Antiviral Activity of Active Materials: Standard and Finger-Pad-Based Innovative Experimental Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses and Cell Lines
2.2. Active and Reference Materials
2.3. Antiviral Activity of Active Materials
2.4. Finger-Pad Experiment
3. Results
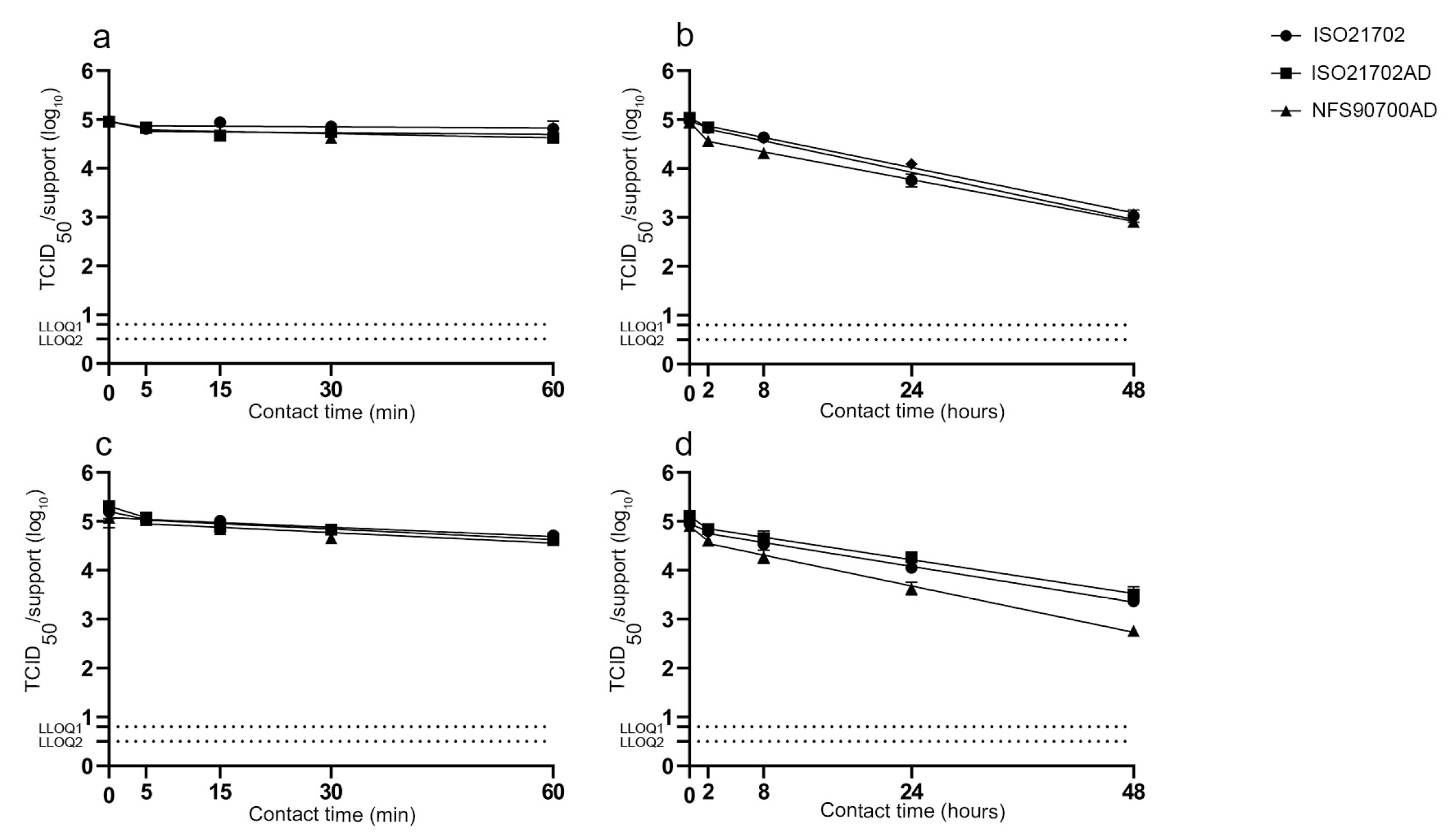
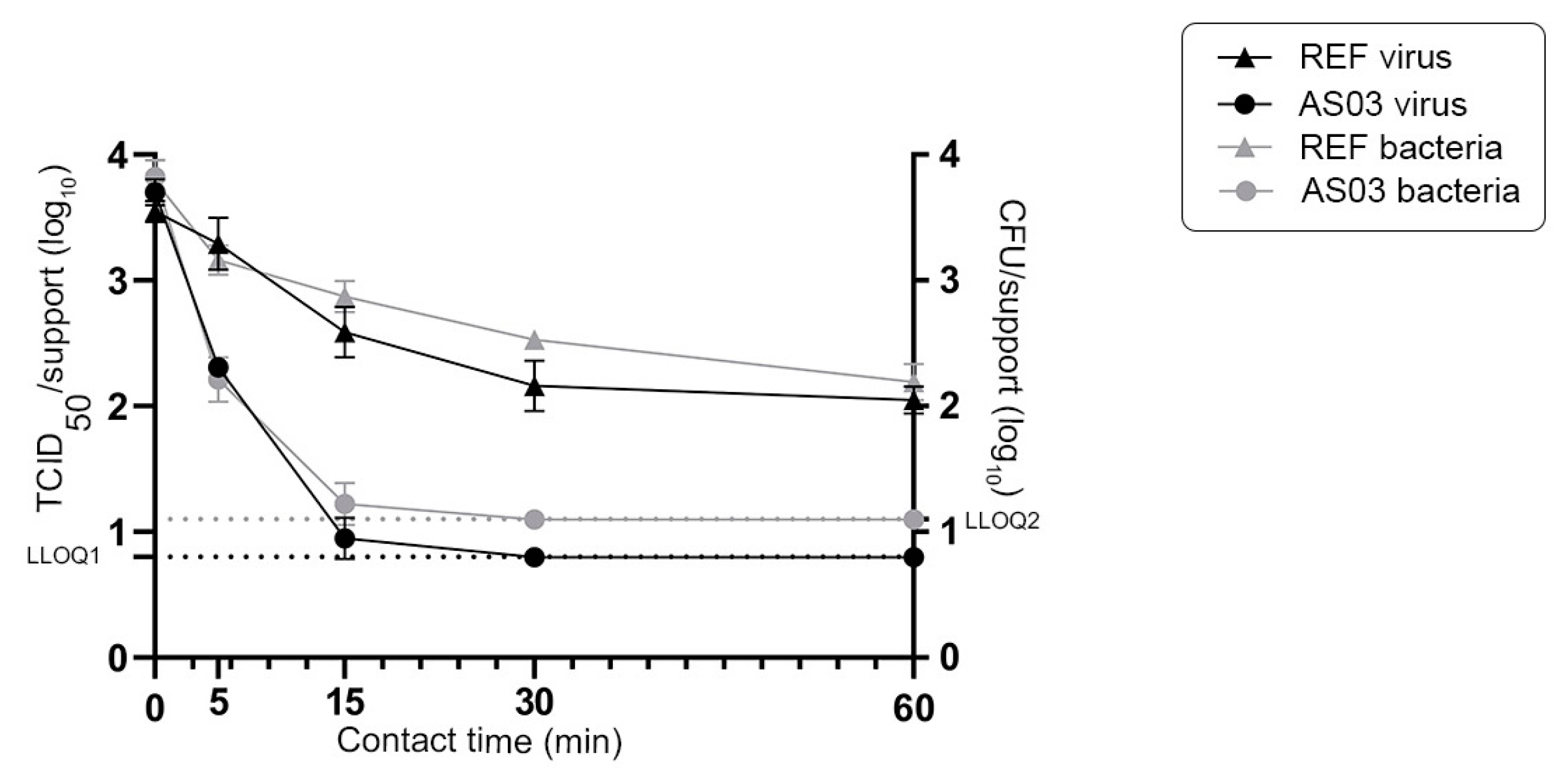
3.1. Impact of Experimental Parameters on the Survival of Enveloped and Non-Enveloped Viruses on the Stainless Steel Reference Surface
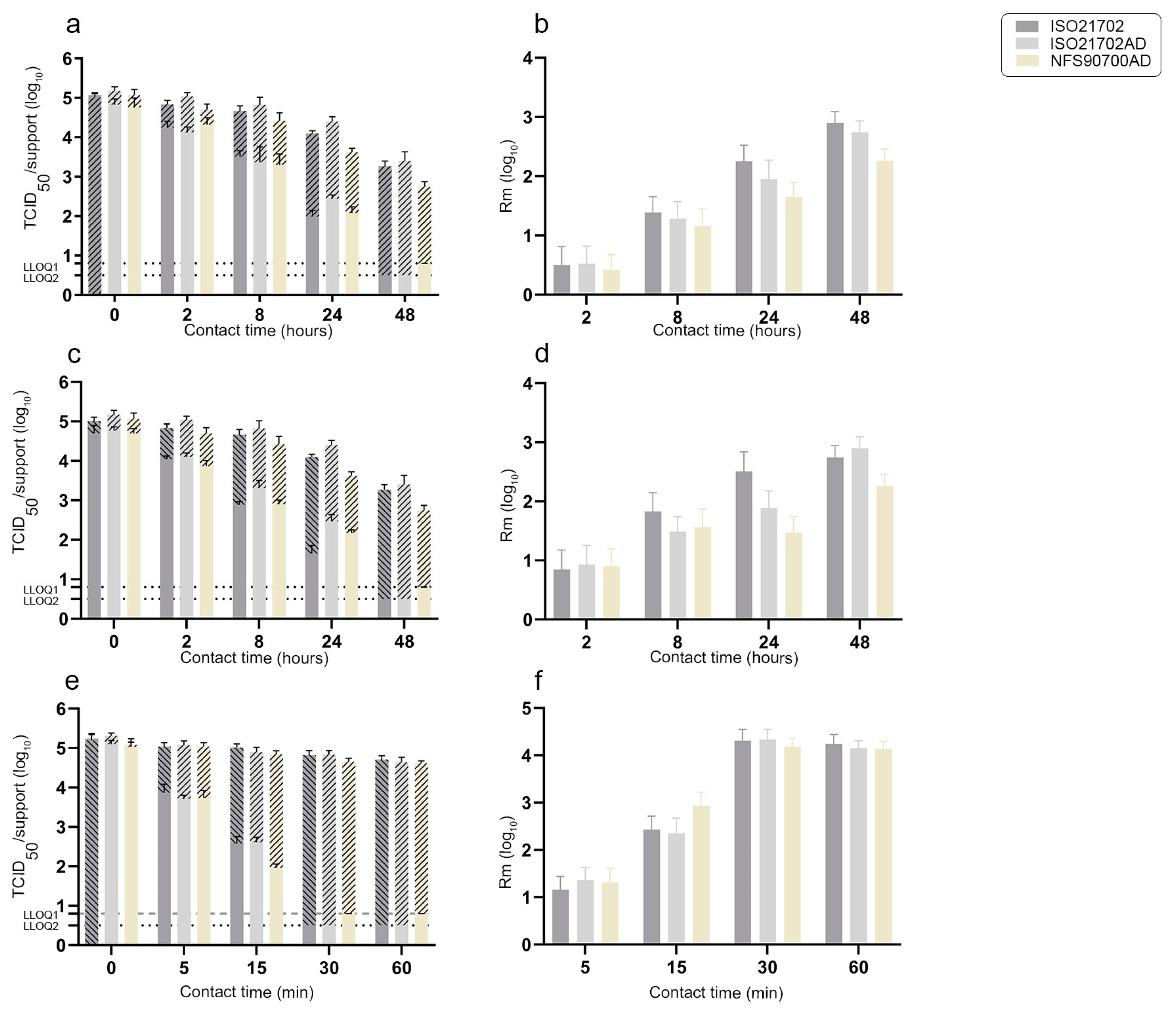
3.2. Residual Infectious Virus on Surfaces over Time and Antiviral Activities of Active Materials According to Experimental Protocols
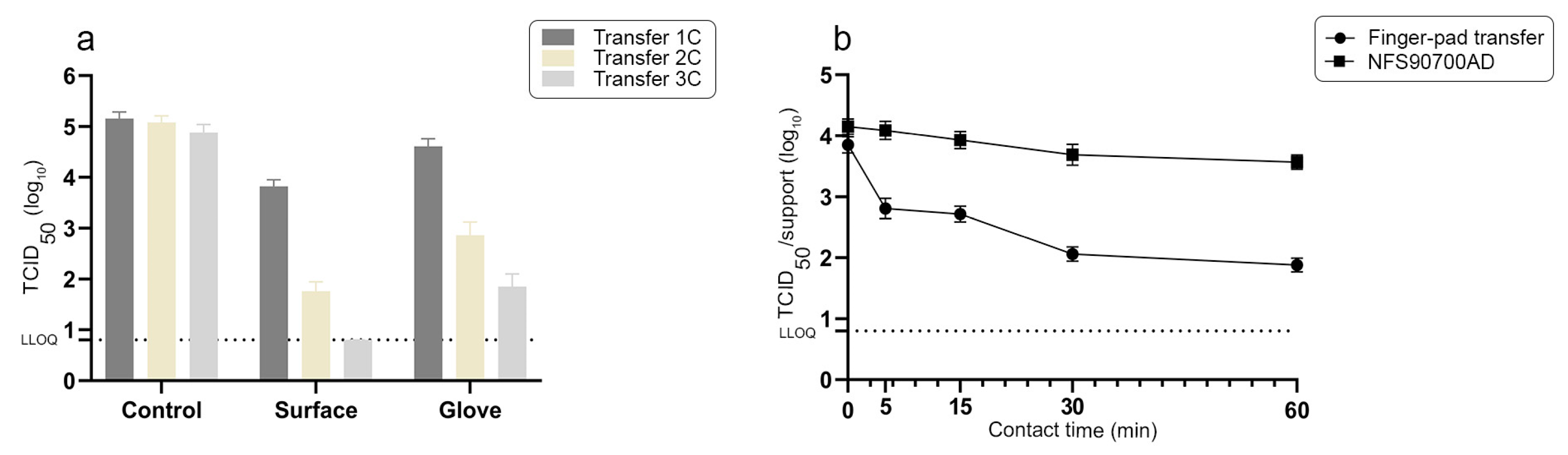
3.3. Antiviral Activity of High-Touch Active Surfaces According to a Finger-Pad Transfer Experimental Method
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashour, H.M.; Elkhatib, W.F.; Rahman, M.M.; Elshabrawy, H.A. Insights into the Recent 2019 Novel Coronavirus (SARS-CoV-2) in Light of Past Human Coronavirus Outbreaks. Pathogens 2020, 9, 186. [Google Scholar] [CrossRef]
- Bean, B.; Moore, B.M.; Sterner, B.; Peterson, L.R.; Gerding, D.N.; Balfour, H.H. Survival of influenza viruses on environmental surfaces. J. Infect. Dis. 1982, 146, 47–51. [Google Scholar] [CrossRef]
