Fluoride Evaporation of Low-Fluoride CaF2-CaO-Al2O3-MgO-TiO2-(Na2O-K2O) Slag for Electroslag Remelting
Abstract
1. Introduction
2. Experiment
2.1. Materials and Sample Preparation
2.2. Thermodynamic Calculations
2.3. XRD and FTIR Spectra Measurement
2.4. Simultaneous Thermal Analysis Measurement
3. Results and Discussion
3.1. Thermodynamic Analysis
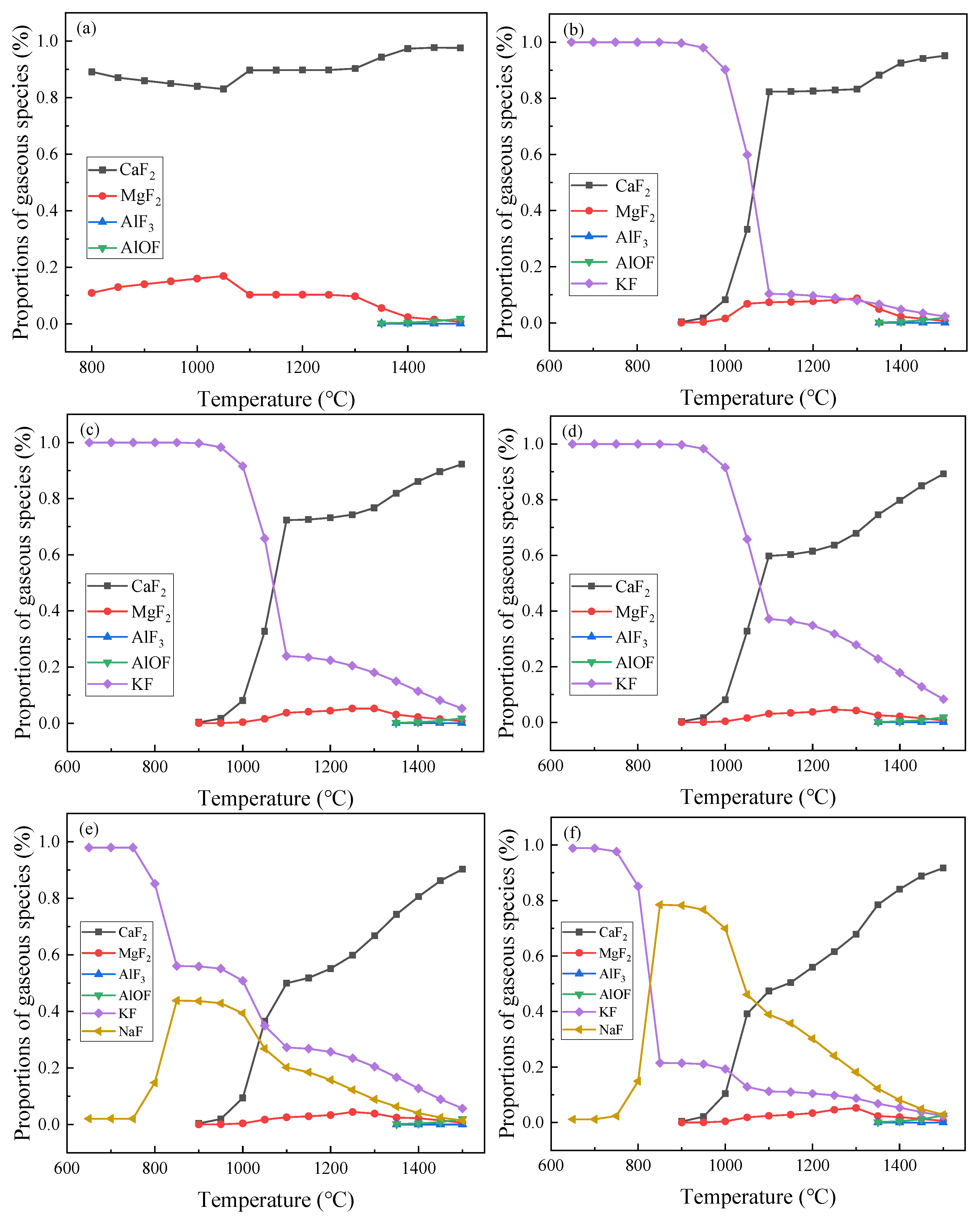
3.1.1. Proportion of Fluorinated Gas Evaporation
3.1.2. Vapor Pressure
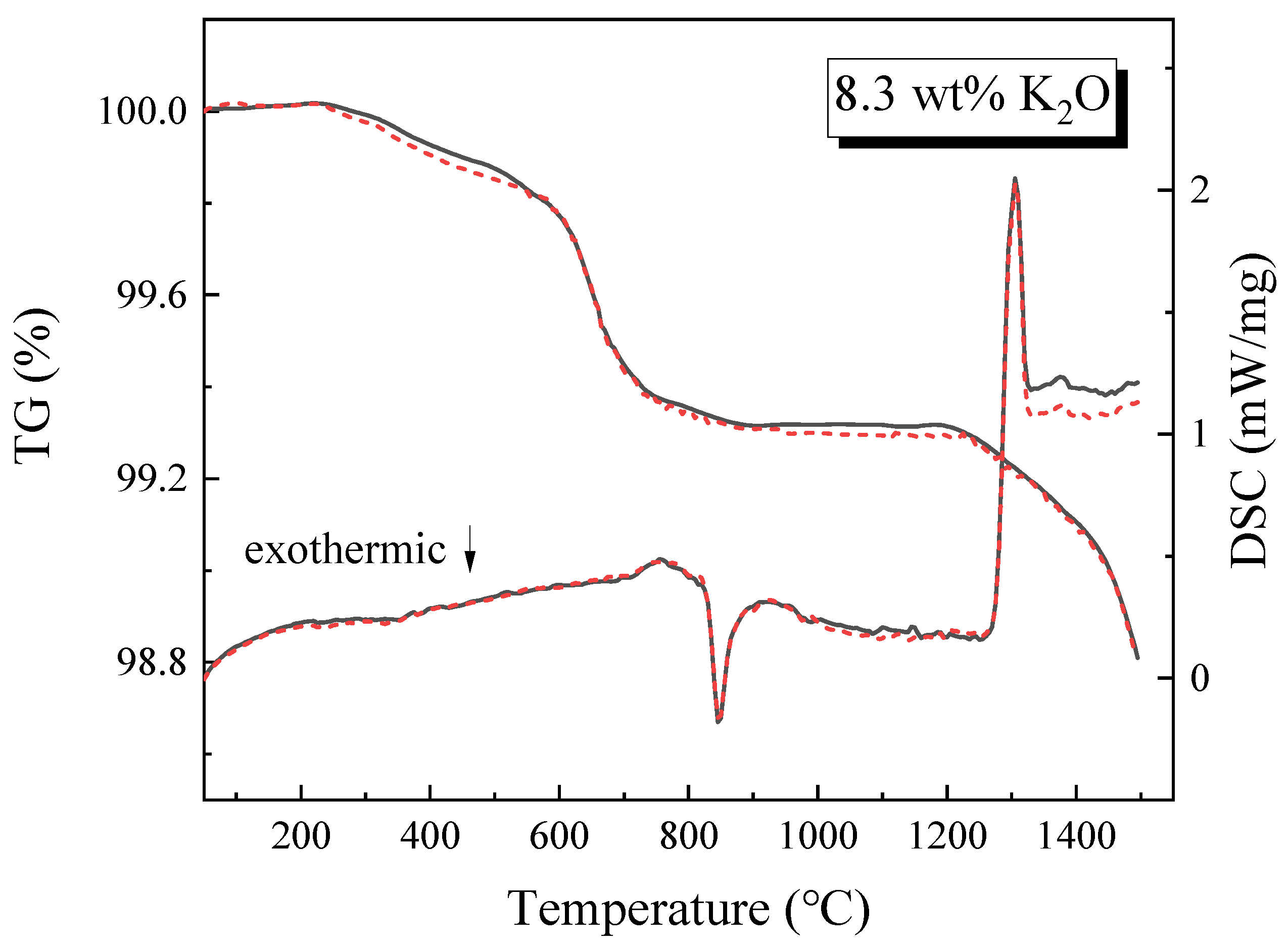
3.2. TG and DSC Analysis of the Slag
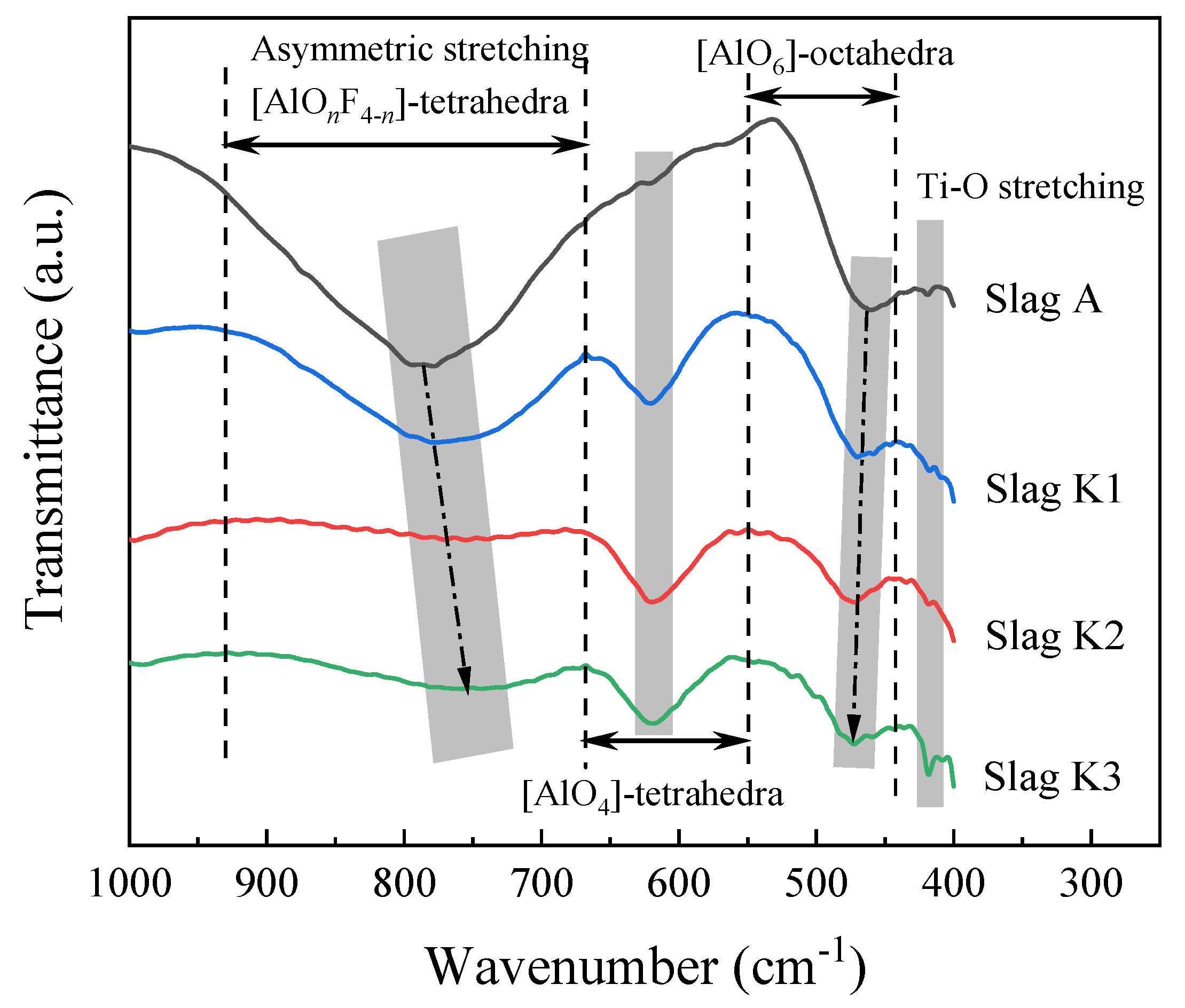
