A Comparative Study of Grain Refining of Al-(7–17%) Si Cast Alloys Using Al-10% Ti and Al-4% B Master Alloys
Abstract
1. Introduction
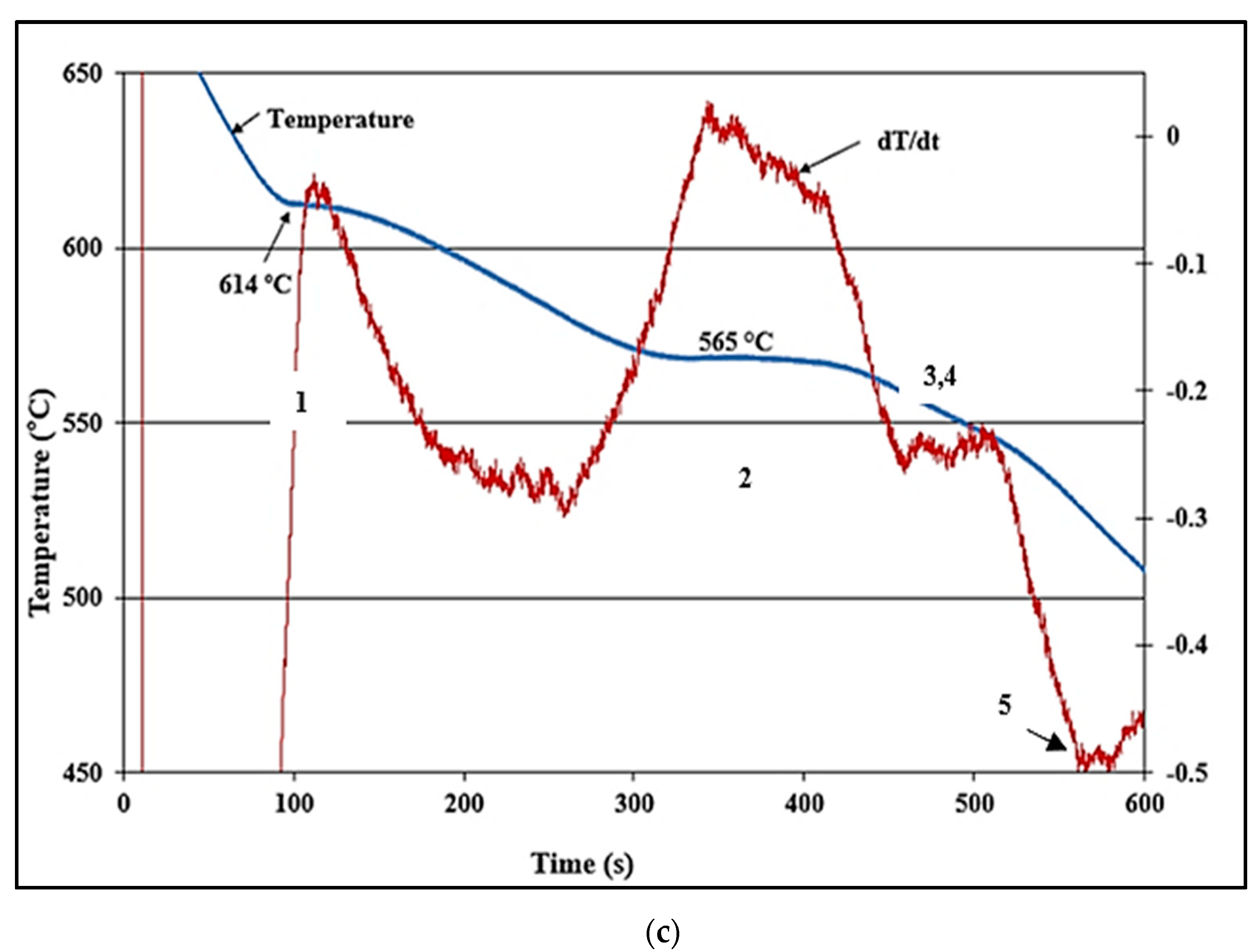
1.1. Thermal Analysis
- -
- ∆TS: undercooling (°C);
- -
- TE: temperature at equilibrium (°C);
- -
- TS: temperature at undercooling (°C);
- -
- K1: constant as a function of solidification rate (s);
- -
- K2: constant as a function of alloying (s);
- -
- : solidification rate (°C/s).

1.2. Grain Nucleation
1.3. Poisoning of Grain Refining
1.4. Objectives
- Analysis of the effect of increasing the concentration on the grain size. One of the particularities of Ti is to further reduce the size of the grains, compared with other elements. Several variables affect grain refining. The different variables that will be studied specifically are the refiner concentration, as well as the overheating and casting temperature. The solidification rate remains identical (≈1 °C/s) for all of the tests.
- Study the different effects of increasing the grain refining concentration on solidification using thermal analysis. The latter makes it possible to study different phenomena and parameters related to (during) solidification.
- Study the different effects of increasing the concentration of the added grain refiner on the microstructure. If there is an impact on the microstructure, there is necessarily an influence on the mechanical properties.
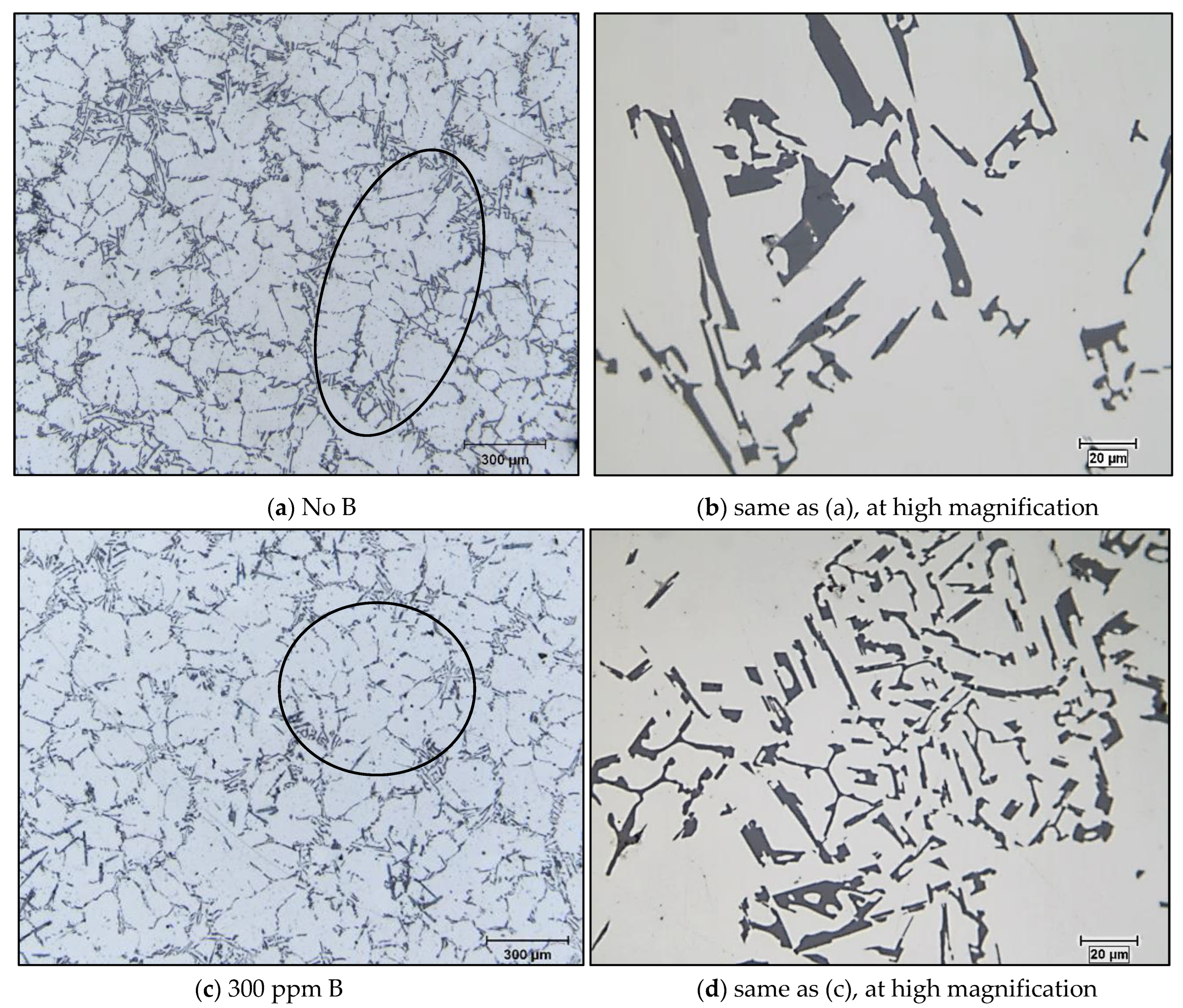
- The introduction of AlB2 in the Al-4% B form in alloys containing traces of Ti leads to the reaction between B and Ti to form TiB2 which is a poor grain refiner.
