Magnesium Ortho-Vanadate/Magnesium Oxide/Graphene Oxide Embedded through Cellulose Acetate-Based Films for Wound Healing Applications
Abstract
1. Introduction
2. Experimental Techniques
2.1. Preparation of Scaffold with Different Contents of Oxides
2.2. Material Characterization
2.3. In Vitro Cell Viability Tests
3. Results and Discussions
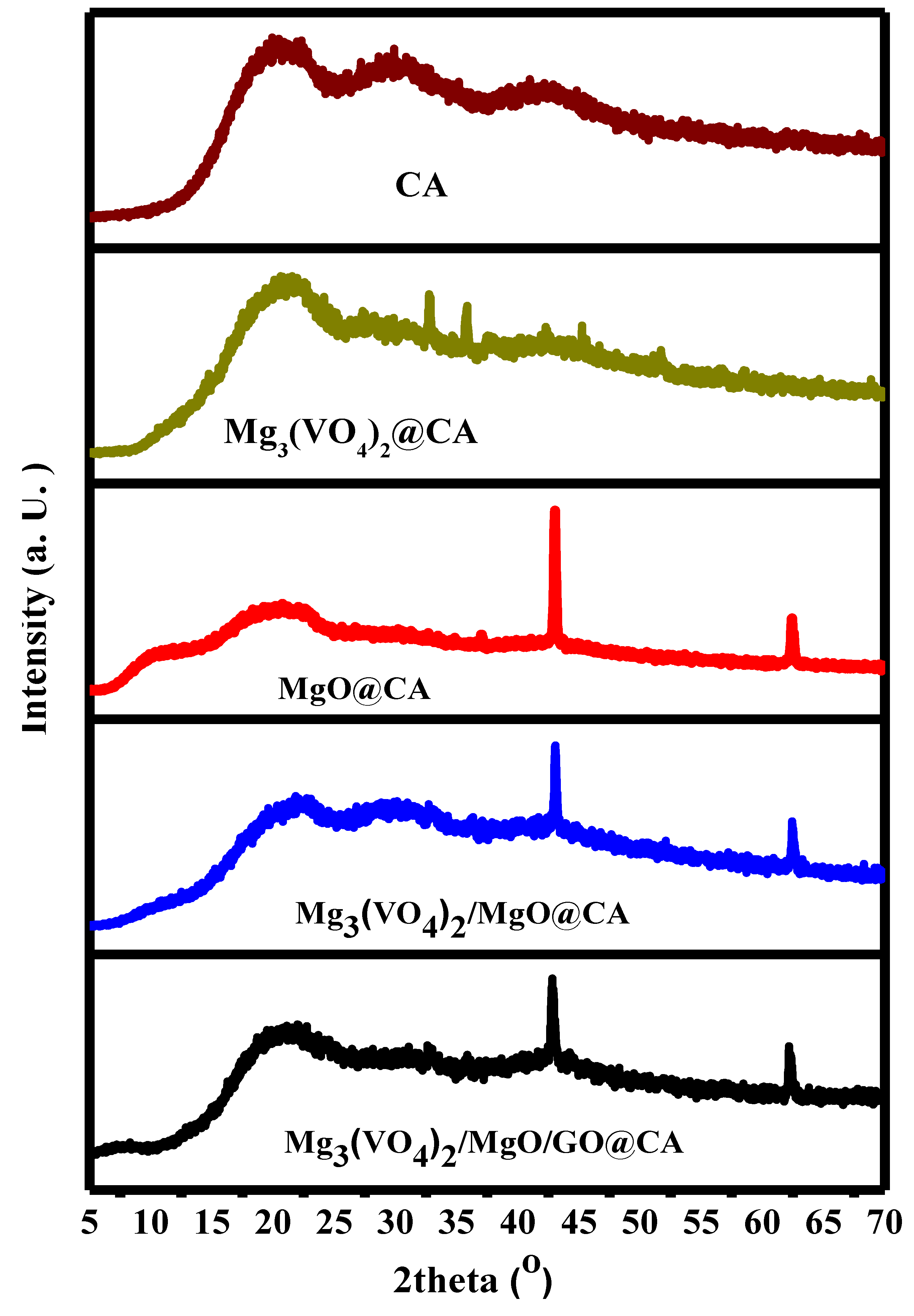
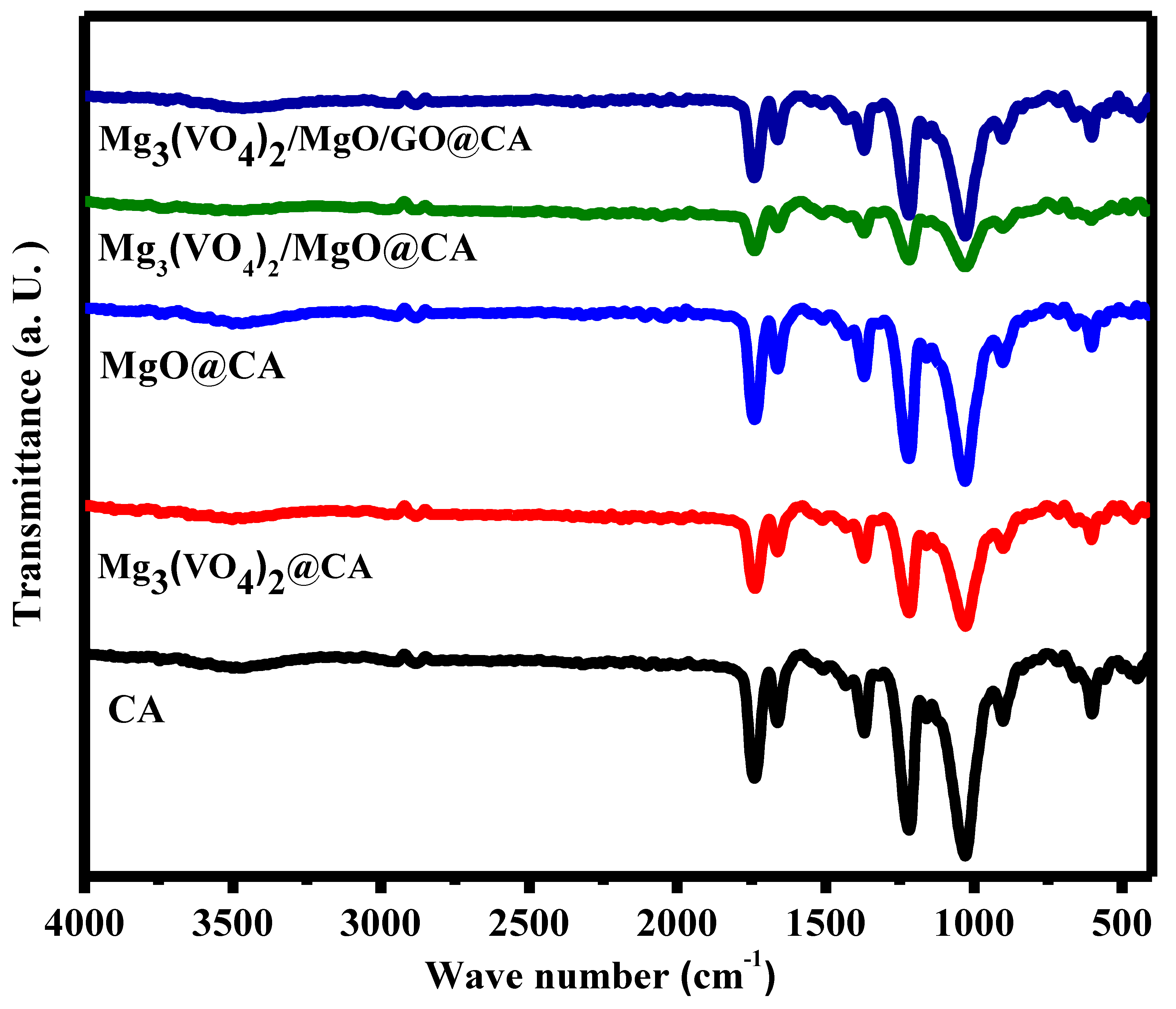
3.1. Qualitative and Quantitative Structural Investigation
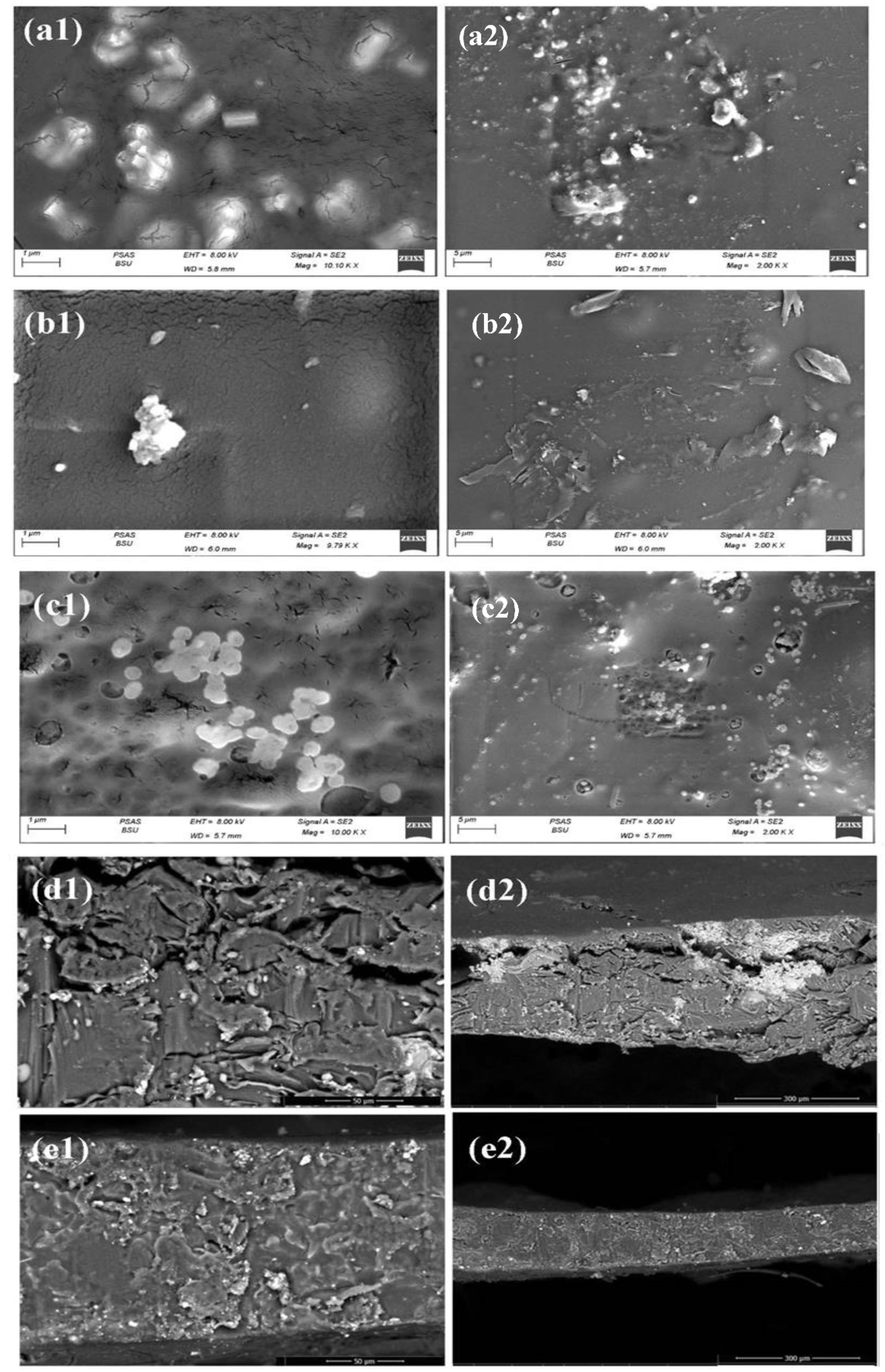
3.2. Morphological Study
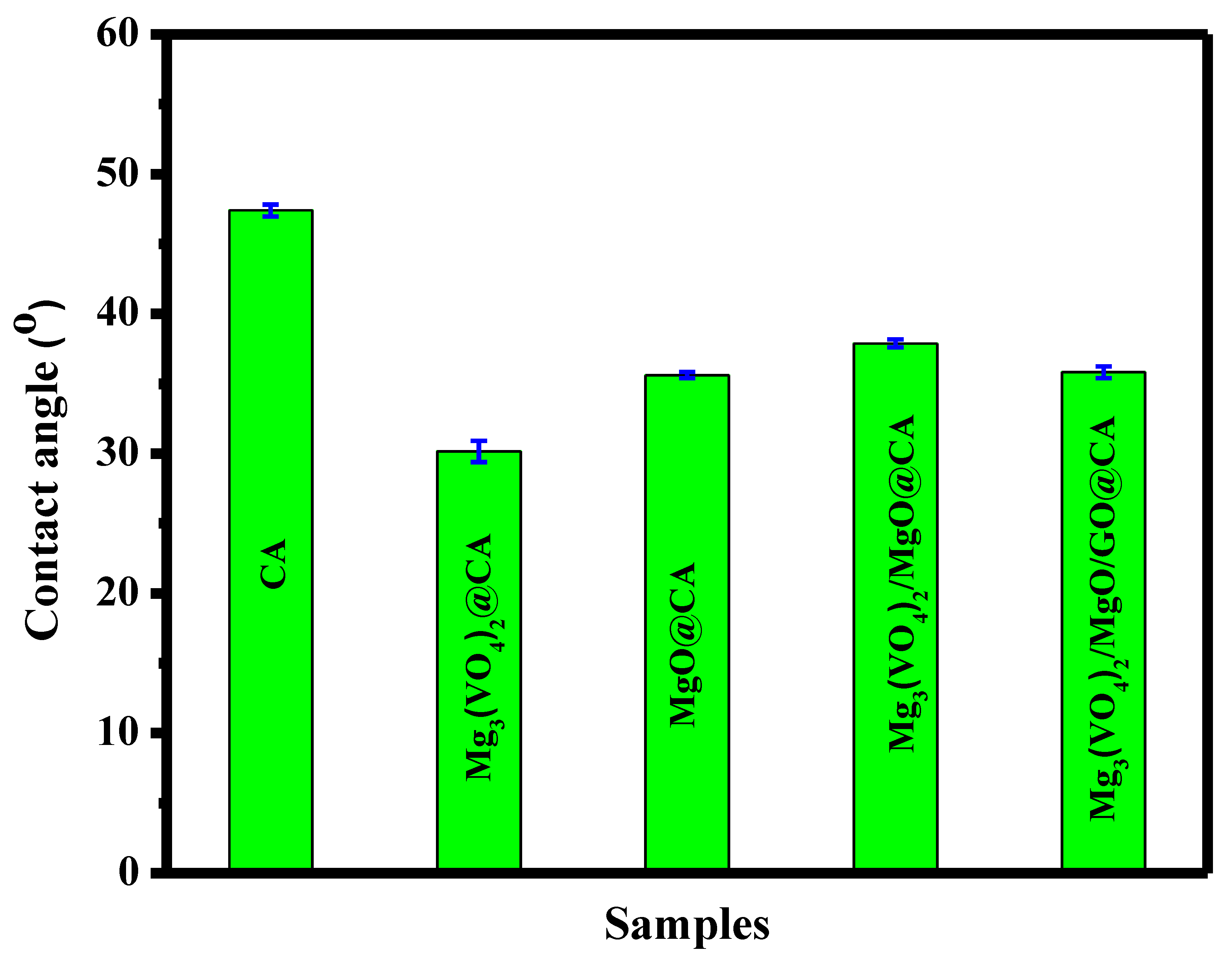
3.3. Wettability Properties
3.4. Cell Viability Percentage upon the Usage of Mg3(VO4)2/MgO/GO@CA against Normal Lung Cells
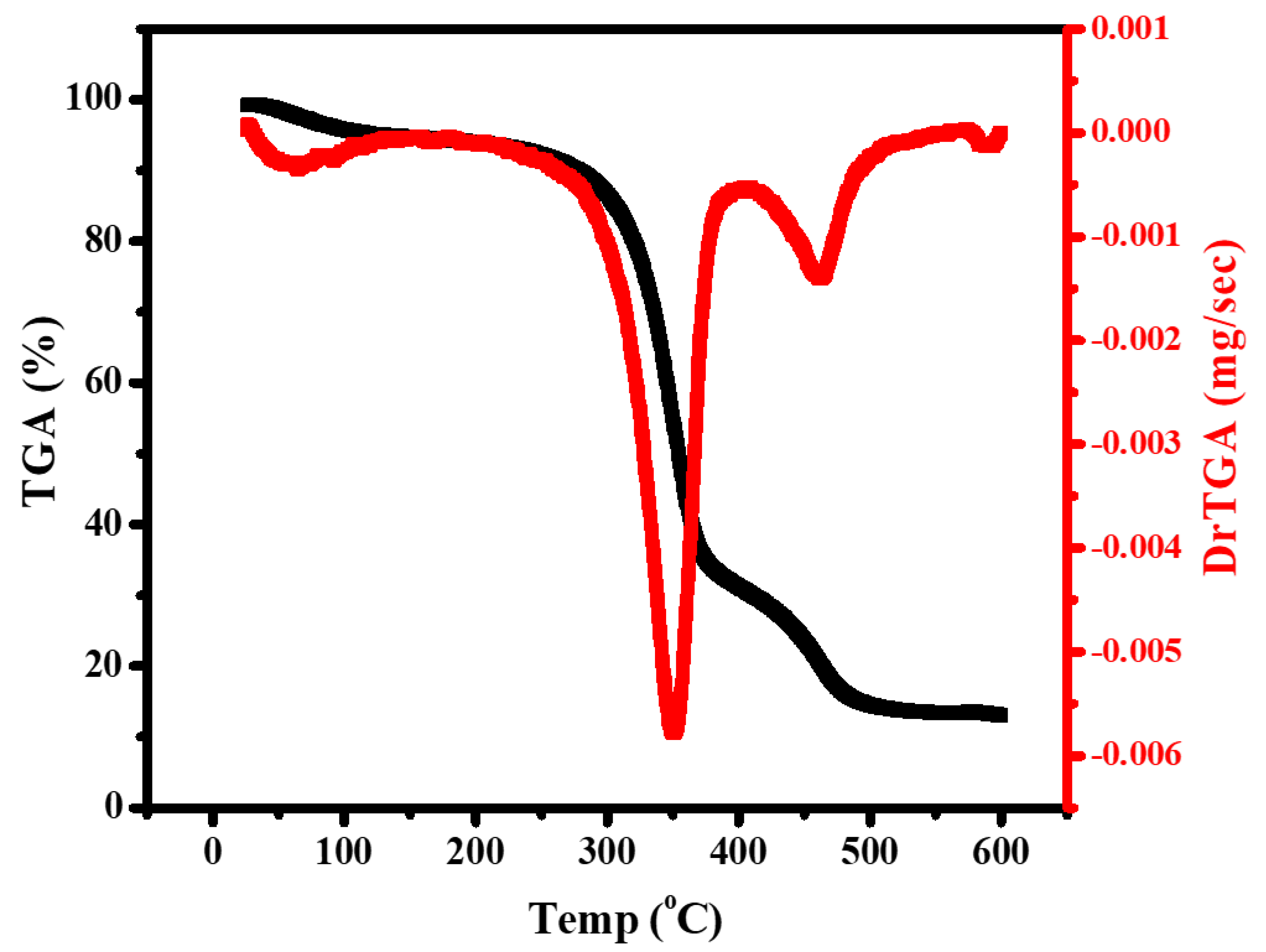
3.5. Thermal Study
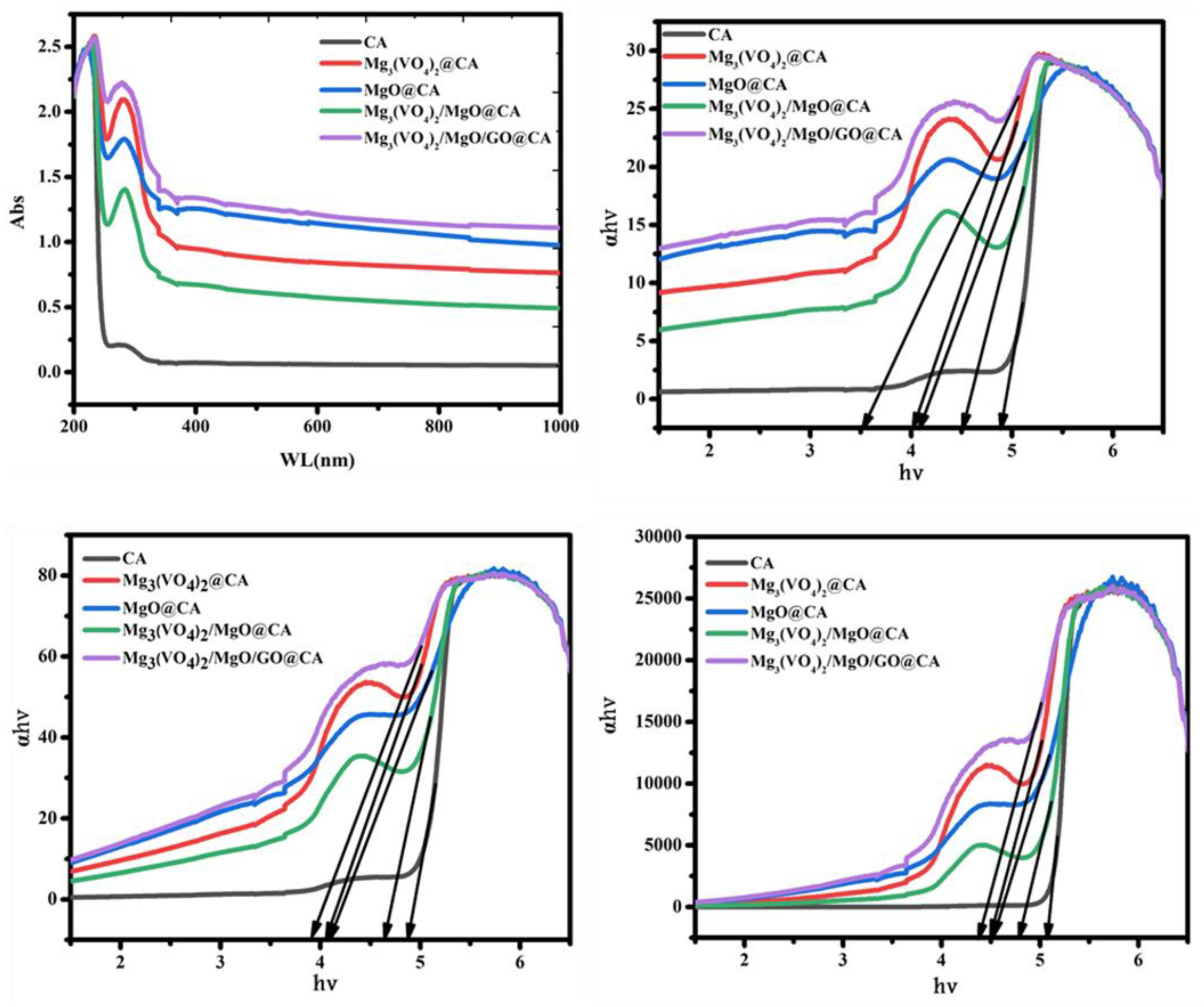
3.6. Optical Behavior
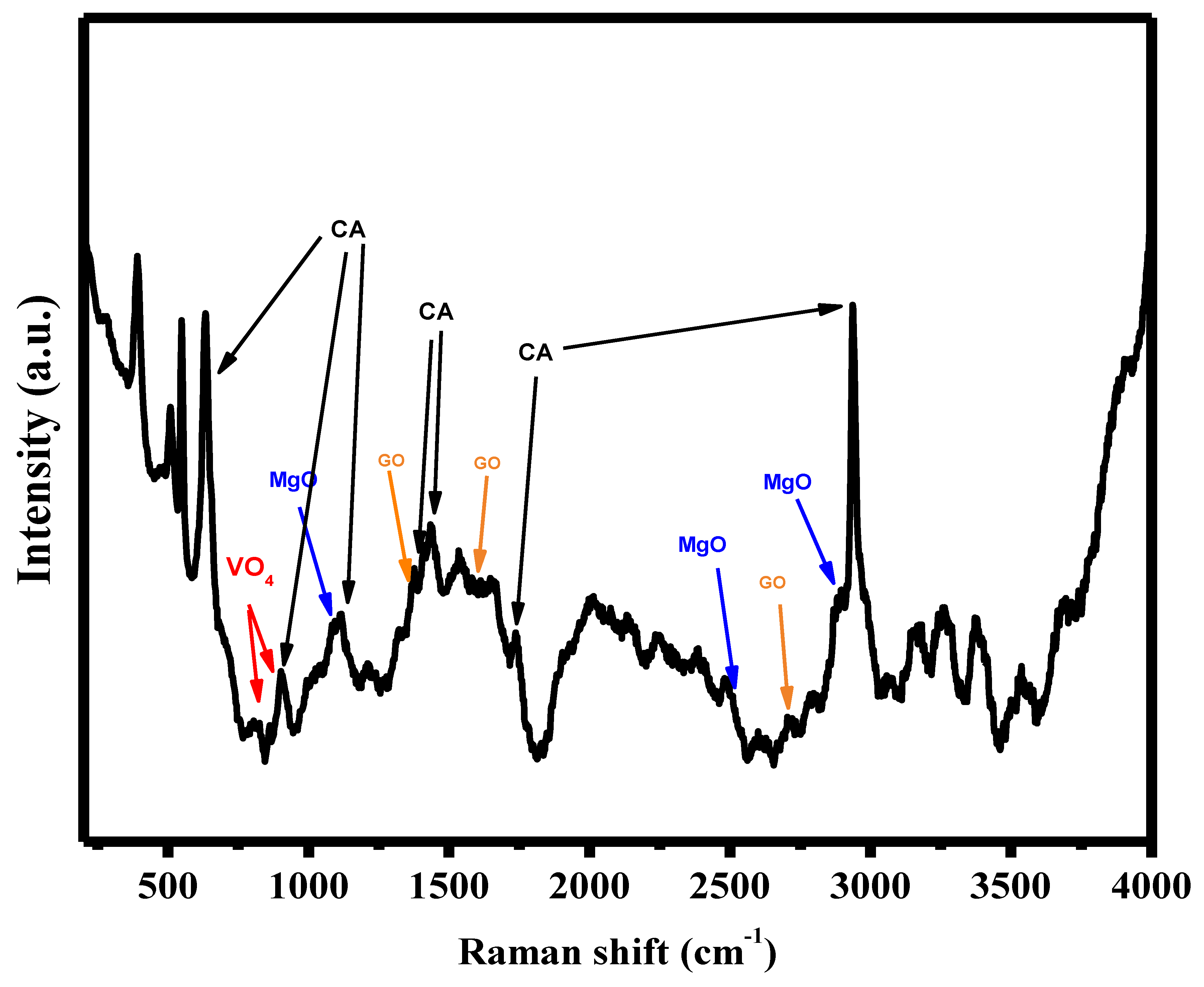
3.7. Raman Spectroscopy
3.8. Ionic Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joseph, J.; Deshmukh, K.; Tung, T.T.; Chidambaram, K.; Khadheer Pasha, S.K. 3D Printing Technology of Polymer Composites and Hydrogels for Artificial Skin Tissue Implementations. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 205–233. [Google Scholar] [CrossRef]
- Taha, M.A.; Youness, R.A.; Zawrah, M.F. Phase composition, sinterability and bioactivity of amorphous nano-CaO-SiO2-CuO powder synthesized by sol-gel technique. Ceram. Int. 2020, 46, 24462–24471. [Google Scholar] [CrossRef]
- Yu, N.; Wang, X.; Qiu, L.; Cai, T.; Jiang, C.; Sun, Y.; Li, Y.; Peng, H.; Xiong, H. Bacteria-triggered hyaluronan/AgNPs/gentamicin nanocarrier for synergistic bacteria disinfection and wound healing application. Chem. Eng. J. 2020, 380, 122582. [Google Scholar] [CrossRef]
- Venault, A.; Lin, K.-H.; Tang, S.-H.; Dizon, G.V.; Hsu, C.-H.; Maggay, I.V.B.; Chang, Y. Zwitterionic electrospun PVDF fibrous membranes with a well-controlled hydration for diabetic wound recovery. J. Membr. Sci. 2020, 598, 117648. [Google Scholar] [CrossRef]
- Abulyazied, D.E.; Alturki, A.M.; Youness, R.A.; Abomostafa, H.M. Synthesis, Structural and Biomedical Characterization of Hydroxyapatite/Borosilicate Bioactive Glass Nanocomposites. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4077–4092. [Google Scholar] [CrossRef]
- Murphy, C.A.; Costa, J.B.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L.; Collins, M.N. Biopolymers and polymers in the search of alternative treatments for meniscal regeneration: State of the art and future trends. Appl. Mater. Today 2018, 12, 51–71. [Google Scholar] [CrossRef]
- Kucharzewski, M.; Rojczyk, E.; Wilemska-Kucharzewska, K.; Wilk, R.; Hudecki, J.; Los, M.J. Novel trends in application of stem cells in skin wound healing. Eur. J. Pharmacol. 2019, 843, 307–315. [Google Scholar] [CrossRef]