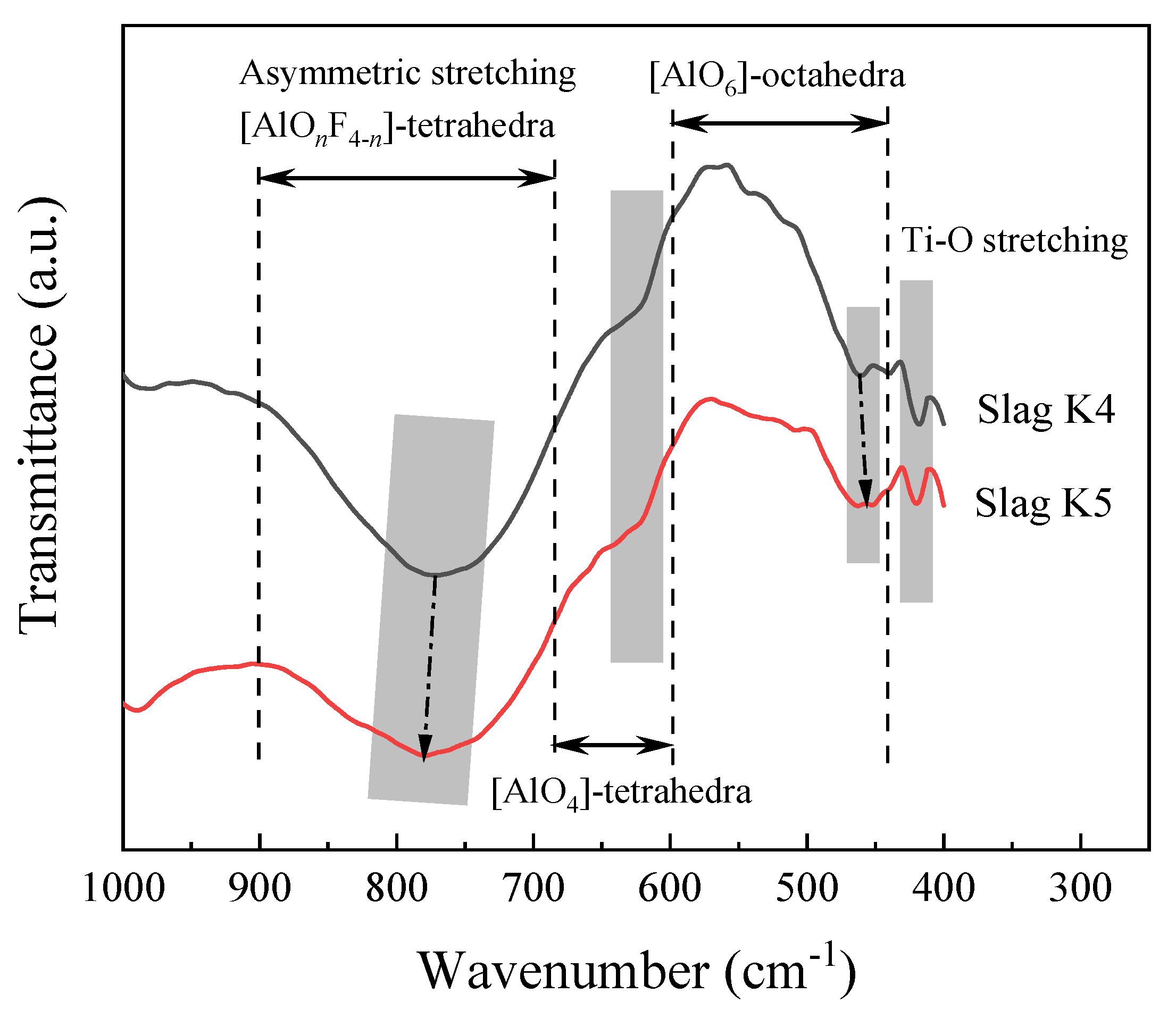
3.3. FTIR Spectra of the Slag Sample
4. Conclusions
- When the slag contains both K2O and Na2O, the main evaporating substances are CaF2, KF, and NaF. In comparison, MgF2, AlF3, and AlOF seldom ever evaporate. KF and NaF begin to evaporate at 650 °C. NaF increases and subsequently decreases as the temperature rises, while KF decreases as temperature rises. At 900 °C, CaF2 tends to evaporate, and the evaporation intensifies with the increase of temperature. At the beginning of the reaction, KF dominates absolutely, while CaF2 dominates when it exceeds 1050 °C.