2. Experimental Procedure
3. Results and Discussion
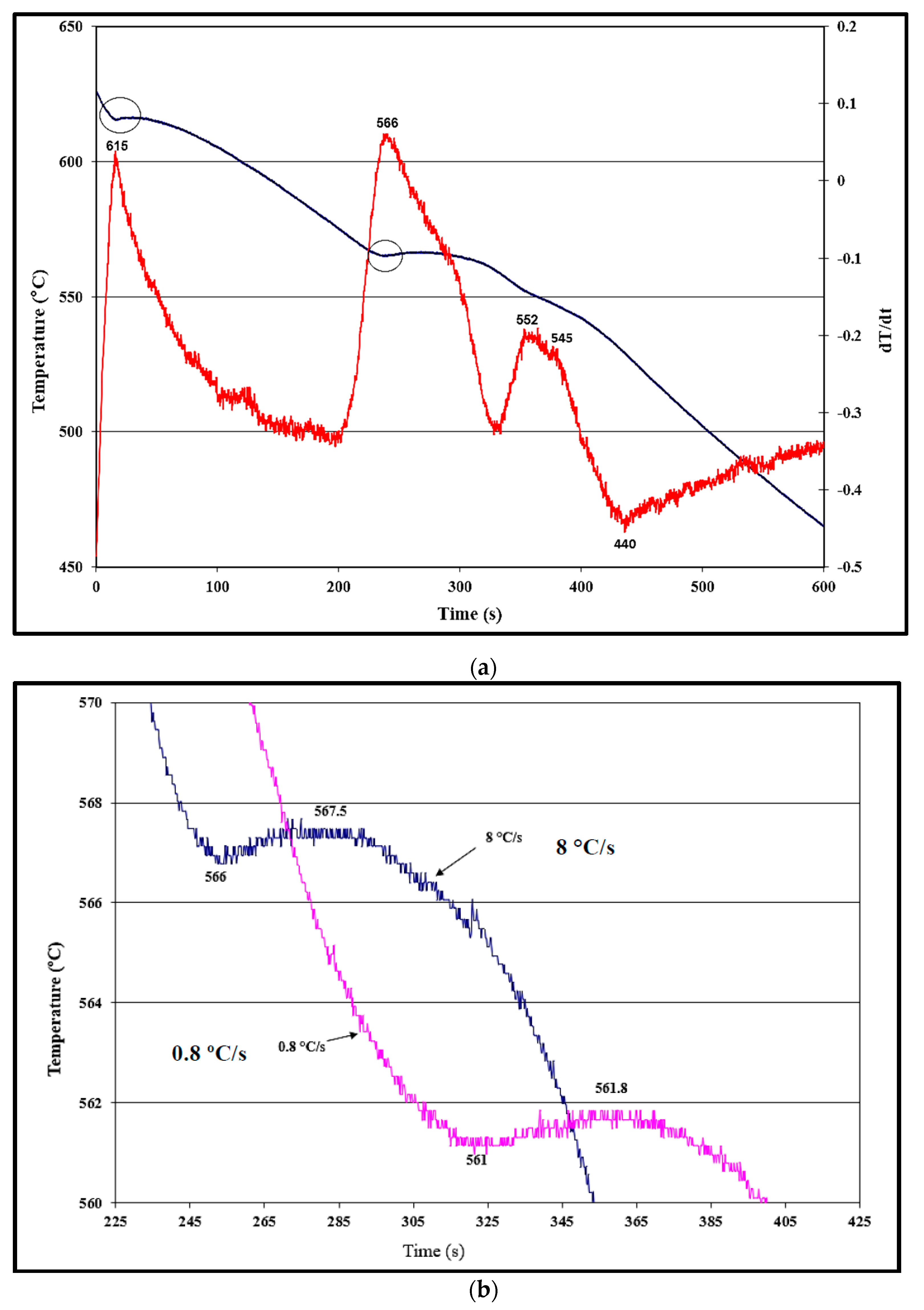
3.1. Solidification Aspects for the A356.2 Alloy
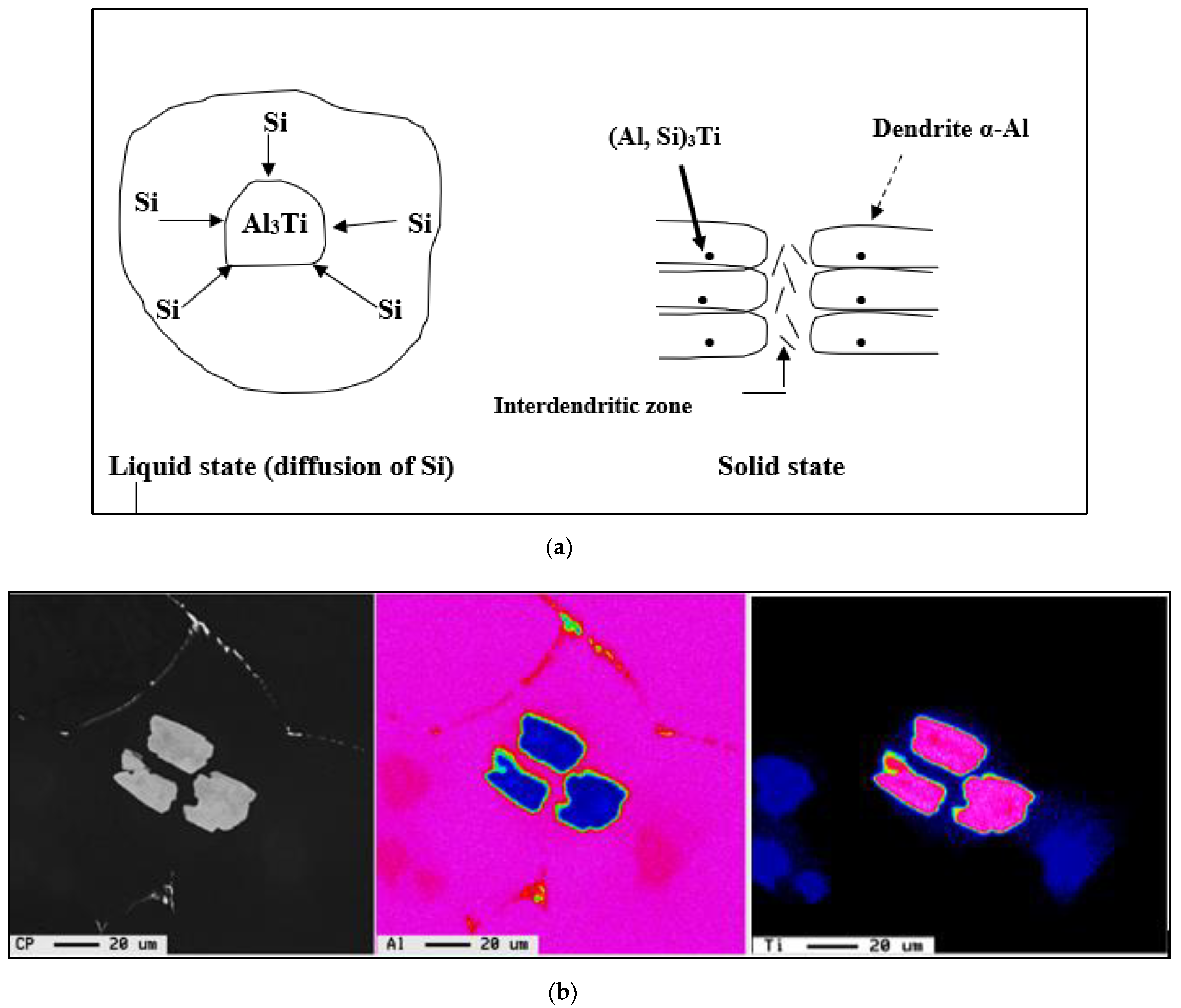
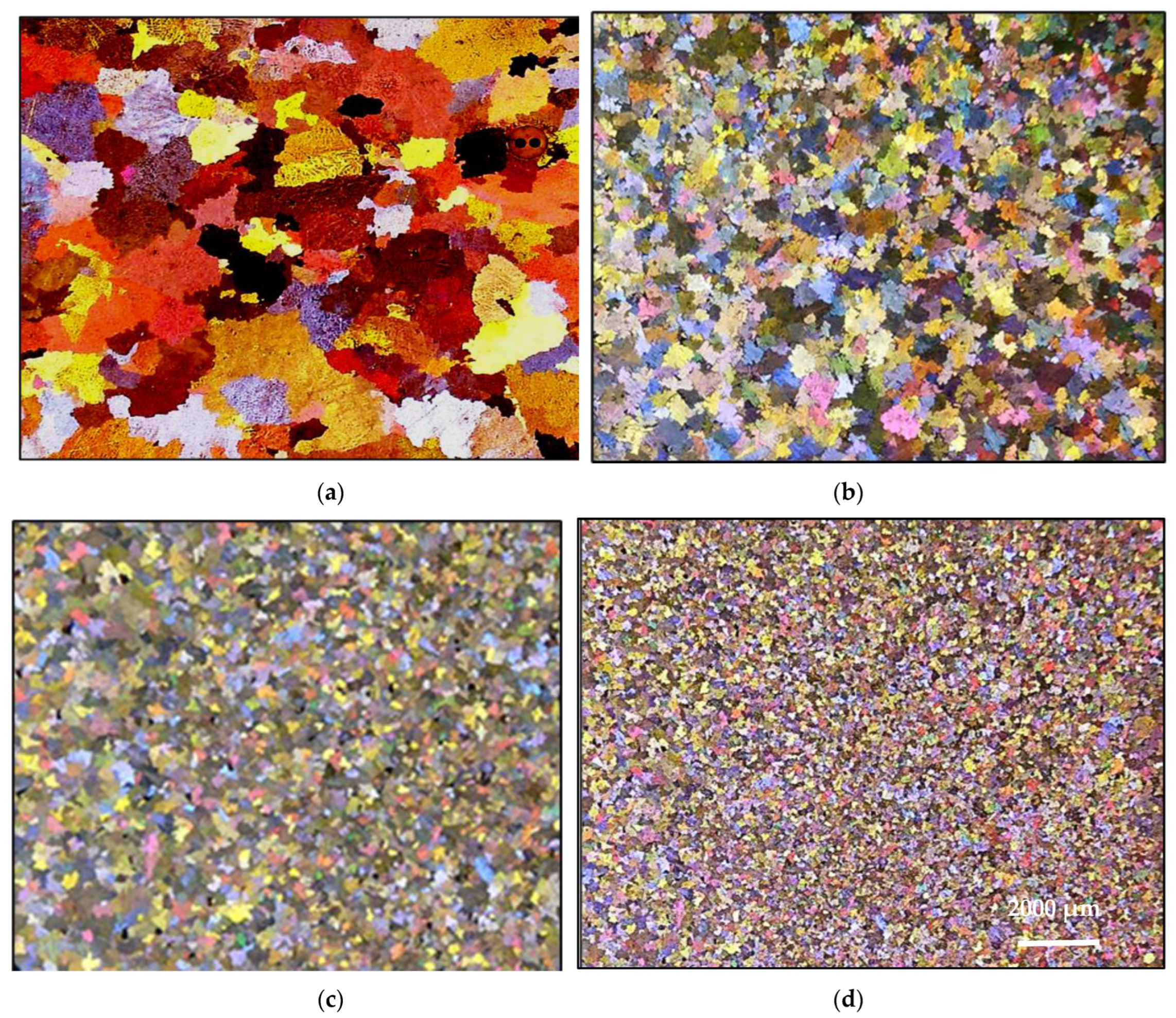
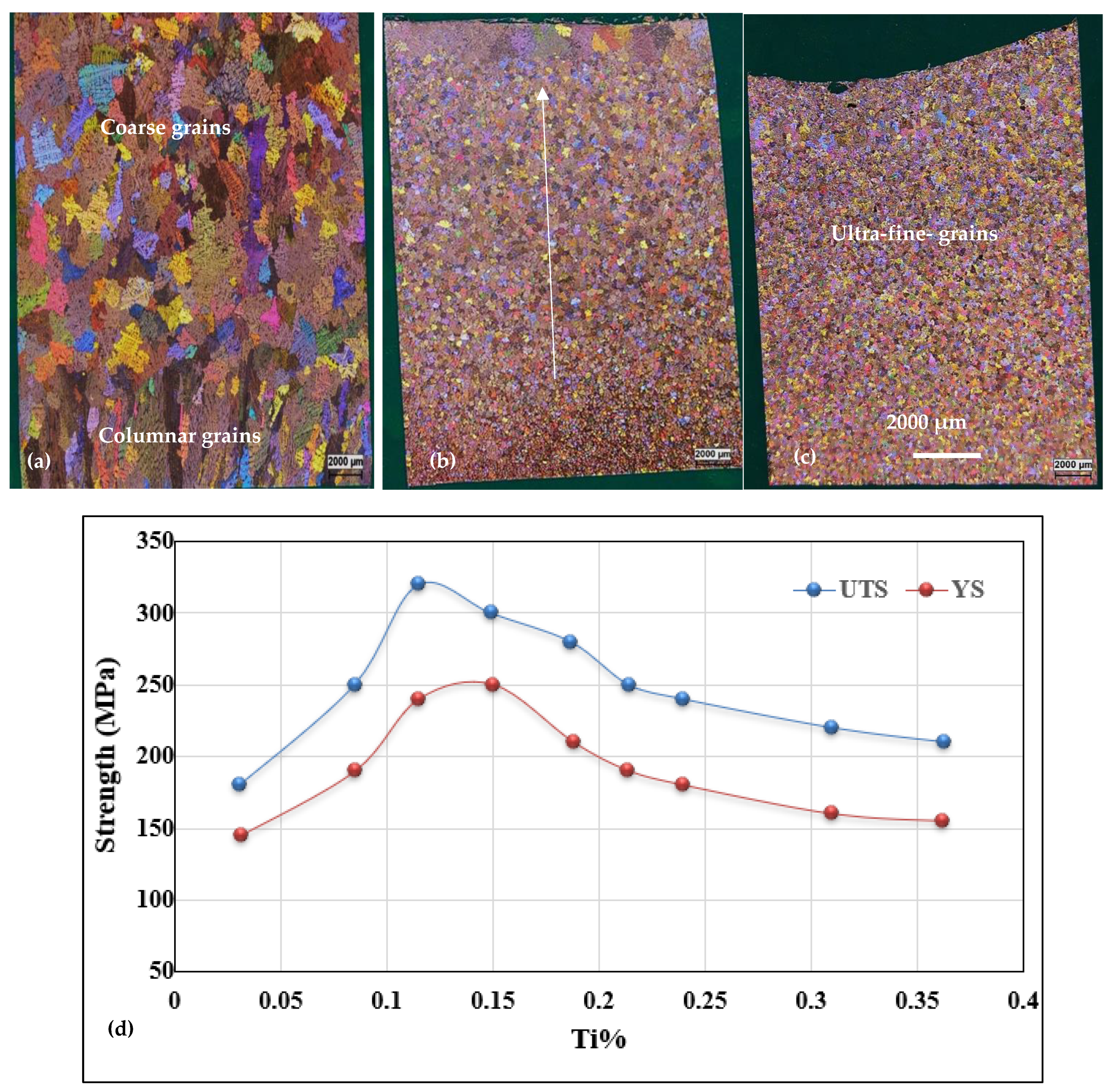
