Photovoltaic Glass Waste Recycling in the Development of Glass Substrates for Photovoltaic Applications
Abstract
1. Introduction
2. Materials and Methods
3. Results
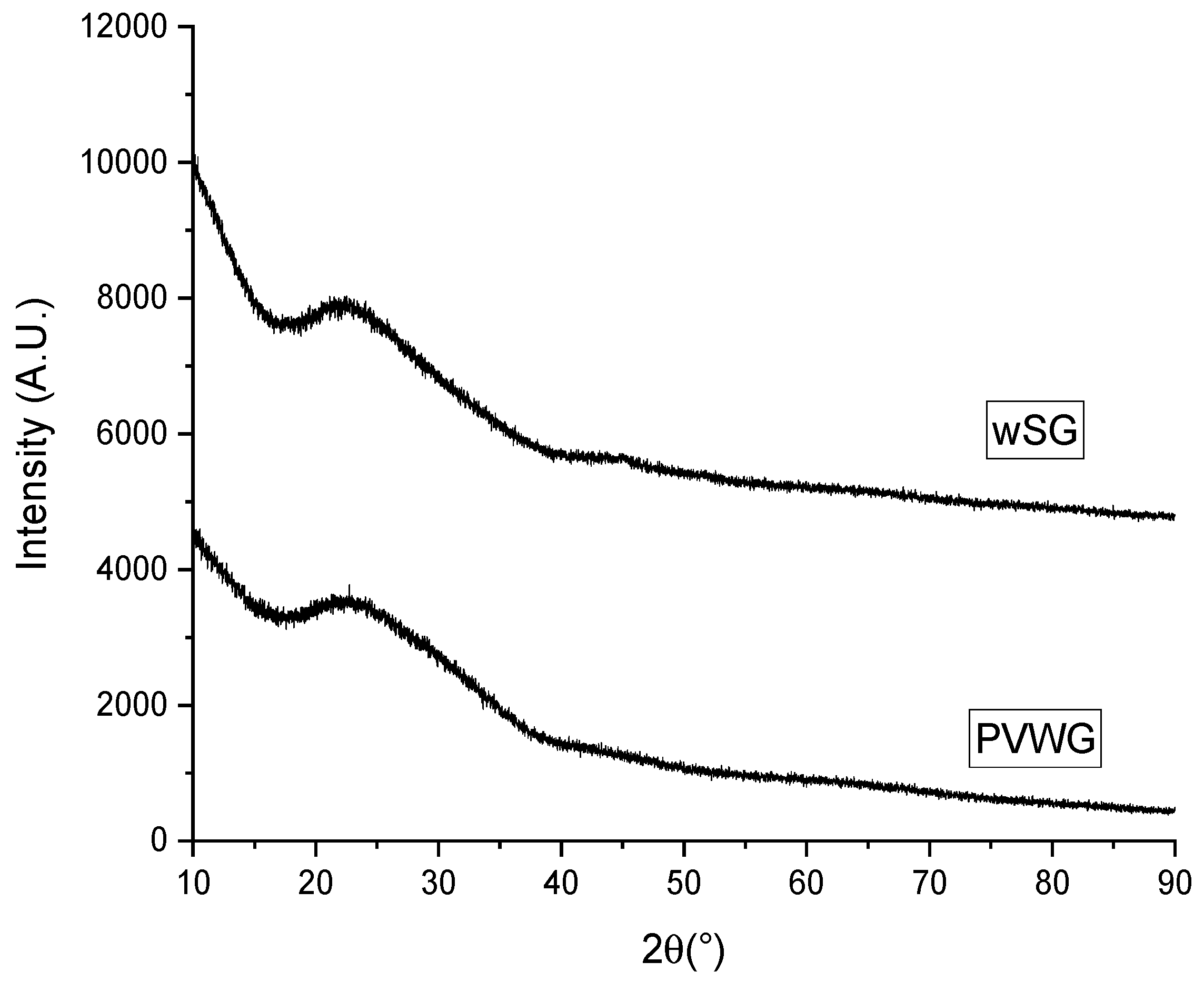
3.1. PVWG Separation and Characterization
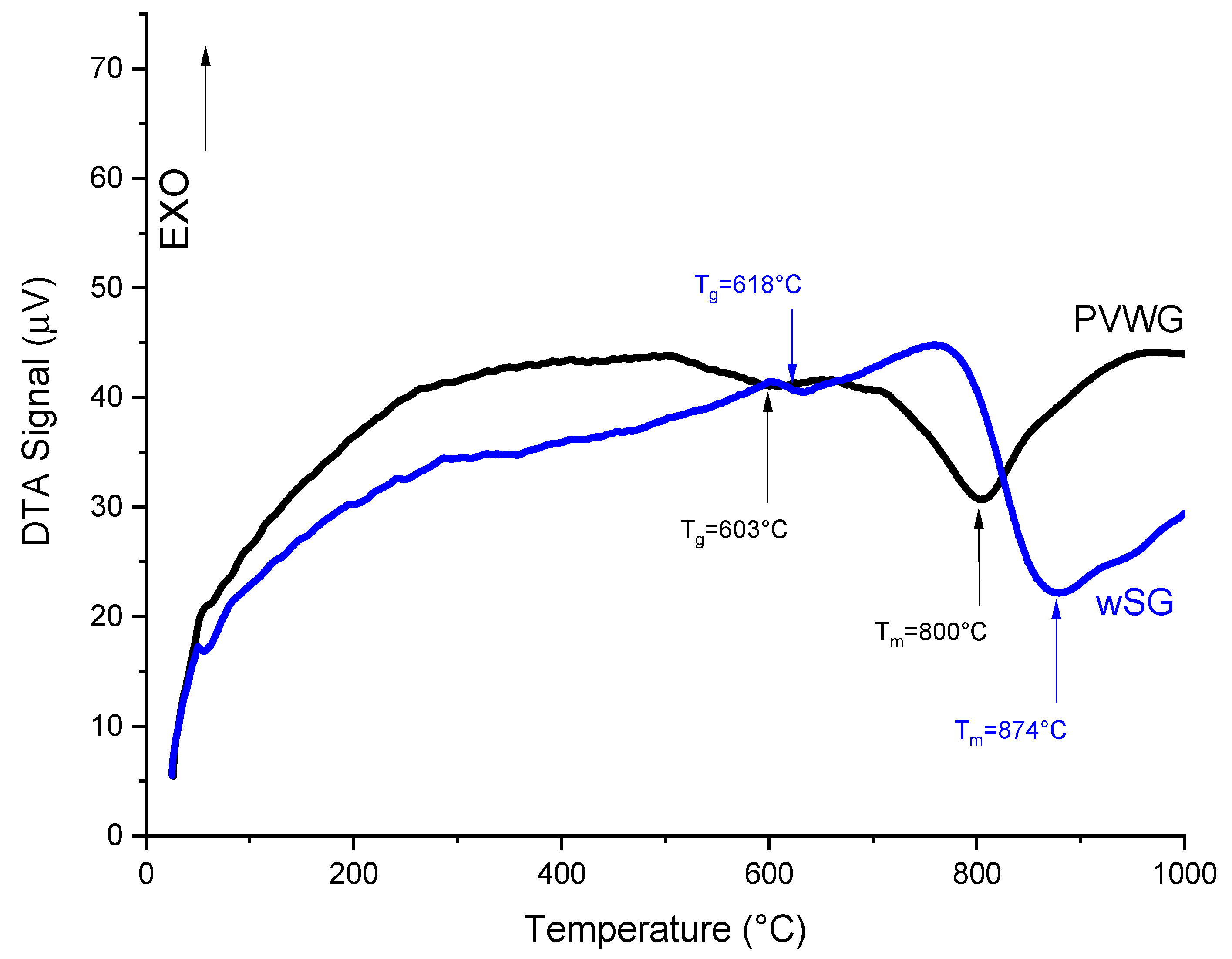
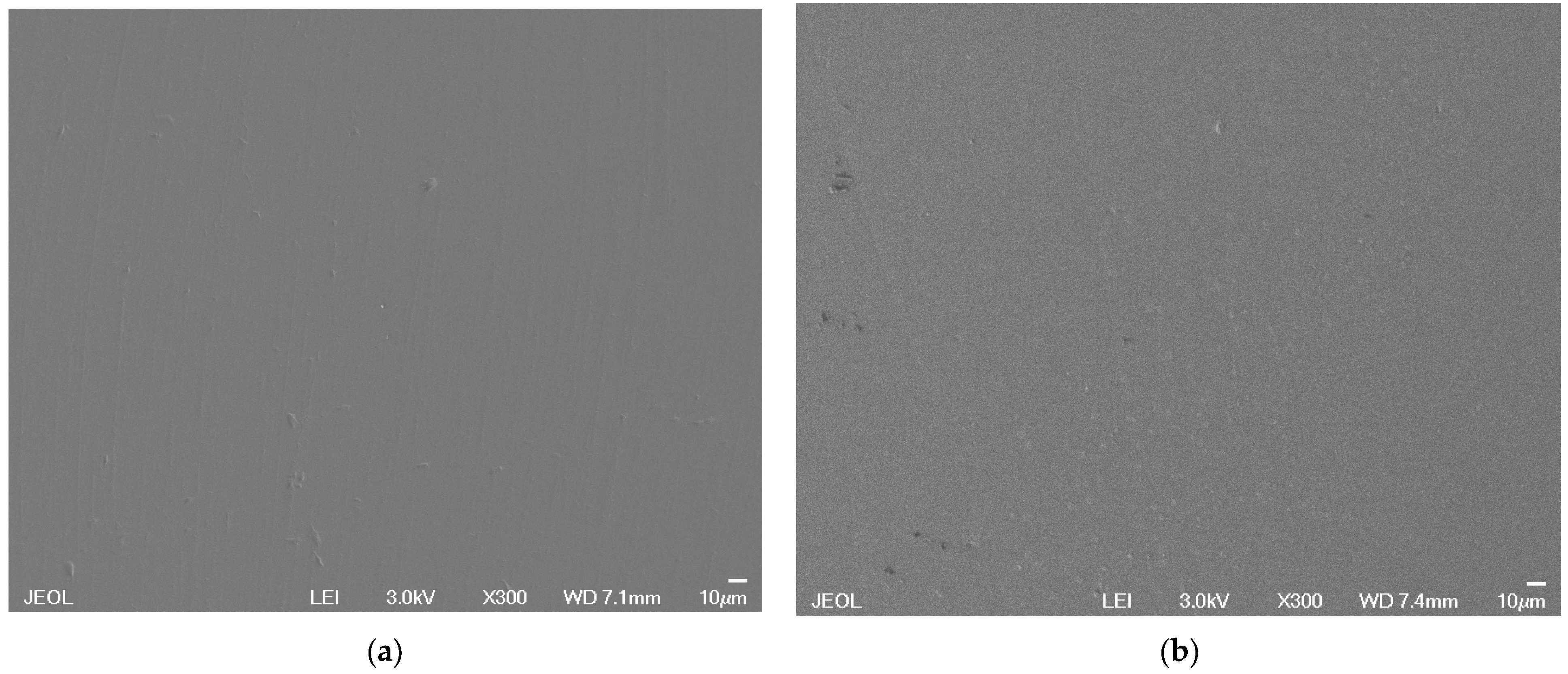
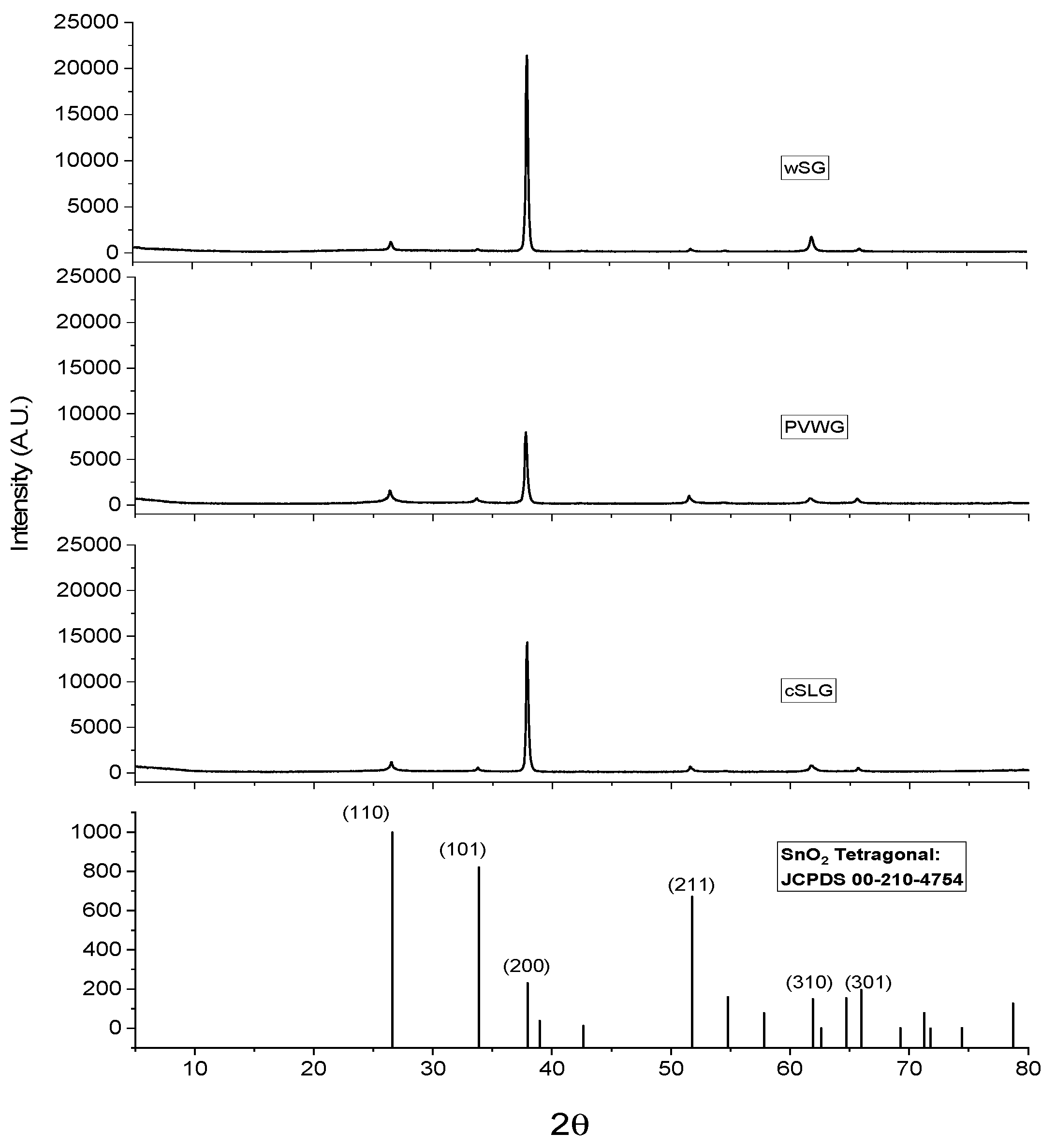
3.2. Glass Quenching, Substrate Preparation and Characterization
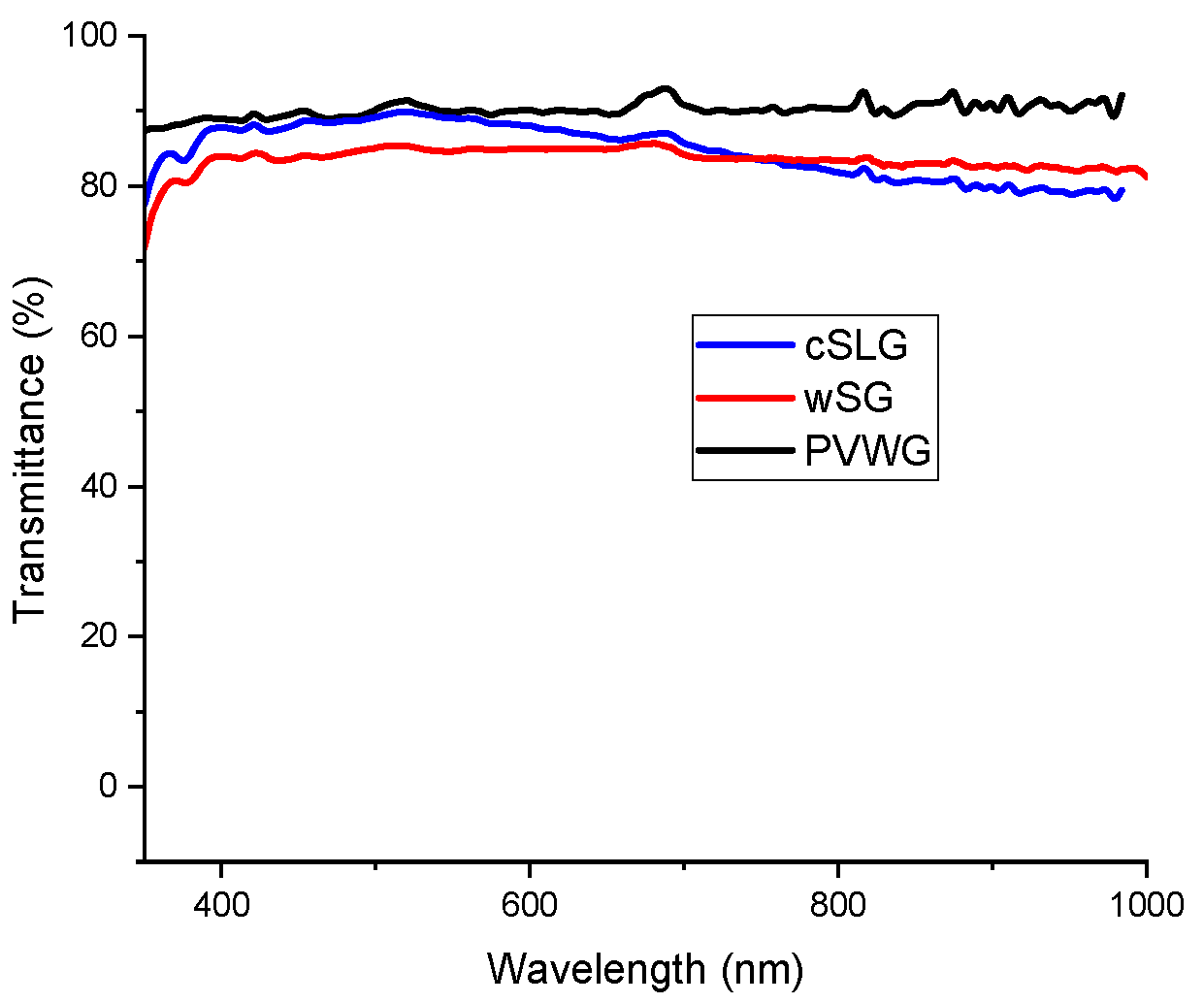
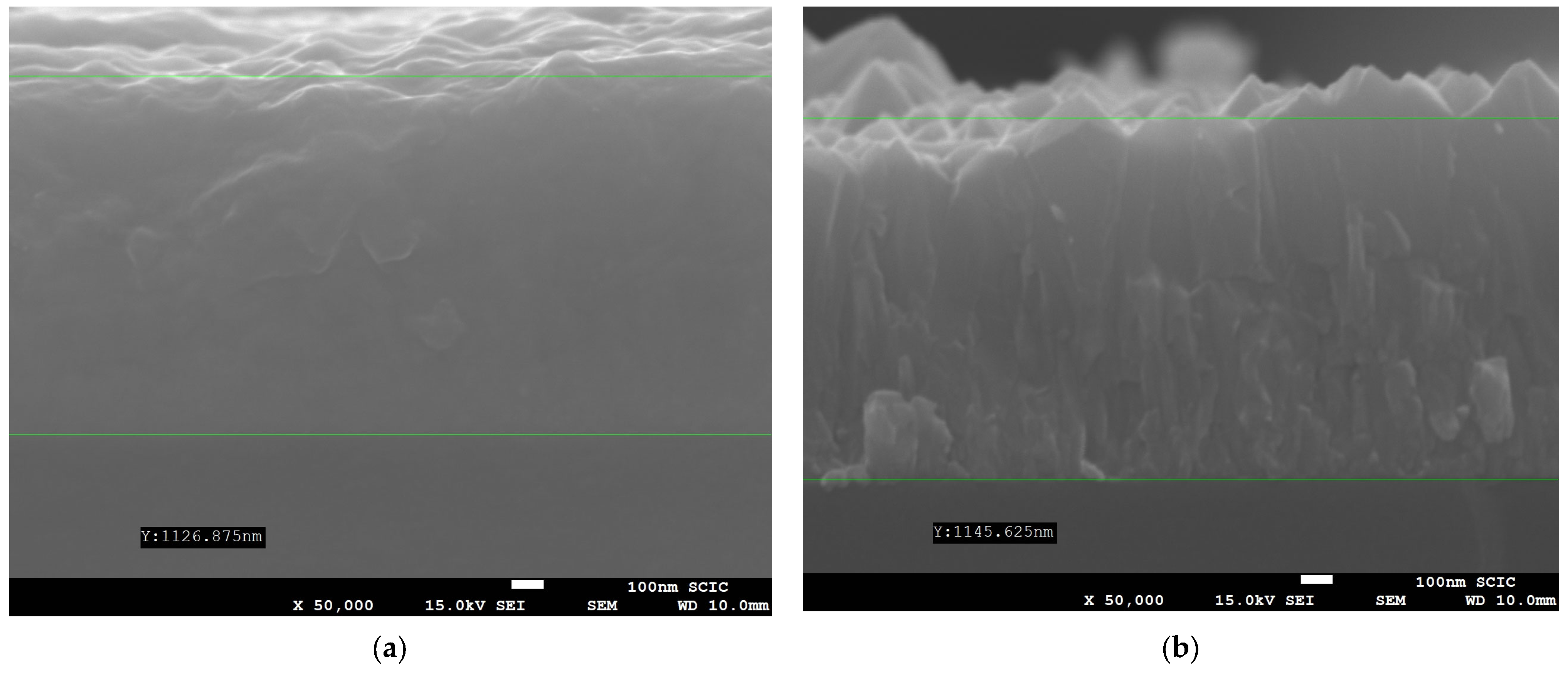
3.3. FTO Deposition and Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Domínguez, A.; Geyer, R. Photovoltaic Waste Assessment in Mexico. Resour. Conserv. Recycl. 2017, 127, 29–41. [Google Scholar] [CrossRef]
- IRENA. Future of Solar Photovoltaic: Deployment, Investment, Technology, Grid Integration and Socio-Economic Aspects (A Global Energy Transformation: Paper); IRENA: Bonn, Germany, 2019. [Google Scholar]
- Weckend, S.; Wade, A.; Heath, G. End-of-Life Management Solar Photovoltaic Panels; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2016. [Google Scholar]
- Peplow, M. Solar Panels Face Recycling Challenge. 2022, 8, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Korniejenko, K.; Kozub, B.; Bąk, A.; Balamurugan, P.; Uthayakumar, M.; Furtos, G. Tackling the Circular Economy Challenges—Composites Recycling: Used Tyres, Wind Turbine Blades, and Solar Panels. J. Compos. Sci. 2021, 5, 243. