Effects of Hypochlorous Acid and Hydrogen Peroxide Treatment on Bacterial Disinfection Treatments in Implantoplasty Procedures
Abstract
:1. Introduction
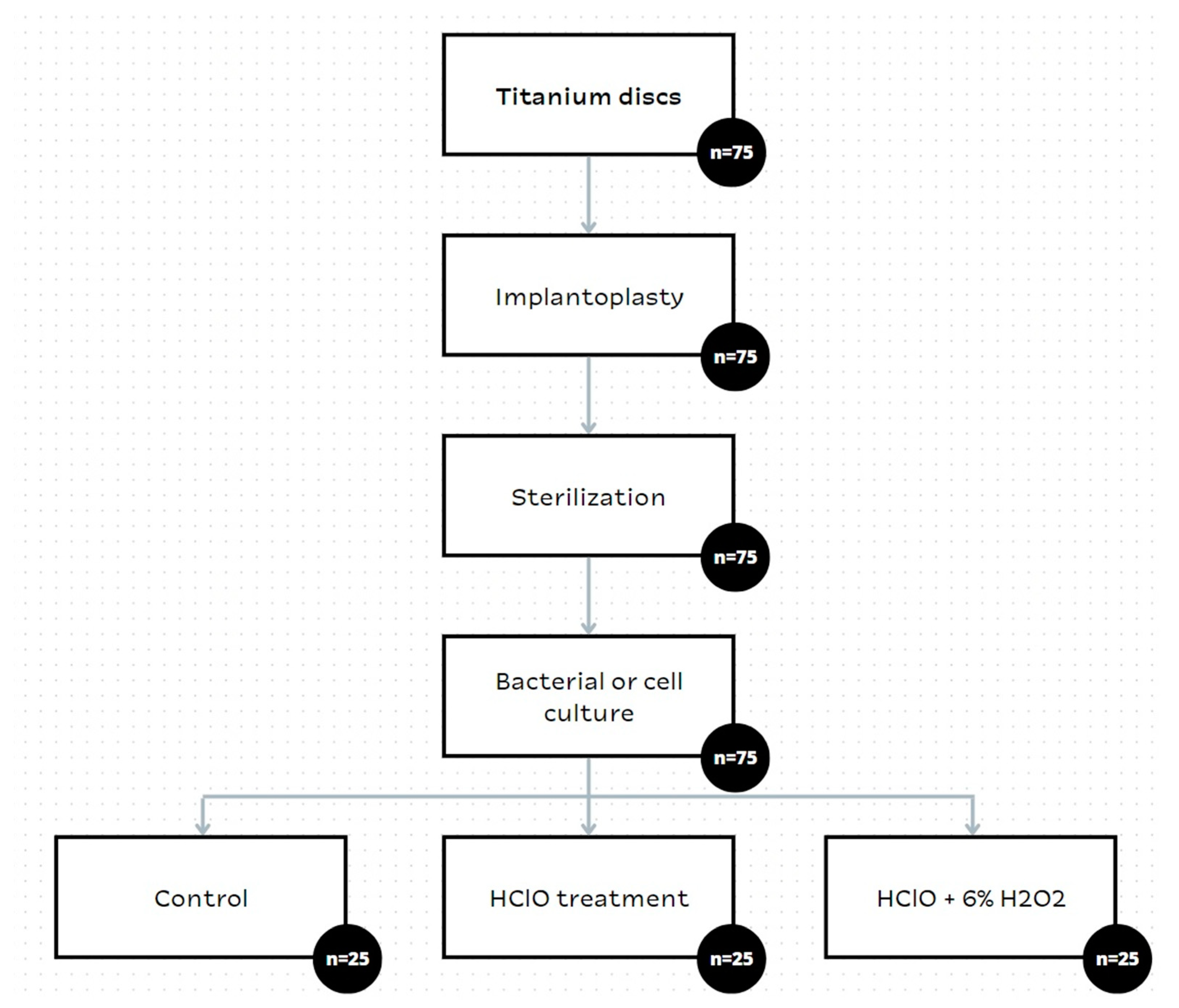
2. Materials and Methods
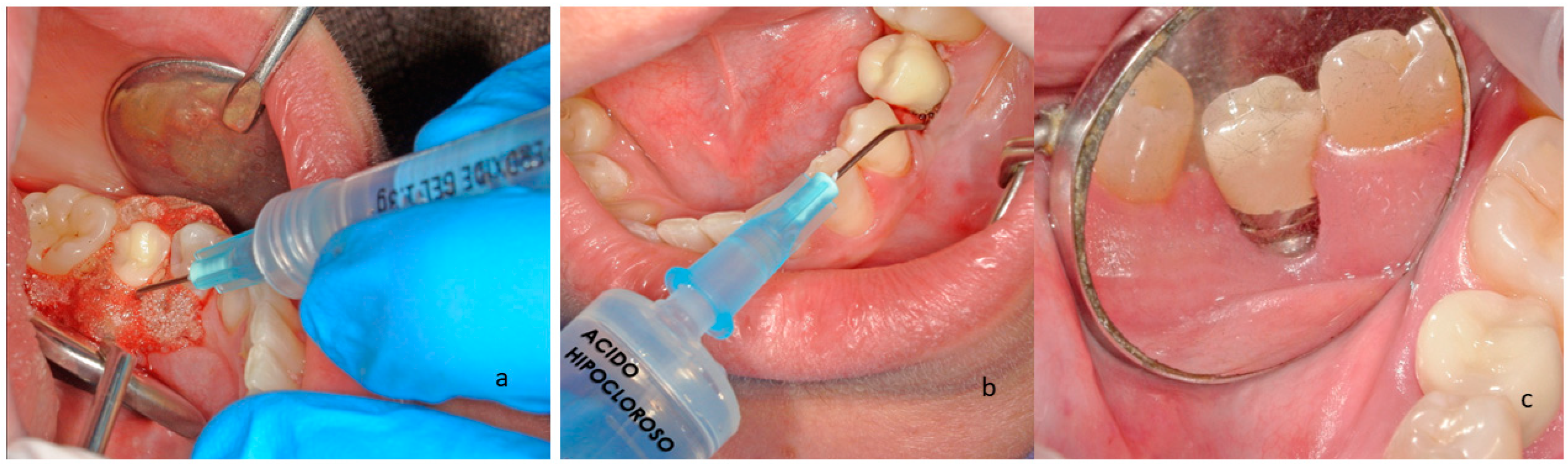
2.1. Materials
2.2. Characterization of the Surfaces
2.3. Cytotoxicity Assay
- -
- Study samples: SAOS-2 cells cultured on discs with different treatments explained above at two time periods (5 s and 1 min);
- -
- Control treatment: SAOS-2 cells cultured on untreated discs (washed with PBS to simulate sample conditions);
- -
- Negative control: SAOS-2 cells seeded directly on the plate.
2.4. Microbiological Response Assay
2.5. Statistical Analysis
3. Results
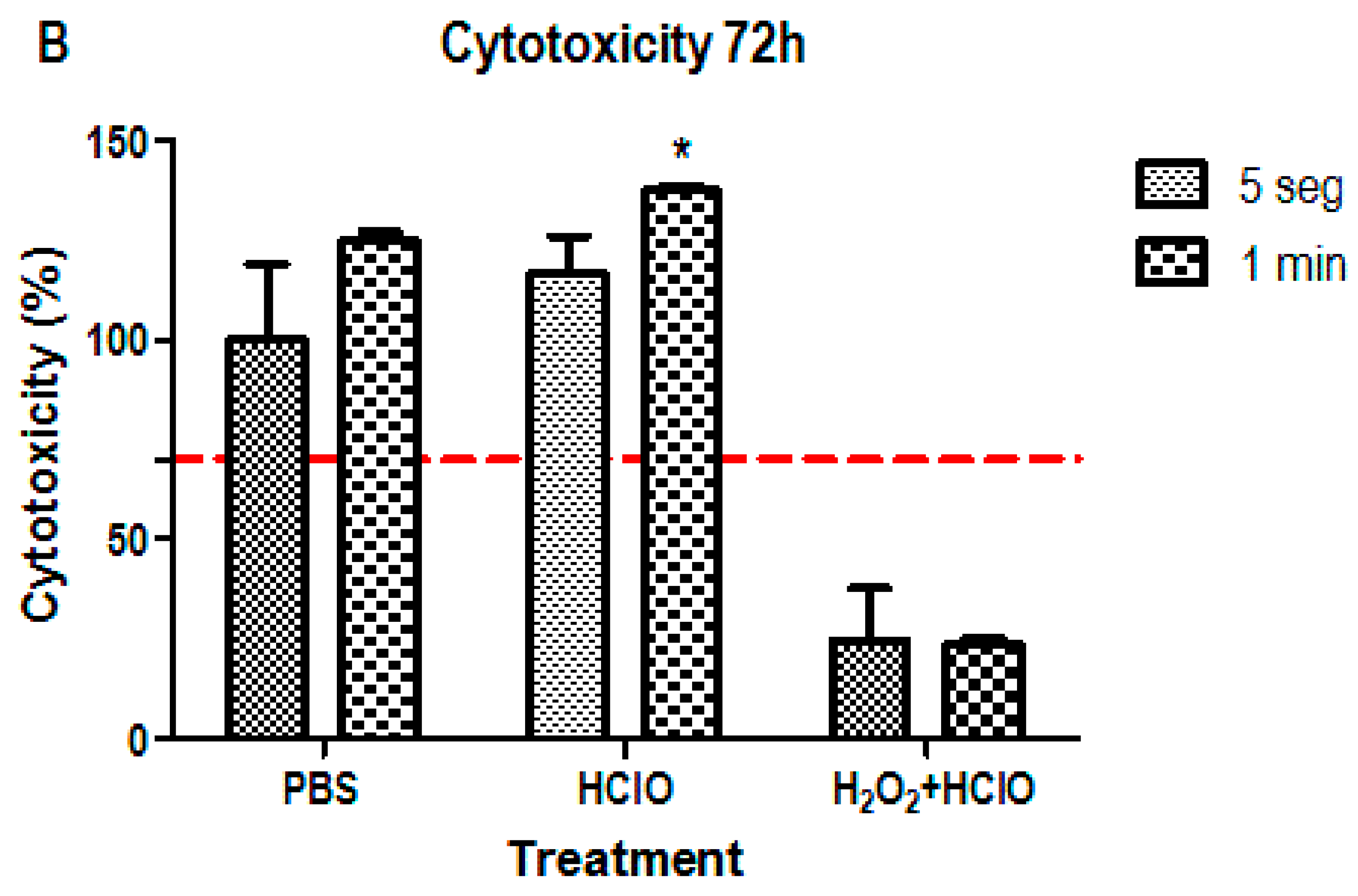
3.1. Cytotoxicity Assay
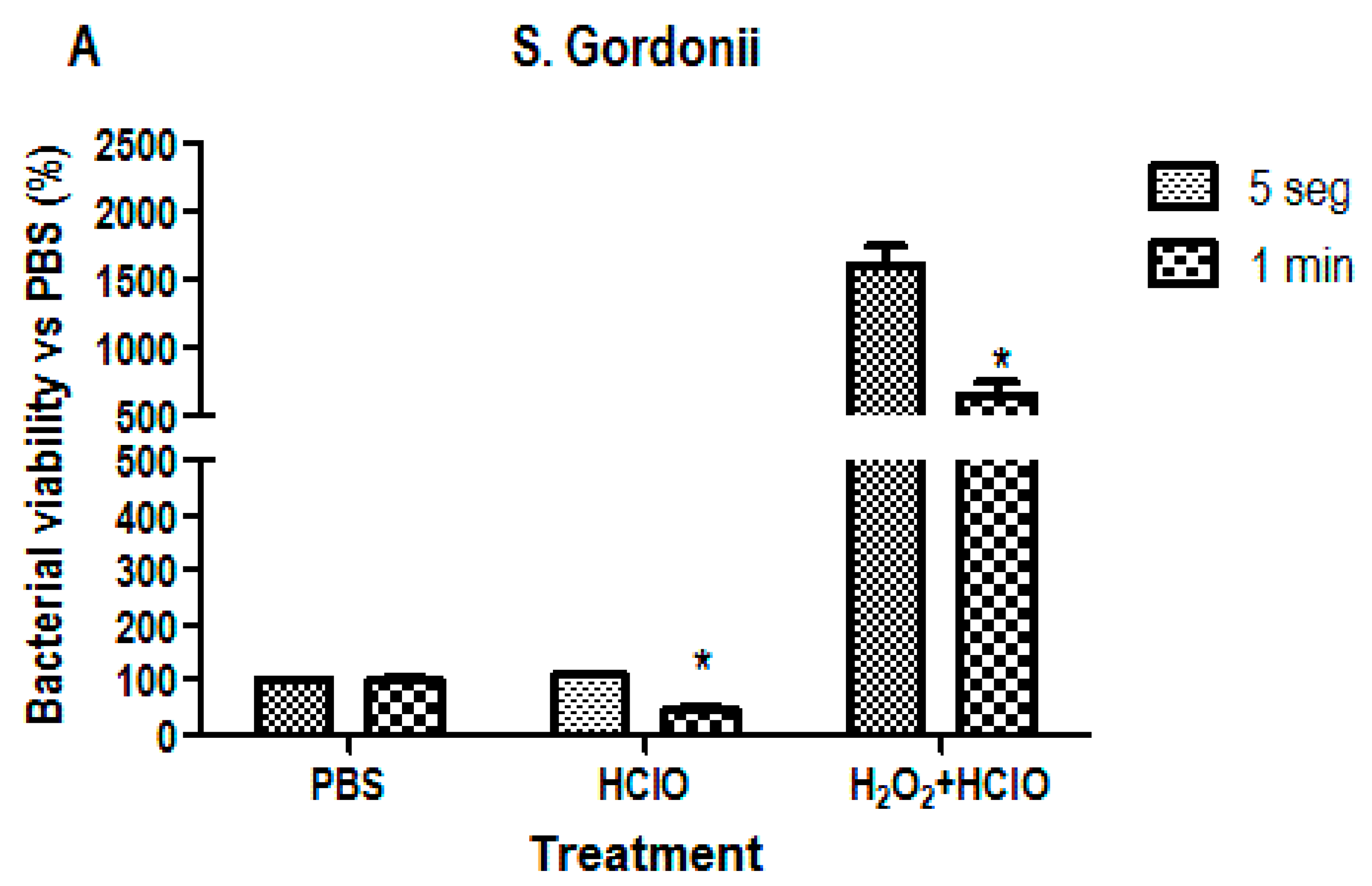
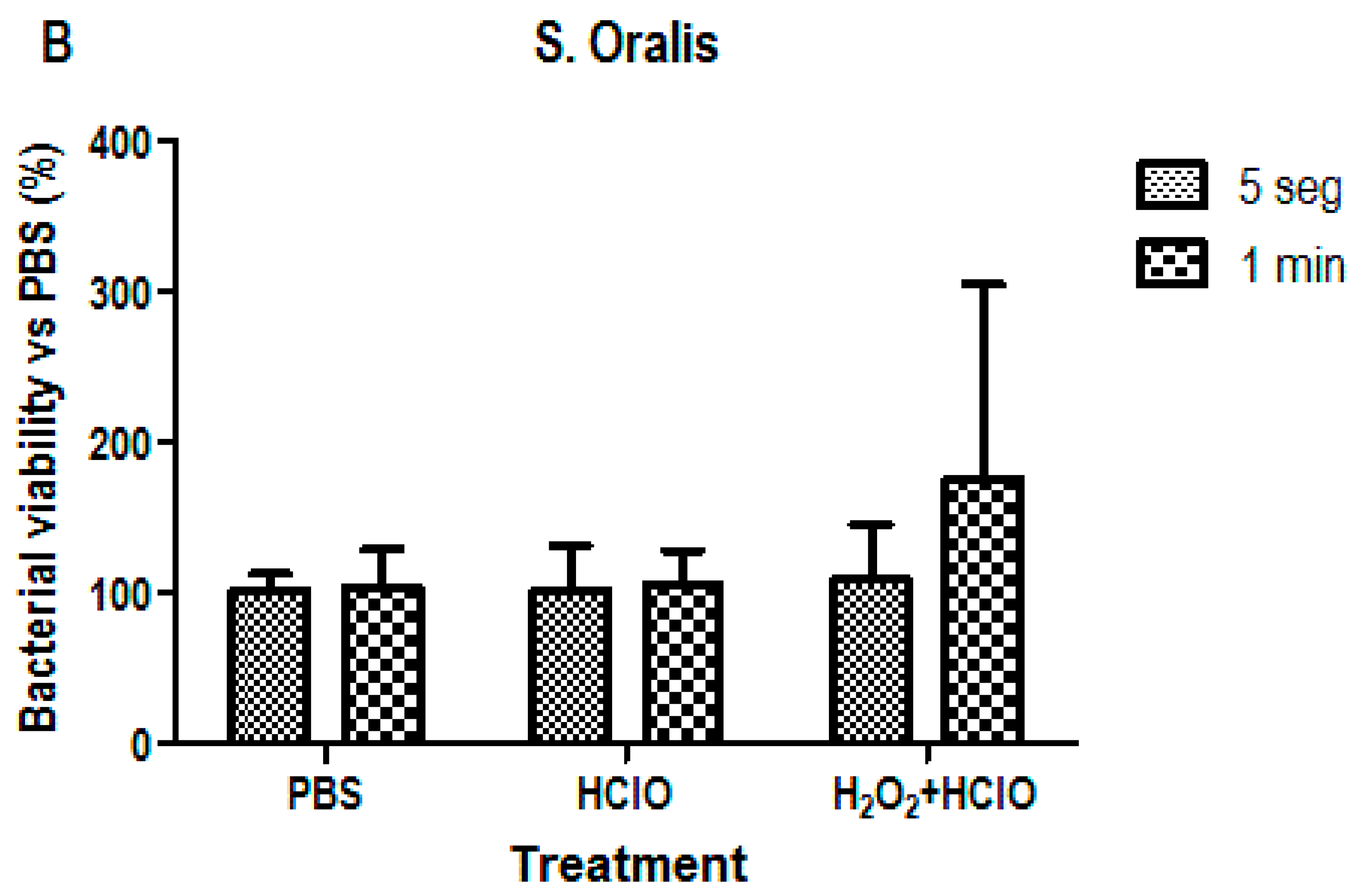
3.2. Bacterial Viability Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S286–S291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Affeld, K.; Grosshauser, J.; Goubergrits, L.; Kertzscher, U. Percutaneous devices: A review of applications, problems and possible solutions. Expert Rev. Med. Devices 2012, 9, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.-N.; Badran, Z.; Ciobanu, O.; Hamdan, N.; Tamimi, F. Strategies for Optimizing the Soft Tissue Seal around Osseointegrated Implants. Adv. Health Mater. 2017, 6, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Zaid, M.B.; O’Donnell, R.J.; Potter, B.K.; Forsberg, J.A. Orthopaedic Osseointegration: State of the Art. J. Am. Acad. Orthop. Surg. 2019, 27, E977–E985. [Google Scholar] [CrossRef]