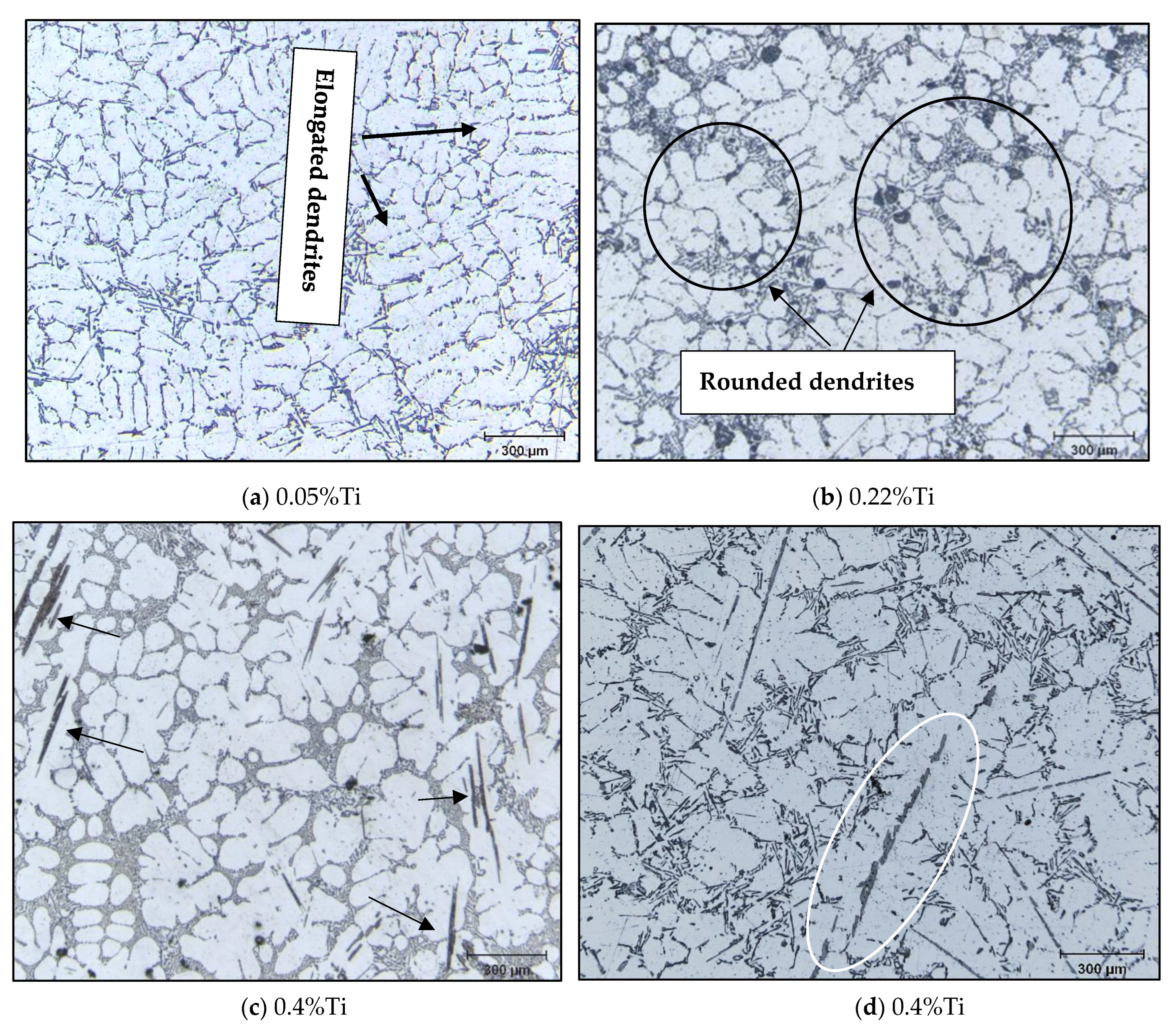
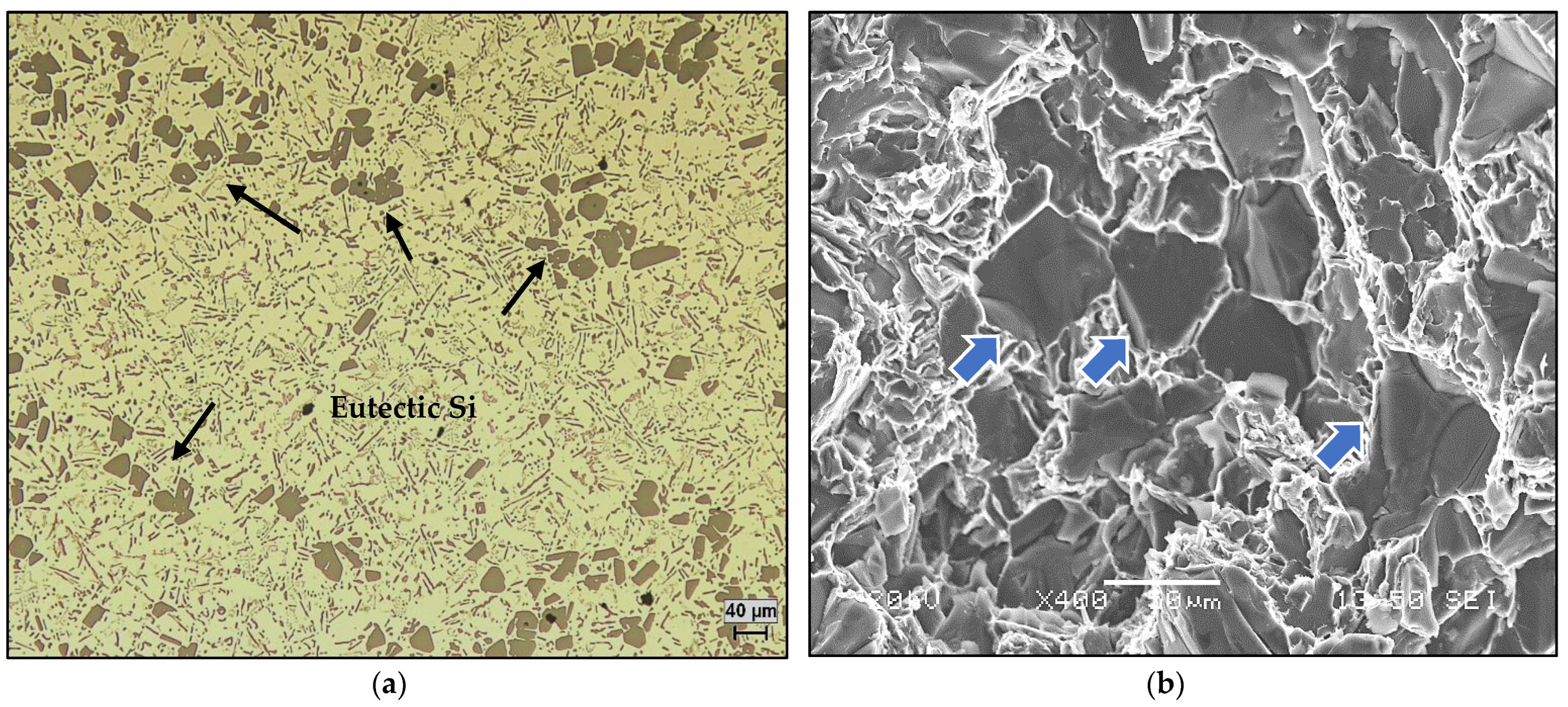
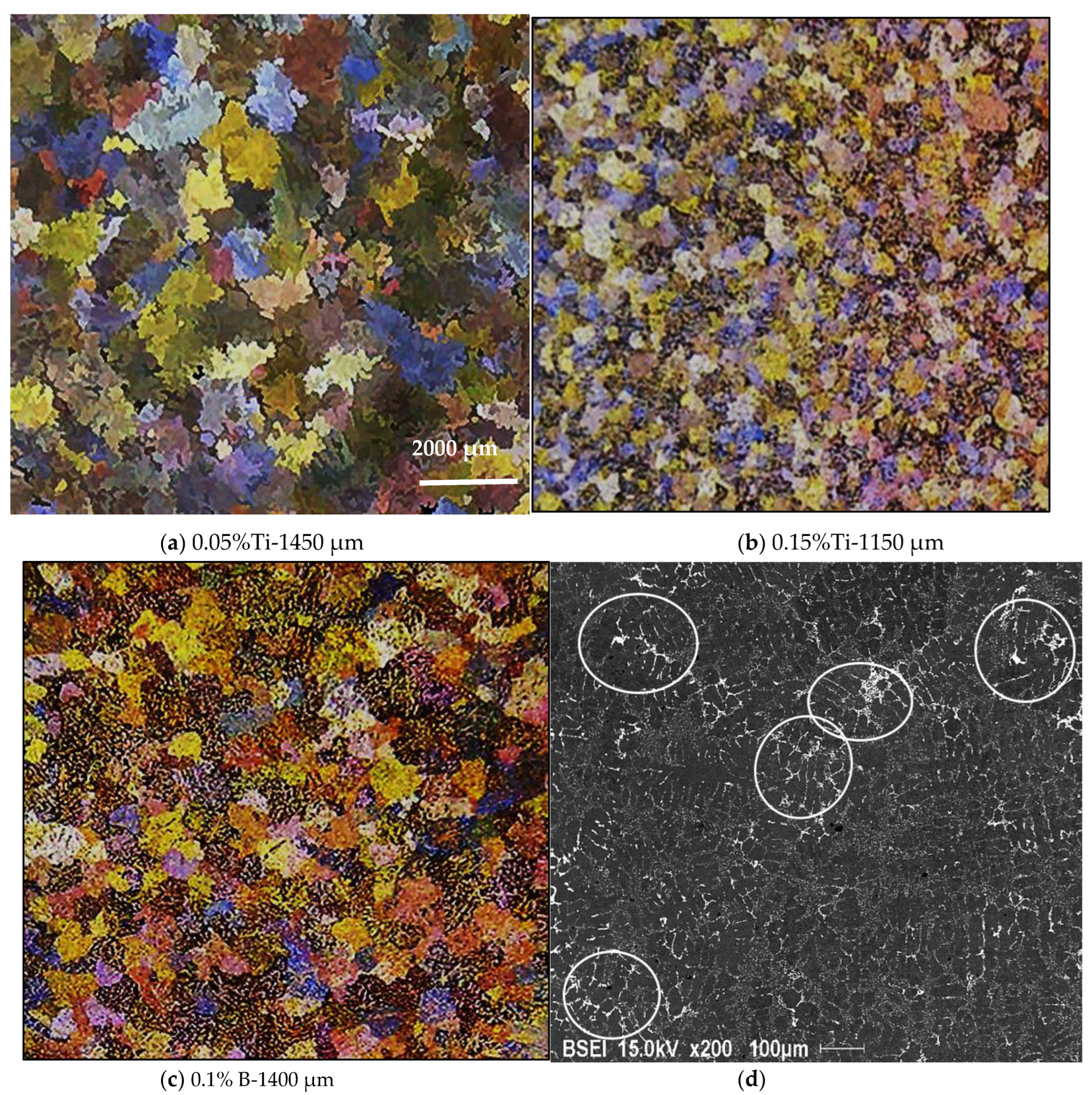
3.2. Macro and Microstructure Characterization
3.3. A390.1 Alloy
4. Conclusions
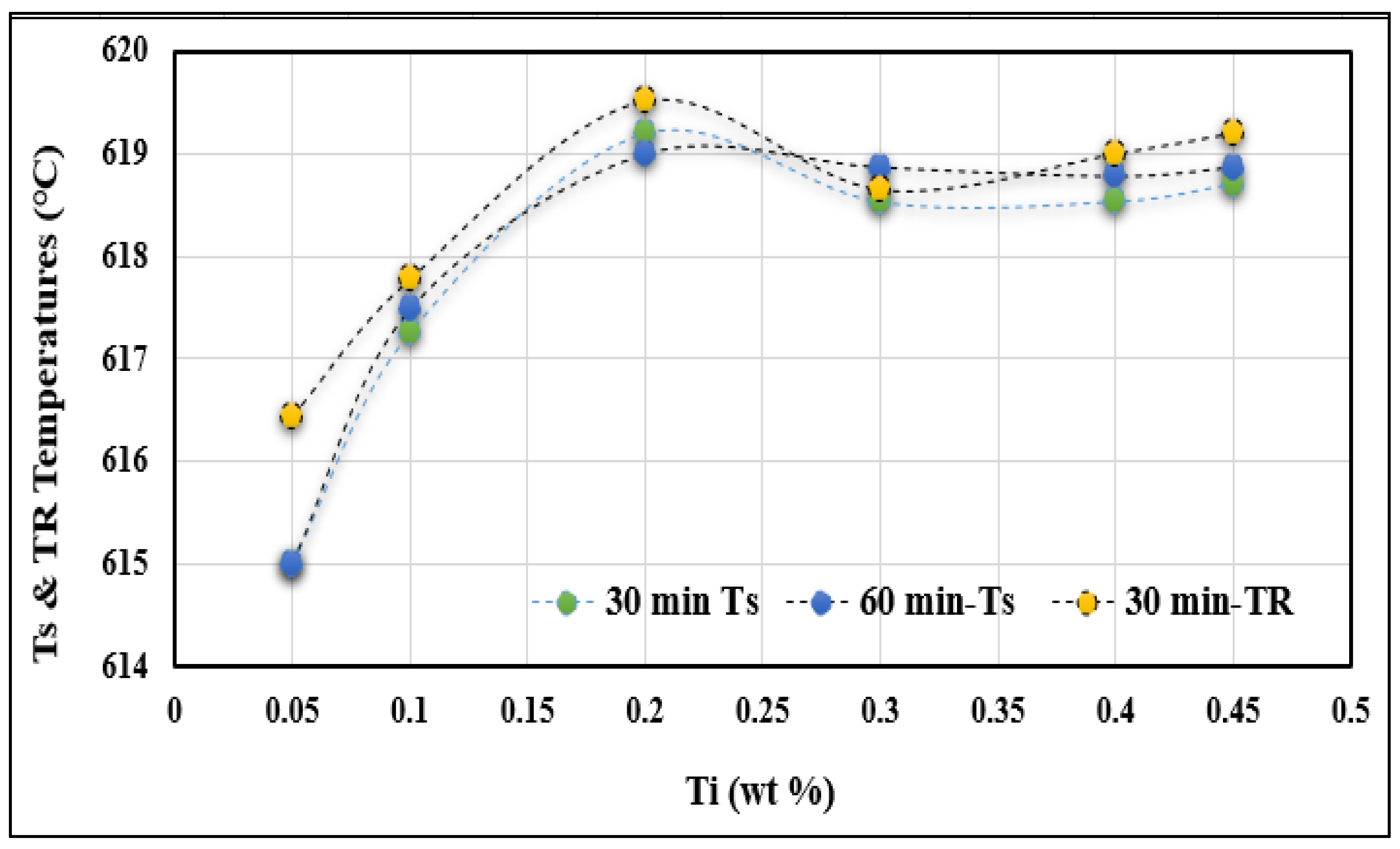