- Otter, J.A.; Yezli, S.; Salkeld, J.A.G.; French, G.L. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am. J. Infect. Control. 2013, 41 (Suppl. S5), S6–S11. [Google Scholar] [CrossRef]
- Bidawid, S.; Malik, N.; Adegbunrin, O.; Sattar, S.A.; Farber, J.M. Norovirus cross-contamination during food handling and interruption of virus transfer by hand antisepsis: Experiments with feline calicivirus as a surrogate. J. Food Prot. 2004, 67, 103–109. [Google Scholar] [CrossRef]
- Kambhampati, A.; Koopmans, M.; Lopman, B.A. Burden of norovirus in healthcare facilities and strategies for outbreak control. J. Hosp. Infect. 2015, 89, 296–301. [Google Scholar] [CrossRef]
- Bidawid, S.; Farber, J.M.; Sattar, S.A. Contamination of foods by food handlers: Experiments on hepatitis A virus transfer to food and its interruption. Appl. Environ. Microbiol. 2000, 66, 2759–2763. [Google Scholar] [CrossRef]
- Santarpia, J.L.; Rivera, D.N.; Herrera, V.L.; Morwitzer, M.J.; Creager, H.M.; Santarpia, G.W.; Crown, K.K.; Brett-Major, D.M.; Schnaubelt, E.R.; Broadhurst, M.J.; et al. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci. Rep. 2020, 10, 12732. [Google Scholar] [CrossRef]
- Dabisch, P.A.; Biryukov, J.; Beck, K.; Boydston, J.A.; Sanjak, J.S.; Herzog, A.; Green, B.; Williams, G.; Yeager, J.; Bohannon, J.K.; et al. Seroconversion and fever are dose-dependent in a nonhuman primate model of inhalational COVID-19. PLoS Pathog. 2021, 17, e1009865. [Google Scholar] [CrossRef]