- Fu, J.; Wang, H. Breaking the wave of peri-implantitis. Periodontology 2000 2020, 84, 145–160. [Google Scholar] [CrossRef]
- Hu, B.; Tao, L.; Rosenthal, V.D.; Liu, K.; Yun, Y.; Suo, Y.; Gao, X.; Li, R.; Su, D.; Wang, H.; et al. Device-associated infection rates, device use, length of stay, and mortality in intensive care units of 4 Chinese hospitals: International Nosocomial Control Consortium findings. Am. J. Infect. Control. 2013, 41, 301–306. [Google Scholar] [CrossRef]
- Astolfi, V.; Ríos-Carrasco, B.; Gil-Mur, F.J.; Ríos-Santos, J.V.; Bullón, B.; Herrero-Climent, M.; Bullón, P. Incidence of Peri-Implantitis and Relationship with Different Conditions: A Retrospective Study. Int. J. Environ. Res. Public Health 2022, 19, 4147. [Google Scholar] [CrossRef]
- Nathan, C. Resisting antimicrobial resistance. Nat. Rev. Microbiol. 2020, 18, 259–260. [Google Scholar] [CrossRef]
- Toledano-Serrabona, J.; Bosch, B.M.; Díez-Tercero, L.; Gil, F.J.; Camps-Font, O.; Valmaseda-Castellón, E.; Gay-Escoda, C.; Sánchez-Garcés, M. Evaluation of the inflammatory and osteogenic response induced by titanium particles released during implantoplasty of dental implants. Sci. Rep. 2022, 12, 15790. [Google Scholar] [CrossRef]
- Callejas, J.A.; Brizuela, A.; Ríos-Carrasco, B.; Gil, J. The Characterization of Titanium Particles Released from Bone-Level Titanium Dental Implants: Effect of the Size of Particles on the Ion Release and Cytotoxicity Behaviour. Materials 2022, 15, 3636. [Google Scholar] [CrossRef]
