fatigue for Peripheral Nitinol Stents. J. Mater. Eng. Perform. 2014, 23, 2606–2613. [Google Scholar] [CrossRef]
- Ho, K.L.; Hung, M.Y.; Chen, J.H.; Jian, Y.M.; Hsiao, H.M. Design and Testing of a New Vascular Stent with Enhanced Fatigue Life. IOP Conf. Ser. Mater. Sci. Eng. 2019, 644, 012015. [Google Scholar] [CrossRef]
- Lei, L.; Qi, X.; Li, S.; Yang, Y.; Hu, Y.; Li, B.; Zhao, S.; Zhang, Y. Finite Element Analysis for Fatigue Behaviour of a Self-Expanding Nitinol Peripheral Stent under Physiological Biomechanical Conditions. Comput. Biol. Med. 2019, 104, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Mahtabi, M.J.; Shamsaei, N.; Mitchell, M.R. Fatigue of Nitinol: The State-of-the-Art and Ongoing Challenges. J. Mech. Behav. Biomed. Mater. 2015, 50, 228–254. [Google Scholar] [CrossRef] [PubMed]
- Susmel, L. Multiaxial Notch Fatigue; Woodhead Publishing Limited: Sawston, UK, 2009; ISBN 9781845693008. [Google Scholar]
- Suresh, S. Fatigue of Materials; Elsevier: Amsterdam, The Netherlands, 1998; ISBN 9780511806575. [Google Scholar]
- Fatemi, A.; Socie, D.F. A Critical Plane Approach to Multiaxial Fatigue Damage Including Out-of-Phase Loading. Fatigue Fract. Eng. Mater. Struct. 1988, 11, 149–165. [Google Scholar] [CrossRef]
- Gates, N.R.; Fatemi, A. Interaction of Shear and Normal Stresses in Multiaxial Fatigue Damage Analysis. Frat. Ed Integrita Strutt. 2016, 10, 160–165. [Google Scholar] [CrossRef]
- Shahriar, S.; Fatemi, A. On the Interaction of Normal and Shear Stress in Multiaxial Fatigue Damage. Fatigue Fract. Eng. Mater. Struct. 2019, 42, 2000–2016. [Google Scholar] [CrossRef]
- Brown, M.W.; Miller, K.J. A Theory for Fatigue Failure under Multiaxial Stress-Strain Conditions. Proc. Inst. Mech. Eng. 1973, 187, 745. [Google Scholar] [CrossRef]
- Papadopoulos, I.V. Critical Plane Approaches in High-Cycle Fatigue: On the Definition of the Amplitude and Mean Value of the Shear Stress Acting on the Critical Plane. Fatigue Fract. Eng. Mater. Struct. 1998, 21, 269–285. [Google Scholar] [CrossRef]
- Carpinteri, A.; Ronchei, C.; Spagnoli, A.; Vantadori, S. On the Use of the Prismatic Hull Method in a Critical Plane-Based Multiaxial Fatigue Criterion. Int. J. Fatigue 2014, 68, 159–167. [Google Scholar] [CrossRef]
- Smith, K.N.; Topper, T.H.; Watson, P. A Stress–Strain Function for the Fatigue of Metals (Stress-Strain Function for Metal Fatigue Including Mean Stress Effect). J Mater. 1970, 5, 767–778. [Google Scholar] [CrossRef]
- Socie, D. Multiaxial Fatigue Damage Models. J. Eng. Mater. Technol. 1987, 109, 293. [Google Scholar] [CrossRef]
- Calhoun, C.; Wheeler, R.; Baxevanis, T.; Lagoudas, D.C. Actuation Fatigue Life Prediction of Shape Memory Alloys under the Constant-Stress Loading Condition. Scr. Mater. 2015, 95, 58–61. [Google Scholar] [CrossRef]
- Auricchio, F.; Constantinescu, A.; Menna, C.; Scalet, G. A Shakedown Analysis of High Cycle Fatigue of Shape Memory Alloys. Int. J. Fatigue 2016, 87, 112–123. [Google Scholar] [CrossRef]
- Scalet, G.; Menna, C.; Constantinescu, A.; Auricchio, F. A Computational Approach Based on a Multiaxial Fatigue Criterion Combining Phase Transformation and Shakedown Response for the Fatigue Life Assessment of Nitinol Stents. J. Intell. Mater. Syst. Struct. 2018, 29, 3710–3724. [Google Scholar] [CrossRef]
- Robertson, S.W.; Pelton, A.R.; Ritchie, R.O. Mechanical Fatigue and Fracture of Nitinol. Int. Mater. Rev. 2012, 57, 1–37. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berti, F.; Brambilla, A.; Pennati, G.; Petrini, L. Relevant Choices Affecting the Fatigue Analysis of Ni-Ti Endovascular Devices. Materials 2023, 16, 3178. https://doi.org/10.3390/ma16083178
Berti F, Brambilla A, Pennati G, Petrini L. Relevant Choices Affecting the Fatigue Analysis of Ni-Ti Endovascular Devices. Materials. 2023; 16(8):3178. https://doi.org/10.3390/ma16083178
Chicago/Turabian StyleBerti, Francesca, Alma Brambilla, Giancarlo Pennati, and Lorenza Petrini. 2023. "Relevant Choices Affecting the Fatigue Analysis of Ni-Ti Endovascular Devices" Materials 16, no. 8: 3178. https://doi.org/10.3390/ma16083178
APA StyleBerti, F., Brambilla, A., Pennati, G., & Petrini, L. (2023). Relevant Choices Affecting the Fatigue Analysis of Ni-Ti Endovascular Devices. Materials, 16(8), 3178. https://doi.org/10.3390/ma16083178