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, M.; Yang, H. A Review of the Energy Performance and Life-Cycle Assessment of Building-Integrated Photovoltaic (BIPV) Systems. Energies 2018, 11, 3157. [Google Scholar] [CrossRef]
- Belançon, M.P.; Sandrini, M.; Tonholi, F.; Herculano, L.S.; Dias, G.S. Towards Long Term Sustainability of C-Si Solar Panels: The Environmental Benefits of Glass Sheet Recovery. Renew. Energy Focus 2022, 42, 206–210. [Google Scholar] [CrossRef]
- Li, M.; Widijatmoko, S.D.; Wang, Z.; Hall, P. A Methodology to Liberate Critical Metals in Waste Solar Panel. Appl. Energy 2023, 337, 120900. [Google Scholar] [CrossRef]
- Masoumian, M.; Kopacek, P. End-of-Life Management of Photovoltaic Modules. IFAC-PapersOnLine 2015, 48, 162–167. [Google Scholar] [CrossRef]
- Lin, K.L.; Huang, L.S.; Shie, J.L.; Cheng, C.J.; Lee, C.H.; Chang, T.C. Elucidating the Effects of Solar Panel Waste Glass Substitution on the Physical and Mechanical Characteristics of Clay Bricks. Environ. Technol. 2013, 34, 15–24. [Google Scholar] [CrossRef]
- Jimenez-Millan, J.; Abad, I.; Jimenez-Espinosa, R.; Yebra-Rodriguez, A. Assessment of Solar Panel Waste Glass in the Manufacture of Sepiolite Based Clay Bricks. Mater. Lett. 2018, 218, 346–348. [Google Scholar] [CrossRef]
