Formation of Ag-Fe Bimetallic Nano-Species on Mordenite Depending on the Initial Ratio of Components
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization Methods
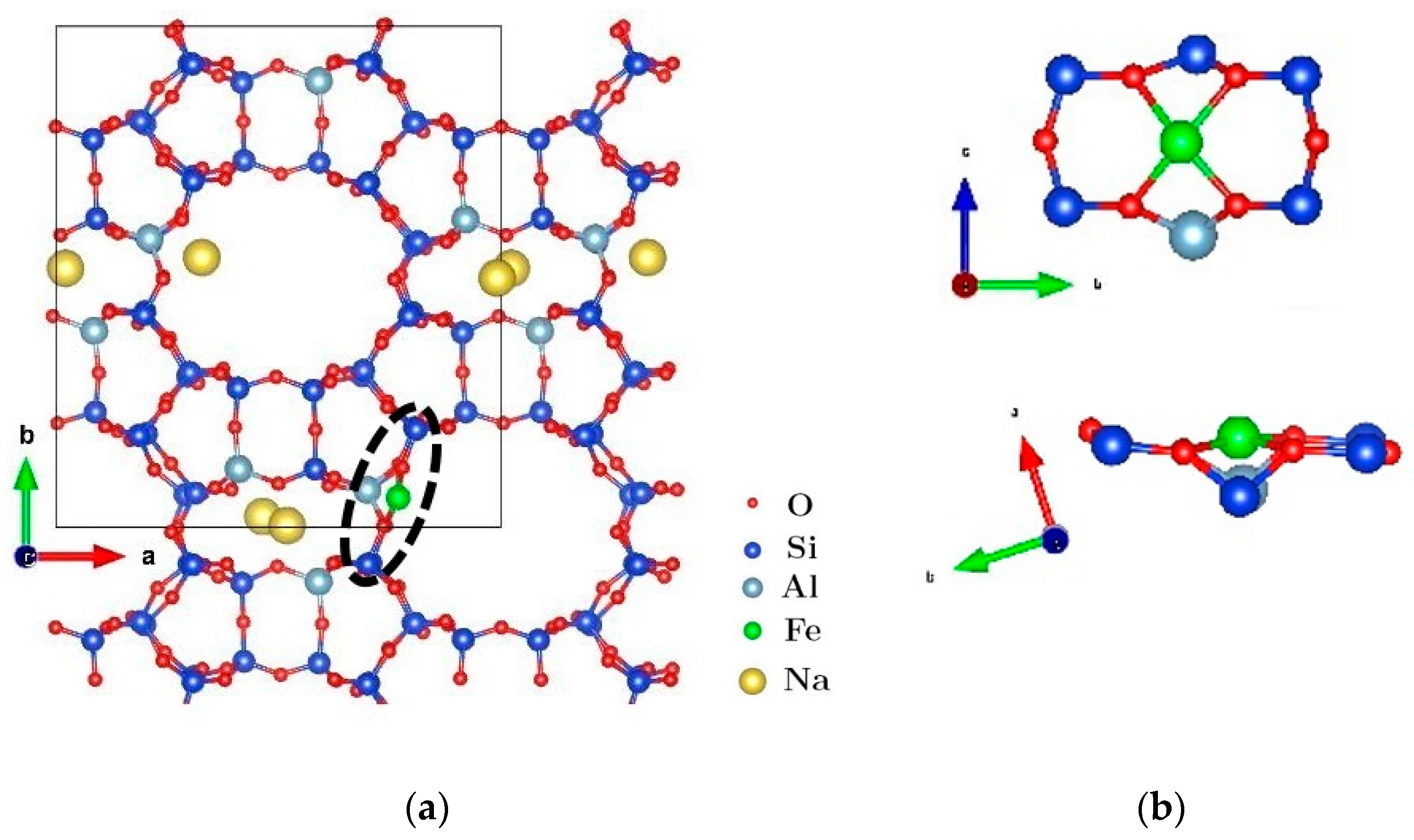
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rashid, T.; Iqbal, D.; Hazafa, A.; Hussain, S.; Sher, F.; Sher, F. Formulation of Zeolite Supported Nano-Metallic Catalyst and Applications in Textile Effluent Treatment. J. Environ. Chem. Eng. 2020, 8, 104023. [Google Scholar] [CrossRef]
- Guan, Q.; Zhu, C.; Lin, Y.; Vovk, E.I.; Zhou, X.; Yang, Y.; Yu, H.; Cao, L.; Wang, H.; Zhang, X.; et al. Bimetallic Monolayer Catalyst Breaks the Activity–Selectivity Trade-off on Metal Particle Size for Efficient Chemoselective Hydrogenations. Nat. Catal. 2021, 4, 840–849. [Google Scholar] [CrossRef]
- Bogdanchikova, N.; Pestryakov, A.; Tuzovskaya, I.; Zepeda, T.A.; Farias, M.H.; Tiznado, H.; Martynyuk, O. Effect of Redox Treatments on Activation and Deactivation of Gold Nanospecies Supported on Mesoporous Silica in CO Oxidation. Fuel 2013, 110, 40–47. [Google Scholar] [CrossRef]
- Sánchez-López, P.; Kotolevich, Y.; Yocupicio-Gaxiola, R.I.; Antúnez-García, J.; Chowdari, R.K.; Petranovskii, V.; Fuentes-Moyado, S. Recent Advances in Catalysis Based on Transition Metals Supported on Zeolites. Front. Chem. 2021, 9, 716745. [Google Scholar] [CrossRef]
- Cui, W.; Hu, T. Incorporation of Active Metal Species in Crystalline Porous Materials for Highly Efficient Synergetic Catalysis. Small 2020, 17, 2003971. [Google Scholar] [CrossRef]
- Gates, B.C.; Flytzani-Stephanopoulos, M.; Dixon, D.A.; Katz, A. Atomically Dispersed Supported Metal Catalysts: Perspectives and Suggestions for Future Research. Catal. Sci. Technol. 2017, 7, 4259–4275. [Google Scholar] [CrossRef]
- Ding, J.; Liu, Q.; Ye, R.; Gong, W.; Zhang, F.; He, X.; Zhang, Y.; Zhong, Q.; Argyle, M.D.; Fan, M. Metal–Support Interactions in Fe–Cu–K Admixed with SAPO-34 Catalysts for Highly Selective Transformation of CO2 and H2 into Lower Olefins. J. Mater. Chem. A 2021, 9, 21877–21887. [Google Scholar] [CrossRef]
- Hamdan, M.; Halawy, L.; Abdel Karim Aramouni, N.; Ahmad, M.N.; Zeaiter, J. Analytical Review of the Catalytic Cracking of Methane. Fuel 2022, 324, 124455. [Google Scholar] [CrossRef]
- Crisafulli, C.; Scirè, S.; Minicò, S.; Solarino, L. Ni–Ru Bimetallic Catalysts for the CO2 Reforming of Methane. Appl. Catal. A Gen. 2002, 225, 1–9. [Google Scholar]