- Dong, W.-H.; Liu, J.-X.; Mou, X.-J.; Liu, G.-S.; Huang, X.-W.; Yan, X.; Ning, X.; Russell, S.J.; Long, Y.-Z. Performance of polyvinyl pyrrolidone-isatis root antibacterial wound dressings produced in situ by handheld electrospinner. Colloids Surf. B Biointerfaces 2019, 188, 110766. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in nanofibers for wound dressing: A review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Alatawi, A.S.; Alturki, A.M.; Soliman, G.M.; Abulyazied, D.E.; Taha, M.A.; Youness, R.A. Improved toughness, electrical conductivity and optical properties of bioactive borosilicate glasses for orthopedic applications. Appl. Phys. A 2021, 127, 971. [Google Scholar] [CrossRef]
- Notodihardjo, S.C.; Morimoto, N.; Kakudo, N.; Mitsui, T.; Le, T.M.; Tabata, Y.; Kusumoto, K. Comparison of the efficacy of cryopreserved human platelet lysate and refrigerated lyophilized human platelet lysate for wound healing. Regen. Ther. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Mauro, N.; Drago, S.E.; Cavallaro, G.; Giammona, G. Near-Infrared, Light-Triggered, On-Demand Anti-inflammatories and Antibiotics Release by Graphene Oxide/Elecrospun PCL Patch for Wound Healing. C 2019, 5, 63. [Google Scholar] [CrossRef]
- Prakash, J.; Venkataprasanna, K.S.; Bharath, G.; Banat, F.; Niranjan, R.; Venkatasubbu, G.D. In-vitro evaluation of electrospun cellulose acetate nanofiber containing Graphene oxide/TiO2/Curcumin for wound healing application. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127166. [Google Scholar] [CrossRef]
- Alghuwainem, Y.A.A.; Gouda, M.; Khalaf, M.M.; Elmushyakhi, A.; Taleb, M.F.A.; El-Lateef, H.M.A. Synthesis and Characterization of Chitosan-Containing ZnS/ZrO(2)/Graphene Oxide Nanocomposites and Their Application in Wound Dressing. Polymers 2022, 14, 5195. [Google Scholar] [CrossRef] [PubMed]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Alturki, A.M.; Abulyazied, D.E.; Taha, M.A.; Abomostafa, H.M.; Youness, R.A. A Study to Evaluate the Bioactivity Behavior and Electrical Properties of Hydroxyapatite/Ag2O-Borosilicate Glass Nanocomposites for Biomedical Applications. J. Inorg. Organomet. Polym. Mater. 2021, 32, 169–179. [Google Scholar] [CrossRef]
- Wang, X.; Chang, J.; Wu, C. Bioactive inorganic/organic nanocomposites for wound healing. Appl. Mater. Today 2018, 11, 308–319. [Google Scholar] [CrossRef]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef]
- Alavi, M.; Karimi, N. Ultrasound assisted-phytofabricated Fe3O4 NPs with antioxidant properties and antibacterial effects on growth, biofilm formation, andspreading ability of multidrug resistant bacteria. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2405–2423. [Google Scholar] [CrossRef]