- Lin, K.-L.; Chu, T.-C.; Cheng, C.-J.; Lee, C.-H.; Chang, T.-C.; Kuen-Sheng, W. Recycling Solar Panel Waste Glass Sintered as Glass-Ceramics. Environ. Prog. Sustain. Energy 2012, 31, 613–618. [Google Scholar] [CrossRef]
- Savvilotidou, V.; Kritikaki, A.; Stratakis, A.; Komnitsas, K.; Gidarakos, E. Energy Efficient Production of Glass-Ceramics Using Photovoltaic (P/V) Glass and Lignite Fly Ash. Waste Manag. 2019, 90, 46–58. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Lee, W.-H.; Lin, K.-L. A Novel Approach for Preparing Ecological Zeolite Material from Solar Panel Waste Lass and Sandblasting Waste: Microscopic Characteristics and Humidity Control Performance. J. Mater. Res. Technol. 2022, 19, 4128–4140. [Google Scholar] [CrossRef]
- Aslam, A.; Ahmed, N.; Qureshi, S.A.; Assadi, M.; Ahmed, N. Advances in Solar PV Systems; A Comprehensive Review of PV Performance, Influencing Factors, and Mitigation Techniques. Energies 2022, 15, 7595. [Google Scholar] [CrossRef]
- Breyer, C.; Koskinen, O.; Blechinger, P. Profitable Climate Change Mitigation: The Case of Greenhouse Gas Emission Reduction Benefits Enabled by Solar Photovoltaic Systems. Renew. Sustain. Energy Rev. 2015, 49, 610–628. [Google Scholar] [CrossRef]
- Martín-Chivelet, N.; Kapsis, K.; Wilson, H.R.; Delisle, V.; Yang, R.; Olivieri, L.; Polo, J.; Eisenlohr, J.; Roy, B.; Maturi, L.; et al. Building-Integrated Photovoltaic (BIPV) Products and Systems: A Review of Energy-Related Behavior. Energy Build 2022, 262, 111998. [Google Scholar] [CrossRef]