- The vapor pressure of KF is stronger than that of CaF2 at 1500 °C. When K2O and Na2O are added to the residue sample at the same time, the evaporation ability of KF is stronger than CaF2 and NaF.
- Evaporation increases from 0.76% to 1.21% when K2O content rises from 0% to 8.3 wt%. The evaporation rates of samples K4 and K5 are 1.48% and 1.32%, respectively. Under the premise that the total amount of K2O and Na2O added is equal, when the amount of K2O added is greater than Na2O, the evaporation rate is relatively large, indicating that K2O has a significant influence on evaporation.
- FTIR results show that with the addition of K2O, the (AlOnF4−n)-tetrahedral complexes, (AlO4)-tetrahedra, and (AlO6)-octahedra network structures are depolymerized; this has little effect on the stretching vibration of Ti-O. Comparing the effects of Na2O and K2O addition under the same conditions, it is found that the slag with higher K2O content can better depolymerize the (AlOnF4−n)-tetrahedral complexes and (AlO4)-tetrahedra network structures, and has little effect on the (AlO6)-octahedra and Ti-O stretching vibration.
- Although the addition of a small amount of alkali metals can promote the partial evaporation of slag, it can also change the melting characteristics of the ESR slag system, including viscosity and melting temperature, which are conducive to the melting of slag system. The applicability of these characteristics in industry should be considered comprehensively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weber, V.; Jardy, A.; Dussoubs, B.; Ablitzer, D.; Rybéron, S.; Schmitt, V.; Hans, S.; Poisson, H. A Comprehensive Model of the Electroslag Remelting Process: Description and Validation. Metall. Mater. Trans. B 2009, 40, 271–280. [Google Scholar] [CrossRef]
- Shi, C.; Huang, Y.; Zhang, J.; Li, J.; Zheng, X. Review on Desulfurization in Electroslag Remelting. Int. J. Miner. Metall. Mater. 2021, 28, 18–29. [Google Scholar] [CrossRef]
- Kharicha, A.; Karimi-Sibaki, E.; Wu, M.; Ludwig, A.; Bohacek, J. Review on Modeling and Simulation of Electroslag Remelting. Steel Res. Int. 2017, 89, 1700100. [Google Scholar] [CrossRef]
- Vaish, A.K.; Iyer, G.V.R.; De, P.K.; Lakra, B.A.; Chakrabarti, A.K.; Ramachandrarao, P. Electroslag Remelting-Its Status, Mechanism and Refining Aspects in the Production of Quality Steels. J. Metall. Mater. Sci. 2000, 42, 11–29. [Google Scholar]
- Maity, S.K.; Ballal, N.B.; Goldhahn, G.; Kawalla, R. Development of Ultrahigh Strength Low Alloy Steel through Electroslag Refining Process. ISIJ Int. 2009, 49, 902–910. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Li, G.; Yuan, C.; Lu, R.; Li, B. Investigation on the Structure, Fluoride Vaporization and Crystallization Behavior of CaF2-CaO-Al2O3-(SiO2) Slag for Electroslag Remelting. J. Therm. Anal. Calorim. 2020, 139, 923–931. [Google Scholar] [CrossRef]