- Aldossary, H.A.; Khalaf, M.M.; Gouda, M.; Elmushyakhi, A.; Abou Taleb, M.F.; Abd El-Lateef, H.M. Wound dressing candidate materials based on casted films of cellulose acetate modified with zirconium oxide (ZrO2), and gallium oxide (Ga2O3). Mater. Today Commun. 2023, 34, 105299. [Google Scholar] [CrossRef]
- Gouda, M.; Khalaf, M.M.; Elmushyakhi, A.; Abou Taleb, M.F.; Abd El-Lateef, H.M. Bactericidal activities of Sm2O3/Sb2O3/graphene oxide loaded cellulose acetate film. J. Mater. Res. Technol. 2022, 21, 4419–4427. [Google Scholar] [CrossRef]
- Pinto, M.D.C.E.; da Silva, D.D.; Gomes, A.L.A.; Leite, V.D.S.A.; e Moraes, A.R.F.; de Novais, R.F.; Tronto, J.; Pinto, F.G. Film based on magnesium impregnated biochar/cellulose acetate for phosphorus adsorption from aqueous solution. RSC Adv. 2019, 9, 5620–5627. [Google Scholar] [CrossRef] [PubMed]
- Almaieli, L.M.A.; Khalaf, M.M.; Gouda, M.; Elmushyakhi, A.; Taleb, M.F.A.; El-Lateef, H.M.A. Fabrication of Bio-Based Film Comprising Metal Oxide Nanoparticles Loaded Chitosan for Wound Dressing Applications. Polymers 2022, 15, 211. [Google Scholar] [CrossRef] [PubMed]
- Alghuwainem, Y.A.A.; Gouda, M.; Khalaf, M.M.; Heakal, F.E.; Albalwi, H.A.; Elmushyakhi, A.; El-Lateef, H.M.A. Highlighting the Compositional Changes of the Sm(2)O(3)/MgO-Containing Cellulose Acetate Films for Wound Dressings. Polymers 2022, 14, 4964. [Google Scholar] [CrossRef] [PubMed]
- Rather, A.H.; Khan, R.S.; Wani, T.U.; Rafiq, M.; Jadhav, A.H.; Srinivasappa, P.M.; Abdal-Hay, A.; Sultan, P.; Rather, S.-U.; Macossay, J.; et al. Polyurethane and cellulose acetate micro-nanofibers containing rosemary essential oil, and decorated with silver nanoparticles for wound healing application. Int. J. Biol. Macromol. 2023, 226, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Al-Saeedi, S.I.; Al-Kadhi, N.S.; Al-Senani, G.M.; Almaghrabi, O.A.; Nafady, A. Antibacterial potency, cell viability and morphological implications of copper oxide nanoparticles encapsulated into cellulose acetate nanofibrous scaffolds. Int. J. Biol. Macromol. 2021, 182, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Ullah, I.; Li, N.; Niu, S.; Sun, R.; Xia, D.; Yang, R.; Zhang, X. Plasma spray of biofunctional (Mg, Sr)-substituted hydroxyapatite coatings for titanium alloy implants. J. Mater. Sci. Technol. 2019, 35, 719–726. [Google Scholar] [CrossRef]
- Qiao, W.; Yang, Z. Forecast the electricity price of U.S. using a wavelet transform-based hybrid model. Energy 2020, 193, 116704. [Google Scholar] [CrossRef]