- Xu, H.; Wu, P. New Progress in Zeolite Synthesis and Catalysis. Natl. Sci. Rev. 2022, 9, nwac045. [Google Scholar] [CrossRef]
- Iwasawa, Y. In Situ Characterization of Supported Metal Catalysts and Model Surfaces by Time-Resolved and Three-Dimensional XAFS Techniques. J. Catal. 2003, 216, 165–177. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y. Research advances on strong metal-support interactions at metal-oxide interfaces and their roles in regulating catalytic properties of noble metal-ceria supported catalysts. J. Chin. Rare Earth Soc. 2014, 32, 129–142. [Google Scholar]
- Sánchez-López, P.; Kotolevich, Y.; Miridonov, S.; Chávez-Rivas, F.; Fuentes, S.; Petranovskii, V. Bimetallic AgFe Systems on Mordenite: Effect of Cation Deposition Order in the NO Reduction with C3H6/CO. Catalysts 2019, 9, 58. [Google Scholar] [CrossRef]
- Sánchez-López, P.; Kotolevich, Y.; Khramov, E.; Chowdari, R.K.; Estrada, M.A.; Berlier, G.; Zubavichus, Y.; Fuentes, S.; Petranovskii, V.; Chávez-Rivas, F. Properties of Iron-Modified-by-Silver Supported on Mordenite as Catalysts for NOx Reduction. Catalysts 2020, 10, 1156. [Google Scholar] [CrossRef]
- Sánchez-López, P.; Kotolevich, Y.; Antúnez-García, J.; Chávez-Rivas, F.; Khramov, E.; Berlier, G.; Moreno-Ruiz, L.; Zubavichus, Y.; Petranovskii, V.; Fuentes-Moyado, S.; et al. Influence of Components Deposition Order on Silver Species Formation in Bimetallic Ag-Fe System Supported on Mordenite. Catalysts 2022, 12, 1453. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Ding, G.; Zheng, H.; Li, X.; Li, Y.; Zhu, Y. One-step Conversion of Fructose to Furfuryl Alcohol in a Continuous Fixed-bed Reactor: The Important Role of Supports. ChemCatChem 2019, 11, 2118–2125. [Google Scholar] [CrossRef]
- Naraki, Y.; Ariga, K.; Oka, H.; Kurashige, H.; Sano, T. An Isomorphously Substituted Fe-BEA Zeolite with High Fe Content: Facile Synthesis and Characterization. J. Nanosci. Nanotechnol. 2018, 18, 11–19. [Google Scholar] [CrossRef]
- Kumon, A.; Abidin, Z.; Matsue, N. Synthesis of Iron Substituted Zeolite with Na-P1 Framework. J. Porous Mater. 2016, 24, 1061–1068. [Google Scholar] [CrossRef]
