Abstract
Bone tissue engineering integrates biomaterials, cells, and bioactive agents to propose sophisticated treatment options over conventional choices. Scaffolds have central roles in this scenario, and precisely designed and fabricated structures with the highest similarity to bone tissue have shown promising outcomes. On the other hand, using nanotechnology and nanomaterials as the enabling options confers fascinating properties to the scaffolds, such as precisely tailoring the physicochemical features and better interactions with cells and surrounding tissues. Among different nanomaterials, polymeric nanofibers and carbon nanofibers have attracted significant attention due to their similarity to bone extracellular matrix (ECM) and high surface-to-volume ratio. Moreover, bone ECM is a biocomposite of collagen fibers and hydroxyapatite crystals; accordingly, researchers have tried to mimic this biocomposite using the mineralization of various polymeric and carbon nanofibers and have shown that the mineralized nanofibers are promising structures to augment the bone healing process in the tissue engineering scenario. In this paper, we reviewed the bone structure, bone defects/fracture healing process, and various structures/cells/growth factors applicable to bone tissue engineering applications. Then, we highlighted the mineralized polymeric and carbon nanofibers and their fabrication methods.
1. Introduction
Bone defects are a high prevalence and devastating injuries, generally caused by trauma, debridement for bone tumors, infection, or nonunion (a bone that is not healing). The effective treatment options include bone transport distraction osteogenesis, shortening the bone, and bone grafting. Despite their efficacy, they have substantial drawbacks and could not be considered as a completely effective treatment. Accordingly, researchers have been seeking alternatives to circumvent the shortcomings of conventional treatments for large bone defects [1,2,3]. Tissue engineering is an emerging concept aiming to combine engineering, cells, biomaterial concepts, and proper biochemical and physicochemical factors to repair, replace, or improve damaged tissues [4,5,6]. Bone tissue engineering has attracted enormous attention during the last few years due to promising results obtained using this approach. Scaffolds play a central role in the bone tissue engineering approach as the supports and modulator for cell attachment, proliferation, and differentiation, and as the carrier for osteogenic substances. It is critical to point out that the scaffold’s morphology, microstructure, porosity, mechanical, and physicochemical characteristics must be similar to the natural bone as much as possible [7,8,9].
A wide range of materials such as strontium- and cobalt-substituted bioactive glasses, hydroxyapatite/tricalcium phosphate (HA/TCP), gelfoam surgical sponges, and nanofibers have been investigated for bone tissue engineering applications [10,11]. Among them, nanofibrous structures have shown fascinating performance and attracted considerable attention. Nanofibers are 2D structures with a high surface-to-volume ratio that mimic the extracellular matrix (ECM) of bone. Moreover, it is possible to prepare 3D constructs using nanofibers, applicable for bone tissue engineering applications [12,13,14]. Different methods have been developed to fabricate nanofibers, such as molecular self-assembly, template synthesis, temperature-induced phase separation, drawing, and electrospinning, which have their pros and cons. Electrospinning is a sophisticated and straightforward method for preparing nanofibrous structures with various morphology, structure, porosity, and geometry [15,16,17,18].
Bone is a nanocomposite structure composed of collagen nanofibers, the organic phase of bone, and mineralized with hydroxyapatite crystals, the bone’s inorganic phase. Accordingly, researchers have proposed that composite constructs made from artificial nanofibers mineralized with bioceramics crystals would be a proper scaffold mimicking the native structure of bone. Hence, various polymeric nanofibers such as carbon nanofibers (CNFs) have been fabricated and composited with different bioceramics types to develop bone healing materials [19,20,21].
Because of their biocompatibility, ease of manufacture, and good electrical conductivity, CNFs are frequently employed in bone tissue engineering applications. This is because they provide the most favorable microenvironment for osteoblasts to proliferate and speed up the regeneration process [22,23]. Many applications, such as selective adsorption, polymer reinforcement, electrochemical catalysis, and hydrogen storage, are made possible by the special features of CNFs [23]. For the purpose of bone tissue engineering, a study by El-Aziz et al. in 2017 focused on the creation of carbon nanofiber sheets functionalized with hydroxyapatite (HA) and BSA. The biocompatible functionalized sheets that were altered with HA or HA and BSA were successfully prepared. For a period of three weeks, the functionalized sheets containing both HA and BSA were more biocompatible and contained less inflammatory cells (neutrophils and lymphocytes) than those with simply HA [24]. Aoki et al. discussed the application of electrospun CNFs in bone regeneration in their 2020 study. These nanofibers were created by electrospinning polyacrylonitrile, then thermally treating them at 1000 °C while being supplied with argon gas to create a thin, three-dimensional matrix. The trials were conducted utilizing rhBMP-2, an anabolic signaling molecule also utilized to treat numerous bone diseases, and the results demonstrated subsequent bone development [25].
This paper aims to discuss various types of nanofibrous scaffolds mineralized with different bioceramics by distinct methods and applied for bone tissue engineering. First, we introduce the structure and mechanical properties of bone and its healing process, and then discuss the basic principles of bone tissue engineering and nanofibers’ applications in bone tissue engineering. Next, we review the mineralized nanofibrous nanocomposites applied as the tissue engineering applications. Finally, the challenges, solutions, and prospective are presented. Although there are some review papers on the application of nanofibers in bone tissue engineering, there is room for more insight, especially on the combination of nanofibers’ technology with bioceramics chemistry.
2. Hierarchical Structure of Bone
Bone is a heterogeneous and anisotropic bio-composite structure that supports and protects the body. The multi-scale structural geometry that organizes this structure into hierarchical levels affects how well it can carry out functions [26,27]. It is known today that bone tissue structure can be classified into five structural levels, from macro- to nanocomponents [26,28,29]
The macroscopic or organ level actually forms the anatomical shape of the bone tissue and includes the outer geometry and internal architecture of the trabecular and cortical bone, which affect the mechanical properties of this tissue. At the next structure level, which defines the micro- and sub-microstructures, the osteons, lamellae and single trabecula structures can be seen, which actually create the extracellular matrix (ECM) of bone. The osteons are composed of concentrated lamellae of mineralized collagen fibrils. This level has a highly porous structure and is surrounded by small cavities containing blood vessels and nerves. The porosity and channels are very important for cell migration and vascularization in the bone. On the lowest level of structural bone, nano- and sub-nanostructures represent the components of the nanocomposite materials. The mineralized collagen fibrils that are the main building block of all types of bone have a diameter of about 0.1 μm. Collagen is made by osteoblasts, which are bone-forming cells that then secrete collagen molecules into the extracellular space, which later forms into a fibril structure [26,27,28,29,30,31,32].
The collagen type 1 molecule is a large protein in a bone organic matrix and the mineralized fibrils are based on parallel collagen molecules reinforced with thin calcium phosphate mineral platelets. The bone mineral has a non-static moiety due to poorly crystalline hydroxyapatite, so calcium (Ca2+) and phosphate ions (Pi) can be easily substituted [33,34,35]. On the other hand, the non-collagenous organic proteins provide natural bone with its remarkable mechanical properties. Osteons in both microscopic and molecular levels create the extracellular matrix (ECM) of bone, whose organic matrix primarily contains collagen (Type I) [36,37]. Overlaid on this, each of these scales or hierarchical levels, molecular (e.g., collagen type and hydroxyapatite (HAP) stoichiometry) (Figure 1) and microscopic (e.g., microstructure and porosity), will have an influence on the biomechanical characteristics of bone and cell behavior [38,39].
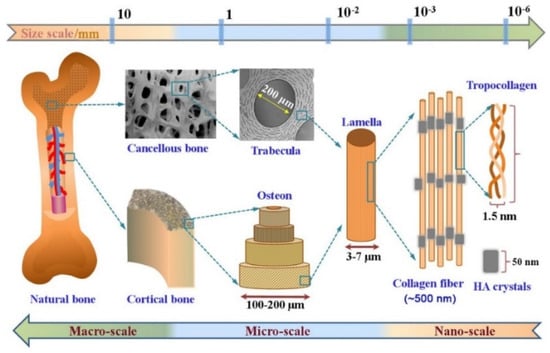
Figure 1.