- Zheng, D.; Li, J.; Shi, C.; Ju, J. Crystallization Characteristics and In-Mold Performance of Electroslag Remelting-Type TiO2-Bearing Slag. Metall. Mater. Trans. B 2019, 50, 1148–1160. [Google Scholar] [CrossRef]
- Roshchin, V.E.; Malkov, N.V.; Roshchin, A.V. Usage of Worked-Out Metallurgical Slag in Electroslag Remelting Processes. In Proceedings of the CIS Iron Steel Rev., Ninth International Conference on Slag, Solvents and Molten Salts MOLTEN12, Beijing, China, 27 May 2012. [Google Scholar]
- Liu, Y.; Zhang, Z.; Li, G.; Wu, Y.; Xu, D.; Li, B. Investigation of Fluoride Vaporization from CaF2-CaO-Al2O3 Slag for Vacuum Electroslag Remelting. Vacuum 2018, 158, 6–13. [Google Scholar] [CrossRef]
- Brandaleze, E.; Valentini, M.; Santini, L.; Benavidez, E. Study on Fluoride Evaporation from Casting Powders. J. Therm. Anal. Calorim. 2018, 133, 271–277. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, C.; Wan, X.; Li, J.; Zheng, D.; Li, J. Effect of SiO2 and B2O3 on Crystallization and Structure of CaF2-CaO-Al2O3-Based Slag for Electroslag Remelting of Ultra-Supercritical Rotor Steel. J. Iron Steel Res. Int. 2021, 28, 1530–1540. [Google Scholar] [CrossRef]
- Zaitsev, A.I.; Leites, A.V.; Lrtvina, A.D.; Mogutnov, B.M. Investigation of the Mould Powder Volatiles During Continuous Casting. Steel Res. 1994, 65, 368–374. [Google Scholar] [CrossRef]
- Shimizu, K.; Cramb, A.W. Fluoride Evaporation from CaF2-SiO2-CaO Slags and Mold Fluxes in Dry and Humid Atmospheres. High Temp. Mater. Process. 2003, 22, 237–246. [Google Scholar] [CrossRef]
- Persson, M.; Seetharaman, S.; Seetharaman, S. Kinetic Studies of Fluoride Evaporation from Slags. ISIJ Int. 2007, 47, 1711–1717. [Google Scholar] [CrossRef]
- Park, J.H.; Min, D.J.; Song, H.S. The Effect of CaF2 on the Viscosities and Structures of CaO-SiO2(-MgO)-CaF2 Slags. Metall. Mater. Trans. B 2002, 33, 723–729. [Google Scholar] [CrossRef]
- Chong, J.; Shen, Y.; Yang, P.; Tian, J.; Zhang, W.; Tang, X.; Du, X. Effects of B2O3 on Melting Characteristics and Temperature-Dependent Viscosity of High-Basicity CaO-SiO2-FeOx-MgO Slag. Materials 2020, 13, 1214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, W.; Wei, J.; Zhou, K. Melting and Heat Transfer Behavior of Fluorine-Free Mold Fluxes for Casting Medium Carbon Steels. ISIJ Int. 2015, 55, 821–829. [Google Scholar] [CrossRef]
- Shi, C.; Shin, S.H.; Zheng, D.; Cho, J.W.; Li, J. Development of Low-Fluoride Slag for Electroslag Remelting: Role of Li2O on the Viscosity and Structure of the Slag. Metall. Mater. Trans. B 2016, 47, 3343–3349. [Google Scholar] [CrossRef]