- Snyder, B.E.R.; Vanelderen, P.; Bols, M.L.; Hallaert, S.D.; Böttger, L.H.; Ungur, L.; Pierloot, K.; Schoonheydt, R.A.; Sels, B.F.; Solomon, E.I. The Active Site of Low-Temperature Methane Hydroxylation in Iron-Containing Zeolites. Nature 2016, 536, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Shelyapina, M.G.; Gurgul, J.; Łątka, K.; Sánchez-López, P.; Bogdanov, D.; Kotolevich, Y.; Petranovskii, V.; Fuentes, S. Mechanism of Formation of Framework Fe3+ in Bimetallic Ag-Fe Mordenites—Effective Catalytic Centers for deNOx Reaction. Microporous Mesoporous Mater. 2020, 299, 109841. [Google Scholar] [CrossRef]
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. The State of the Art in Selective Catalytic Reduction of NOx by Ammonia Using Metal-Exchanged Zeolite Catalysts. Catal. Rev. 2008, 50, 492–531. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data Analysis for X-ray Absorption Spectroscopy Using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Newville, M. IFEFFIT: Interactive XAFS Analysis and FEFF Fitting. J. Synchrotron Radiat. 2001, 8, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, P.; Antúnez-García, J.; Fuentes-Moyado, S.; Galván, D.H.; Petranovskii, V.; Chávez-Rivas, F. Analysis of theoretical and experimental X-ray diffraction patterns for distinct mordenite frameworks. J. Mater. Sci. 2019, 54, 7745–7757. [Google Scholar] [CrossRef]
- Kuroda, Y.; Mori, T.; Sugiyama, H.; Uozumi, Y.; Ikeda, K.; Itadani, A.; Nagao, M. On the Possibility of AgZSM-5 Zeolite Being a Partial Oxidation Catalyst for Methane. J. Colloid Interface Sci. 2009, 333, 294–299. [Google Scholar] [CrossRef]
- Matsuoka, M.; Ju, W.S.; Yamashita, H.; Anpo, M. In situ characterization of the Ag+ ion-exchanged zeolites and their photocatalytic activity for the decomposition of N2O into N2and O2 at 298 K. J. Photochem. Photobiol. A Chem. 2003, 160, 43–46. [Google Scholar] [CrossRef]
- Matsuoka, M.; Matsuda, E.; Tsuji, K.; Yamashita, H.; Anpo, M. The photocatalytic decomposition of nitric oxide on Ag+/ZSM-5 catalyst prepared by ion-exchange. J. Mol. Catal. A Chem 1996, 107, 399–403. [Google Scholar] [CrossRef]
- Aspromonte, S.G.; Serra, R.M.; Miró, E.E.; Boix, A.V. AgNaMordenite catalysts for hydrocarbon adsorption and deNOx processes. Appl. Catal. A Gen. 2011, 407, 134–144. [Google Scholar] [CrossRef]
- Spivey, J.J.; Dooley, K.M. (Eds.) Catalysis; Royal Society of Chemistry: London, UK, 2010. [Google Scholar]