- Popa, A.; Genger, J.-W.; Nicholson, M.D.; Penz, T.; Schmid, D.; Aberle, S.W.; Agerer, B.; Lercher, A.; Endler, L.; Colaço, H.; et al. Genomic epidemiology of superspreading events in Austria reveals mutational dynamics and transmission properties of SARS-CoV-2. Sci. Transl. Med. 2020, 12, eabe2555. [Google Scholar] [CrossRef]
- Fan, C.; Wu, Y.; Rui, X.; Yang, Y.; Ling, C.; Liu, S.; Liu, S.; Wang, Y. Animal models for COVID-19: Advances, gaps and perspectives. Signal Transduct. Target. Ther. 2022, 7, 220. [Google Scholar] [CrossRef]
- Fankhauser, R.L.; Monroe, S.S.; Noel, J.S.; Humphrey, C.D.; Bresee, J.S.; Parashar, U.D.; Ando, T.; Glass, R.I. Epidemiologic and molecular trends of “Norwalk-like viruses” associated with outbreaks of gastroenteritis in the United States. J. Infect. Dis. 2002, 186, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Monroe, S.S.; Ando, T.; Glass, R.I. Introduction: Human enteric caliciviruses-an emerging pathogen whose time has come. J. Infect. Dis. 2000, 181 (Suppl. 2), S249–S251. [Google Scholar] [CrossRef]
- Lopez, G.U.; Gerba, C.P.; Tamimi, A.H.; Kitajima, M.; Maxwell, S.L.; Rose, J.B. Transfer efficiency of bacteria and viruses from porous and nonporous fomites to fingers under different relative humidity conditions. Appl. Environ. Microbiol. 2013, 79, 5728–5734. [Google Scholar] [CrossRef]