- Zhang, Z.; Sridhar, S.; Cho, J.W. An Investigation of the Evaporation of B2O3 and Na2O in F-Free Mold Slags. ISIJ Int. 2011, 51, 80–87. [Google Scholar] [CrossRef]
- Sukenaga, S.; Haruki, S.; Nomoto, Y.; Saito, N.; Nakashima, K. Density and Surface Tension of CaO-SiO2-Al2O3-R2O (R = Li, Na, K) Melts. ISIJ Int. 2011, 51, 1285–1289. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, G.; Chou, K.C.; Fan, D. Mixed Alkali Effect in Viscosity of CaO-SiO2-Al2O3-R2O Melts. Metall. Mater. Trans. B 2020, 51, 985–1002. [Google Scholar] [CrossRef]
- Chang, Z.; Jiao, K.; Ning, X.; Zhang, J. Novel Approach to Studying Influences of Na2O and K2O Additions on Viscosity and Thermodynamic Properties of BF Slags. Metall. Mater. Trans. B 2019, 50, 1399–1406. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, W.; Jiao, S.; Chou, K.C. Influences of Na2O and K2O Additions on Electrical Conductivity of CaO-SiO2-(Al2O3) Melts. ISIJ Int. 2017, 57, 2091–2096. [Google Scholar] [CrossRef]
- Zheng, D.; Li, J.; Shi, C. Development of Low-fluoride Slag for Electroslag Remelting: Role of Li2O on the Crystallization and Evaporation of the Slag. ISIJ Int. 2020, 60, 840–847. [Google Scholar] [CrossRef]
- Ju, J.; Yang, K.; Gu, Y.; He, K. Effect of Na2O on Viscosity, Structure and Crystallization of CaF2-CaO-Al2O3-MgO-TiO2 Slag in Electroslag Remelting. Russ. J. Non-Ferr. Met. 2022, 63, 599–609. [Google Scholar] [CrossRef]
- Suk, M.O.; Park, J.H. Corrosion Behavior of Zirconia Refractory By CaO-SiO2-MgO-CaF2 Slag. J. Am. Ceram. Soc. 2009, 92, 717–723. [Google Scholar] [CrossRef]
- Mao, H.; Hu, H.; Ma, G. Contamination of Fluorine in CC Mould Powder to Environment and Countermeasures. Steelmaking 1999, 15, 41–45. [Google Scholar] [CrossRef]
- Wu, P.; Eriksson, G.; Pelton, A.D. Critical Evaluation and Optimization of the Thermodynamic Properties and Phase Diagrams of the CaO-FeO, CaO-MgO, CaO-MnO, FeO-MgO, FeO-MnO, and MgO-MnO Systems. J. Am. Ceram. Soc. 1993, 76, 2065–2075. [Google Scholar] [CrossRef]
- Zhang, L.; Zhai, B.; Wang, W.; Sohn, I. Effects of (CaO+BaO)/Al2O3 Ratio on the Melting, Crystallization, and Melt Structure of CaO-Al2O3-10 wt% SiO2-Based Mold Fluxes for Advanced High-Strength Steels. J. Sustain. Metall. 2021, 7, 559–568. [Google Scholar] [CrossRef]
- Ju, J.; Yang, K.; Zhu, Z.; Gu, Y.; Chang, L. Effect of CaF2 and CaO/Al2O3 on Viscosity and Structure of TiO2-Bearing Slag for Electroslag Remelting. J. Iron Steel Res. Int. 2021, 28, 1541–1550. [Google Scholar] [CrossRef]