- Texter, J.; Hastrelter, J.J.; Hall, J.L. Spectroscopic Confirmation of the Tetrahedral Geometry of Ag(H2O)4+. J. Phys. Chem. 1983, 87, 4690–4693. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Davydov, A.A. Study of supported silver states by the method of electron spectroscopy of diffuse reflectance. J. Electron. Spectrosc. Relat. Phenom. 1995, 74, 195–199. [Google Scholar] [CrossRef]
- Ju, W.-S.; Matsuoka, M.; Iino, K.; Yamashita, H.; Anpo, M. Surface Structure of Hydroxylated and Sulfated Zirconia. A Periodic Density-Functional Study. J. Phys. Chem. B 2004, 108, 14652–14662. [Google Scholar]
- Sklenak, S.; Andrikopoulos, P.C.; Boekfa, B.; Jansang, B.; Nováková, J.; Benco, L.; Bucko, T.; Hafner, J.; Dědeček, J.; Sobalík, Z. N2O Decomposition over Fe-Zeolites: Structure of the Active Sites and the Origin of the Distinct Reactivity of Fe-Ferrierite, Fe-ZSM-5, and Fe-Beta. A Combined Periodic DFT and Multispectral Study. J. Catal. 2010, 272, 262–274. [Google Scholar] [CrossRef]
- Chávez-Rivas, F.; Rodríguez-Fuentes, G.; Berlier, G.; Rodríguez-Iznaga, I.; Petranovskii, V.; Zamorano-Ulloa, R.; Coluccia, S. Evidence for Controlled Insertion of Fe Ions in the Framework of Clinoptilolite Natural Zeolites. Microporous Mesoporous Mater. 2013, 167, 76–81. [Google Scholar] [CrossRef]
- Kumar, M.S.; Schwidder, M.; Grünert, W.; Bentrup, U.; Brückner, A. Selective reduction of NO with Fe-ZSM-5 catalysts of low Fe content: Part II. Assessing the function of different Fe sites by spectroscopic in situ studies. J. Catal. 2006, 239, 173–186. [Google Scholar]
- Bordiga, S.; Buzzoni, R.; Geobaldo, F.; Lamberti, C.; Giamello, E.; Zecchina, A.; Leofanti, G.; Petrini, G.; Tozzola, G.; Vlaic, G. Structure and Reactivity of Framework and Extraframework Iron in Fe-Silicalite as Investigated by Spectroscopic and Physicochemical Methods. J. Catal. 1996, 158, 486–501. [Google Scholar] [CrossRef]
- Setyawati, I.A.; Rettig, S.J.; Orvig, C. Cationic Iron(III) Complex with a Hexadentate N2,N′2′,O2-Aminopyridylphenolate Ligand. Canadian. J. Chem. 1999, 77, 2033–2038. [Google Scholar]
- Ameur, N.; Bachir, R.; Bedrane, S.; Choukchou-Braham, A. A Green Route to Produce Adipic Acid on TiO2-Fe2O3 Nanocomposites. J. Chin. Chem. Soc. 2017, 64, 1096–1103. [Google Scholar] [CrossRef]