- Toledano-Serrabona, J.; Gil, F.J.; Camps-Font, O.; Valmaseda-Castellón, E.; Gay-Escoda, C.; Sánchez-Garcés, M. Physicochemical and Biological Characterization of Ti6Al4V Particles Obtained by Implantoplasty: An In Vitro Study. Part I. Materials 2021, 14, 6507. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Serrabona, J.; Sánchez-Garcés, M.; Gay-Escoda, C.; Valmaseda-Castellón, E.; Camps-Font, O.; Verdeguer, P.; Molmeneu, M.; Gil, F.J. Mechanical Properties and Corrosion Behavior of Ti6Al4V Particles Obtained by Implantoplasty: An In Vitro Study. Part II. Materials 2021, 14, 6519. [Google Scholar] [CrossRef]
- Wu, X.; Cai, C.; Gil, J.; Jantz, E.; Al Sakka, Y.; Padial-Molina, M.; Suárez-López del Amo, F. Characteristics of Particles and Debris Released after Implantoplasty: A Comparative Study. Materials 2022, 15, 602. [Google Scholar] [CrossRef] [PubMed]
- Lozano, P.; Peña, M.; Herrero-Climent, M.; Rios-Santos, J.V.; Rios-Carrasco, B.; Brizuela, A.; Gil, J. Corrosion Behavior of Titanium Dental Implants with Implantoplasty. Materials 2022, 15, 1563. [Google Scholar] [CrossRef] [PubMed]
- Vara, J.C.; Delgado, J.; Estrada-Martínez, A.; Pérez-Pevida, E.; Brizuela, A.; Bosch, B.; Pérez, R.; Gil, J. Effect of the Nature of the Particles Released from Bone Level Dental Implants: Physicochemical and Biological Characterization. Coatings 2022, 12, 219. [Google Scholar] [CrossRef]
- Lindhe, J.; Meyle, J.; on behalf of Group D of the European Workshop on Periodontology. Peri-implant diseases: Consensus report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 282–285. [Google Scholar] [CrossRef] [Green Version]