- Crans, D.C. Antidiabetic, Chemical, and Physical Properties of Organic Vanadates as Presumed Transition-State Inhibitors for Phosphatases. J. Org. Chem. 2015, 80, 11899–11915. [Google Scholar] [CrossRef]
- Crans, D.C.; Smee, J.J.; Gaidamauskas, E.; Yang, L. The Chemistry and Biochemistry of Vanadium and the Biological Activities Exerted by Vanadium Compounds. Chem. Rev. 2004, 104, 849–902. [Google Scholar] [CrossRef]
- Treviño, A.D.S.; Sánchez-Lara, E.; Sanchez-Gaytan, B.L.; Perez-Aguilar, J.M.; González-Vergara, E. Vanadium in biological action: Chemical, pharmacological aspects, and metabolic implications in diabetes mellitus. Biol. Trace Elem. Res. 2019, 188, 68–98. [Google Scholar] [CrossRef]
- Bogden, J.D.; Higashino, H.; Lavenhar, M.A.; Bauman, J.W.; Kemp, F.W.; Aviv, A. Balance and Tissue Distribution of Vanadium after Short-Term Ingestion of Vanadate. J. Nutr. 1982, 112, 2279–2285. [Google Scholar] [CrossRef] [PubMed]
- Wiegmann, T.B.; Day, H.D.; Patak, R.V. Intestinal absorption and secretion of radioactive vanadium (48VO3-) in rats and effect of Al(OH)3. J. Toxicol. Environ. Health 1982, 10, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Crans, D.C.; Yang, L.; Haase, A.; Yang, X. Health Benefits of Vanadium and Its Potential as an Anticancer Agent. Met. Ions Life Sci. 2018, 18, 251–280. [Google Scholar] [CrossRef]
- Iordachescu, D.; Dinu, D.; Bonoiu, A.; Bucata, G.; Ciubar, R.; Homeghiu, C.; Iancu, C.; Stan, C.; Nae, S. Time and dose-response study of the effects of vanadate on human skin fibroblasts. Rom. J. Biophys. 2002, 12, 69–76. [Google Scholar]
- Zou, R.; Xu, T.; Lei, X.; Wu, Q.; Xue, S. Novel design of porous hollow hydroxyapatite microspheres decorated by reduced graphene oxides with superior photocatalytic performance for tetracycline removal. Solid State Sci. 2020, 99, 106067. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Cao, X.; Xue, J.; Zhang, Q.; Tian, J.; Li, X.; Qiu, X.; Pan, B.; Gu, A.Z.; et al. Effect of carboxyl and hydroxyl groups on adsorptive polysaccharide fouling: A comparative study based on PVDF and graphene oxide (GO) modified PVDF surfaces. J. Membr. Sci. 2020, 595, 117514. [Google Scholar] [CrossRef]
- Zhang, Y.; Ruan, H.; Guo, C.; Liao, J.; Shen, J.; Gao, C. Thin-film nanocomposite reverse osmosis membranes with enhanced antibacterial resistance by incorporating p-aminophenol-modified graphene oxide. Sep. Purif. Technol. 2020, 234, 116017. [Google Scholar] [CrossRef]
- Xie, Y.Y.; Hu, X.H.; Zhang, Y.W.; Wahid, F.; Chu, L.Q.; Jia, S.R.; Zhong, C. Development and antibacterial activities of bacterial cellulose/graphene oxide-CuO nanocomposite films. Carbohydr. Polym. 2020, 229, 115456. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, X.; Qin, L.; Li, X.; Meng, Q.; Shen, C.; Zhang, G. Enhanced MPBR with polyvinylpyrrolidone-graphene oxide/PVDF hollow fiber membrane for efficient ammonia nitrogen wastewater treatment and high-density Chlorella cultivation. Chem. Eng. J. 2020, 379, 122368. [Google Scholar] [CrossRef]