- Concepción-Rosabal, B.; Rodríguez-Fuentes, G.; Bogdanchikova, N.; Bosch, P.; Avalos, M.; Lara, V.H. Comparative Study of Natural and Synthetic Clinoptilolites Containing Silver in Different States. Microporous Mesoporous Mater. 2005, 86, 249–255. [Google Scholar] [CrossRef]
- Gurin, V.S.; Petranovskii, V.P.; Bogdanchikova, N.E. Metal Clusters and Nanoparticles Assembled in Zeolites: An Example of Stable Materials with Controllable Particle Size. Mater. Sci. Eng. C 2002, 19, 327–331. [Google Scholar] [CrossRef]
- López Bastidas, C.; Smolentseva, E.; Machorro, R.; Petranovskii, V. Optical Spectra of Noble Metal Nanoparticles Supported on Zeolites; SPIE Proceedings: Bellingham, WA, USA, 2014. [Google Scholar]
- Smolentseva, E.; López-Bastidas, C.; Petranovskii, V.; Machorro, R. Plasmon Resonance of Gold Nanoparticles Supported on Y-Zeolite in the Presence of Various Co-Cations. Appl. Surf. Sci. 2014, 321, 136–143. [Google Scholar] [CrossRef]
- Gurin, V.S.; Petranovskii, V.P.; Hernandez, M.-A.; Bogdanchikova, N.E.; Alexeenko, A.A. Silver and Copper Clusters and Small Particles Stabilized within Nanoporous Silicate-Based Materials. Mater. Sci. Eng. A 2005, 391, 71–76. [Google Scholar] [CrossRef]
- Li, Z.; Flytzani-Stephanopoulos, M. On the Promotion of Ag–ZSM-5 by Cerium for the SCR of NO by Methane. J. Catal. 1999, 182, 313–327. [Google Scholar] [CrossRef]
- Sazama, P.; Capek, L.; Drobna, H.; Sobalik, Z.; Dedecek, J.; Arve, K.; Wichterlova, B. Enhancement of Decane-SCR-NO over Ag/Alumina by Hydrogen. Reaction Kinetics and in Situ FTIR and UV–Vis Study. J. Catal. 2005, 232, 302–317. [Google Scholar] [CrossRef]
- Lysenko, V.S.; Mal’nev, A.F. Optical characteristics of metal blacks. J. Appl. Spectrosc. 1969, 10, 838–845. [Google Scholar] [CrossRef]
- Rodríguez-Iznaga, I.; Petranovskii, V.; Castillón-Barraza, F.; Concepción-Rosabal, B. Copper-Silver Bimetallic System on Natural Clinoptilolite: Thermal Reduction of Cu2+ and Ag+ Exchanged. J. Nanosci. Nanotechnol. 2011, 11, 5580–5586. [Google Scholar] [CrossRef] [PubMed]
- Rtimi, S.; Baghriche, O.; Sanjines, R.; Pulgarin, C.; Bensimon, M.; Kiwi, J. TiON and TiON-Ag Sputtered Surfaces Leading to Bacterial Inactivation under Indoor Actinic Light. J. Photochem. Photobiol. A Chem. 2013, 256, 52–63. [Google Scholar] [CrossRef]