- Cerón, I.; Caamaño-Martín, E.; Neila, F.J. “State-of-the-Art” of Building Integrated Photovoltaic Products. Renew. Energy 2013, 58, 127–133. [Google Scholar] [CrossRef]
- ONIX-SOLAR Photovoltaic Glass for Biuldings. Available online: https://www.onyxsolar.com/ (accessed on 6 February 2020).
- TESLA Tesla Solar Roof. Available online: https://www.tesla.com/es_MX/solarroof (accessed on 24 February 2020).
- Alim, M.A.; Tao, Z.; Hassan, M.K.; Rahman, A.; Wang, B.; Zhang, C.; Samali, B. Is It Time to Embrace Building Integrated Photovoltaics? A Review with Particular Focus on Australia. Sol. Energy 2019, 188, 1118–1133. [Google Scholar] [CrossRef]
- Castillo, M.S.; Liu, X.; Abd-AlHamid, F.; Connelly, K.; Wu, Y. Intelligent Windows for Electricity Generation: A Technologies Review. In Building Simulation; Springer: Berlin/Heidelberg, Germany, 2022; Volume 15, pp. 1747–1773. [Google Scholar]
- Li, D.H.W.; Aghimien, E.I.; Alshaibani, K. An Analysis of Real-Time Measured Solar Radiation and Daylight and Its Energy Implications for Semi-Transparent Building-Integrated Photovoltaic Façades. Buildings 2023, 13, 386. [Google Scholar] [CrossRef]