- Nu, Z.; Berglundh, T. Definition and prevalence of peri-implant diseases. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 286–291. [Google Scholar]
- Segura, G.; Gil, R.; Vicente, F.; Ferreiroa, A.; Faus, J.; Agustín, R. Periimplantitis y mucositis periimplantaria. Factores de riesgo, diagnóstico y tratamiento. Av. Periodoncia 2015, 27, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Padullés, E. Patología Periimplantaria. In Efecto de las Bacterias en el Ecosistema Implantológico; Esteban Padullés-Roig; Editorial Quintessence: Madrid, Spain, 2016; pp. 124–132. ISBN 978-84-89873-64-4. [Google Scholar]
- Gil, F.J.; Planell, J.A. Aplicaciones Biomédicas del titanio y sus aleaciones. Soc. Ibérica Biomecánica Biomater. 1993, 1–9. [Google Scholar] [CrossRef]
- Godoy-gallardo, M.; Wang, Z.; Shen, Y.; Manero, J.M.; Gil, F.J.; Rodriguez, D.; Haapasalo, M. Antibacterial coatings on titanium surfaces: A comparison study between in vitro single-species and multispecies biofilm. ACS Appl. Mater. Interfaces 2015, 7, 5992–6001. [Google Scholar] [CrossRef] [Green Version]
- Ungvári, K.; Pelsöczi, I.K.; Kormos, B.; Oszkó, A.; Rakonczay, Z.; Kemény, L.; Radnai, M.; Nagy, K.; Fazekas, A.; Turzó, K. Effects on titanium implant surfaces of chemical agents used for the treatment of peri-implantitis. J. Clin. Periodontol. 