- Wu, M.; Qi, X.; Xie, R.; Bai, Z.; Qin, S.; Zhong, W.; Deng, C. Graphene oxide/carbon nanotubes/Co Fe3-O4 ternary nanocomposites: Controllable synthesis and their excellent microwave absorption capabilities. J. Alloys Compd. 2020, 813, 151996. [Google Scholar] [CrossRef]
- Wang, S.-D.; Wang, K.; Ma, Q.; Qu, C.-X. Fabrication of the multifunctional durable silk fabric with synthesized graphene oxide nanosheets. Mater. Today Commun. 2020, 23, 100893. [Google Scholar] [CrossRef]
- Vijesh, K.R.; Musfir, P.N.; Thomas, T.; Vaishakh, M.; Nampoori, V.; Thomas, S. Enhanced nonlinear optical properties of solution dispersed carbon dots decorated graphene oxide with varying viscosity. Opt. Laser Technol. 2020, 121, 105776. [Google Scholar] [CrossRef]
- Irshad, M.S.; Arshad, N.; Zhang, J.; Song, C.; Mushtaq, N.; Alomar, M.; Shamim, T.; Dao, V.-D.; Wang, H.; Wang, X.; et al. Wormlike Perovskite Oxide Coupled with Phase-Change Material for All-Weather Solar Evaporation and Thermal Storage Applications. Adv. Energy Sustain. Res. 2023, 4, 2200158. [Google Scholar] [CrossRef]
- Tan, S.; Wu, X.; Xing, Y.; Lilak, S.; Wu, M.; Zhao, J.X. Enhanced synergetic antibacterial activity by a reduce graphene oxide/Ag nanocomposite through the photothermal effect. Colloids Surf. B Biointerfaces 2020, 185, 110616. [Google Scholar] [CrossRef]
- Qiao, W.; Yang, Z. Solving Large-Scale Function Optimization Problem by Using a New Metaheuristic Algorithm Based on Quantum Dolphin Swarm Algorithm. IEEE Access 2019, 7, 138972–138989. [Google Scholar] [CrossRef]
- Shu, R.; Zhang, J.; Guo, C.; Wu, Y.; Wan, Z.; Shi, J.; Liu, Y.; Zheng, M. Facile synthesis of nitrogen-doped reduced graphene oxide/nickel-zinc ferrite composites as high-performance microwave absorbers in the X-band. Chem. Eng. J. 2020, 384, 123266. [Google Scholar] [CrossRef]
- Sheng, J.; Yin, H.; Qian, F.; Huang, H.; Gao, S.; Wang, J. Reduced graphene oxide-based composite membranes for in-situ catalytic oxidation of sulfamethoxazole operated in membrane filtration. Sep. Purif. Technol. 2020, 236, 116275. [Google Scholar] [CrossRef]
- Rivera-Briso, A.L.; Aachmann, F.L.; Moreno-Manzano, V.; Serrano-Aroca, A. Graphene oxide nanosheets versus carbon nanofibers: Enhancement of physical and biological properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) films for biomedical applications. Int. J. Biol. Macromol. 2020, 143, 1000–1008. [Google Scholar] [CrossRef]
