Theoretical Prediction of the Sublimation Behavior by Combining Ab Initio Calculations with Statistical Mechanics
Abstract
1. Introduction
2. Theory
2.1. Sublimation Function
2.1.1. Monoatomic Molecule
2.1.2. Diatomic Molecule
2.1.3. Monoatomic Molecule Approximation
2.2. Chemical Reaction
2.3. Computational Details
3. Results
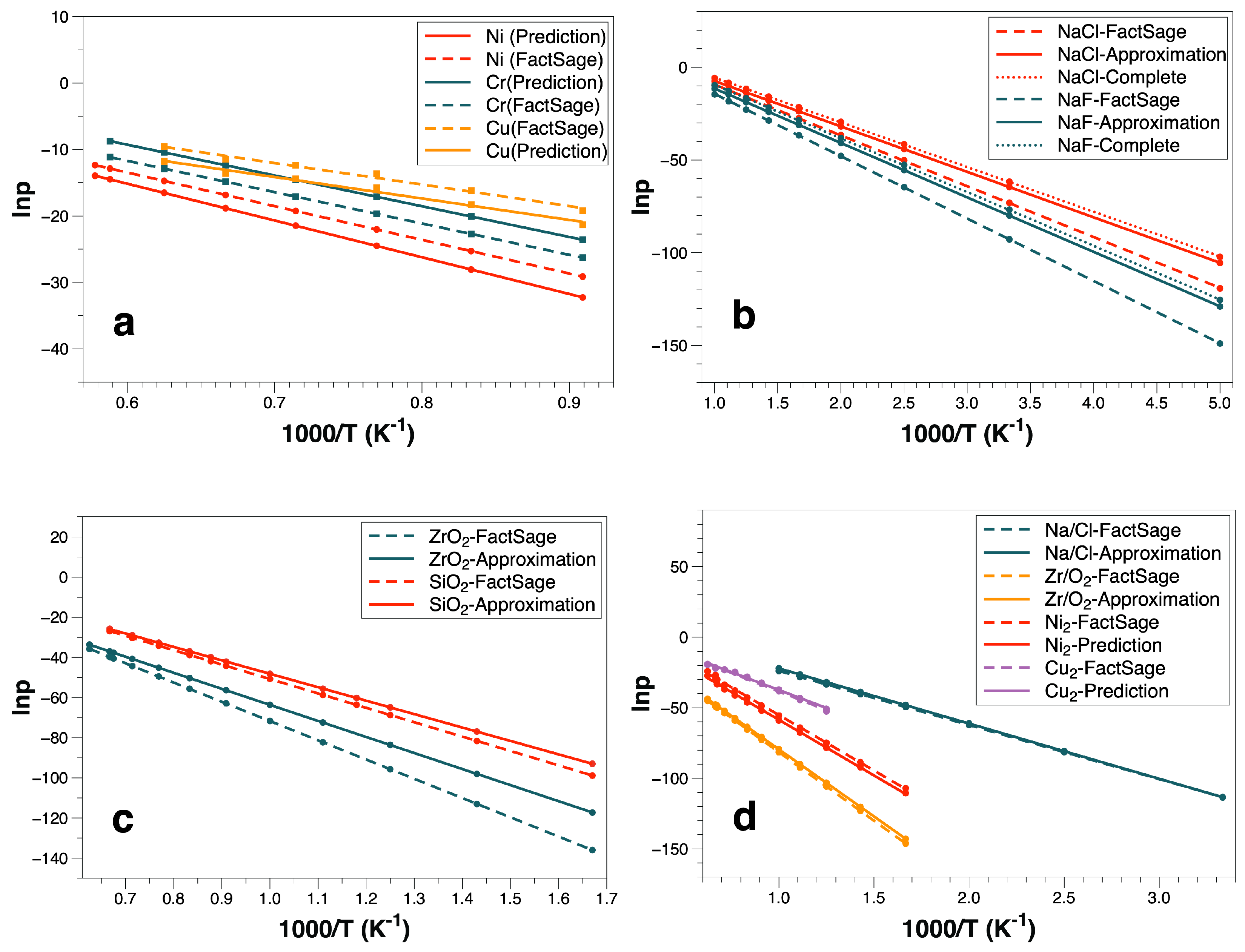
3.1. Monoatomic Molecule
3.2. Monoatomic Molecule Approximation
3.3. Chemical Reaction
4. Conclusions
- The comparison of the predictions to thermodynamic databases (FactPS database) has proven that the theoretical prediction of the vapor pressure is promising and precise.