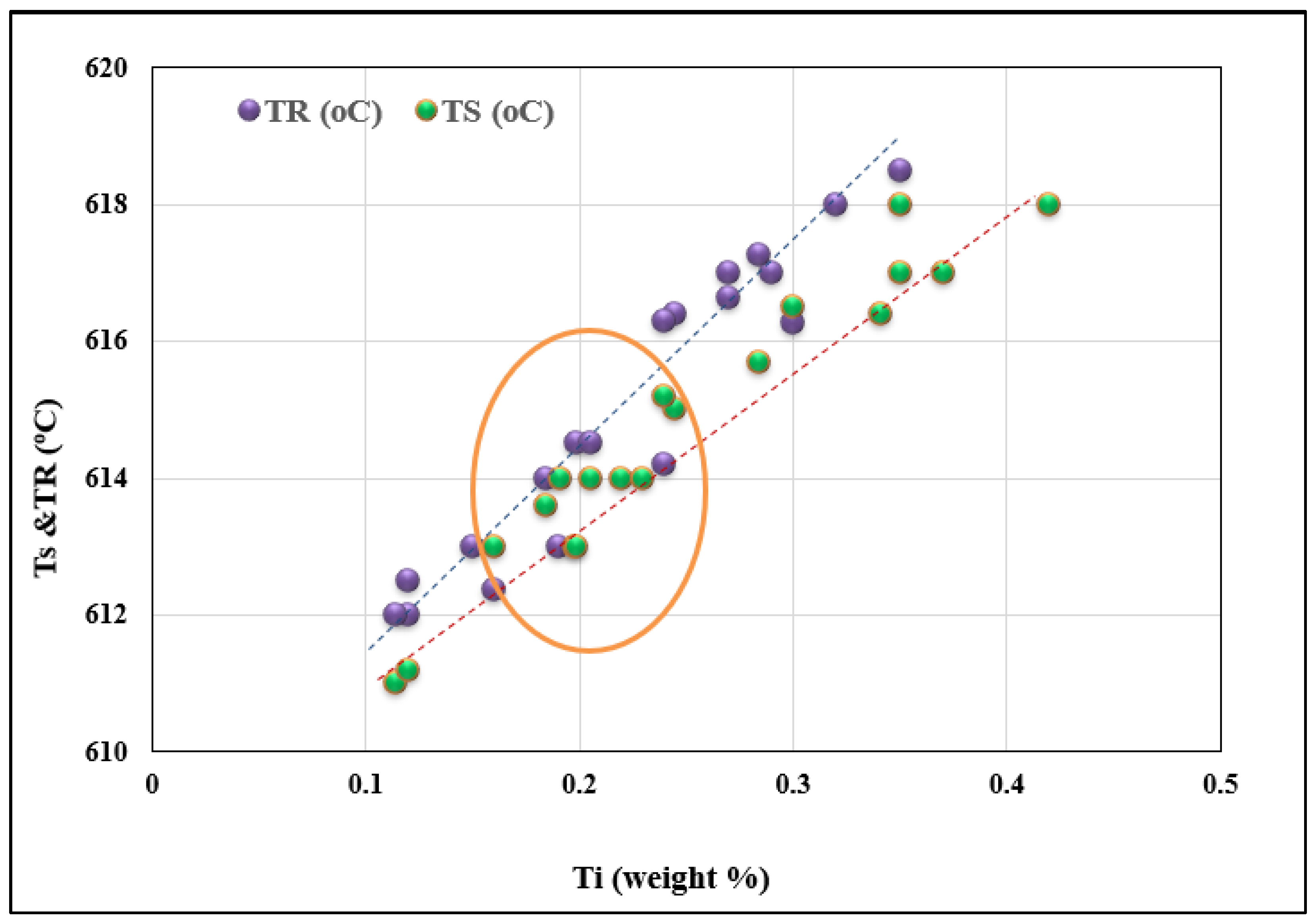
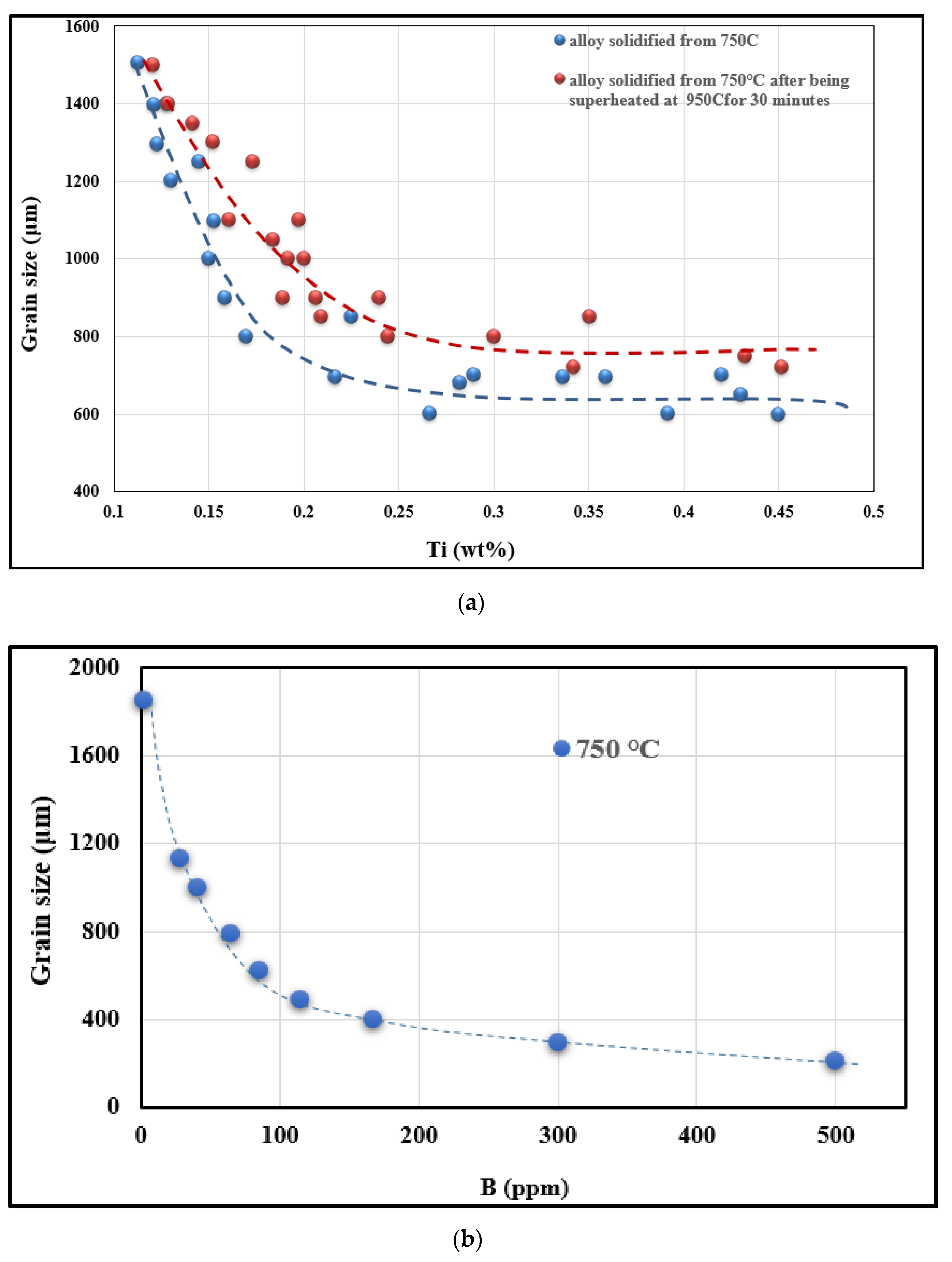
- Undercooling (TS) and recalescence (TR) temperatures increase with the initial increase in Ti concentration. At approximately 0.25%, a rapid decrease in these temperatures is observed.
- The minimum grain size is achieved when the Ti concentration exceeds about 0.20%.
- Superheating has a negative effect on the structure of the alloy. In general, high temperature casting leads to a coarse grain size.