- Yu, G.; Yang, H.; Luo, D.; Cheng, X.; Ansah, M.K. A Review on Developments and Researches of Building Integrated Photovoltaic (BIPV) Windows and Shading Blinds. Renew. Sustain. Energy Rev. 2021, 149, 111355. [Google Scholar] [CrossRef]
- Powalla, M.; Paetel, S.; Ahlswede, E.; Wuerz, R.; Wessendorf, C.D.; Friedlmeier, T.M. Thin-Film Solar Cells Exceeding 22% Solar Cell Efficiency: An Overview on CdTe-, Cu(In,Ga)Se2-, and Perovskite-Based Materials. Appl. Phys. Rev. 2018, 5. [Google Scholar] [CrossRef]
- Becerril-Romero, I.; Giraldo, S.; López-Marino, S.; Placidi, M.; Sánchez, Y.; Sylla, D.; Pérez-Rodríguez, A.; Saucedo, E.; Pistor, P. Vitreous Enamel as Sodium Source for Efficient Kesterite Solar Cells on Commercial Ceramic Tiles. Sol. Energy Mater. Sol. Cells 2016, 154, 11–17. [Google Scholar] [CrossRef]
- Fraga, D.; Barrachina, E.; Calvet, I.; Lyubenova, T.S.S.; Carda, J.B. Developing CIGS Solar Cells on Glass-Ceramic Substrates. Mater. Lett. 2018, 221, 104–106. [Google Scholar] [CrossRef]
- Fraga, D.; Lyubenova, T.S.; Martí, R.; Calvet, I.; Barrachina, E.; Carda, J.B. Ecologic Ceramic Substrates for CIGS Solar Cells. Ceram. Int. 2016, 42, 7148–7154. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, T.; Gupta, A.K. End-of-Life Management of Solar PV Waste in India: Situation Analysis and Proposed Policy Framework. Renew. Sustain. Energy Rev. 2022, 153, 111774. [Google Scholar] [CrossRef]