2010, 37, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Hauser-Gerspach, I.; Vadaszan, J.; Deronjic, I.; Gass, C.; Meyer, J.; Dard, M.; Waltimo, T.; Stübinger, S.; Mauth, C. Influence of gaseous ozone in peri-implantitis: Bactericidal efficacy and cellular response. An in vitro study using titanium and zirconia. Clin. Oral Investig. 2012, 16, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Becker, J. Periimplant Infection. Etiology, Diagnosis and Treatment; Quintessence Publishing Co Ltd.: London, UK, 2010. [Google Scholar]
- Ungvári, K.; Pelsoczi, K.I.; Kormos, B.; Oszkó, A.; Radnai, M.; Nagy, K.; Fazekas, A.; Turzó, K. Effect of decontaminating solutions on titanium surface: An in vitro study of human epithelial cell culture. Fogorvosi Szle. 2011, 104, 9–18. [Google Scholar]
- Padullés IRoig, E.; Arano Sesma, J.M.; Padullés Gaspar, C. Periimplantitis, protocolo de tratamiento clínico. A propósito de un caso. Rev. Esp. Odontoestom. Impl. 2009, 17, 231–241. [Google Scholar]
- Gosau, M.; Hahnel, S.; Schwarz, F.; Gerlach, T.; Reichert, T.E.; Bürgers, R. Effect of six different peri-implantitis disinfection methods on in vivo human oral biofilm. Int. J. Oral Maxillofac. Implants 2010, 25, 831–833. [Google Scholar]
- Cabanes-Gumbau, G.; Amengual-Lorenzo, J.; Padullés-Roig, E.; Gil-Mur, J. Descontaminación química en periimplantitis mediante gel de peróxido de hidrógeno. Gaceta Dental. 2016, 7, 180–192. [Google Scholar]
- Wennström, J.; Lindhe, J. Effect of hydrogen peroxide on developing plaque and gingivitis in man. J. Clin. Periodontol. 1979, 6, 115–130. [Google Scholar] [CrossRef]