The hierarchical multi-scale structure of natural bone ranging from the nanoscale to macroscale. Reproduced with permission from Ref. [38].
3. Mechanical Properties of Bone
The bones in the human skeleton have a variety of functions; hence, they are certainly not mechanically identical. The mechanical properties of bone are essential for the ability of skeletons to support the movement and protect the vital organs of the body [40,41]. In fact, bones represent a system with a complex function and structure. The biomechanical properties of bone tissue are affected by the physiological and metabolic conditions of the body. In addition, there are tissues such as fat, blood vessels, cartilage, and nerves in this tissue. In general, the bone structure has a decisive influence on its mechanical properties. As we presented, bone is a biological composite material that is made of two major components: organic and inorganic; organic components (ossein protein and collagen fibers) are responsible for flexibility and elasticity, and the hardness of the bone tissue is due to the inorganic part (phosphoric and carbonic acids) [41,42,43,44,45].
Bone is a heterogeneous composite material with a mineral phase, hydroxyapatite (Ca10(PO4)6(OH)2), an organic phase (90% type I collagen, 5% noncollagenous proteins (NCPs), 2% lipids by weight), and water as its least abundant components. There are two main types of proteins found in the bone extracellular matrix: (a) structural proteins (such as collagen and fibronectin) and (b) proteins with specialized functions, such as those that (i) control the diameter of collagen fibrils, (ii) act as signaling molecules, (iii) act as growth factors, (iv) act as enzymes, and (v) have other functions.
Previous studies based on mechanical models have shown the important mechanical properties of bone and the role of mineral platelets in the strengthening of bone material [42,43,46].
At the molecular scale, the interaction between collagen molecules and inorganic hydroxyapatite (HAP) as well as the amount of minerals have been identified to play an important role in providing bone strength and toughness [46,47,48]. For this, an important characteristic of this material is the mechanical properties of bone. Because of the complex composite structure, bone has excellent mechanical properties for its functions that affect load-bearing capacity. Bone properties depend on the constituent properties and the geometrical and topological characteristics of the complex. For these reasons, understanding how bone components are arranged is important to the successful engineering of this tissue. Therefore, the analysis of the mechanical properties of the bones should be able to characterize the geometric and topological relationships between all components [49,50].
Besides the effects of the amounts of cortical and trabecular bone on whole-bone mechanical properties, structural properties, and geometries, such as bone shape, bone size, cortical bone thickness, and the cross-sectional spatial distribution of trabecular bone, are key factors that change with aging and disease. However, research has shown that the amount of minerals in the bone changes little with aging, and this trend is also reflected in their stiffness [41,51]. Therefore, the study of the mechanical behavior of whole bones can be more complex than the study of cortical or trabecular bone. It can be said that the mechanical properties of bone are intermediate between type I collagen and HAP [52]. HAP crystals are arranged in the length of collagen fibrils. Thus, this structure combines the mechanical advantages of both materials. HAP provides structural rigidity and compression strength, while the more flexible collagen fibers prevent brittle cracking and provide improved tensile properties. Interestingly, the bone is a very weak organ against tension but is strongest in stress tolerance [52,53,54].
The ideal mechanical characteristics of bone are the outcome of its complex hierarchical structure from a nano- to macro-surface, which is described further. In general, the mechanical properties of the trabecular bone are due to the high-porosity structure and the mechanical characteristics of a single trabecular bone. Bone porosity is also arranged hierarchically, and this porosity has a significant effect on bone mechanical properties [39,55]. As the basic block of bone is the mineralized collagen fibril, more attention has been paid to understand its mechanical function. The organic matrix is under pressure because of how the mineral particles react to the pressure [56,57]. In a mechanical model, it was shown that the reaction of the inorganic particles and the organic matrix is such that under tensile loading, these particles bear the load, and the role of the organic matrix is to carry the charge between the particles through the shear [58].
Hence, in order to compensate for the high elasticity of the organic elements, the shape of mineral particles is relatively large in size such that, at this scale, the sensitivity of these particles to flow decreases, which could be an advantage. Generally, it can be expected that the degree of stiffness and hardness of the whole structure can be adjusted by the number of mineral platelets in the elastic collagen fiber. Increased bone mineral density (BMD) increases bone hardness and reduces its fragility [59]. Overall, extensive efforts have been made to elucidate the structure of bone nanocomposite materials, their relationship to the mechanical properties, and the mechanism of deformation in the mineral tissue of this organ [60].
4. Bone Healing Process
Bone is a living organ and can grow; it can also regenerate itself and heal in case of injury. Bone can be defined as a unique tissue and, due to the importance of this organ in the stability of the whole body, its healing is very important. On the other side, the body can facilitate the repair or healing of bone. It is known that bone is one of a few tissues that can heal without forming a fibrous scar [61,62]. In this way, the process of bone healing recapitulates bone regrowth and can be considered a kind of tissue regeneration. However, bone healing is a dynamic biological process that results from the balance between mechanical and physiological stimuli [63,64,65,66].
In the classic histological terms, healing has been divided into two types including direct and indirect healing models [67]. The most common form of bone repair is indirect fracture healing, which involves multiple stages. The induction of hematoma and inflammation is the first stage at the time of bone fracture and can last for about 5 days. The organization of hematoma in an injured site that contains blood cells, mesenchymal stem cells, fibroblasts, osteoclasts, osteoblasts, cytokines, growth factors, and other hormones is an immediate response to fracture. The inflammatory cells of the acute immune system migrate to the fracture site and activate the upregulation of angiogenic factors to support the vascularization of the injured site. The inflammatory response, around 24 h after the fracture, is then balanced by the release of anti-inflammatory cytokines involved in the recruitment of cells to begin tissue healing. If the level of the acute inflammatory response is not reduced by the interaction of the immune system (for example, due to bacterial infection at the site of injury or chronic inflammatory disease), the healing process can be disrupted or inhibited and entered into the chronic phase [66,68,69,70].
In the next phase, the fracture healing process continues with the replacement of the gradual evolution of the hematoma into granular tissue, followed by a soft callus composed of fibrous tissue and cartilage. This stage, called the anabolic phase, is characterized by an increase in the volume of mesenchymal precursor cells. These cells are then differentiated into osteoblasts or cartilaginous cells for forming the extracellular matrix and cartilage. This process is induced by the replacement of the hematoma and the filling of the fracture gap [66,71,72,73]. Importantly, with the formation of soft callus tissue and the initial stability of the cells in the fracture area, the process of bone healing is not yet complete. Progressively, the process of endochondral ossification in soft callus tissue begins under mineralization and becomes a structure with a stronger mechanical property that is eventually replaced by tissue bone. In the final stage, which is the remodeling phase of the fracture area, the woven bone is slowly replaced by the lamellar bone. In this process, the remodeling is performed by the balance of callus absorption by osteoclasts and the deposition of the layered bone by the osteoblasts. Eventually, this process is completed by restoring the mechanical and biological functions of the bone [66,74,75].
In contrast, the direct healing process (Figure 2), which is usually not a normal fracture repair process, aims to recover the correct fracture anatomy without creating a gap and is often needed after internal stabilization surgery. However, callus tissue does not form here and the two plates of bone at the site of fracture connect together, and the healing occurs to produce the bone by osteoclasts and osteoblast activities directly. The process of direct or primary, complete healing of the bones can take several months to several years, depending on the species [61,76,77].