- Burrows, K.; Fthenakis, V. Glass Needs for a Growing Photovoltaics Industry. Sol. Energy Mater. Sol. Cells 2015, 132, 455–459. [Google Scholar] [CrossRef]
- Kang, S.; Yoo, S.; Lee, J.; Boo, B.; Ryu, H. Experimental Investigations for Recycling of Silicon and Glass from Waste Photovoltaic Modules. Renew. Energy 2012, 47, 152–159. [Google Scholar] [CrossRef]
- Lin, K.-L.; Lee, T.-C.; Hwang, C.-L. Effects of Sintering Temperature on the Characteristics of Solar Panel Waste Glass in the Production of Ceramic Tiles. J. Mater. Cycles Waste Manag. 2015, 17, 194–200. [Google Scholar] [CrossRef]
- Alvarez-Mendez, A.; Torres-González, L.C.; Alvarez, N.; Torres-Martínez, L.M. Kinetic Thermal Analysis of Glass Ceramics from Industrial Wastes. J. Non. Cryst. Solids 2003, 329, 73–76. [Google Scholar] [CrossRef]
- Morris, G.C.; McElnea, A.E. Fluorine Doped Tin Oxide Films from Spray Pyrolysis of Stannous Fluoride Solutions. Appl. Surf. Sci. 1996, 92, 167–170. [Google Scholar] [CrossRef]
- Koirala, M.P.; Joshi, L.P. Structural and Optical Properties of Fluorine Doped Tin Oxide Thin Film Deposited by Home Built Spray Pyrolysis Unit. Himal. Phys. 2017, 6, 58–60. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Rafferty, A.; Sajjia, M.; Olabi, A.-G. Properties of Glass Materials. In Reference Module in Materials Science and Materials Engineering; Hashmi, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Navarro, J.M.F. El Vidrio; Consejo Superior de Investigaciones Científicas: Madrid, Spain, 2003; ISBN 8400081587. [Google Scholar]