- Anderson, C.E.; Boehm, A.B. Transfer Rate of Enveloped and Nonenveloped Viruses between Fingerpads and Surfaces. Appl. Environ. Microbiol. 2021, 87, e0121521. [Google Scholar] [CrossRef]
- Ansari, S.A.; Springthorpe, V.S.; Sattar, S.A.; Rivard, S.; Rahman, M. Potential role of hands in the spread of respiratory viral infections: Studies with human parainfluenza virus 3 and rhinovirus 14. J. Clin. Microbiol. 1991, 29, 2115–2119. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Chattopadhyay, S.; Lyon, W.G.; Wilson, J.T. Effect of surfactants on the survival and sorption of viruses. Environ. Sci. Technol. 2002, 36, 4017–4024. [Google Scholar] [CrossRef]
- Tiwari, A.; Patnayak, D.P.; Chander, Y.; Parsad, M.; Goyal, S.M. Survival of two avian respiratory viruses on porous and nonporous surfaces. Avian Dis. 2006, 50, 284–287. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Pratsinis, S.E. Engineering nanosilver as an antibacterial, biosensor and bioimaging material. Curr. Opin. Chem. Eng. 2011, 1, 3–10. [Google Scholar] [CrossRef]
- Pop, C.S.; Hussien, M.D.; Popa, M.; Mares, A.; Grumezescu, A.M.; Grigore, R.; Lazar, V.; Chifiriuc, M.C.; Sakizlian, M.; Bezirtzoglou, E.; et al. Metallic-based micro and nanostructures with antimicrobial activity. Curr. Top. Med. Chem. 2015, 15, 1577–1582. [Google Scholar] [CrossRef]