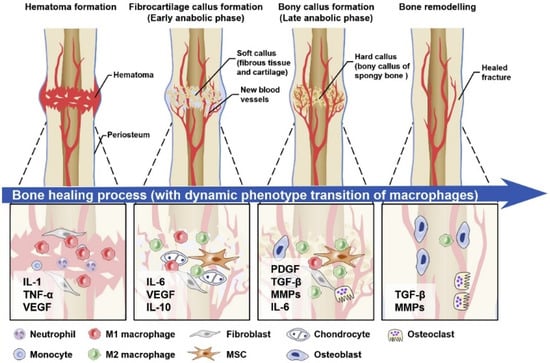
Figure 2.
A graphical view of the direct bone healing process.
5. Bone Tissue Engineering
Bone tissue engineering is a multidisciplinary approach aiming to accelerate bone regeneration. Bone tissue is considered the field of active interaction between biology and engineering. Tissue engineering and, specifically, bone tissue engineering combine cells, growth factors, and various biomaterials to provide a suitable microenvironment to regenerate tissue and its proper function. Scaffolds have a central role in bone tissue engineering application as the substrate to support and guide cell growth and as the vehicle for bioactive agents’ delivery. Various types of materials have been evaluated as the bone tissue engineering scaffold, such as synthetic and natural polymers, ceramics, and metals.
Due to the nature of each material, it has advantages or disadvantages, and the choice of a biomaterial is influenced by how closely the biomechanical qualities match those of the implantation location [78]. In addition, the optimal biomaterial needs to be osteoconductive in vivo, printable, biodegradable, and non-cytotoxic [2].
Biomaterials that are naturally derived (such as collagen, gelatin, chitosan, and starch) can provide cells with a variety of active stimuli and have positive characteristics such as biocompatibility and ECM resemblance [9,79], but even these could cause immunogenic problems, and controlling the biodegradability rate can be challenging [80]. Due to the greater control over the degradation rate and mechanical qualities, synthetic polymers, such as polylactic acid (PLA), polycaprolactone (PCL), and polyurethane (PU), are more frequently taken into consideration [7,81,82]. High strength and biocompatibility can be found in metals (such as titanium alloys) and inert ceramics (such as alumina and zirconia), but their usefulness in tissue engineering applications is limited due to their inability to degrade. It has been suggested to employ biodegradable metallic alloys, such as those based on magnesium, iron, and zinc, to get around this restriction [78,83]. Because of their resemblance to the mineral phase of bone, bioactive ceramics (such as hydroxyapatite (HA), tricalcium phosphate, and bioactive glasses) and their combinations have been used extensively. However, it should be noted that biological HA is non-stoichiometric and contains several impurities, most notably carbonates but also other anions and cations [84,85].
From a practical perspective, because of the unique properties of the biomaterial, scaffolds made from that material may have a number of disadvantages. The goal of composite biomaterials is to increase the processability, mechanical qualities, and bioactivity of the final scaffolds in order to better mimic certain traits of the target tissue (e.g., bone), as biological tissues can be thought of as natural composites [78,86]. So, in order to mimic the chemical makeup and mechanical characteristics of bone, composite bone scaffolds may blend inorganic compounds such as bioceramics with organic polymers, such as collagen [78].
6. Mineralized Nanofibers in Bone Tissue
Nanofibers are fascinating structures with a myriad of applications in various aspects of biomedicine. The similarity to ECM of tissues, the high surface-to-volume ratio, the ability to load multiple bioactive molecules onto/into nanofibers, and the availability of different fabrication methods have made nanofibers promising structures for tissue engineering applications. Electrospun nanofibers have found a critical role in bone tissue engineering as scaffolding materials. Electrospinning is a sophisticated, flexible, and straightforward technique capable of fabricating nanofibers from various natural, synthetic, and semisynthetic polymers with different geometry and conformation [17,22]. The critical challenge in bone tissue engineering is to fabricate scaffolds with the highest similarity to structure and mechanical properties of bone, which exhibit osteoconductivity and osteoinductivity. Nanofibers can be loaded with various osteogenic substances, such as bone morphogenetic proteins (BMPs). Moreover, it is shown that calcium phosphate bioceramics, such as hydroxyapatite (HA), bioglasses, β-tricalcium phosphate (β-TCP), and biphasic calcium phosphate, can confer osteoconductivity and osteoinductivity to the scaffolds [87,88]. CNFs are also thought of as reinforcing agents for polymers such as PCL, PLA, and others to improve or increase their mechanical, biocompatibility, and cellular responses and also mimic the nanoscale architecture of natural ECM [89,90]. Accordingly, mineralized nanofibrous composites are the main focus of bone tissue engineering. Electrospinning enables researchers to prepare mineralized nanofibrous composites with a high resemblance to bone structure. The mineralization of electrospun nanofibers can be conducted by various approaches, which have their advantages and disadvantages.
Samadian et al. described the creation of hydroxyapatite (HA)-crystal-decorated osteoconductive electrospun CNFs for use as the scaffold for bone tissue engineering in an animal model. In this study, CNFs were produced by heating electrospun polyacrylonitrile (PAN) nanofibers, and a biomimetic method was used to mineralize the carbonized nanofibers. By considerably promoting in vivo bone formation in the rat femur defect site, the osteoconductive properties of the CNFs/HA nanocomposite were seen by computed tomography (CT) scan images and histological analysis (Figure 3). Additionally, the histomorphometric analysis revealed that the modified CNFs-treated group had the highest new bone production (61.3 ± 4.2%), which was significantly higher than that of the negative control group (the defect without treatment) (p < 0.05) [91].
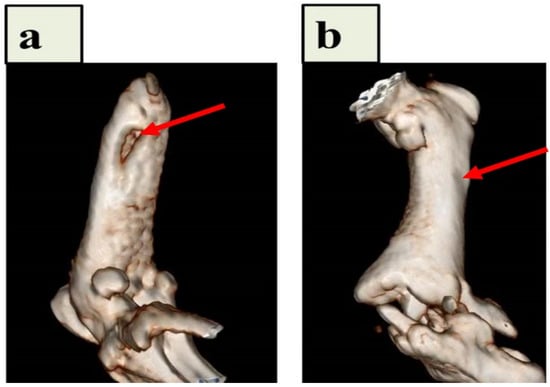
Figure 3.
CT pictures of the implanted CNFs/HA nanocomposite that repaired the damaged femur in vivo. After 8 weeks following the accident, diagnostic 3D imaging (CT scan) was conducted of the femur bone defects. The arrow indicates the deficient area that was not healed in the control group (a) and the bone defect that was corrected as a result of the implanted CNFs/HA nanocomposite stimulating the growth of normal tissue (b). Reproduced with permission from Ref. [91].
Nekounam et al. used the electrospinning process and thermal treatments to create the various ECNFs/silica nanoparticles (ECNFs/SNPs) composites. SNPs were included into ECNFs to increase their hydrophilicity while lowering their electrical conductivity. Additionally, the inclusion of SNPs increased the biological activity of the ECNFs, including cell attachment, viability, and proliferation rate. It was determined that the enhanced MG-63 proliferation rate observed in the ECNFs/SNPs composite was caused by the composite’s potent osteoactive activity [22].
In another study, conductive scaffolds made of carbon nanofiber and gold nanoparticles (CNF/AuNP) were created utilizing two different techniques. These techniques included electrospinning with blending, in which the electrospinning solution and AuNPs were blended, and electrospinning/electrospraying, in which AuNPs were electrosprayed concurrently with electrospinning. A stabilization/carbonization technique was used to create the electrospun mats. In the electrospraying and mixing electrospinning modes, respectively, electrical conductivity increased by up to 29.2% and 81%. Indirect MTT and LDH toxicity assays were used to evaluate the toxicity of MG63 cells, but no discernible toxicity was found, and the scaffolds had no negative effects on cell growth. We can draw the conclusion that these scaffolds could be used in bone tissue engineering [92]. Table 1 summarizes the advantages and limitations of the above-mentioned nanofibers-based scaffolds prepared for bone tissue regeneration.

Table 1.
Examples of some nanofibers-based scaffolds for bone tissue regeneration.