- Theoretical predictions of and with two different methods reveal the feasibility of the monoatomic molecule approximation. The deviations of the prefactor are 5.02% and 7.08%, respectively. The application to triatomic molecules ( and ) indicates the benefit for complex situations, especially , where the deviations of the sublimation enthalpy and the prefactor are 7.06% and 10.43%, respectively.
- The additional exploration of the formation of , , , , and indicates the rationality of our theoretical calculation to explain further chemical reactions. Except for the predicted prefactor of and , the deviations are below 5%.
- The determination of unknown gaseous molecule structures, the approximation of the molecules’ motion, and inaccuracies of the thermodynamic database may lead to deviations.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SOFC | Solid oxide fuel cell |
| KEMS | Knudsen effusion mass spectrometry |
| DFT | Density functional theory |
| VASP | Vienna ab initio simulation package |
| PAW | Project augmented wave |
| PBE | Perdew, Burke and Ernzerhof |
| GGA | Generalized gradient approximation |
References
- Drozd, K.V.; Manin, A.N.; Voronin, A.P.; Perlovich, G.L. Sublimation thermodynamics of pyrazinoic, dipicolinic and quinolinic acids: Experiment and theoretical prediction. J. Chem. Thermodyn. 2021, 155, 106369–106382. [Google Scholar] [CrossRef]
- Yin, X.Y.; Bencze, L.; Motalov, V.; Spatschek, R.; Singheiser, L. Thermodynamic perspective of Sr-related degradation issues in SOFCs. Int. J. Appl. Ceram. Technol. 2018, 15, 380–390. [Google Scholar] [CrossRef]
- Liu, Y.; Motalov, V.; Baumann, S.; Sergeev, D.; Müller, M.; Sohn, Y.J.; Guillon, O. Thermochemical stability of Fe- and Co-functionalized perovskite-type SrTiO3 oxygen transport membrane materials in syngas conditions. J. Eur. Ceram. Soc. 2019, 39, 4874–4881. [Google Scholar] [CrossRef]
- Sergeev, D.; Yazhenskikh, E.; Kobertz, D.; Müller, M. Vaporization behavior of Na2CO3 and K2CO3. Calphad 2019, 65, 42–49. [Google Scholar] [CrossRef]
- Dong, Z.H.; Sergeev, D.; Kobertz, D.; D’Souza, N.; Feng, S.; Müller, M.; Dong, H.B. Vaporization of Ni, Al and Cr in Ni-Base alloys and its influence on surface defect formation during manufacturing of single-crystal components. Metall. Mater. Trans. A 2019, 65, 309–322. [Google Scholar]
- Pu, C.; Wang, Z.; Tang, X.; Zhou, D.; Cheng, J. A novel two-dimensional ZnSiP2 monolayer as an anode material for K-ion batteries and NO2 gas sensing. Molecules 2022, 27, 6726. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, R.; Yu, X.Y.; Wang, Y.J. NO2 adsorption and decomposition ZnO (0001) doped graphene: Density functional theory calculations. Appl. Sur. Sci. 2017, 420, 944–953. [Google Scholar] [CrossRef]
- Yu, B.J.; Ren, H.; Piao, X.L. Towards absorptive enrichment of flavonoids from honey using h-BN monolayer. ChemPhysChem 2022, 23, e202100828. [Google Scholar] [CrossRef]
- Sholl, D.S.; Steckel, J.A. Density Functional Theory: A Practical Introduction; John Wiley & Sons, Inc.: New York, NY, USA, 2011. [Google Scholar]
- Cervinka, C.; Fulem, M. State-of-the-art calculations of sublimation enthalpies for selected molecular crystals and their computational uncertainty. J. Chem. Theory Comput. 2017, 13, 2840–2850. [Google Scholar] [CrossRef]
- Lopes Jesus, A.J.; Tomé, L.I.N.; Ermelinda Eusébio, M.; Redinha, J.S. Enthalpy of sublimation in the study of the solid state of organic compounds. Application to erythritol and threitol. J. Phys. Chem. B 2005, 109, 18055–18060. [Google Scholar] [CrossRef]
- Halpern, A.M.; Marzzacco, C.J. Using the principles of classical and statistical thermodynamics to calculate the melting and boiling points, enthalpies and entropies of fusion and vaporization of water, and the freezing point depression and boiling point elevation of ideal and nonideal aqueous solutions. J. Chem. Educ. 2018, 95, 2205–2211. [Google Scholar]
- Zaby, P.; Ingenmey, J.; Kirchner, B.; Grimme, S.; Ehlert, S. Calculation of improved enthalpy and entropy of vaporization by a modified partition function in quantum cluster equilibrium theory. J. Chem. Phys. 2021, 155, 104101. [Google Scholar] [CrossRef] [PubMed]