- Selvam, N.C.S.; Kumar, R.T.; Kennedy, L.J.; Vijaya, J.J. Comparative study of microwave and conventional methods for the preparation and optical properties of novel MgO-micro and nano-structures. J. Alloys Compd. 2011, 509, 9809–9815. [Google Scholar] [CrossRef]
- Gao, X.; Ruiz, P.; Xin, Q.; Guo, X.; Delmon, B. Preparation and characterization of three pure magnesium vanadate phases as catalysts for selective oxidation of propane to propene. Catal. Lett. 1994, 23, 321–337. [Google Scholar] [CrossRef]
- Ramis, G.; Busca, G.; Lorenzelli, V. Spectroscopic Characterization of Magnesium Vanadate Catalysts, Part 2.t-FTIR Study of the Surface Properties of Pure and Mixed-phase Powders. J. Chem. Soc. Faraday Trans. 1994, 90, 1293–1299. [Google Scholar] [CrossRef]
- Wong, C.W.; Chan, Y.S.; Jeevanandam, J.; Pal, K.; Bechelany, M.; Elkodous, M.A.; El-Sayyad, G.S. Response Surface Methodology Optimization of Mono-dispersed MgO Nanoparticles Fabricated by Ultrasonic-Assisted Sol–Gel Method for Outstanding Antimicrobial and Antibiofilm Activities. J. Clust. Sci. 2019, 31, 367–389. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Umasuthan, N.; Mohan, R.; Lee, J.; Kim, S.-J. Antibacterial Activity of Graphene Oxide Nanosheets. Sci. Adv. Mater. 2012, 4, 1111–1117. [Google Scholar] [CrossRef]
- Ghotekar, S.; Pansambal, S.; Bilal, M.; Pingale, S.S.; Oza, R. Environmentally friendly synthesis of Cr2O3 nanoparticles: Characterization, applications and future perspective—A review. Case Stud. Chem. Environ. Eng. 2021, 3, 100089. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Mariappan, A.; Neyvasagam, K.; Ayeshamariam, A.; Pandi, P.; Palanichamy, R.R.; Gopinathan, C.; Mola, G.T.; Maaza, M. Photocatalytic performance and antimicrobial activities of HAp-TiO 2 nanocomposite thin films by sol-gel method. Surf. Interfaces 2017, 6, 247–255. [Google Scholar] [CrossRef]
- Khoshsima, S.; Yilmaz, B.; Tezcaner, A.; Evis, Z. Structural, mechanical and biological properties of hydroxyapatite-zirconia-lanthanum oxide composites. Ceram. Int. 2016, 42, 15773–15779. [Google Scholar] [CrossRef]
- Yu, Q.; Jiang, W.; Li, D.; Gu, M.; Liu, K.; Dong, L.; Wang, C.; Jiang, H.; Dai, W. Sodium orthovanadate inhibits growth and triggers apoptosis of human anaplastic thyroid carcinoma cells in vitro and in vivo. Oncol. Lett. 2019, 17, 4255–4262. [Google Scholar] [CrossRef]
- Trotter, P. Wound Dressings Comprising Vanadate Complexed with Organic Ligands. U.S. Patent Application No. 10/538,315, 27 July 2006. [Google Scholar]
- Chen, Y.; Qiu, Y.; Chen, W.; Wei, Q. Electrospun thymol-loaded porous cellulose acetate fibers with potential biomedical applications. Mater. Sci. Eng. C 2020, 109, 110536. [Google Scholar] [CrossRef]