A critical step in the application of mineralized nanofibers is the characterization of the fabricated nanofibers. In this concept, the morphology of nanofibers (using SEM, TEM, and AFM microscopy), mechanical properties, swelling capacity, degradation state, hydrophilicity/hydrophobicity state, bioactivity/osteoactivity, hemocompatibility, cell viability/toxicity, immunogenicity, osteoconductivity, and osteoinductivity of the fabricated nanofibers must be evaluated.
7. Mineralization Process
7.1. Biomimetic Mineralization Approach
The biomimetic approach is considered as the mineralization process is performed in the aqueous solutions phase in ambient conditions or nearly ambient conditions using simulated body fluids (SBFs). These approaches are powerful methods that enable us to fabricate advanced mineralized nanofibers with complex shape, hierarchical organization, and controlled polymorph architecture and size under ambient conditions in aqueous environments. The biomimetic mineralization relies on the nucleation and crystal growth, where the crystals’ shape, size, aggregation, orientation, and texture can be controlled by thermodynamic or kinetic methods [95]. For complex structures to become mineralized, nucleation is the first and most important stage. The nuclei are usually unstable due to their high surface energy and grow soon after their formation, which makes this step difficult to study. It has been shown that the presence or induction of monodisperse nuclei on the nanofibers for the formation of nanocrystals with nearly monodisperse size while in the fast growth regime is critical for the formation of nanocrystals with the anisotropic shape.
In the nucleation step, the ions of the mineralizing medium anchor on the nanofibers to form nuclei, and the presence or induction of proper surface functional groups determines the homogenous and monodisperse formation of the nuclei. In a previous study, we observed that the surface treatment of carbon nanofibers (CNFs) using concentrated NaOH and the formation of carboxyl groups resulted in the uniform and monodispersed formation of HA crystals [96]. In another study, Wu et al. [97] reported that the surface treatment and induction of carboxyl functional groups on CNFs not only induced the uniform mineralization but also the mineralization of fibers located at deeper parts (Figure 4).
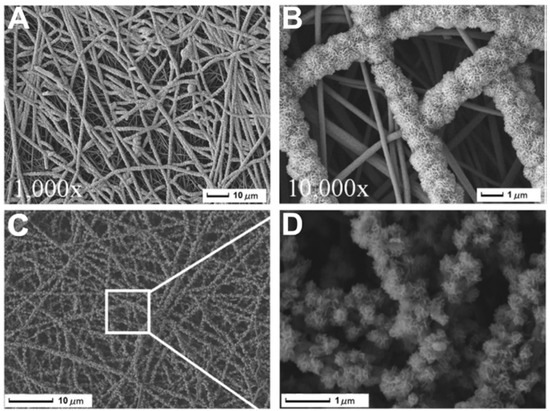
Figure 4.
SEM photos show the development of HAp particles on T-CNF mats treated with NaOH aq. solution at concentrations of (A) 8%, (B) 16%, (C) 20%, and (D) the crystals that resemble flowers. One day for cultivation. Reproduced with permission from Ref. [97].
7.2. Sequential Approach
The sequential mineralization approach is based on alternate dipping of the scaffold into the mineralization solutions containing calcium or phosphate ions. Using this method, it is possible to control the amount of mineralized phase formation, as well as the ratio of calcium/phosphate. Accordingly, the concentration of ions in the mineralization solution and the incubation time is determinant along with the surface properties of nanofibers. Several studies applied this technique to mineralize the nanofibrous scaffolds. The presence or induction of nucleation sites is critical for mineral phase deposition on the nanofibers. Luickx et al. [98] induced the sequential mineralization process to improve the osteoactivity of electrospun poly(D,L-lactide) (PLA) nanofibers. They used ethanol and demineralized water incubation along with ultrasonication to activate the surface of the nanofibers (Figure 5).
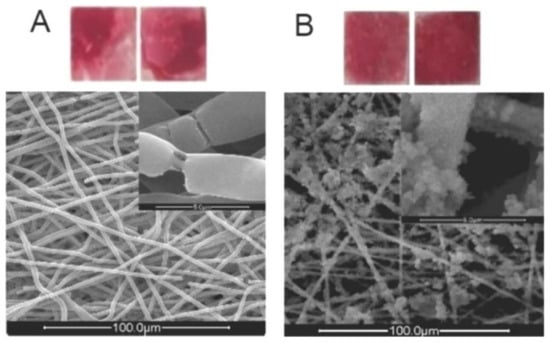
Figure 5.
(A): Digital image of an ARS-stained sample that has been treated with the standard nucleation procedure (SNP) after being dipped in EtOH; bottom: SEM image of the same sample. (B): Digital image of an ARS-stained sample that has been treated with the SNP after being activated by ultrasonic waves; top: SEM image of the same sample. Reproduced with permission from Ref. [98].
Then, the activated nanofibers were alternately immersed in the mineralization solutions (20 mL/cm2 1000 mM CaCl2.2H2O and 20 mL/cm2 600 mM Na2HPO4 solutions) and washed with water between the intervals. They reported that the nucleation step along with the mineralization parameters has significant effects on the physicochemical and biological properties of the final structure. The cell viability studies on MC3T3-E1 cells showed that the mineralization process significantly increased the viability of cells on the treated nanofibers compared with pristine nanofibers. Using the same approach, Luickx et al. [99] coated poly(e-caprolactone) nanofibers with a calcium phosphate layer to improve the hydrophilicity and biocompatibility of the nanofibers. The authors reported similar results and pointed out that the developed coating method can be applied for different nanofibers.
In another study, Kothapalli et al. [100] reported that the washing step parameters, such as the duration of washing and induction of agitation using the stirring process, also have determinant effects on the quantity of mineralization. In this study, they used 1N NaOH to activate the PLA nanofibers’ surface and induce –COO- and -OH functional groups on the nanofibers as the nucleation sites. They also observed that increasing the soaking time as well as soaking repetitions increased the net amount of mineral phase deposition. The results showed that around 35 wt% of the mineral phase was deposited on the nanofibrous mat after six cycles of dipping. Itoh et al. [101] reported that the mineral phase deposition on chitosan tubes reached around 57 wt% after 15 cycles of soaking. Cui et al. [102] grafted chitosan on PLA nanofibers and applied the sequential mineralization approach to grow hydroxyapatite (HA) crystals on the nanofibers. According to their findings, the degree of chitosan grafting and the amount of HA development on the nanofibers both directly correlate with each other and depend on the aminolysis parameters (e.g., time and reagent). The in vitro studies showed that grafting and mineralization significantly improved the cytocompatibility of the scaffold.
7.3. Sol–Gel Approach
The combination of the electrospinning technique with the sol–gel approach is another fascinating method for fabricating mineralized nanofibrous scaffolds [103,104]. The conventional sol–gel process includes two different phases of solution (sol) and gelation (gel) [105,106]. For instance, the formation of a silica network using this approach follows a well-established three-stage process:
Hydrolysis: Si(OR)4 + nH2O → (OH)nSi(OR)4−n + nROH
Condensation: 2Si(OH)4 → (OH)3Si–O–Si(OH)3 + H2O
Gelation: Si(OR)4 + Si(OH)4 → (OH)3Si–O–Si(OR)3 + ROH
In this three-stage process, silanol groups form through hydrolysis of the silica precursor (usually tetraethyl orthosilicate (TEOS) and/or triethyl phosphate (TEP)) and condense through the silicon atoms’ cross-linking to form silica nanoparticles. This process is the basic principle of the sol–gel process that can be integrated into other approaches, such as particulate leaching and polymer polymerization, from 3D porous scaffolds (Figure 6).
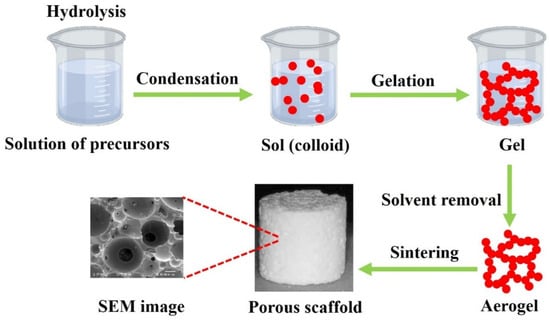
Figure 6.