- Lang, N.P.; Brecx, M.C. Chlorhexidine digluconate—An agent for chemical plaque control and prevention of gingival inflammation. J. Periodont. Res. 1986, 21, 74–89. [Google Scholar] [CrossRef]
- Quirynen, M.; Avontroodt, P.; Peeters, W. Effect of different chlorhexidine formulations in mouthrinses on de novo plaque formation. J. Clin. Periodontol. 2001, 28, 1127–1136. [Google Scholar] [CrossRef]
- Salvi, G.E.; Persson, G.R.; Heitz-Mayfield, L.J.; Frei, M.; Lang, N.P. Adjunctive local antibiotic therapy in the treatment of peri-implantitis II: Clinical and radiographic outcomes. Clin. Oral Implants Res. 2007, 18, 281–285. [Google Scholar] [CrossRef]
- Scientific Committee on Consumer Products. Opinion on Hydrogen Peroxide in Tooth Whitening Products; European Commission, Ed.; Health & Consume Protection Directorate General: Brussels, Belgium, 2005; pp. 234–236. [Google Scholar]
- Amengual, J.; Giménez, A.; Torregrosa, M.B.; Forner, L. Actualización de los procedimientos de protección tisular en el tratamiento de las discoloraciones en dientes vitales. Lab Dent. 2005, 6, 226–232. [Google Scholar]
- Powell, L.V.; Bales, D.J. Tooth Bleaching: Its Effect on Oral Tissues. J. Am. Dent. Assoc. 1991, 122, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Alovisi, M.; Carossa, M.; Mandras, N.; Roana, J.; Costalonga, M.; Cavallo, L.; Pira, E.; Putzu, M.G.; Bosio, D.; Roato, I.; et al. Disinfection and Biocompatibility of Titanium Surfaces Treated with Glycine Powder Airflow and Triple Antibiotic Mixture: An In Vitro Study. Materials 2022, 15, 4850. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneve, Switzerland, 2009.