- Chromčíková, M.; Liška, M.; Macháček, J.; Chovanec, J. Thermodynamic Model and Viscosity of Na2O–MgO–CaO–SiO2 Glasses. J. Non. Cryst. Solids 2014, 401, 237–240. [Google Scholar] [CrossRef]
- Uchino, T.; Nakaguchi, K.; Nagashima, Y.; Kondo, T. Prediction of Optical Properties of Commercial Soda–Lime-Silicate Glasses Containing Iron. J. Non. Cryst. Solids 2000, 261, 72–78. [Google Scholar] [CrossRef]
- Vogt, M.R.; Hahn, H.; Holst, H.; Winter, M.; Schinke, C.; Köntges, M.; Brendel, R.; Altermatt, P.P. Measurement of the Optical Constants of Soda-Lime Glasses in Dependence of Iron Content and Modeling of Iron-Related Power Losses in Crystalline Si Solar Cell Modules. IEEE J. Photovolt. 2015, 6, 111–118. [Google Scholar] [CrossRef]
- Muniramaiah, R.; Reddy, N.P.; Santhosh, R.; Fernandes, J.M.; Padmanaban, D.B.; Maharana, G.; Kovendhan, M.; Veerappan, G.; Laxminarayana, G.; Banavoth, M.; et al. Solvent Effect on the Optoelectronic Properties of Fluorine Doped SnO2 Thin Films Prepared by Spray-Pyrolysis. Surf. Interfaces 2022, 33, 102174. [Google Scholar] [CrossRef]
- Ossila FTO Glass Substrates (Unpatterned). Available online: https://www.ossila.com/products/fto-glass-unpatterned (accessed on 14 February 2023).