The conventional porous bioglass scaffold fabrication.
Fabricating the mineralized electrospun nanofibers using the sol–gel method can be classified as the template-assisted sol–gel method that requires a precursor/carrying polymer. In this scenario, the precursor substances of the mineral phase are mixed with a carrier polymer (e.g., Polyvinylpyrrolidone (PVP) [107], Poly(vinyl alcohol) (PVA) [108], or Polyacrylonitrile (PAN) [109]) and converted to nanofibers. The calcination/sintering is the critical step to transform the fabricated nanofibers into mineralized nanofibers. The benefits of this method over the coating-based methods are the preservation of the original morphology and diameter of precursor nanofibers that provide a high surface-to-volume ratio, pore size, and porosity. These properties provide a higher adsorption of proteins, rapid dissolution of ions, controlled drug delivery, and higher osteogenic potential that subsequently result in a higher osseointegration and osteoconductivity. Liu et al. [110] blended the mineral phase precursor (triethylphosphate (TEP), calcium nitrate tetrahydrate) to the carrier polymer (PAN) and fabricated the electrospun precursor nanofibers. The created nanofibers were then carbonized in highly pure N2 and stabilized in air. The results showed that the formed mineral phase comprised calcium pyrophosphate (Ca2P2O7), HA, and β-tricalcium phosphate (β-TCP) crystals. The SEM images (Figure 7) clearly showed the transformation of the precursor nanofibers to mineralized nanofibers. The cell culture studies showed that the synthesized nanofibers supported the attachment and proliferation of MG-63 cells.
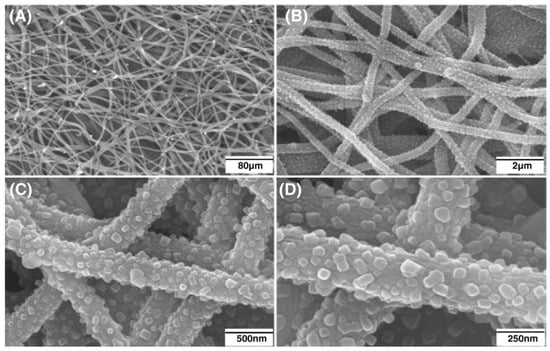
Figure 7.
SEM photos of the distribution of well-dispersed β-TCP nanoparticles that firmly adhered to CNFs at various magnifications (A) 80 µm, (B) 2 µm, (C) 500 nm, (D) 250 nm. (Whereas the CNFs had an average diameter of about 300 nm, the average size of the β-TCP particles ranged from 30 to 60 nm). Reproduced with permission from Ref. [110].
In another study, Han et al. [111] applied a similar method with slight modifications. They hydrolyzed the TEP solution before the incorporation into the polymeric solution. They also added TEOS to the precursor polymeric solution and applied the electrospinning. The results showed the formation of mineral crystals within and onto the nanofibers. The biological evaluations confirmed the bioactivity and biocompatibility of the fabricated nanofibers. Liu et al. [112] also combined the sol–gel mineralization process with the electrospun carbon nanofibers (CNFs) fabrication method. They used TEP as the phosphorus source and calcium nitrate tetrahydrate as the calcium source to fabricate β-TCP-decorated CNFs. The toxicity of the fabricated nanofibers against human periodontal ligament cells (PDLCs) revealed the biocompatibility of the nanofiber. The SEM and confocal laser scanning microscope (CLSM) imaging (Figure 8) showed that the cells grew on nanofibers along with the axis of aligned nanofibers.
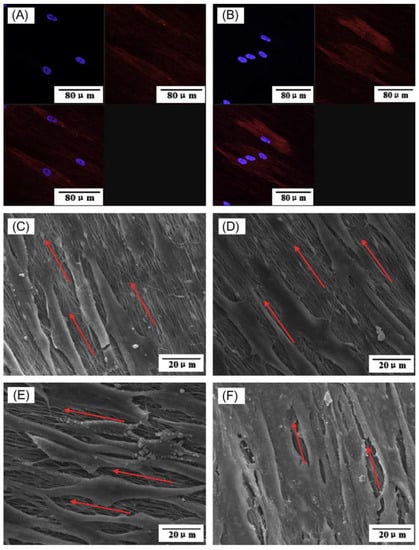
Figure 8.
Morphology of PDLCs on the fabricated nanofibers. (A) CLSM image of cells on pristine CNFs, (B) CLSM image of cells on β-TCP-decorated CNFs, (C) SEM image of cells on pristine CNFs after 1 day of cell seeding, (D) SEM image of cells on pristine β-TCP-decorated CNFs after 1 day of cell seeding, (E) SEM image of cells on pristine CNFs after 7 days of cell seeding, and (F) SEM image of cells on pristine β-TCP-decorated CNFs after 7 days of cell seeding. Reproduced with permission from Ref. [112].
8. Conclusions and Future Remarks
Bone tissue engineering is an alternative approach aiming to improve the healing outcomes of the conventional bone regeneration methods. In this scenario, the combination of nanomaterials, especially nanofibers, with bioceramic concepts can be promising to propose a sophisticated scaffold for bone tissue engineering. The current article reviews and discusses the application of mineralized nanofibers for bone tissue engineering applications, their structures, properties, and fabrication methods over the past few years. Despite their beneficial properties, mineralized nanofibers suffer from their 2D structure that limits translating them to the clinic. Alternatively, the combination of the mineralized nanofibers with 3D scaffolds as the nanocomposite can tailor them for clinical applications. Typical fabrication techniques for 3D scaffold preparations that have the potential to be combined with the mineralization process include rapid prototyping, hydrogels, thermal-induced phase separation, gas foaming, and particle leaching. From the authors’ point of view, the combination of an innovative and emerging manufacturing concept (electrospinning) with the partially long-lasting bone regeneration concept (bioceramics and mineralization) can provide scaffolds with the highest similarity to native bone structure and, subsequently, the highest healing/regeneration outcomes. However, more precise studies must be conducted to translate the proposed structure to the clinic.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [PubMed]
- Samadian, H.; Khastar, H.; Ehterami, A.; Salehi, M. Bioengineered 3D nanocomposite based on gold nanoparticles and gelatin nanofibers for bone regeneration: In vitro and in vivo study. Sci. Rep. 2021, 11, 13877. [Google Scholar] [CrossRef] [PubMed]
- Nekounam, H.; Gholizadeh, S.; Allahyari, Z.; Samadian, H.; Nazeri, N.; Shokrgozar, M.A.; Faridi-Majidi, R. Electroconductive Scaffolds for Tissue Regeneration: Current opportunities, pitfalls, and potential solutions. Mater. Res. Bull. 2021, 134, 111083. [Google Scholar] [CrossRef]
- Nazarnezhada, S.; Abbaszadeh-Goudarzi, G.; Samadian, H.; Khaksari, M.; Ghatar, J.M.; Khastar, H.; Rezaei, N.; Mousavi, S.R.; Shirian, S.; Salehi, M. Alginate hydrogel containing hydrogen sulfide as the functional wound dressing material: In vitro and in vivo study. Int. J. Biol. Macromol. 2020, 164, 3323–3331. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Mohammad-Rezaei, R.; Massoumi, B.; Abbasian, M.; Rezaei, A.; Samadian, H.; Jaymand, M. Conducting polymer-based electrically conductive adhesive materials: Design, fabrication, properties, and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 10947–10961. [Google Scholar] [CrossRef]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current concepts in scaffolding for bone tissue engineering. Arch. Bone Jt. Surg. 2018, 6, 90. [Google Scholar]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- De Witte, T.-M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef]
- Winkler, T.; Sass, F.; Duda, G.; Schmidt-Bleek, K. A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: The unsolved challenge. Bone Jt. Res. 2018, 7, 232–243. [Google Scholar] [CrossRef]