- Chase, M.W. National Information Standards Organization (US). NIST-JANAF Thermochemical Tables, 4th ed.; American Chemical Society: Washington, DC, USA, 1998. [Google Scholar]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 454, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kittel, C. Kittel’s Introduction to Solid State Physics, Global ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2018. [Google Scholar]
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.C.; Tang, G.; Geng, W.T. VASPKIT: A user-friendly interface facilitating high-throughput computing; analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartr, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. Reprint of: FactSage thermochemical software and databases, 2010–2016. Calphad 2016, 55, 1–19. [Google Scholar] [CrossRef]
- Blundell, S.J.; Blundell, K.M. Concepts in Thermal Physics, 2nd ed.; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Pathria, P.K.; Beale, P.D. Statistical Mechanics, 3rd ed.; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Atkins, P.; De Paula, J.; Keeler, J. Atkins’ Physical Chemistry, 11th ed.; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Lee, J.G. Computational Materials Science An Introduction, 2nd ed.; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 264–266. [Google Scholar]
- Freysoldt, C.; Grabowski, B.; Hickel, T.; Neugebauer, J.; Kresse, G.; Janotti, A.; Van de Walle, C.G. First-principles calculations for point defects in solids. Rev. Mod. Phys. 2014, 86, 253–305. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Blöch, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- John, P.P.; Kieron, B.; Matthias, E. Generalized gradient approximation made simple. Phys. Rev. B 1996, 77, 3865–3868. [Google Scholar]
- Krause, D.; Thörnig, P. JURECA: Modular supercomputer at Jülich supercomputing centre. J. Large-Scale Res. Facil. 2018, 4, A132. [Google Scholar] [CrossRef]
| Substance | Crystal System | Space Group | Number | Chemical Bond |
|---|---|---|---|---|
| cubic | Fm3m | 225 | Metallic | |
| cubic | Im3m | 229 | Metallic | |
| cubic | Fm3m | 225 | Metallic | |
| cubic | Fm3m | 225 | Ionic | |
| cubic | Fm3m | 225 | Ionic | |
| monoclinic | P21/c | 14 | Ionic | |
| tetragonal | I42d | 122 | Covalent |
| Substance | FactSage | Monoatomic Molecule Approximation | Complete Model | |||
|---|---|---|---|---|---|---|
| A | A | A | ||||
| −50.677 | 16.94 | − | − | −55.354 | 18.068 | |
| −76.544 | 20.81 | − | − | −77.080 | 18.02 | |
| −47.163 | 16.59 | − | − | −46.420 | 18.55 | |
| −39.847 | 14.88 | − | − | −39.795 | 16.95 | |
| −56.628 | 18.41 | − | − | −54.532 | 18.02 | |
| −27.404 | 18.069 | −24.520 | 17.162 | −24.117 | 18.559 | |
| Na/Cl(NaCl) | −38.358 | 14.464 | −39.139 | 16.38 | − | − |
| NaF | −33.624 | 19.271 | −29.364 | 17.906 | −29.000 | 19.6 |
| −93.144 | 21.84 | −80.045 | 17.61 | − | − | |
| Zr/ | −97.014 | 15.56 | −95.113 | 15.67 | − | − |
| −71.753 | 20.99 | −66.9 | 18.8 | − | − | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Wang, K.; Müller, M.; Wessel, E.; Spatschek, R. Theoretical Prediction of the Sublimation Behavior by Combining Ab Initio Calculations with Statistical Mechanics. Materials 2023, 16, 2826. https://doi.org/10.3390/ma16072826
Hu Y, Wang K, Müller M, Wessel E, Spatschek R. Theoretical Prediction of the Sublimation Behavior by Combining Ab Initio Calculations with Statistical Mechanics. Materials. 2023; 16(7):2826. https://doi.org/10.3390/ma16072826
Chicago/Turabian StyleHu, Yang, Kai Wang, Michael Müller, Egbert Wessel, and Robert Spatschek. 2023. "Theoretical Prediction of the Sublimation Behavior by Combining Ab Initio Calculations with Statistical Mechanics" Materials 16, no. 7: 2826. https://doi.org/10.3390/ma16072826
APA StyleHu, Y., Wang, K., Müller, M., Wessel, E., & Spatschek, R. (2023). Theoretical Prediction of the Sublimation Behavior by Combining Ab Initio Calculations with Statistical Mechanics. Materials, 16(7), 2826. https://doi.org/10.3390/ma16072826