- Youness, R.A.; Amer, M.S.; Taha, M.A. Tribo-mechanical measurements and in vivo performance of zirconia-containing biphasic calcium phosphate material implanted in a rat model for bone replacement applications. Mater. Chem. Phys. 2022, 285, 126085. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Giovanardi, R.; Veronesi, P. Corrosion behavior and mechanical properties of bioactive sol-gel coatings on titanium implants. Mater. Sci. Eng. C 2014, 43, 375–382. [Google Scholar] [CrossRef]
- Youness, R.A.; Ibrahim, M.A.; Taha, M.A. Evaluation of the electrical and dielectric behavior of the apatite layer formed on the surface of hydroxyapatite/hardystonite/copper oxide hybrid nanocomposites for bone repair applications. Ceram. Int. 2022, 48, 19837–19850. [Google Scholar] [CrossRef]
- Abushanab, W.S.; Moustafa, E.B.; Youness, R.A. Evaluation of the dynamic behavior, elastic properties, and in vitro bioactivity of some borophosphosilicate glasses for orthopedic applications. J. Non-Crystalline Solids 2022, 586, 121539. [Google Scholar] [CrossRef]
- Khalaf, M.M.; Gouda, M.; Mohamed, I.M.A.; Abd El-Lateef, H.M. Different additives of gold nanoparticles and lithium oxide loaded chitosan-based films; controlling optical and structural properties, evaluating cell viability. Biochem. Biophys. Res. Commun. 2023, 49, 118–124. [Google Scholar] [CrossRef]
- Orelma, H.; Hokkanen, A.; Leppänen, I.; Kammiovirta, K.; Kapulainen, M.; Harlin, A. Optical cellulose fiber made from regenerated cellulose and cellulose acetate for water sensor applications. Cellulose 2019, 27, 1543–1553. [Google Scholar] [CrossRef]
- Zak, A.K.; Majid, W.H.A.; Abrishami, M.E.; Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson–Hall and size–strain plot methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar]
- Huy, B.T.; Kwon, D.H.; Lee, S.-S.; Dao, V.-D.; Truong, H.B.; Lee, Y.-I. Optical properties of Sr2YF7 material doped with Yb3+, Er3+, and Eu3+ ions for solar cell application. J. Alloys Compd. 2022, 897, 163189. [Google Scholar] [CrossRef]
- Li, Y.; Pan, D.; Zhang, M.; Xie, J.; Yan, Z. Ultrafine Co3O4embedded in nitrogen-doped graphene with synergistic effect and high stability for supercapacitors. RSC Adv. 2016, 6, 48357–48364. [Google Scholar] [CrossRef]
- Di Pietro, P.; Forte, G.; D’Urso, L.; Satriano, C. The hybrid nanobiointerface between nitrogen-doped graphene oxide and lipid membranes: A theoretical and experimental study. AIMS Mater. Sci. 2016, 4, 43–60. [Google Scholar] [CrossRef]
- Sánchez-Márquez, J.A.; Fuentes-Ramírez, R.; Cano-Rodríguez, I.; Gamiño-Arroyo, Z.; Rubio-Rosas, E.; Kenny, J.M.; Rescignano, N. Membrane Made of Cellulose Acetate with Polyacrylic Acid Reinforced with Carbon Nanotubes and Its Applicability for Chromium Removal. Int. J. Polym. Sci. 2015, 2015, 320631. [Google Scholar] [CrossRef]