- Imani, S.M.; Maclachlan, R.; Rachwalski, K.; Chan, Y.; Lee, B.; McInnes, M.; Grandfield, K.; Brown, E.D.; Didar, T.F.; Soleymani, L. Flexible Hierarchical Wraps Repel Drug-Resistant Gram-Negative and Positive Bacteria. ACS Nano 2020, 14, 454–465. [Google Scholar] [CrossRef]
- Leslie, D.C.; Waterhouse, A.; Berthet, J.B.; Valentin, T.M.; Watters, A.L.; Jain, A.; Kim, P.; Hatton, B.D.; Nedder, A.; Donovan, K.; et al. A bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling. Nat. Biotechnol. 2014, 32, 1134–1140. [Google Scholar] [CrossRef]
- Nguyen, D.H.K.; Pham, V.T.H.; Truong, V.K.; Sbarski, I.; Wang, J.; Balčytis, A.; Juodkazis, S.; Mainwaring, D.E.; Crawford, R.J.; Ivanova, E.P. Role of topological scale in the differential fouling of Pseudomonas aeruginosa and Staphylococcus aureus bacterial cells on wrinkled gold-coated polystyrene surfaces. Nanoscale 2018, 10, 5089–5096. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Pratsinis, S.E. Antibacterial activity of nanosilver ions and particles. Environ. Sci. Technol. 2010, 44, 5649–5654. [Google Scholar] [CrossRef]
- Gunawan, C.; Faiz, M.B.; Mann, R.; Ting, S.R.S.; Sotiriou, G.A.; Marquis, C.P.; Amal, R. Nanosilver Targets the Bacterial Cell Envelope: The Link with Generation of Reactive Oxygen Radicals. ACS Appl. Mater. Interfaces 2020, 12, 5557–5568. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Deshmukh, S.D.; Ingle, A.P.; Gupta, I.R.; Galdiero, M.; Galdiero, S. Metal nanoparticles: The protective nanoshield against virus infection. Crit. Rev. Microbiol. 2016, 42, 46–56. [Google Scholar] [CrossRef]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef]
- Hu, R.L.; Li, S.R.; Kong, F.J.; Hou, R.J.; Guan, X.L.; Guo, F. Inhibition effect of silver nanoparticles on herpes simplex virus 2. Genet. Mol. Res. GMR 2014, 13, 7022–7028. [Google Scholar] [CrossRef]
- Lu, L.; Sun, R.W.-Y.; Chen, R.; Hui, C.-K.; Ho, C.-M.; Luk, J.M.; Lau, G.K.K.; Che, C.-M. Silver nanoparticles inhibit hepatitis B virus replication. Antivir. Ther. 2008, 13, 253–262. [Google Scholar] [CrossRef]
- Speshock, J.L.; Murdock, R.C.; Braydich-Stolle, L.K.; Schrand, A.M.; Hussain, S.M. Interaction of silver nanoparticles with Tacaribe virus. J. Nanobiotechnol. 2010, 8, 19. [Google Scholar] [CrossRef]
- Trefry, J.C.; Wooley, D.P. Silver nanoparticles inhibit vaccinia virus infection by preventing viral entry through a macropinocytosis-dependent mechanism. J. Biomed. Nanotechnol. 2013, 9, 1624–1635. [Google Scholar] [CrossRef]
- Xiang, D.; Chen, Q.; Pang, L.; Zheng, C. Inhibitory effects of silver nanoparticles on H1N1 influenza A virus in vitro. J. Virol. Methods 2011, 178, 137–142. [Google Scholar] [CrossRef]
- Hosseini, M.; Chin, A.W.H.; Behzadinasab, S.; Poon, L.L.M.; Ducker, W.A. Cupric Oxide Coating That Rapidly Reduces Infection by SARS-CoV-2 via Solids. ACS Appl. Mater. Interfaces 2021, 13, 5919–5928. [Google Scholar] [CrossRef]