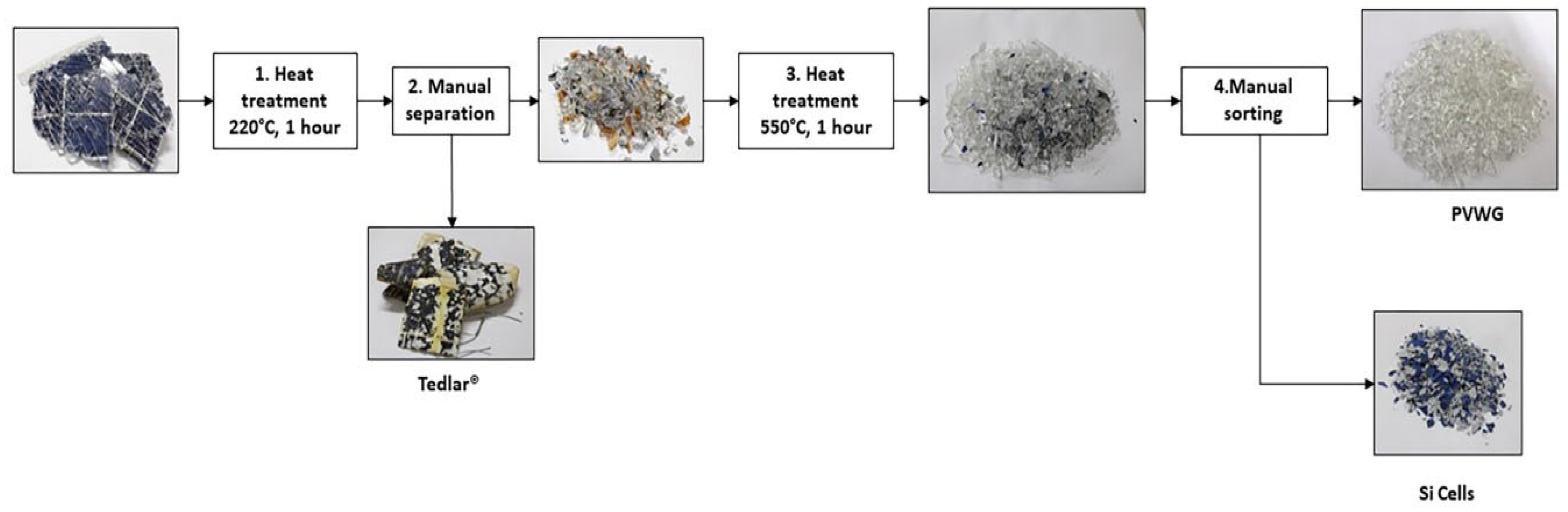

| Wastes | Wt. (%) |
|---|---|
| PVWG | 48.08 |
| Quartz Sand | 15.86 |
| Dolomite | 36.06 |
| Material Recovered | Wt. (%) |
|---|---|
| PVWG | 80.83 |
| Poly-Si cell chunks | 2.95 |
| Tedlar® | 11.95 |
| Evaporated EVA | 4.27 |
| Component | PVWG | SLG | wSG |
|---|---|---|---|
| SiO2 | 71.63 | 70.5 | 70.9 |
| Na2O | 14.20 | 13.6 | 7.18 |
| CaO | 8.98 | 9.82 | 10.68 |
| MgO | 3.58 | 3.9 | 4.91 |
| Al2O3 | 1.13 | 1.4 | 5.65 |
| SO3 | 0.17 | N.D. | N.D. |
| K2O | N.D. | 0.39 | 0.41 |
| TiO2 | N.D. | N.D. | 0.27 |
| L.O.I * | 0.29 | 0.39 | 0.44 |
| Total | 99.98 | 100.00 | 100.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Treviño Rodríguez, K.; Sánchez Vázquez, A.I.; Ruiz Valdés, J.J.; Ibarra Rodríguez, J.; Paredes Figueroa, M.G.; Porcar García, S.; Carda Castelló, J.B.; Álvarez Méndez, A. Photovoltaic Glass Waste Recycling in the Development of Glass Substrates for Photovoltaic Applications. Materials 2023, 16, 2848. https://doi.org/10.3390/ma16072848
Treviño Rodríguez K, Sánchez Vázquez AI, Ruiz Valdés JJ, Ibarra Rodríguez J, Paredes Figueroa MG, Porcar García S, Carda Castelló JB, Álvarez Méndez A. Photovoltaic Glass Waste Recycling in the Development of Glass Substrates for Photovoltaic Applications. Materials. 2023; 16(7):2848. https://doi.org/10.3390/ma16072848
Chicago/Turabian StyleTreviño Rodríguez, Karina, Astrid Iriana Sánchez Vázquez, Juan Jacobo Ruiz Valdés, Jorge Ibarra Rodríguez, María Guadalupe Paredes Figueroa, Samuel Porcar García, Juan Bautista Carda Castelló, and Anabel Álvarez Méndez. 2023. "Photovoltaic Glass Waste Recycling in the Development of Glass Substrates for Photovoltaic Applications" Materials 16, no. 7: 2848. https://doi.org/10.3390/ma16072848
APA StyleTreviño Rodríguez, K., Sánchez Vázquez, A. I., Ruiz Valdés, J. J., Ibarra Rodríguez, J., Paredes Figueroa, M. G., Porcar García, S., Carda Castelló, J. B., & Álvarez Méndez, A. (2023). Photovoltaic Glass Waste Recycling in the Development of Glass Substrates for Photovoltaic Applications. Materials, 16(7), 2848. https://doi.org/10.3390/ma16072848