- Velasco-Ortega, E.; Alfonso-Rodríguez, C.; Monsalve-Guil, L.; España-López, A.; Jiménez-Guerra, A.; Garzón, I.; Alaminos, M.; Gil, F. Relevant aspects in the surface properties in titanium dental implants for the cellular viability. Mater. Sci. Eng. C 2016, 64, 1–10. [Google Scholar] [CrossRef]
- Aparicio, C.; Rodriguez, D.; Gil, F.J. Variation of roughness and adhesion strength of deposited apatite layers on titanium dental implants. Mater. Sci. Eng. C 2011, 31, 320–324. [Google Scholar] [CrossRef]
- McDonnell, G. The Use of Hydrogen Peroxide for Disinfection and Sterilization Applications. In PATAI’S Chemistry of Functional Groups; Rappoport, Z., Ed.; Vaisala: Saclay, France, 2014. [Google Scholar]
- Kasemo, B. Biological surface science. Surf. Sci. 2002, 500, 656–677. [Google Scholar] [CrossRef]
- Kasemo, B.; Lausma, J. Surface science aspects on inorganic biomaterials. CRC Crit. Rev. Biocompat. 2006, 2, 335–380. [Google Scholar]
- Moreira, S.D.; Puy, C.L.; Navarro, L.F.; Canut, P.M.; Lorenzo, J.A. Gingival bleeding reduction using a carbamide peroxide based tooth paste with lactoperoxidase. J. Clin. Exp. Dent. 2011, 3, e452–e455. [Google Scholar] [CrossRef]
- Renvert, S.; Lessem, J.; Dahlén, G.; Renvert, H.; Lindahl, C. Mechanical and Repeated Antimicrobial Therapy Using a Local Drug Delivery System in the Treatment of Peri-Implantitis: A Randomized Clinical Trial. J. Periodontol. 2008, 79, 836–844. [Google Scholar] [CrossRef]
- Renvert, S.; Lessem, J.; Dahlen, G.; Lindahl, C.; Svensson, M. Topical minocycline microspheres versus topical chlorhexidine gel as an adjunct to mechanical debridement of incipient peri-implant infections: A randomized clinical trial. J. Clin. Periodontol. 2006, 33, 362–369. [Google Scholar] [CrossRef]
- Tonetti, M.; Cugini, M.A.; Goodson, J.M. Zero-order delivery with periodontal placement of tetracycline-loaded ethylene vinyl acetate fibers. J. Periodontal Res. 1990, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Garrett, S.; Johnson, L.; Drisko, C.H. Two multi-center clinical studies evaluating locally delivered doxycycline hyclate, placebo control, oral hygiene, and scaling and root planing in the treatment of periodontitis. J. Periodontol. 1999, 70, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Radvar, M. A six-month comparison of three periodontal local antimicrobial therapies in persistent periodontal pockets. J. Periodontol. 1999, 70, 1–7. [Google Scholar] [CrossRef]
- Dennison, D.K.; Huerzeler, M.B.; Quinones, C.; Caffesse, R.G. Contaminated Implant Surfaces: An In Vitro Comparison of Implant Surface Coating and Treatment Modalities for Decontamination. J. Periodontol. 1994, 65, 942–948. [Google Scholar] [CrossRef]
- Zablotsky, N.H.; Diedrich, D.L.; Meffert, R.M.; Wittrig, E. The hability of various chemotherapeutic agents to detoxify the endotoxin infected HA-coated implant surface. Int. J. Oral Implant. 1991, 8, 45–51. [Google Scholar]
- Afflito, J.; Gaffar, A.; Marcus, E.C. Effects of tetrapotassium peroxydiphosphate on plaque –gingivitis in vivo. J. Dent. Res. 1988, 67, 401. [Google Scholar]
- Taller, S.H. The effect of baking soda/hydrogen peroxide dentifrice (Mentadent) and a 0.12% percent clorhexidine glu-conato mouthrinse (Peridex) in reducing gingival bleeding. J. New Jersey Dent. Assoc. 1993, 64, 23–25. [Google Scholar]
- Epstein, F.H.; Weiss, S.J. Tissue Destruction by Neutrophils. New Engl. J. Med. 1989, 320, 365–376. [Google Scholar] [CrossRef]