- Mejía, M.I.; Restrepo, G.; Marín, J.M.; Sanjines, R.; Pulgarín, C.; Mielczarski, E.; Mielczarski, J.; Kiwi, J. Magnetron-Sputtered Ag Surfaces. New Evidence for the Nature of the Ag Ions Intervening in Bacterial Inactivation. ACS Appl. Mater. Interfaces 2010, 2, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D. Handbook of X-ray Photoelectron Spectroscopy; Wanger, C.D., Riggs, W.M., Davis, L.E., Moulder, J.F., Muilenberg, G.E., Eds.; Perkin-Elmer Corp.: Eden Prairie, MN, USA, 1979; 190p. [Google Scholar]
- Lopez-Salido, I.; Lim, D.C.; Kim, Y.D. Ag Nanoparticles on Highly Ordered Pyrolytic Graphite (HOPG) Surfaces Studied Using STM and XPS. Surf. Sci. 2005, 588, 6–18. [Google Scholar] [CrossRef]
- Lim, D.C.; Lopez-Salido, I.; Kim, Y.D. Size Selectivity for CO-Oxidation of Ag Nanoparticles on Highly Ordered Pyrolytic Graphite (HOPG). Surf. Sci. 2005, 598, 96–103. [Google Scholar] [CrossRef]
- Wu, H.; Feng, Q.; Yang, H.; Alam, E.; Gao, B.; Gu, D. Modified Biochar Supported Ag/Fe Nanoparticles Used for Removal of Cephalexin in Solution: Characterization, Kinetics and Mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2017, 517, 63–71. [Google Scholar] [CrossRef]
- Shibata, J.; Shimizu, K.; Takada, Y.; Shichi, A.; Yoshida, H.; Satokawa, S.; Satsuma, A.; Hattori, T. Structure of active Ag clusters in Ag zeolites for SCR of NO by propane in the presence of hydrogen. J. Catal. 2004, 227, 367–374. [Google Scholar] [CrossRef]
- Suzuki, Y.; Miyanaga, T.; Hoshino, H.; Matsumoto, N.; Ainai, T. In-Situ XAFS Study of Ag Clusters in Zeolite 4A. Phys. Scr. 2005, T115, 765–768. [Google Scholar] [CrossRef]
- Yamamoto, T.; Takenaka, S.; Tanaka, T.; Baba, T. Stability of silver cluster in zeolite A and Y catalysts. J. Phys. Conf. Ser. 2009, 190, 012171. [Google Scholar] [CrossRef]
- Ju, W.-S.; Matsuoka, M.; Iino, K.; Yamashita, H.; Anpo, M. The Local Structures of Silver(I) Ion Catalysts Anchored within Zeolite Cavities and Their Photocatalytic Reactivities for the Elimination of N2O into N2 and O2. J. Phys. Chem. 2004, 108, 2128–2133. [Google Scholar] [CrossRef]
- Antúnez-García, J.; Galván, D.H.; Petranovskii, V.; Murrieta-Rico, F.N.; Yocupicio-Gaxiola, R.I.; Shelyapina, M.G.; Fuentes-Moyado, S. Aluminum Distribution in Mordenite-Zeolite Framework: A New Outlook Based on Density Functional Theory Calculations. J. Solid State Chem. 2022, 306, 122725. [Google Scholar] [CrossRef]
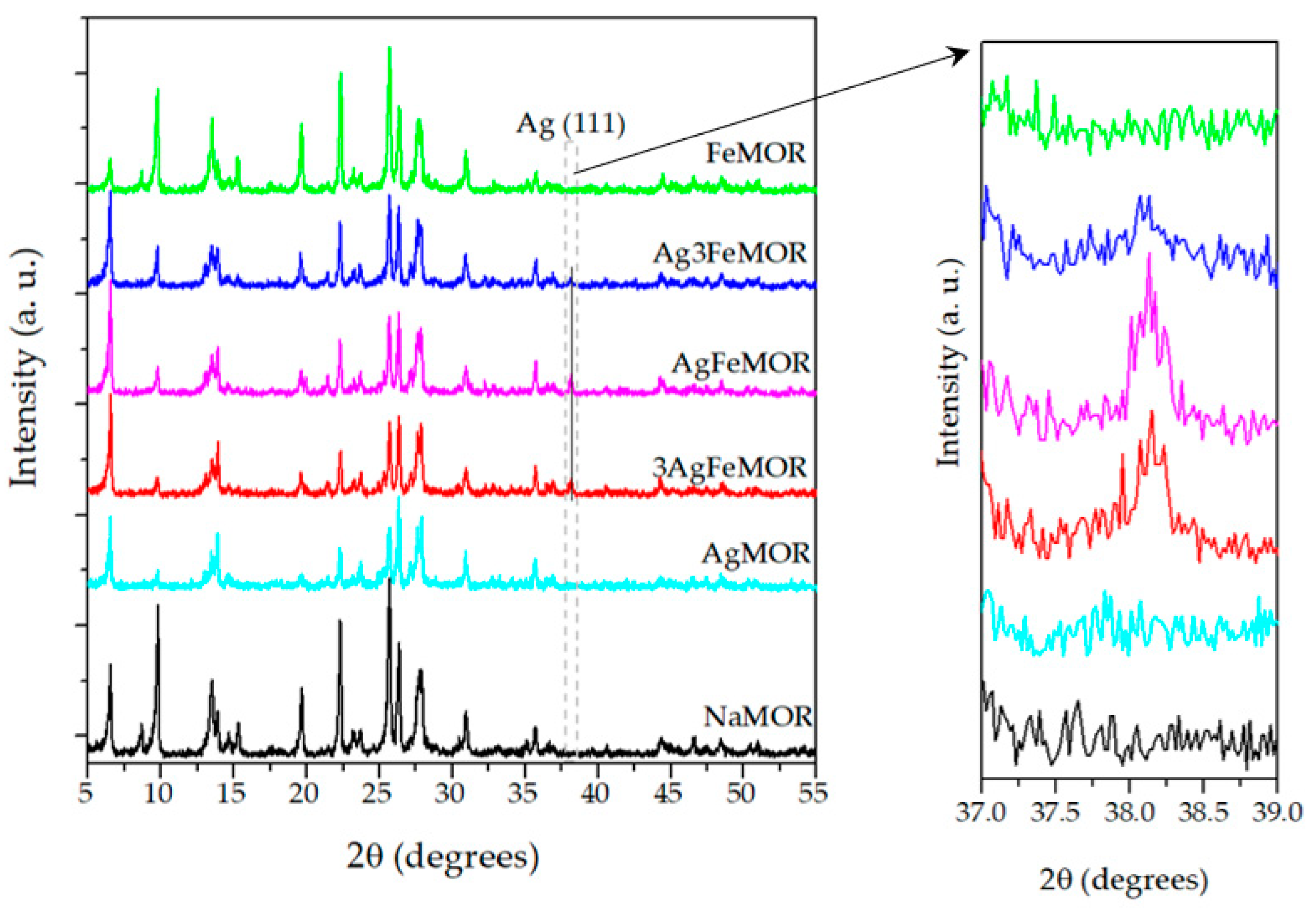

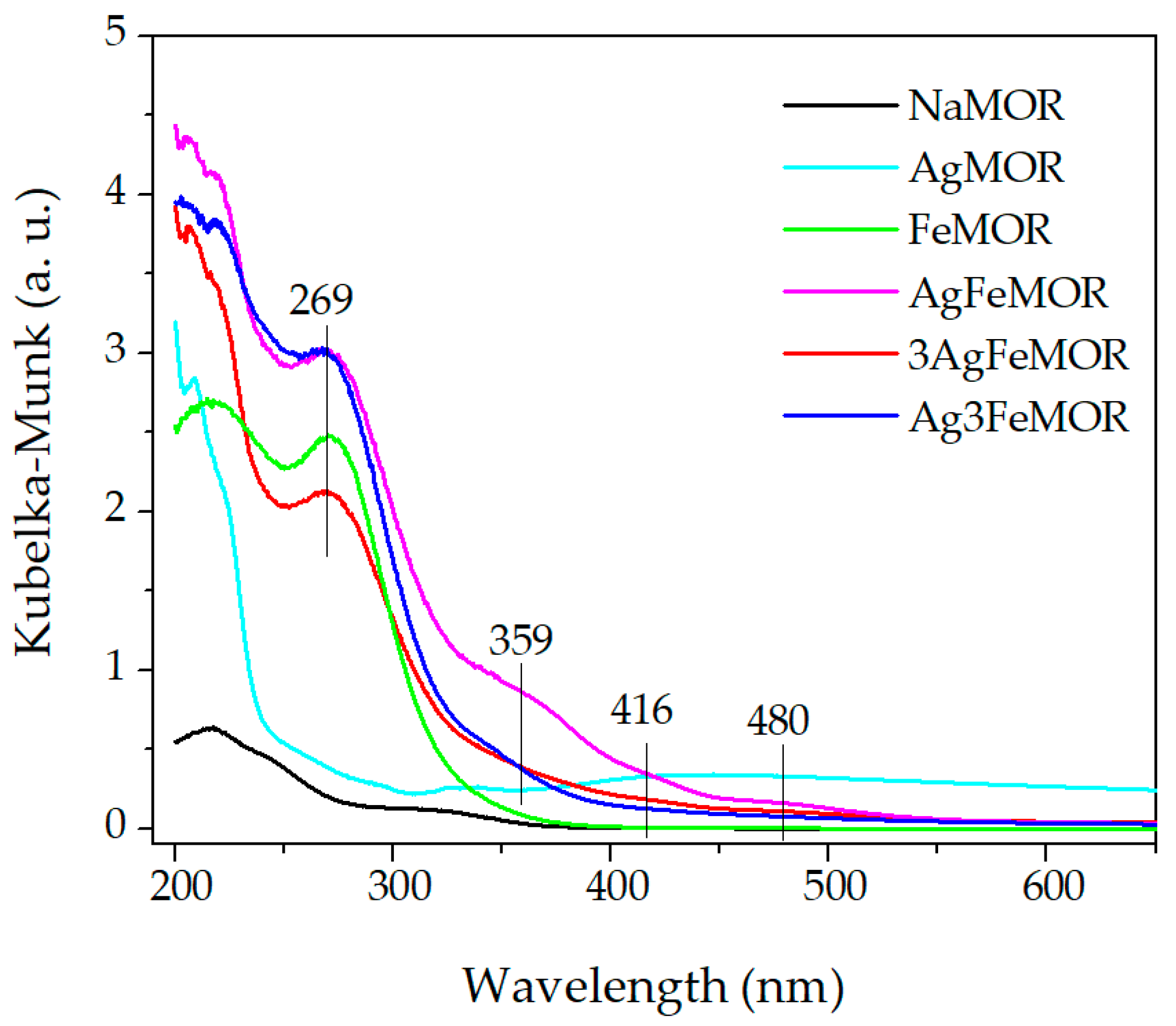
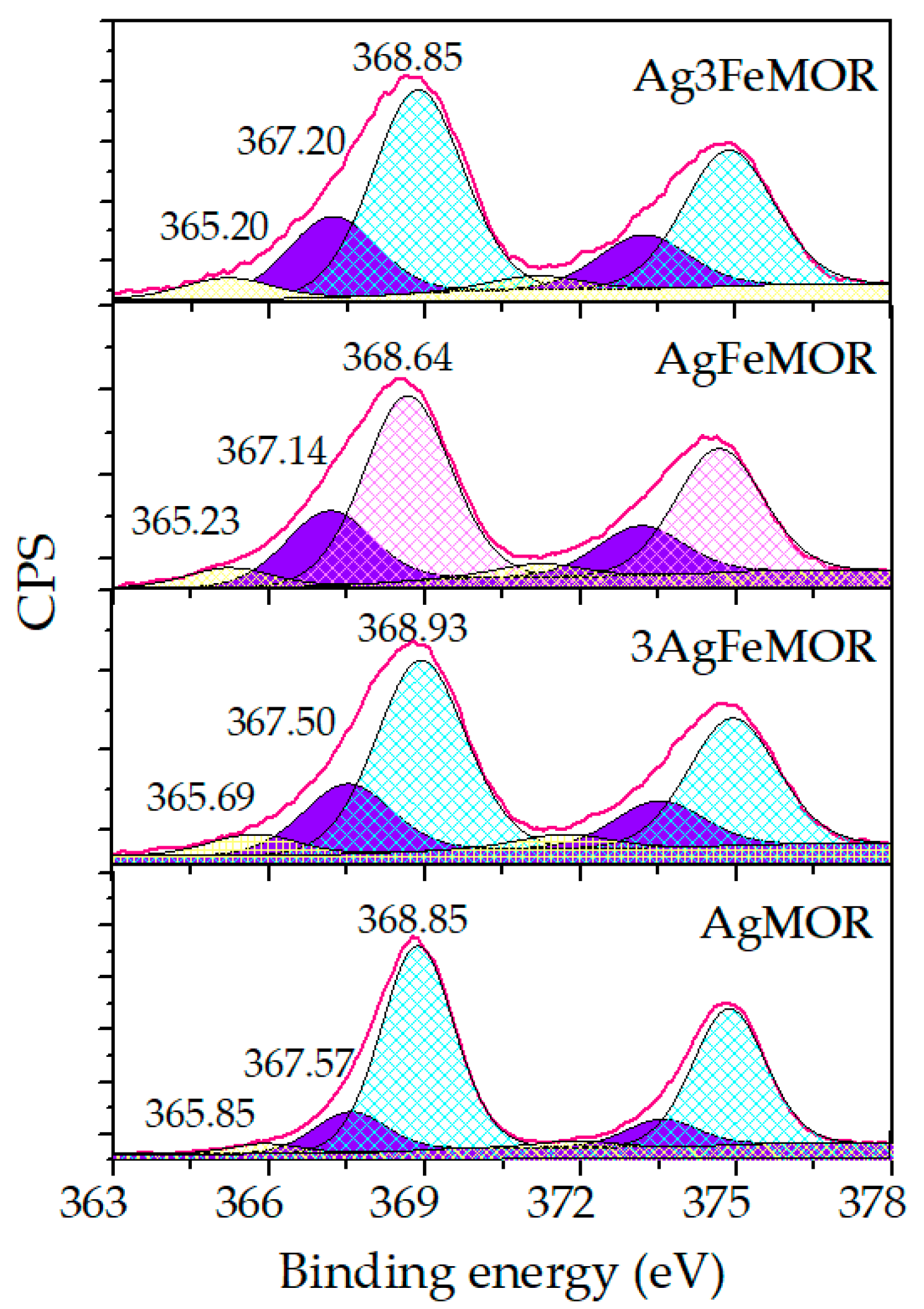