- Chen, P.; Liu, L.; Pan, J.; Mei, J.; Li, C.; Zheng, Y. Biomimetic composite scaffold of hydroxyapatite/gelatin-chitosan core-shell nanofibers for bone tissue engineering. Mater. Sci. Eng. C 2019, 97, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Udomluck, N.; Lee, H.; Hong, S.; Lee, S.-H.; Park, H. Surface functionalization of dual growth factor on hydroxyapatite-coated nanofibers for bone tissue engineering. Appl. Surf. Sci. 2020, 520, 146311. [Google Scholar] [CrossRef]
- Samadian, H.; Zakariaee, S.S.; Adabi, M.; Mobasheri, H.; Azami, M.; Faridi-Majidi, R. Effective parameters on conductivity of mineralized carbon nanofibers: An investigation using artificial neural networks. RSC Adv. 2016, 6, 111908–111918. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, W.; Chou, J.; Wen, S.; Sun, Y.; Zhang, H. Electrospun nanosilicates-based organic/inorganic nanofibers for potential bone tissue engineering. Colloids Surf. B Biointerfaces 2018, 172, 90–97. [Google Scholar] [CrossRef]
- Udomluck, N.; Koh, W.-G.; Lim, D.-J.; Park, H. Recent developments in nanofiber fabrication and modification for bone tissue engineering. Int. J. Mol. Sci. 2020, 21, 99. [Google Scholar] [CrossRef] [PubMed]
- Samadian, H.; Mobasheri, H.; Hasanpour, S.; Faridi-Majid, R. Needleless electrospinning system, an efficient platform to fabricate carbon nanofibers. J. Nano Res. 2017, 50, 78–89. [Google Scholar] [CrossRef]
- Iacob, A.-T.; Drăgan, M.; Ionescu, O.-M.; Profire, L.; Ficai, A.; Andronescu, E.; Confederat, L.G.; Lupașcu, D. An overview of biopolymeric electrospun nanofibers based on polysaccharides for wound healing management. Pharmaceutics 2020, 12, 983. [Google Scholar] [CrossRef]
- Maghsoudlou, M.A.; Nassireslami, E.; Saber-Samandari, S.; Khandan, A. Bone regeneration using bio-nanocomposite tissue reinforced with bioactive nanoparticles for femoral defect applications in medicine. Avicenna J. Med. Biotechnol. 2020, 12, 68. [Google Scholar]
- Christy, P.N.; Basha, S.K.; Kumari, V.S.; Bashir, A.; Maaza, M.; Kaviyarasu, K.; Arasu, M.V.; Al-Dhabi, N.A.; Ignacimuthu, S. Biopolymeric nanocomposite scaffolds for bone tissue engineering applications–A review. J. Drug Deliv. Sci. Technol. 2020, 55, 101452. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Ahmad, S.A.; Chaudhary, N.; Hoda, M.N.; Nayak, A.K. Biodegradable polymer matrix nanocomposites for bone tissue engineering. In Applications of Nanocomposite Materials in Orthopedics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–37. [Google Scholar]
- Nekounam, H.; Kandi, M.R.; Shaterabadi, D.; Samadian, H.; Mahmoodi, N.; Hasanzadeh, E.; Faridi-Majidi, R. Silica nanoparticles-incorporated carbon nanofibers as bioactive biomaterial for bone tissue engineering. Diam. Relat. Mater. 2021, 115, 108320. [Google Scholar] [CrossRef]
- Yadav, D.; Amini, F.; Ehrmann, A. Recent advances in carbon nanofibers and their applications—A review. Eur. Polym. J. 2020, 138, 109963. [Google Scholar] [CrossRef]
- Abd El-Aziz, A.M.; El Backly, R.M.; Taha, N.A.; El-Maghraby, A.; Kandil, S.H. Preparation and characterization of carbon nanofibrous/hydroxyapatite sheets for bone tissue engineering. Mater. Sci. Eng. C 2017, 76, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Haniu, H.; Kim, Y.A.; Saito, N. The use of electrospun organic and carbon nanofibers in bone regeneration. Nanomaterials 2020, 10, 562. [Google Scholar] [CrossRef] [PubMed]
- Reznikov, N.; Shahar, R.; Weiner, S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014, 10, 3815–3826. [Google Scholar] [CrossRef]
- Fratzl, P.; Weinkamer, R. Hierarchical structure and repair of bone: Deformation, remodelling, healing. In Self Healing Materials; Springer: Berlin/Heidelberg, Germany, 2007; pp. 323–335. [Google Scholar]
- Rho, J.-Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- McCreadie, B.R.; Goulet, R.W.; Feldkamp, L.A.; Goldstein, S.A. Hierarchical structure of bone and micro-computed tomography. In Noninvasive Assessment of Trabecular Bone Architecture and the Competence of Bone; Springer: Berlin/Heidelberg, Germany, 2001; pp. 67–83. [Google Scholar]
- Cui, F.Z.; Ge, J. New observations of the hierarchical structure of human enamel, from nanoscale to microscale. J. Tissue Eng. Regen. Med. 2007, 1, 185–191. [Google Scholar] [CrossRef]
- Bini, F.; Pica, A.; Marinozzi, A.; Marinozzi, F. Percolation networks inside 3D model of the mineralized collagen fibril. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Bini, F.; Pica, A.; Marinozzi, A.; Marinozzi, F. 3D random walk model of diffusion in human Hypo-and Hyper-mineralized collagen fibrils. J. Biomech. 2021, 125, 110586. [Google Scholar] [CrossRef]
- Goto, T.; Sasaki, K. Effects of trace elements in fish bones on crystal characteristics of hydroxyapatite obtained by calcination. Ceram. Int. 2014, 40, 10777–10785. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Annabi, N.; Nikkhah, M.; Bae, H.; Binan, L.; Park, S.; Kang, Y.; Yang, Y.; Khademhosseini, A. Vascularized bone tissue engineering: Approaches for potential improvement. Tissue Eng. Part B Rev. 2012, 18, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Fratzl, P. Collagen: Structure and mechanics, an introduction. In Collagen; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–13. [Google Scholar]
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F.Z.; Watari, F. Nanostructured scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 2424–2435. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef]
- Faingold, A.; Cohen, S.R.; Reznikov, N.; Wagner, H.D. Osteonal lamellae elementary units: Lamellar microstructure, curvature and mechanical properties. Acta Biomater. 2013, 9, 5956–5962. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone mechanical properties in healthy and diseased states. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef]
- Weiner, S.; Wagner, H.D. The material bone: Structure-mechanical function relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Sharir, A.; Barak, M.M.; Shahar, R. Whole bone mechanics and mechanical testing. Vet. J. 2008, 177, 8–17. [Google Scholar] [CrossRef]
- Hang, F.; Barber, A.H. Nano-mechanical properties of individual mineralized collagen fibrils from bone tissue. J. R. Soc. Interface 2011, 8, 500–505. [Google Scholar] [CrossRef]
- Currey, J. Measurement of the mechanical properties of bone: A recent history. Clin. Orthop. Relat. Res. 2009, 467, 1948–1954. [Google Scholar] [CrossRef]