- Park, J.H.; Min, D.J.; Song, H.S. Structural Investigation of CaO-Al2O3 and CaO-Al2O3-CaF2 Slags Via Fourier Transform Infrared Spectra. ISIJ Int. 2002, 42, 38–43. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.H.; Park, J.H.; Min, D.J. A Study on the Effect of Na2O on the Viscosity for Ironmaking Slags. Steel Res. Int. 2010, 81, 17–24. [Google Scholar] [CrossRef]
- Ju, J.; Ji, G.; Tang, C.; Yang, K.; Zhu, Z. The Effect of Li2O on the Evaporation and Structure of Low-Fluoride Slag for Vacuum Electroslag Remelting. Vacuum 2021, 183, 109920. [Google Scholar] [CrossRef]



| Slag | Pre-Experimental Composition (Designed) | Post-Experimental Composition (XRF) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CaF2 | CaO | Al2O3 | MgO | TiO2 | Na2O | K2O | CaF2 | CaO | Al2O3 | MgO | TiO2 | Na2O | K2O | |
| A | 30.0 | 30.0 | 30.0 | 2.0 | 8.0 | 0.0 | 0.0 | 29.8 | 31.2 | 29.5 | 1.8 | 7.7 | 0.0 | 0.0 |
| K1 | 30.0 | 28.5 | 28.5 | 2.0 | 8.0 | 0.0 | 3.0 | 29.7 | 29.8 | 29.2 | 1.7 | 7.3 | 0.0 | 2.3 |
| K2 | 30.0 | 27.0 | 27.0 | 2.0 | 8.0 | 0.0 | 6.0 | 27.9 | 29.9 | 27.3 | 1.8 | 7.8 | 0.0 | 5.3 |
| K3 | 30.0 | 25.5 | 25.5 | 2.0 | 8.0 | 0.0 | 9.0 | 26.8 | 28.9 | 26.2 | 1.9 | 7.9 | 0.0 | 8.3 |
| K4 | 30.0 | 25.5 | 25.5 | 2.0 | 8.0 | 3.0 | 6.0 | 25.9 | 29.5 | 26.5 | 1.9 | 7.3 | 3.2 | 5.7 |
| K5 | 30.0 | 25.5 | 25.5 | 2.0 | 8.0 | 6.0 | 3.0 | 25.6 | 29.7 | 27.3 | 1.7 | 7.2 | 6.3 | 2.2 |
| CaF2 | KF | NaF | |
|---|---|---|---|
| A | 3.86 × 10−4 | - | - |
| K1 | 3.89 × 10−4 | 4.75 × 10−2 | - |
| K2 | 3.90 × 10−4 | 6.18 × 10−2 | - |
| K3 | 3.92 × 10−4 | 6.21 × 10−2 | - |
| K4 | 3.92 × 10−4 | 4.87 × 10−2 | 1.15 × 10−2 |
| K5 | 3.93 × 10−4 | 3.09 × 10−2 | 2.76 × 10−2 |
| CaF2 | KF | |
|---|---|---|
| A | 3.86 × 10−4 | - |
| K1 | 3.89 × 10−4 | 4.75 × 10−2 |
| K2 | 3.90 × 10−4 | 6.18 × 10−2 |
| K3 | 3.92 × 10−4 | 6.21 × 10−2 |
| CaF2 | KF | NaF | |
|---|---|---|---|
| K4 | 3.92 × 10−4 | 4.87 × 10−2 | 1.15 × 10−2 |
| K5 | 3.93 × 10−4 | 3.09 × 10−2 | 2.76 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, B.; Gu, Y.; Ju, J.; He, K. Fluoride Evaporation of Low-Fluoride CaF2-CaO-Al2O3-MgO-TiO2-(Na2O-K2O) Slag for Electroslag Remelting. Materials 2023, 16, 2777. https://doi.org/10.3390/ma16072777
An B, Gu Y, Ju J, He K. Fluoride Evaporation of Low-Fluoride CaF2-CaO-Al2O3-MgO-TiO2-(Na2O-K2O) Slag for Electroslag Remelting. Materials. 2023; 16(7):2777. https://doi.org/10.3390/ma16072777
Chicago/Turabian StyleAn, Bo, Yue Gu, Jiantao Ju, and Kun He. 2023. "Fluoride Evaporation of Low-Fluoride CaF2-CaO-Al2O3-MgO-TiO2-(Na2O-K2O) Slag for Electroslag Remelting" Materials 16, no. 7: 2777. https://doi.org/10.3390/ma16072777
APA StyleAn, B., Gu, Y., Ju, J., & He, K. (2023). Fluoride Evaporation of Low-Fluoride CaF2-CaO-Al2O3-MgO-TiO2-(Na2O-K2O) Slag for Electroslag Remelting. Materials, 16(7), 2777. https://doi.org/10.3390/ma16072777