- Visweswaran, S.; Venkatachalapathy, R.; Haris, M.; Murugesan, R. Characterization of MgO thin film prepared by spray pyrolysis technique using perfume atomizer. J. Mater. Sci. Mater. Electron. 2020, 31, 14838–14850. [Google Scholar] [CrossRef]
- Hadouchi, M.; Assani, A.; Saadi, M.; Lahmar, A.; El Marssi, M.; Sajieddine, M.; El Ammari, L. Synthesis, Characterization, and Magnetic Properties of A2Co2Fe(VO4)3 (A = Ag or Na) Alluaudite-Type Vanadates. J. Supercond. Nov. Magn. 2019, 32, 2437–2446. [Google Scholar] [CrossRef]
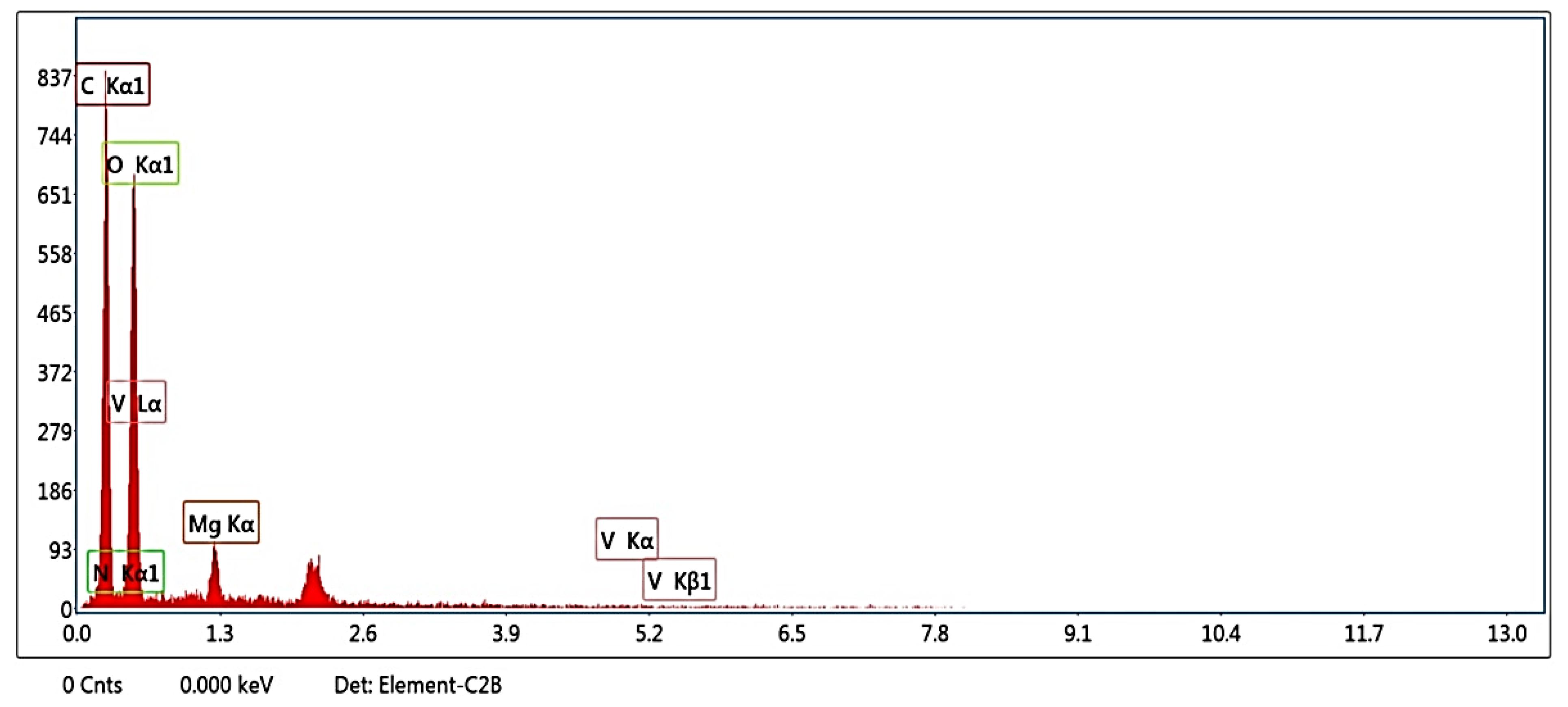

| Element | Weight % | Atomic % |
|---|---|---|
| C K | 43.05 | 50.66 |
| N K | 2.56 | 2.59 |
| O K | 50.68 | 44.77 |
| MgK | 3.12 | 1.81 |
| V K | 0.59 | 0.16 |
| Composition | Thickness (mm) | Absorption Edge (eV) | Bandgap (eV) | n | |
|---|---|---|---|---|---|
| Direct | Indirect | ||||
| CA | 0.1 | 4.9 | 4.9 | 5.1 | 1.73 |
| Mg3(VO4)2@CA | 0.3 | 4.05 | 4.05 | 4.5 | 1.8 |
| MgO@CA | 0.25 | 4.1 | 4.1 | 4.55 | 1.78 |
| Mg3(VO4)2/MgO@CA | 0.12 | 4.5 | 4.7 | 4.75 | 1.75 |
| Mg3(VO4)2/MgO/GO@CA | 0.31 | 3.45 | 3.9 | 4.4 | 1.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taher, F.A.; Gouda, M.; Khalaf, M.M.; Shaaban, S.; Al Bosager, A.Y.A.; Algafly, D.A.A.; Mahfouz, M.K.; Abou Taleb, M.F.; Abd El-Lateef, H.M. Magnesium Ortho-Vanadate/Magnesium Oxide/Graphene Oxide Embedded through Cellulose Acetate-Based Films for Wound Healing Applications. Materials 2023, 16, 3009. https://doi.org/10.3390/ma16083009
Taher FA, Gouda M, Khalaf MM, Shaaban S, Al Bosager AYA, Algafly DAA, Mahfouz MK, Abou Taleb MF, Abd El-Lateef HM. Magnesium Ortho-Vanadate/Magnesium Oxide/Graphene Oxide Embedded through Cellulose Acetate-Based Films for Wound Healing Applications. Materials. 2023; 16(8):3009. https://doi.org/10.3390/ma16083009
Chicago/Turabian StyleTaher, Fatemah A., Mohamed Gouda, Mai M. Khalaf, Saad Shaaban, Alnoor Y. A. Al Bosager, Dania A. A. Algafly, Metwally K. Mahfouz, Manal F. Abou Taleb, and Hany M. Abd El-Lateef. 2023. "Magnesium Ortho-Vanadate/Magnesium Oxide/Graphene Oxide Embedded through Cellulose Acetate-Based Films for Wound Healing Applications" Materials 16, no. 8: 3009. https://doi.org/10.3390/ma16083009
APA StyleTaher, F. A., Gouda, M., Khalaf, M. M., Shaaban, S., Al Bosager, A. Y. A., Algafly, D. A. A., Mahfouz, M. K., Abou Taleb, M. F., & Abd El-Lateef, H. M. (2023). Magnesium Ortho-Vanadate/Magnesium Oxide/Graphene Oxide Embedded through Cellulose Acetate-Based Films for Wound Healing Applications. Materials, 16(8), 3009. https://doi.org/10.3390/ma16083009