- Yerramshetty, J.S.; Akkus, O. The associations between mineral crystallinity and the mechanical properties of human cortical bone. Bone 2008, 42, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Saber-Samandari, S.; Gross, K.A. Micromechanical properties of single crystal hydroxyapatite by nanoindentation. Acta Biomater. 2009, 5, 2206–2212. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shi, Y.; Zhang, X.; Ma, J. 3D printed hydroxyapatite composite scaffolds with enhanced mechanical properties. Ceram. Int. 2019, 45, 10991–10996. [Google Scholar] [CrossRef]
- Saxena, V.; Shukla, I.; Pandey, L.M. Hydroxyapatite: An inorganic ceramic for biomedical applications. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 205–249. [Google Scholar]
- Buehler, M. Hierarchical nanomechanics of collagen fibrils: Atomistic and molecular modeling. In Collagen; Springer: Berlin/Heidelberg, Germany, 2008; pp. 175–247. [Google Scholar]
- Seeman, E.; Delmas, P.D. Bone quality—The material and structural basis of bone strength and fragility. N. Engl. J. Med. 2006, 354, 2250–2261. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Nishida, A.; Koga, A.; Ikeda, S.; Shiraishi, A.; Uetani, M.; Hayashi, K.; Nakamura, T. Contribution of trabecular and cortical components to the mechanical properties of bone and their regulating parameters. Bone 2002, 31, 351–358. [Google Scholar] [CrossRef]
- Paul, C.D. Interface Property of Collagen and Hydroxyapatite in Bone. Bachelor’s Thesis, University of Arkansas, Fayetteville, AR, USA, 2015. [Google Scholar]
- Paul, C. Interface property of collagen and hydroxyapatite in bone and developing bioinspired materials. Inq. Univ. Ark. Undergrad. Res. J. 2015, 19, 10. [Google Scholar]
- Stock, S.R. The mineral–collagen interface in bone. Calcif. Tissue Int. 2015, 97, 262–280. [Google Scholar] [CrossRef]
- Robling, A.G.; Stout, S.D. Morphology of the drifting osteon. Cells Tissues Organs 1999, 164, 192–204. [Google Scholar] [CrossRef]
- Currey, J. The structure and mechanical properties of bone. In Bioceramics and Their Clinical Applications; Elsevier: Amsterdam, The Netherlands, 2008; pp. 3–27. [Google Scholar]
- Bartel, D.L.; Davy, D.T. Orthopaedic Biomechanics: Mechanics and Design in Musculoskeletal Systems; Prentice Hall: Hoboken, NJ, USA, 2006. [Google Scholar]
- Karpiński, R.; Zubrzycki, J. Structural analysis of articular cartilage of the hip joint using finite element method. Adv. Sci. Technol. Res. J. 2016, 10, 240–246. [Google Scholar] [CrossRef]
- Currey, J.D. Bones: Structure and Mechanics; Princeton University Press: Princeton, NJ, USA, 2006. [Google Scholar]
- Liu, Y.; Luo, D.; Wang, T. Hierarchical structures of bone and bioinspired bone tissue engineering. Small 2016, 12, 4611–4632. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Henrich, D. Focus on bone healing: New strategies for improvement of bone healing. Eur. J. Trauma Emerg. Surg. 2020, 46, 229. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, M.S.; Chen, J.; Vaziri, A.; Rodriguez, E.K.; Nazarian, A. Bone fracture healing in mechanobiological modeling: A review of principles and methods. Bone Rep. 2017, 6, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, M.; Mignemi, N.A.; Nyman, J.S.; Duvall, C.L.; Schwartz, H.S.; Okawa, A.; Yoshii, T.; Bhattacharjee, G.; Zhao, C.; Bible, J.E. Fibrinolysis is essential for fracture repair and prevention of heterotopic ossification. J. Clin. Investig. 2015, 125, 3117–3131. [Google Scholar] [CrossRef]
- Bahney, C.S.; Zondervan, R.L.; Allison, P.; Theologis, A.; Ashley, J.W.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular biology of fracture healing. J. Orthop. Res. 2019, 37, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45. [Google Scholar] [CrossRef]
- Einhorn, T.A. The cell and molecular biology of fracture healing. Clin. Orthop. Relat. Res. 1998, 355, S7–S21. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A. Overview of the fracture healing cascade. Injury 2005, 36, S5–S7. [Google Scholar] [CrossRef]
- Hausman, M.; Schaffler, M.; Majeska, R. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone 2001, 29, 560–564. [Google Scholar] [CrossRef]
- Kurdy, N.; Weiss, J.; Bate, A. Endothelial stimulating angiogenic factor in early fracture healing. Injury 1996, 27, 143–145. [Google Scholar] [CrossRef]
- Lee, F.Y.I.; Choi, Y.W.; Behrens, F.F.; DeFouw, D.O.; Einhorn, T.A. Programmed removal of chondrocytes during endochondral fracture healing. J. Orthop. Res. 1998, 16, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Gerstenfeld, L.C.; Cho, T.J.; Kon, T.; Aizawa, T.; Tsay, A.; Fitch, J.; Barnes, G.; Graves, D.; Einhorn, T. Impaired fracture healing in the absence of TNF-α signaling: The role of TNF-α in endochondral cartilage resorption. J. Bone Miner. Res. 2003, 18, 1584–1592. [Google Scholar] [CrossRef]
- Pivonka, P.; Dunstan, C.R. Role of mathematical modeling in bone fracture healing. BoneKEy Rep. 2012, 1, 221. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, M.; Henke, T.; Claes, L.; Augat, P. Revascularisation during fracture healing with soft tissue injury. Arch. Orthop. Trauma Surg. 2008, 128, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Holstein, J.H.; Karabin-Kehl, B.; Scheuer, C.; Garcia, P.; Histing, T.; Meier, C.; Benninger, E.; Menger, M.D.; Pohlemann, T. Endostatin inhibits callus remodeling during fracture healing in mice. J. Orthop. Res. 2013, 31, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Aydin, A.; Memisoglu, K.; Cengiz, A.; Atmaca, H.; Muezzinoglu, B.; Muezzinoglu, U.S. Effects of botulinum toxin A on fracture healing in rats: An experimental study. J. Orthop. Sci. 2012, 17, 796–801. [Google Scholar] [CrossRef]
- Isaksson, H.; Comas, O.; van Donkelaar, C.C.; Mediavilla, J.; Wilson, W.; Huiskes, R.; Ito, K. Bone regeneration during distraction osteogenesis: Mechano-regulation by shear strain and fluid velocity. J. Biomech. 2007, 40, 2002–2011. [Google Scholar] [CrossRef]
- Mochi, F.; Scatena, E.; Rodriguez, D.; Ginebra, M.-P.; Del Gaudio, C. Scaffold-based bone tissue engineering in microgravity: Potential, concerns and implications. npj Microgravity 2022, 8, 45. [Google Scholar] [CrossRef]
- Murphy, C.A.; Costa, J.B.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L.; Collins, M.N. Biopolymers and polymers in the search of alternative treatments for meniscal regeneration: State of the art and future trends. Appl. Mater. Today 2018, 12, 51–71. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef]
- Sofi, H.S.; Ashraf, R.; Beigh, M.A.; Sheikh, F.A. Scaffolds fabricated from natural polymers/composites by electrospinning for bone tissue regeneration. Cut.-Edge Enabling Technol. Regen. Med. 2018, 1078, 49–78. [Google Scholar]
- Pedersen, D.D.; Kim, S.; Wagner, W.R. Biodegradable polyurethane scaffolds in regenerative medicine: Clinical translation review. J. Biomed. Mater. Res. Part A 2022, 110, 1460–1487. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Yang, F.; Zheng, Y.; Liu, Y.; Zhou, Y. Research status of biodegradable metals designed for oral and maxillofacial applications: A review. Bioact. Mater. 2021, 6, 4186–4208. [Google Scholar] [CrossRef]