- The Al-4%B master alloy shows remarkable grain refining effectiveness in comparison with Al-10%Ti. The residual Ti in the A356 alloy reacts with boron B to form TiB2, which subsequently acts as an active seed for the α-Al phase. Grain refining is achieved primarily with TiB2 rather than AlB2, or both, depending on the Ti content in the alloy.
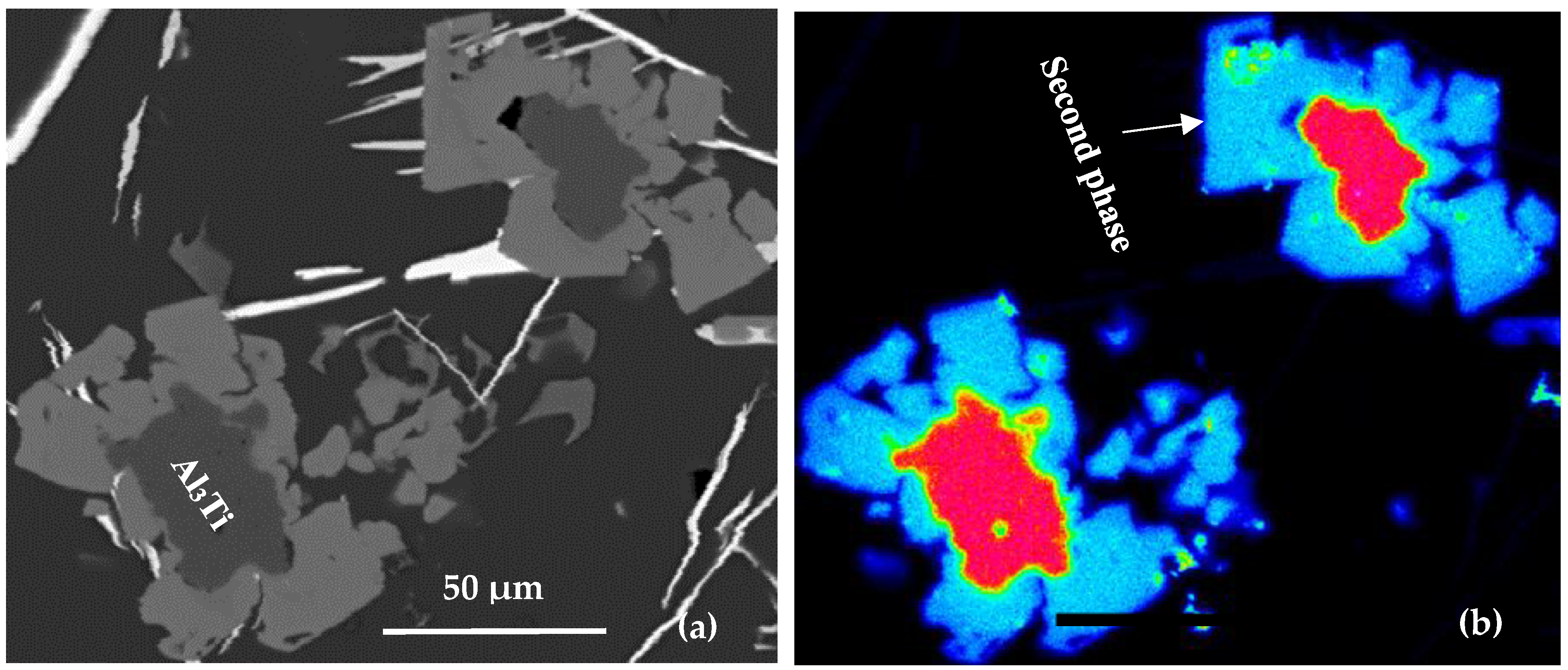
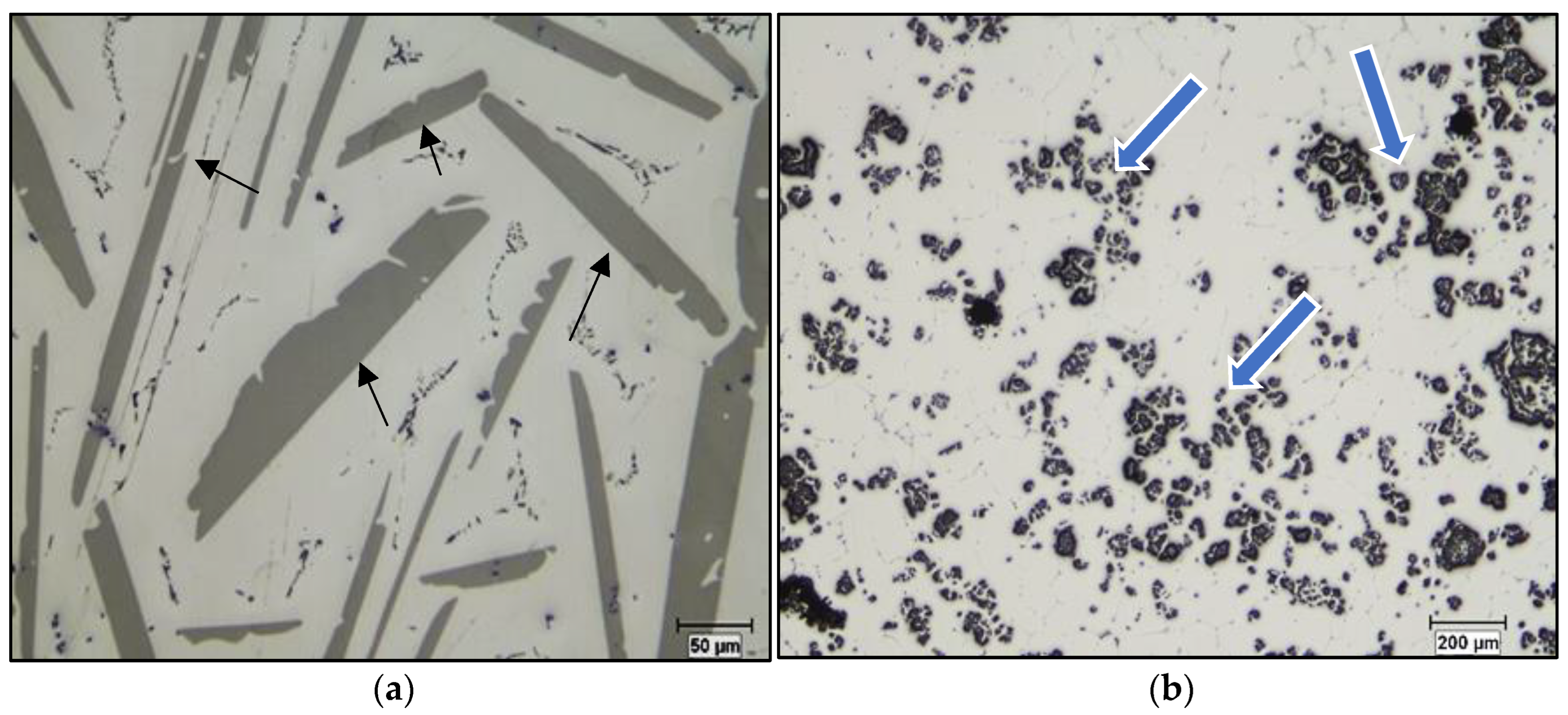
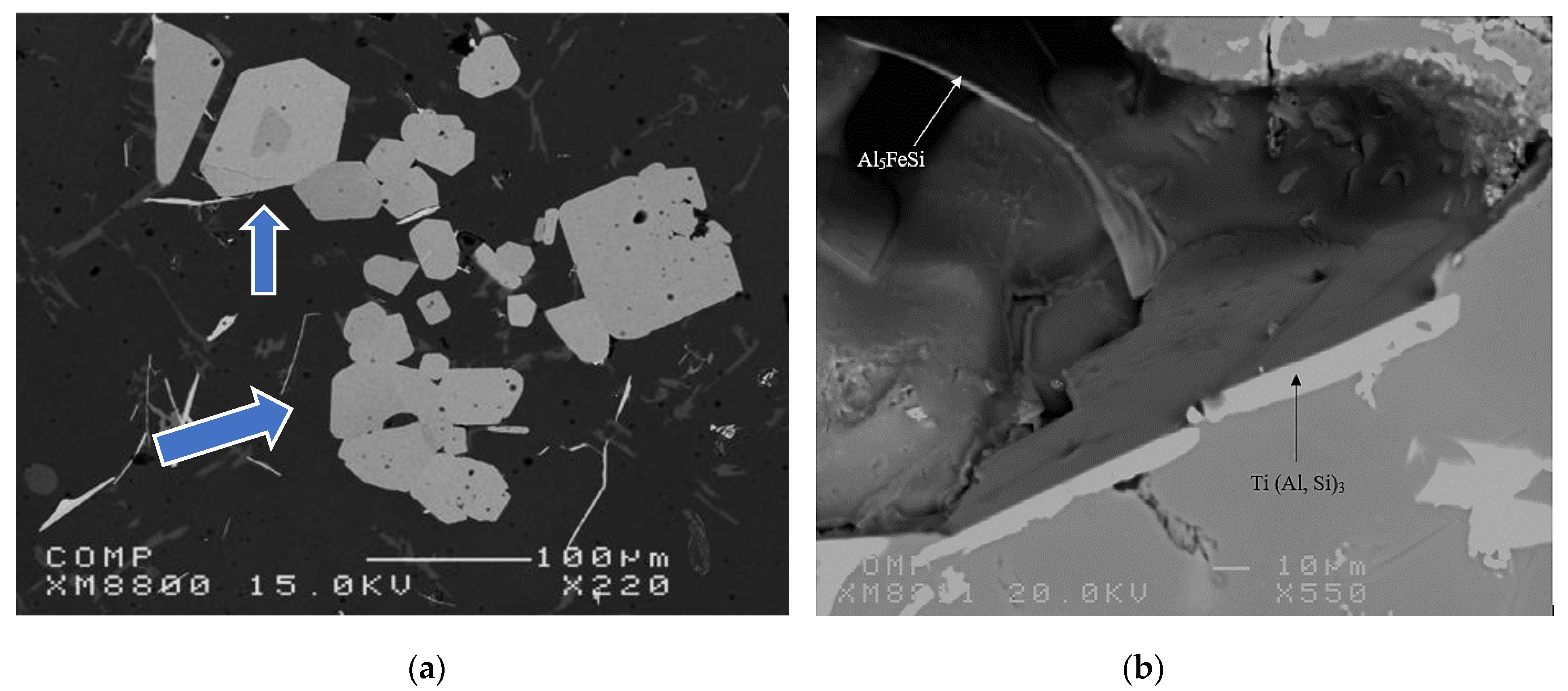
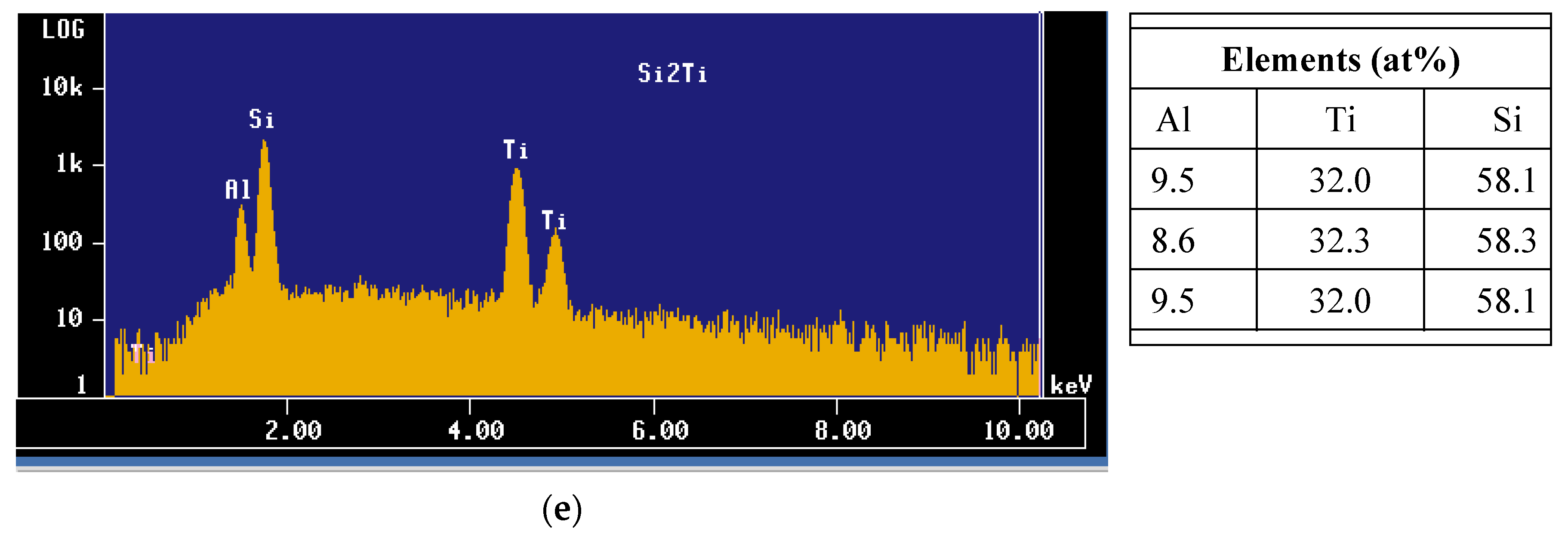
- The Al-10%Ti master alloy is rich in Al3Ti particles. Once added to the molten alloy, these particles convert into other intermetallics (Al,Si)3Ti.
- The term “poisoning”, reported frequently in various studies on grain refining to explain the loss or weakening of the effectiveness of Al3Ti nucleation sites, is misused, as these nucleation sites undergo a phase transformation of the type Al3Ti → (Al,Si)3Ti → (Al,Si)2Ti, which reduces their efficiency to nucleate the pre-eutectic α-Al phase. Hence the removal of the term “poisoning” is sought when describing weakening of the grain refiner in such cases.
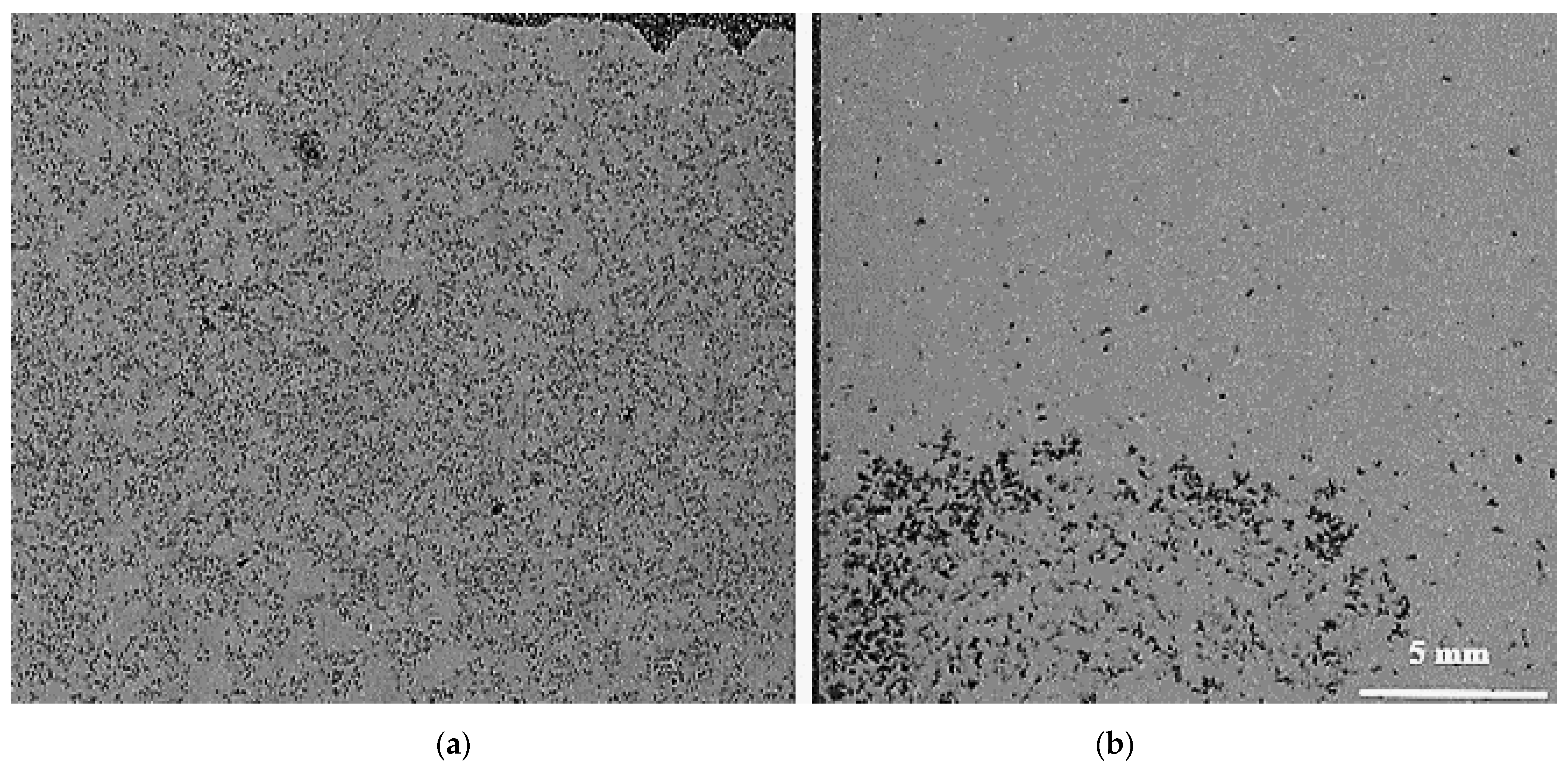
- In the A390.1 alloy with a high Si content (~17%), the grain size varies between 1450 µm and 1600 µm maximum vs. 1850 µm for A356.2 alloy for the base alloys. This suggests that Si plays the role of a grain refiner in the absence of the usual refiners.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramli, R.; Talari, M.K.; Omar Arawi, A.Z. Microstructure and mechanical properties of Al-Si cast alloy grain refined with Ti-B-Sr-Sc-Mg. In Proceedings of the 2011 IEEE Colloquium on Humanities, Science and Engineering, Penang, Malaysia, 5–6 December 2011; pp. 692–695. [Google Scholar] [CrossRef]
- Rathi, S.; Sharma, A.; Di Sabatino, M.D. Effect of mould temperature, grain refinement and modification on hot tearing test in Al-7Si-3Cu alloy. Eng. Fail. Anal. 2017, 79, 592–605. [Google Scholar] [CrossRef]
- Rathi, S.K.; Sharma, A.; Sabatino, M.D. Grain Refinement of Al-Si Alloys: Scientific and Industrial Aspects. J. Mod. Thermodyn. Mech. Syst. 2019, 1, 7–15. [Google Scholar]
- Estrin, Y.; Vinogradov, A. Extreme grain refinement by severe plastic deformation: A wealth of challenging science. Acta Mater. 2013, 61, 782–817. [Google Scholar] [CrossRef]
- Hassanbeygi, V.; Shafyei, A. Investigation on Microstructure and Grain Refining Performance of a New Type of Al-3Ti-1C Master Alloy. Open J. Met. 2014, 4, 49–55. [Google Scholar] [CrossRef]
- Djurdjevic, M.B.; Vicario, I.; Huber, G. Review of thermal analysis applications in aluminium casting plants. Rev. Metal. 2014, 50, e004. [Google Scholar] [CrossRef]
- Djurdjevic, M.; Huber, G. The Determination of Dendrite Coherency Point Characteristics Using Three New Methods for Aluminum Alloys. Appl. Sci. 2018, 8, 1236. [Google Scholar]
- Backerud, L. How does a good grain refiner work? Light Met. Age 1983, 41, 6–12. [Google Scholar]
- Johnsson, M.; Backerud, L.; Sigworth, G.K. Study of the mechanism of grain refinement of aluminium after additions of Ti- and B-containing master alloys. Metall. Trans. A 1993, 24A, 481–491. [Google Scholar] [CrossRef]