- Lafaurie, G.; Calderón, J.; Zaror, C.; Viviana, L.; Castillo, D. Hypochlorous Acid: A New Alternative as Antimicrobial Agent and for Cell Proliferation for Use in Dentistry. Int. J. Odontostomat. 2015, 9, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Frysh, H. Chemistry of Bleaching. In Complete Dental Bleaching; Goldstein, R.E., Garber, R.E., Eds.; Quintessence Publishing Co., Inc.: Chicago, IL, USA, 1995; pp. 25–34. [Google Scholar]
- Fu, X.; Kassim, S.Y.; Parks, W.C.; Heinecke, J.W. Hypochlorous acid generated by myeloperoxidase modifies adjacent tryptophan and glycine residues in the catalytic domain of matrix metalloproteinase-7 (matrilysin): An oxidative mechanism for restraining proteolytic activity during inflammation. J. Biol. Chem. 2003, 278, 28403–28409. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Cha, Y.N. Taurine chloramine produced from taurine under inflammation provides anti-inflammatory and cyto-protective effects. Amino Acids 2014, 46, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bassiri, M.; Najafi, R.; Najafi, K.; Yang, J.; Khosrovi, B.; Hwong, W.; Barati, E.; Belisle, B.; Celeri, C.; et al. Hypochlorous acid as a potential wound care agent: Part I. Stabilized hypochlorous acid: A component of the inorganic armamentarium of innate immunity. J. Burns Wounds 2007, 6, e5. [Google Scholar] [PubMed]
- Mohammadi, Z.; Shalavi, S.; Yazdizadeh, M. Antimicrobial Activity of Calcium Hydroxide in Endodontics: A Review. Chonnam Med. J. 2012, 48, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, G.; Griffith, C.; Peters, A. Bactericidal Properties of Ozone and Its Potential Application as a Terminal Disinfectant. J. Food Prot. 2000, 63, 1100–1106. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Manzanares-Céspedes, M.C.; Sevilla, P.; Nart, J.; Manzanares, N.; Manero, J.M.; Gil, F.J.; Boyd, S.K.; Rodríguez, D. Evaluation of bone loss in antibacterial coated dental implants: An experimental study in dogs. Mater. Sci. Eng. C 2016, 69, 538–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velasco, E.; Monsalve-Guil, L.; Jimenez, A.; Ortiz, I.; Moreno-Muñoz, J.; Nuñez-Marquez, E.; Pegueroles, M.; Pérez, R.A.; Gil, F.J. Importance of the Roughness and Residual Stresses of Dental Implants on Fatigue and Osseointegration Behavior. In Vivo Study in Rabbits. J. Oral Implant. 2016, 42, 469–476. [Google Scholar] [CrossRef] [PubMed]



| Implant | Ra (μm) | Rz (μm) |
|---|---|---|
| Titanium-control | 0.33 ± 0.12 | 3.10 ± 0.69 |
| Titanium + HClO | 0.41 ± 0.11 | 3.20 ± 0.34 |
| Titanium + HClO + H2O2 | 0.68 ± 0.23 * | 3.92 ± 0.36 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padulles-Gaspar, E.; Padulles-Roig, E.; Cabanes, G.; Pérez, R.A.; Gil, J.; Bosch, B.M. Effects of Hypochlorous Acid and Hydrogen Peroxide Treatment on Bacterial Disinfection Treatments in Implantoplasty Procedures. Materials 2023, 16, 2953. https://doi.org/10.3390/ma16082953
Padulles-Gaspar E, Padulles-Roig E, Cabanes G, Pérez RA, Gil J, Bosch BM. Effects of Hypochlorous Acid and Hydrogen Peroxide Treatment on Bacterial Disinfection Treatments in Implantoplasty Procedures. Materials. 2023; 16(8):2953. https://doi.org/10.3390/ma16082953
Chicago/Turabian StylePadulles-Gaspar, Esteban, Esteban Padulles-Roig, Guillermo Cabanes, Román A. Pérez, Javier Gil, and Begoña M. Bosch. 2023. "Effects of Hypochlorous Acid and Hydrogen Peroxide Treatment on Bacterial Disinfection Treatments in Implantoplasty Procedures" Materials 16, no. 8: 2953. https://doi.org/10.3390/ma16082953
APA StylePadulles-Gaspar, E., Padulles-Roig, E., Cabanes, G., Pérez, R. A., Gil, J., & Bosch, B. M. (2023). Effects of Hypochlorous Acid and Hydrogen Peroxide Treatment on Bacterial Disinfection Treatments in Implantoplasty Procedures. Materials, 16(8), 2953. https://doi.org/10.3390/ma16082953