- Warnes, S.L.; Keevil, C.W. Mechanism of copper surface toxicity in vancomycin-resistant enterococci following wet or dry surface contact. Appl. Environ. Microbiol. 2011, 77, 6049–6059. [Google Scholar] [CrossRef]
- Warnes, S.L.; Little, Z.R.; Keevil, C.W. Human Coronavirus 229E Remains Infectious on Common Touch Surface Materials. mBio 2015, 6, e01697-15. [Google Scholar] [CrossRef]
- Behzadinasab, S.; Chin, A.; Hosseini, M.; Poon, L.; Ducker, W.A. A Surface Coating that Rapidly Inactivates SARS-CoV-2. ACS Appl. Mater. Interfaces 2020, 12, 34723–34727. [Google Scholar] [CrossRef]
- Kampf, G. Efficacy of biocidal agents and disinfectants against the monkeypox virus and other orthopoxviruses. J. Hosp. Infect. 2022, 127, 101–110. [Google Scholar] [CrossRef]
- Puchkova, L.V.; Broggini, M.; Polishchuk, E.V.; Ilyechova, E.Y.; Polishchuk, R.S. Silver Ions as a Tool for Understanding Different Aspects of Copper Metabolism. Nutrients 2019, 11, 1364. [Google Scholar] [CrossRef]
- ISO 21702; Measurement of Antiviral Activity on Plastics and other Non-Porous Surfaces. AFNOR: Saint-Denis, France, 2019.
- NFS 90700; Surfaces à Propriétés Biocides-Méthode D’évaluation de L’activité Bactéricide de Base D’une Surface Non Poreuse. AFNOR: Saint-Denis, France, 2019.