- Samuel, A.M.; Alkahtani, S.A.; Abuhasel, K.; Samuel, F.H. Role of Solidification Conditions in Determining the Microstructure of Al-Si Cast Alloys. In Light Metals 2015; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Cibula, A. The Mechanism of Grain Refinement of Sand Castings in Aluminum, Alloys. J. Inst. Met. 1949, 76, 321–360. [Google Scholar]
- Khalifa, W.; Samuel, F.H.; Gruzleski, J.E.; Doty, H.W.; Valtierra, S. Nucleation of Fe-intermetallic phases in the Al-Si-Fe alloys. Metall. Mater. Trans. A 2005, 36, 1017–1032. [Google Scholar] [CrossRef]
- Khalifa, W.; Doty, H.W.; Valtierra Gallardo, S.; Samuel, F.H. Effect of Bismuth and Calcium Additions on the Performance of B319 Al Cast Alloy. In Proceedings of the CastExpo & 120th Metalcasting Congress, Minneapolis, Minnesota, 16–19 April 2016. [Google Scholar]
- Samuel, E.; Golbahar, B.; Samuel, A.M.; Doty, H.W.; Valtierra, S.; Samuel, F.H. Effect of grain refiner on the tensile and impact properties of Al–Si–Mg cast alloys. Mater. Des. 2015, 56, 468–479. [Google Scholar] [CrossRef]
- Singh, L.; Goga, G.; Singh, R. Review of the latest developments in Grain refinement. Int. J. Mod. Eng. Res. (IJMER) 2012, 2, 2724–2727. [Google Scholar]
- Canales, A.A.; Talamantes-Silva, J.; Gloria, D.; Valtierra, S.; Colás, R. Thermal analysis during solidification of cast Al–Si alloys. Thermochim. Acta 2010, 510, 82–87. [Google Scholar] [CrossRef]
- Farahany, S.; Ourdjini, A.; Idris, M.H. The usage of computer-aided cooling curve thermal analysis to optimise eutectic refiner and modifier in Al–Si alloys. J. Therm. Anal. Calorim. 2012, 109, 105–111. [Google Scholar] [CrossRef]
- Khalifa, W.; Samuel, F.H.; Gruzleski, J.E. Nucleation of solid Aluminum on inclusion particles injected Into Al-Si-Fe alloys. Metall. Mater. Trans. A 2004, 35A, 3233–3250. [Google Scholar] [CrossRef]
- Savas, Ö. The production and properties of Al3Ti reinforced functionally graded aluminum matrix composites produced by the centrifugal casting method. Mater. Res. Express. 2019, 6, 126532. [Google Scholar] [CrossRef]
- Zeng, Y.; Himmler, D.; Randelzhofer, P.; Körner, C. Microstructures and Mechanical Properties of Al3Ti/Al Composites Produced In Situ by High Shearing Technology. Light Mater. Sci. Technol. 2019, 21, 1800259. [Google Scholar] [CrossRef]
- Fukahori, R.; Nomura, T.; Zhu, C.; Sheng, N.; Okinaka, N.; Akiyama, T. Thermal analysis of Al–Si alloys as high-temperature phase-change material and their corrosion properties with ceramic materials. Appl. Energy 2016, 163, 1–8. [Google Scholar] [CrossRef]
- Rakhmonov, J.; Timelli, G.; Bonollo, F. Characterization of the solidification path and microstructure of secondary Al-7Si-3Cu-0.3Mg alloy with Zr, V and Ni additions. Mater. Charact. 2017, 128, 100–108. [Google Scholar] [CrossRef]
- Anjosa, V.; Deike, R.; Silva Ribeiro, C. The use of thermal analysis to predict the dendritic coherency point on nodular cast iron melts. Ciênc. Tecnol. Mater. 2017, 29, 27–33. [Google Scholar] [CrossRef]
- Elsharkawi, E.A.; Samuel, E.; Samuel, A.M.; Samuel, F.H. Effects of Mg, Fe, Be additions and solution heat treatment on the pi-AlMgFeSi iron intermetallic phase in Al-7Si-Mg alloys. J. Mater. Sci. 2010, 45, 1528–1539. [Google Scholar] [CrossRef]