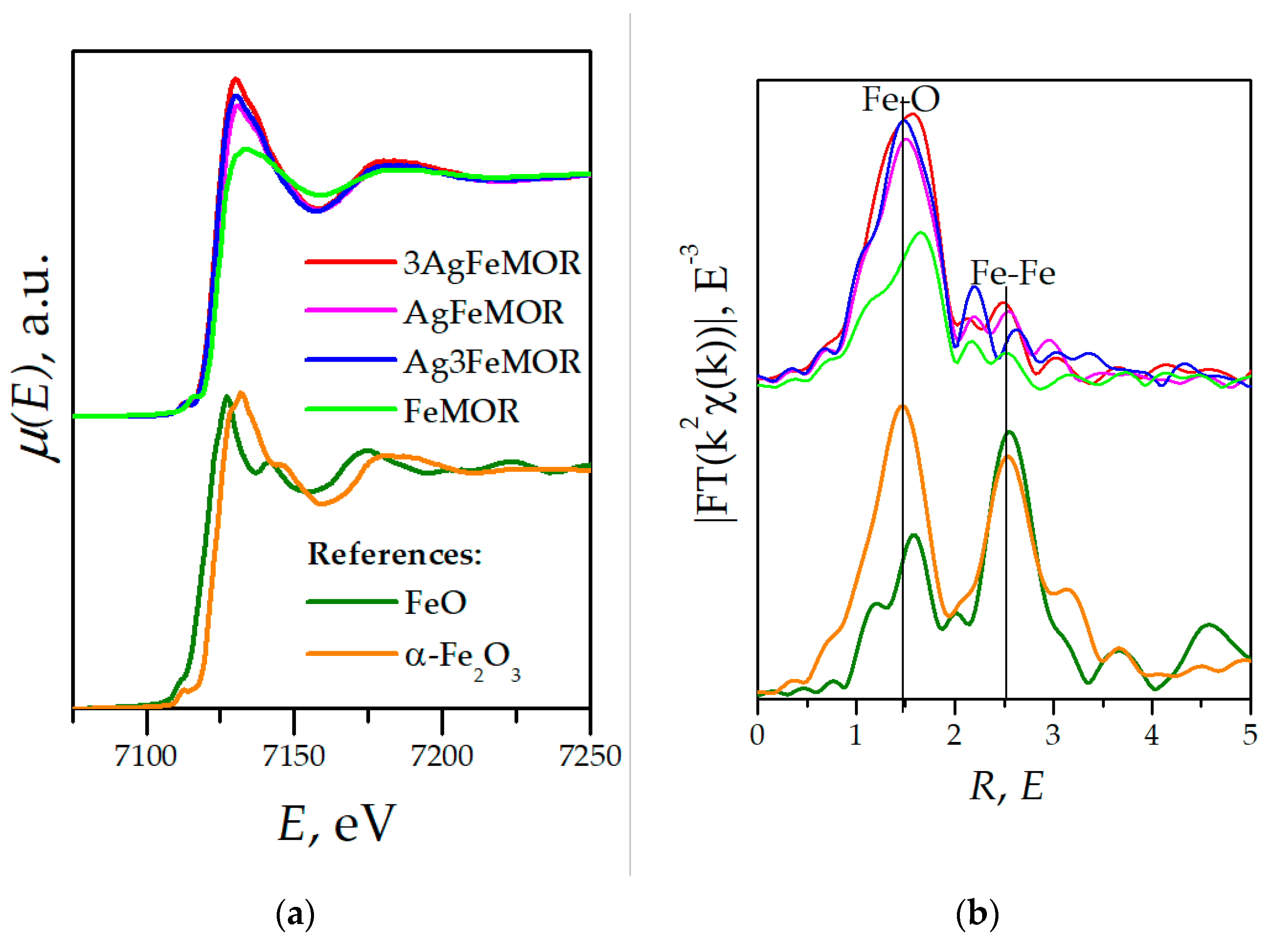

| Sample | Ion Exchange, First Step | Ion Exchange, Second Step | ||||||
|---|---|---|---|---|---|---|---|---|
| Solution, 0.03 N | V, mL | The Accessible Limit of Metal Content, Atomic % | pH | Solution, 0.03 N | V, mL | The Accessible Limit of Metal Content, Atomic % | pH | |
| FeMOR | FeSO4 | 254 | 3.2 | 2 | - | - | - | - |
| Ag3FeMOR | AgNO3 | 64 | 6.4 | 4–5 | FeSO4 | 190 | 3.2 | 2 |
| AgFeMOR | AgNO3 | 127 | 6.4 | 4–5 | FeSO4 | 127 | 3.2 | 2 |
| 3AgFeMOR | AgNO3 | 190 | 6.4 | 4–5 | FeSO4 | 64 | 3.2 | 2 |
| AgMOR | AgNO3 | 254 | 6.4 | 4–5 | - | - | - | - |
| Sample | Atomic % | Ag/Fe | EIEM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Si | Al | O | Ag | Fe | Na | Si:Al | Nomin. | Experim. | EIEM-Fe2+ | EIEM-Fe3+ | |
| NaMOR | 48.9 | 7.5 | 33.9 | 0 | 0 | 9.7 | 6.5 | - | 1.29 | ||
| FeMOR | 44.0 | 6.4 | 45.4 | 0 | 0.8 | 3.4 | 6.8 | - | 0.78 | 0.91 | |
| Ag3FeMOR | 44.2 | 6.5 | 38.0 | 2.3 | 0.9 | 1.3 | 6.8 | 1:3 | 15:3 | 0.83 | 0.97 |
| AgFeMOR | 39.7 | 5.9 | 49.7 | 3.1 | 0.9 | 0.7 | 6.7 | 1:1 | 7:1 | 0.95 | 1.10 |
| 3AgFeMOR | 40.6 | 6.0 | 41.8 | 3.7 | 0.6 | 0.6 | 6.7 | 3:1 | 12:1 | 0.92 | 1.01 |
| AgMOR | 38.1 | 5.8 | 49.9 | 4.6 | 0 | 1.6 | 6.5 | - | 1.07 | ||
| Sample | SBET, m2/g | Porosity | Acidity, % | Total Acidity, μmol/g | |||
|---|---|---|---|---|---|---|---|
| Diameter, Å | Vtotal, cm3∙g−1 | Vmicro, cm3∙g−1 | L-Peak | H-Peak | |||
| NaMOR | 243.3 | 22.8 | 0.19 | 0.16 | 32 | 68 | 1359 |
| AgMOR | 237.2 | 23.2 | 0.19 | 0.14 | 33 | 67 | 1662 |
| 3AgFeMOR | 235.5 | 23.8 | 0.22 | 0.16 | 28 | 72 | 2072 |
| AgFeMOR | 251.5 | 23.7 | 0.20 | 0.15 | 29 | 71 | 2264 |
| Ag3FeMOR | 264.8 | 28.2 | 0.22 | 0.16 | 31 | 69 | 1941 |
| FeMOR | 240.6 | 23.3 | 0.23 | 0.17 | 16 | 84 | 2970 |
| Samples | Ag Ions | Ag-Support Interaction | Ag0 | Ag0 < 2 nm |
|---|---|---|---|---|
| 366.2 eV | 367.4–368 eV | 368.0–368.2 eV | ≥369 eV | |
| Ag3FeMOR | 365.20—(7%) | 367.20—(26%) | - | 368.85—(67%) |
| AgFeMOR | 365.23—(7%) | 367.14—(27%) | 368.64—(66%) | |
| 3AgFeMOR | 365.69—(7%) | 367.50—(25%) | - | 368.93—(68%) |
| AgMOR | 365.85—(4%) | 367.57—(16%) | - | 368.85—(80%) |
| Sample | Scattering Path | N | R, Å | σ2, Å2 | R-Factor, % |
|---|---|---|---|---|---|
| AgMOR | Ag-O | 1.4 | 1.91 | 0.0001 | 3.1 |
| 1.4 | 2.05 | ||||
| 1.4 | 2.12 | ||||
| 1.4 | 2.24 | ||||
| Ag-(Si or Al) | 1.3 | 2.82 | 0.0020 | ||