- Ginebra, M.-P.; Espanol, M.; Maazouz, Y.; Bergez, V.; Pastorino, D. Bioceramics and bone healing. EFORT Open Rev. 2018, 3, 173. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite–-Past, present, and future in bone regeneration. Bone Tissue Regen. Insights 2016, 7. [Google Scholar] [CrossRef]
- Canillas, M.; Pena, P.; Antonio, H.; Rodríguez, M.A. Calcium phosphates for biomedical applications. Boletín Soc. Española Cerámica Vidr. 2017, 56, 91–112. [Google Scholar] [CrossRef]
- Pazarçeviren, A.E.; Tezcaner, A.; Keskin, D.; Kolukısa, S.T.; Sürdem, S.; Evis, Z. Boron-doped biphasic hydroxyapatite/β-tricalcium phosphate for bone tissue engineering. Biol. Trace Elem. Res. 2021, 199, 968–980. [Google Scholar] [CrossRef]
- Cai, Q.; Subramani, K.; Mathew, R.T.; Yang, X. Chapter 18-Carbon nanomaterials for implant dentistry and bone tissue engineering. In Nanobiomaterials in Clinical Dentistry, 2nd ed.; Subramani, K., Ahmed, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 429–468. [Google Scholar]
- Keshavarz, S.; Okoro, O.V.; Hamidi, M.; Derakhshankhah, H.; Azizi, M.; Mohammad Nabavi, S.; Gholizadeh, S.; Amini, S.M.; Shavandi, A.; Luque, R.; et al. Synthesis, surface modifications, and biomedical applications of carbon nanofibers: Electrospun vs vapor-grown carbon nanofibers. Coord. Chem. Rev. 2022, 472, 214770. [Google Scholar] [CrossRef]
- Samadian, H.; Mobasheri, H.; Azami, M.; Faridi-Majidi, R. Osteoconductive and electroactive carbon nanofibers/hydroxyapatite nanocomposite tailored for bone tissue engineering: In vitro and in vivo studies. Sci. Rep. 2020, 10, 14853. [Google Scholar] [CrossRef]
- Nekounam, H.; Allahyari, Z.; Gholizadeh, S.; Mirzaei, E.; Shokrgozar, M.A.; Faridi-Majidi, R. Simple and robust fabrication and characterization of conductive carbonized nanofibers loaded with gold nanoparticles for bone tissue engineering applications. Mater. Sci. Eng. C 2020, 117, 111226. [Google Scholar] [CrossRef] [PubMed]
- Samadian, H.; Mobasheri, H.; Hasanpour, S.; Ai, J.; Azamie, M.; Faridi-Majidi, R. Electro-conductive carbon nanofibers as the promising interfacial biomaterials for bone tissue engineering. J. Mol. Liq. 2020, 298, 112021. [Google Scholar] [CrossRef]
- Nekounam, H.; Samadian, H.; Bonakdar, S.; Asghari, F.; Shokrgozar, M.A.; Majidi, R.F. Electro-conductive carbon nanofibers containing ferrous sulfate for bone tissue engineering. Life Sci. 2021, 282, 119602. [Google Scholar] [CrossRef]
- Xu, A.-W.; Ma, Y.; Cölfen, H. Biomimetic mineralization. J. Mater. Chem. 2007, 17, 415–449. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, R.; Soleimani, K.; Derakhshankhah, H.; Haghshenas, B.; Rezaei, A.; Massoumi, B.; Farnudiyan-Habibi, A.; Samadian, H.; Jaymand, M. Multi-stimuli-responsive magnetic hydrogel based on Tragacanth gum as a de novo nanosystem for targeted chemo/hyperthermia treatment of cancer. J. Mater. Res. 2021, 36, 858–869. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Q.; Liu, X.; Liu, H. Biomimetic synthesis and characterization of carbon nanofiber/hydroxyapatite composite scaffolds. Carbon 2013, 51, 335–345. [Google Scholar] [CrossRef]
- Luickx, N.; Van Den Vreken, N.; Segaert, J.; Declercq, H.; Cornelissen, M.; Verbeeck, R. Optimization of the time efficient calcium phosphate coating on electrospun poly (d, l-lactide). J. Biomed. Mater. Res. Part A 2015, 103, 2720–2730. [Google Scholar] [CrossRef]
- Luickx, N.; Van den Vreken, N.; D’Oosterlinck, W.; Van der Schueren, L.; Declercq, H.; De Clerck, K.; Cornelissen, M.; Verbeeck, R. Optimization of the activation and nucleation steps in the precipitation of a calcium phosphate primer layer on electrospun poly (ɛ-caprolactone). J. Biomed. Mater. Res. Part A 2015, 103, 511–524. [Google Scholar] [CrossRef]
- Kothapalli, C.R.; Shaw, M.T.; Olson, J.R.; Wei, M. Fabrication of novel calcium phosphate/poly (lactic acid) fiber composites. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2008, 84, 89–97. [Google Scholar] [CrossRef]
- Itoh, S.; Yamaguchi, I.; Suzuki, M.; Ichinose, S.; Takakuda, K.; Kobayashi, H.; Shinomiya, K.; Tanaka, J. Hydroxyapatite-coated tendon chitosan tubes with adsorbed laminin peptides facilitate nerve regeneration in vivo. Brain Res. 2003, 993, 111–123. [Google Scholar] [CrossRef]
- Cui, W.; Li, X.; Zhou, S.; Weng, J. In situ growth of hydroxyapatite within electrospun poly (DL-lactide) fibers. J. Biomed. Mater. Res. Part A 2007, 82, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-S.; Lee, S.G.; Im, S.S.; Kim, S.H.; Joo, Y.L. Silica nanofibers from electrospinning/sol-gel process. J. Mater. Sci. Lett. 2003, 22, 891–893. [Google Scholar] [CrossRef]
- Yang, G.; Yan, W.; Wang, J.; Yang, H. Fabrication and characterization of CoTiO3 nanofibers by sol–gel assisted electrospinning. Mater. Lett. 2014, 122, 117–120. [Google Scholar] [CrossRef]
- Esposito, S. “Traditional” sol-gel chemistry as a powerful tool for the preparation of supported metal and metal oxide catalysts. Materials 2019, 12, 668. [Google Scholar] [CrossRef] [PubMed]
- Roy, R. Ceramics by the solution-sol-gel route. Science 1987, 238, 1664–1669. [Google Scholar] [CrossRef]
- Hou, J.; Wang, Y.; Xue, H.; Dou, Y. Biomimetic growth of hydroxyapatite on electrospun CA/PVP core–shell nanofiber membranes. Polymers 2018, 10, 1032. [Google Scholar] [CrossRef]
- Chang, W.; Mu, X.; Zhu, X.; Ma, G.; Li, C.; Xu, F.; Nie, J. Biomimetic composite scaffolds based mineralization of hydroxyapatite on electrospun calcium-containing poly (vinyl alcohol) nanofibers. Mater. Sci. Eng. C 2013, 33, 4369–4376. [Google Scholar] [CrossRef]
- Haider, M.K.; Sun, L.; Ullah, A.; Ullah, S.; Suzuki, Y.; Park, S.; Kato, Y.; Tamada, Y.; Kim, I.S. Polyacrylonitrile/Carbon Black nanoparticle/Nano-Hydroxyapatite (PAN/nCB/HA) composite nanofibrous matrix as a potential biomaterial scaffold for bone regenerative applications. Mater. Today Commun. 2021, 27, 102259. [Google Scholar] [CrossRef]
- Liu, H.; Cai, Q.; Lian, P.; Fang, Z.; Duan, S.; Yang, X.; Deng, X.; Ryu, S. β-Tricalcium phosphate nanoparticles adhered carbon nanofibrous membrane for human osteoblasts cell culture. Mater. Lett. 2010, 64, 725–728. [Google Scholar] [CrossRef]
- Han, B.; Zhang, X.; Liu, H.; Deng, X.; Cai, Q.; Jia, X.; Yang, X.; Wei, Y.; Li, G. Improved bioactivity of PAN-based carbon nanofibers decorated with bioglass nanoparticles. J. Biomater. Sci. Polym. Ed. 2014, 25, 341–353. [Google Scholar] [CrossRef]
- Liu, H.; Cai, Q.; Lian, P.; Fang, Z.; Duan, S.; Ryu, S.; Yang, X.; Deng, X. The biological properties of carbon nanofibers decorated with β-tricalcium phosphate nanoparticles. Carbon 2010, 48, 2266–2272. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).