- Youdelis, W.V.; Yang, C.S. Ti(Al,Si)3 compound formation in non-equilibrated Al-Ti-Si. Met. Sci. 1980, 14, 500–501. [Google Scholar] [CrossRef]
- Tahiri, H.; Mohamed, S.S.; Doty, H.W.; Valtierra, S.; Samuel, F.H. Effect of Sr–Grain Refining–Si Interactions on the Microstructural Characteristics of Al–Si Hypoeutectic Alloys. Int. J. Met. 2018, 12, 343–361. [Google Scholar] [CrossRef]
- Tahiri, H.; Samuel, A.M.; Doty, H.W.; Valtierra, S.; Samuel, F.H. Effect of Sr–Grain Refiner–Si Interactions on the Microstructure Characteristics of Al–Si Hypereutectic Alloys. Int. J. Met. 2018, 12, 307–320. [Google Scholar] [CrossRef]
- Nafisi, S.; Ghomashchi, R. Grain refining of conventional and semi-solid A356 Al–Si alloy. J. Mater. Process. Technol. 2006, 174, 371–383. [Google Scholar] [CrossRef]
- Birch, M.E.J.; Fisher, P. Solidification Processing; The Institute of Metals: London, UK, 1987; pp. 500–502. [Google Scholar]
- Wang, X.J.; Cong, X.; Muhammad, A.; Hanada, S.; Yamagata, H.; Wang, W.H. Effects of Al-Ti-B-RE grain refiner on microstructure and mechanical properties of Al-7.0Si-0.55Mg alloy. Trans. Nonferrous Met. Soc. China 2014, 24, 2244–2250. [Google Scholar] [CrossRef]
- Easton, M.A.; Qian, M.; Prasad, A.; StJohn, D.H. Recent advances in grain refinement of light metals and alloys. Curr. Opin. Solid State Mater. Sci. 2016, 20, 13–24. [Google Scholar] [CrossRef]
- Dong, X.; Ji, S. Si poisoning and promotion on the microstructure and mechanical properties of Al–Si–Mg cast alloys. J. Mater. Sci. 2018, 53, 7778–7792. [Google Scholar] [CrossRef]



| Alloy | Al | Si | Cu | Mg | Fe | Mn | Zn | Ti |
|---|---|---|---|---|---|---|---|---|
| A356.2 | bal. | 6.78 | 0.02 | 0.33 | 0.11 | 0.04 | 0.04 | 0.07 |
| A390.1 | bal. | 17.30 | 4.33 | 0.54 | 0.32 | 0.06 | 0.06 | 0.07 |
| Reactions | Temperature (°C) | |
|---|---|---|
| A | Primary Si | 670 |
| B | Developpement of α-Al network | 561 |
| C | Liq. → Al + Si + Al5FeSi | 575 |
| D | Liq. → Al + Si + Mg2Si | 555 |
| E | Liq. + Mg2Si → Al + Si + Al2Cu + Al5Mg8Cu2Si6 | 512 |
| F | Liq. → Al + Al2Cu + Al5Mg8Cu2Si6 | 502 |
| G | End of splidification | 490 |
| Total solidification time: approximately 750 s | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samuel, A.M.; Samuel, E.; Songmene, V.; Samuel, F.H. A Comparative Study of Grain Refining of Al-(7–17%) Si Cast Alloys Using Al-10% Ti and Al-4% B Master Alloys. Materials 2023, 16, 2867. https://doi.org/10.3390/ma16072867
Samuel AM, Samuel E, Songmene V, Samuel FH. A Comparative Study of Grain Refining of Al-(7–17%) Si Cast Alloys Using Al-10% Ti and Al-4% B Master Alloys. Materials. 2023; 16(7):2867. https://doi.org/10.3390/ma16072867
Chicago/Turabian StyleSamuel, Agnes M., Ehab Samuel, Victor Songmene, and Fawzy H. Samuel. 2023. "A Comparative Study of Grain Refining of Al-(7–17%) Si Cast Alloys Using Al-10% Ti and Al-4% B Master Alloys" Materials 16, no. 7: 2867. https://doi.org/10.3390/ma16072867
APA StyleSamuel, A. M., Samuel, E., Songmene, V., & Samuel, F. H. (2023). A Comparative Study of Grain Refining of Al-(7–17%) Si Cast Alloys Using Al-10% Ti and Al-4% B Master Alloys. Materials, 16(7), 2867. https://doi.org/10.3390/ma16072867