| 1.3 | 2.96 | ||||
| 1.3 | 3.02 | ||||
| 1.3 | 3.15 | ||||
| 3AgFeMOR | Ag-O | 1.0 | 1.93 | 0.0001 | 3.2 |
| 1.0 | 2.08 | ||||
| 1.0 | 2.13 | ||||
| 1.0 | 2.26 | ||||
| Ag-(Si or Al) | 1.0 | 2.92 | 0.0020 | ||
| 1.0 | 3.03 | ||||
| 1.0 | 3.11 | ||||
| 1.0 | 3.23 | ||||
| Ag-Ag | 0.9 | 2.87 | 0.0073 | ||
| AgFeMOR | Ag-O | 1.0 | 1.90 | 0.0001 | 2.3 |
| 1.0 | 2.04 | ||||
| 1.0 | 2.10 | ||||
| 1.0 | 2.22 | ||||
| Ag-(Si or Al) | 1.0 | 2.92 | 0.0028 | ||
| 1.0 | 2.92 | ||||
| 1.0 | 3.12 | ||||
| 1.0 | 3.21 | ||||
| Ag-Ag | 1.6 | 2.82 | 0.0059 | ||
| Ag3FeMOR | Ag-O | 1.0 | 1.91 | 0.0003 | 3.1 |
| 1.0 | 2.05 | ||||
| 1.0 | 2.12 | ||||
| 1.0 | 2.25 | ||||
| Ag-(Si or Al) | 1 | 2.913 | 0.0049 | ||
| 2.913 | |||||
| 3.06 | |||||
| 3.17 | |||||
| Ag-Ag | 0.8 | 2.80 | 0.0076 |
| Sample | N | R, Å | σ2, Å2 | R-Factor, % |
|---|---|---|---|---|
| FeMOR | 0.6 | 1.85 | 0.0061 | 1.7 |
| 1.9 | 2.06 | 0.0061 | ||
| Ag3FeMOR | 1.5 | 1.94 | 0.0015 | 1.3 |
| 1.9 | 2.10 | 0.0015 | ||
| AgFeMOR | 1.6 | 1.94 | 0.0049 | 0.9 |
| 2.0 | 2.10 | 0.0049 | ||
| 3AgFeMOR | 1.4 | 1.91 | 0.0044 | 0.3 |
| 2.7 | 2.07 | 0.0044 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotolevich, Y.; Khramov, E.; Sánchez-López, P.; Pestryakov, A.; Zubavichus, Y.; Antúnez-Garcia, J.; Petranovskii, V. Formation of Ag-Fe Bimetallic Nano-Species on Mordenite Depending on the Initial Ratio of Components. Materials 2023, 16, 3026. https://doi.org/10.3390/ma16083026
Kotolevich Y, Khramov E, Sánchez-López P, Pestryakov A, Zubavichus Y, Antúnez-Garcia J, Petranovskii V. Formation of Ag-Fe Bimetallic Nano-Species on Mordenite Depending on the Initial Ratio of Components. Materials. 2023; 16(8):3026. https://doi.org/10.3390/ma16083026
Chicago/Turabian StyleKotolevich, Yulia, Evgenii Khramov, Perla Sánchez-López, Alexey Pestryakov, Yan Zubavichus, Joel Antúnez-Garcia, and Vitalii Petranovskii. 2023. "Formation of Ag-Fe Bimetallic Nano-Species on Mordenite Depending on the Initial Ratio of Components" Materials 16, no. 8: 3026. https://doi.org/10.3390/ma16083026
APA StyleKotolevich, Y., Khramov, E., Sánchez-López, P., Pestryakov, A., Zubavichus, Y., Antúnez-Garcia, J., & Petranovskii, V. (2023). Formation of Ag-Fe Bimetallic Nano-Species on Mordenite Depending on the Initial Ratio of Components. Materials, 16(8), 3026. https://doi.org/10.3390/ma16083026



_CHAINOK.jpg)