- Julian, T.R.; Leckie, J.O.; Boehm, A.B. Virus transfer between fingerpads and fomites. J. Appl. Microbiol. 2010, 109, 1868–1874. [Google Scholar] [CrossRef]
- Gao, W.; Howden, B.P.; Stinear, T.P. Evolution of virulence in Enterococcus faecium, a hospital-adapted opportunistic pathogen. Curr. Opin. Microbiol. 2018, 41, 76–82. [Google Scholar] [CrossRef]
- Dowd, S.E.; Pillai, S.D.; Wang, S.; Corapcioglu, M.Y. Delineating the specific influence of virus isoelectric point and size on virus adsorption and transport through sandy soils. Appl. Environ. Microbiol. 1998, 64, 405–410. [Google Scholar] [CrossRef]
- Firquet, S.; Beaujard, S.; Lobert, P.-E.; Sané, F.; Caloone, D.; Izard, D.; Hober, D. Survival of Enveloped and Non-Enveloped Viruses on Inanimate Surfaces. Microbes Environ. 2015, 30, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.Y.Y.; Cheng, P.K.C.; Lim, W.W.L. Survival of severe acute respiratory syndrome coronavirus. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2005, 41, e67–e71. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.S.; Yates, M.V. Bacteriophage inactivation at the air-water-solid interface in dynamic batch systems. Appl. Environ. Microbiol. 1999, 65, 1186–1190. [Google Scholar] [CrossRef]
- Thompson, S.S.; Flury, M.; Yates, M.V.; Jury, W.A. Role of the air-water-solid interface in bacteriophage sorption experiments. Appl. Environ. Microbiol. 1998, 64, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.M.; Ladouceur, L.; Marshall, T.; Maclachlan, R.; Soleymani, L.; Didar, T.F. Antimicrobial Nanomaterials and Coatings: Current Mechanisms and Future Perspectives to Control the Spread of Viruses Including SARS-CoV-2. ACS Nano 2020, 14, 12341–12369. [Google Scholar] [CrossRef]
- Bello-Lopez, J.M.; Silva-Bermudez, P.; Prado, G.; Martínez, A.; Ibáñez-Cervantes, G.; Cureño-Díaz, M.A.; Rocha-Zavaleta, L.; Manzo-Merino, J.; Almaguer-Flores, A.; Ramos-Vilchis, C.; et al. Biocide effect against SARS-CoV-2 and ESKAPE pathogens of a noncytotoxic silver-copper nanofilm. Biomed. Mater. 2021, 17, 015002. [Google Scholar] [CrossRef]
- Ansari, S.A.; Sattar, S.A.; Springthorpe, V.S.; Wells, G.A.; Tostowaryk, W. Rotavirus survival on human hands and transfer of infectious virus to animate and nonporous inanimate surfaces. J. Clin. Microbiol. 1988, 26, 1513–1518. [Google Scholar] [CrossRef]
- Lew, J.F.; Glass, R.I.; Petric, M.; Lebaron, C.W.; Hammond, G.W.; Miller, S.E.; Robinson, C.; Boutilier, J.; Riepenhoff-Talty, M.; Payne, C.M. Six-year retrospective surveillance of gastroenteritis viruses identified at ten electron microscopy centers in the United States and Canada. Pediatr. Infect. Dis. J. 1990, 9, 709–714. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szpiro, L.; Bourgeay, C.; Hoareau, A.L.; Julien, T.; Menard, C.; Marie, Y.; Rosa-Calatrava, M.; Moules, V. Antiviral Activity of Active Materials: Standard and Finger-Pad-Based Innovative Experimental Approaches. Materials 2023, 16, 2889. https://doi.org/10.3390/ma16072889
Szpiro L, Bourgeay C, Hoareau AL, Julien T, Menard C, Marie Y, Rosa-Calatrava M, Moules V. Antiviral Activity of Active Materials: Standard and Finger-Pad-Based Innovative Experimental Approaches. Materials. 2023; 16(7):2889. https://doi.org/10.3390/ma16072889
Chicago/Turabian StyleSzpiro, Lea, Clara Bourgeay, Alexandre Loic Hoareau, Thomas Julien, Camille Menard, Yana Marie, Manuel Rosa-Calatrava, and Vincent Moules. 2023. "Antiviral Activity of Active Materials: Standard and Finger-Pad-Based Innovative Experimental Approaches" Materials 16, no. 7: 2889. https://doi.org/10.3390/ma16072889
APA StyleSzpiro, L., Bourgeay, C., Hoareau, A. L., Julien, T., Menard, C., Marie, Y., Rosa-Calatrava, M., & Moules, V. (2023). Antiviral Activity of Active Materials: Standard and Finger-Pad-Based Innovative Experimental Approaches. Materials, 16(7), 2889. https://doi.org/10.3390/ma16072889





