Durability of Concrete with Partial Replacement of Portland Cement by Incorporating Reactive Magnesium Oxide and Fly Ash
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composition of the Mixes
2.3. Tests
3. Results and Discussion
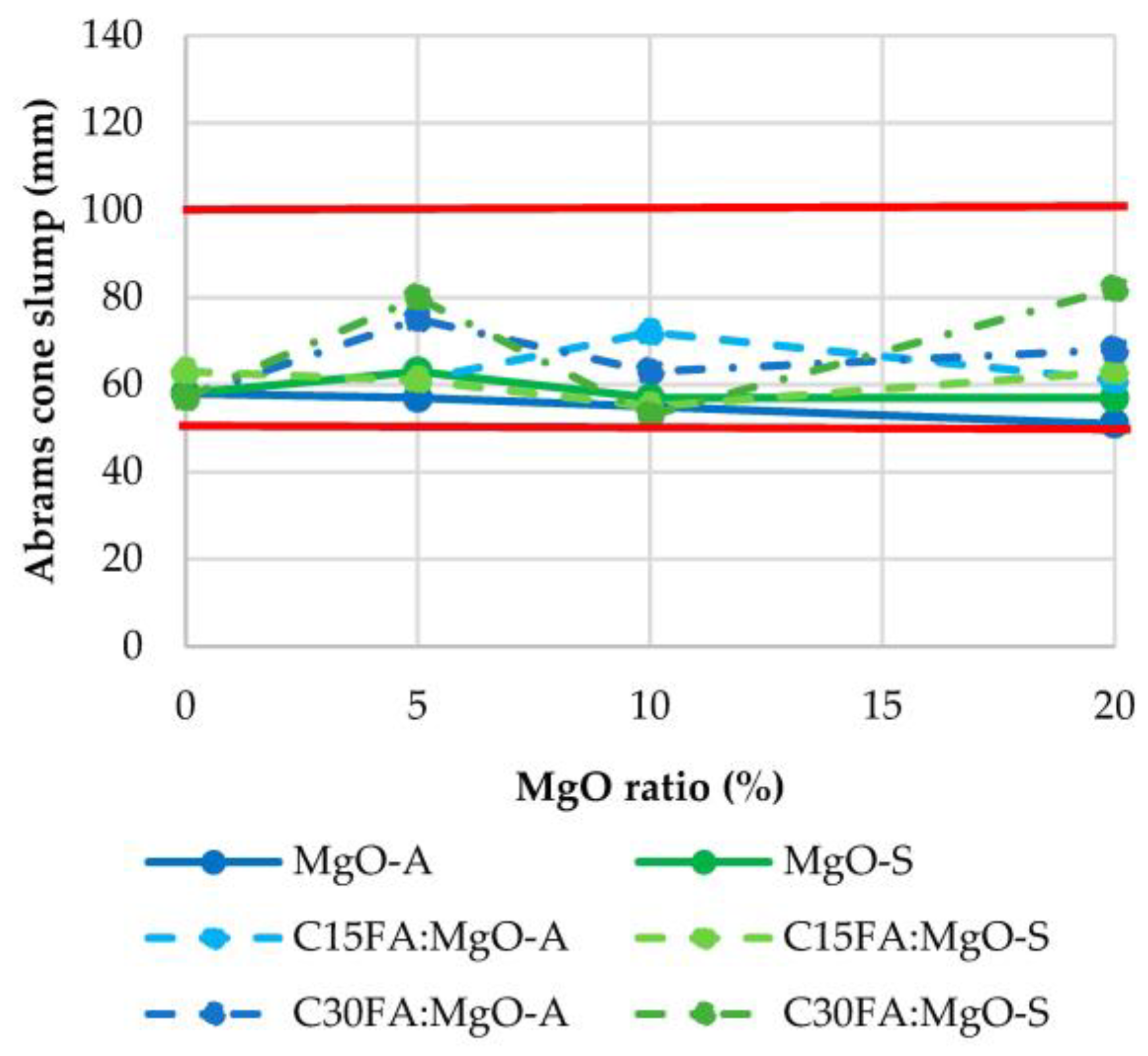
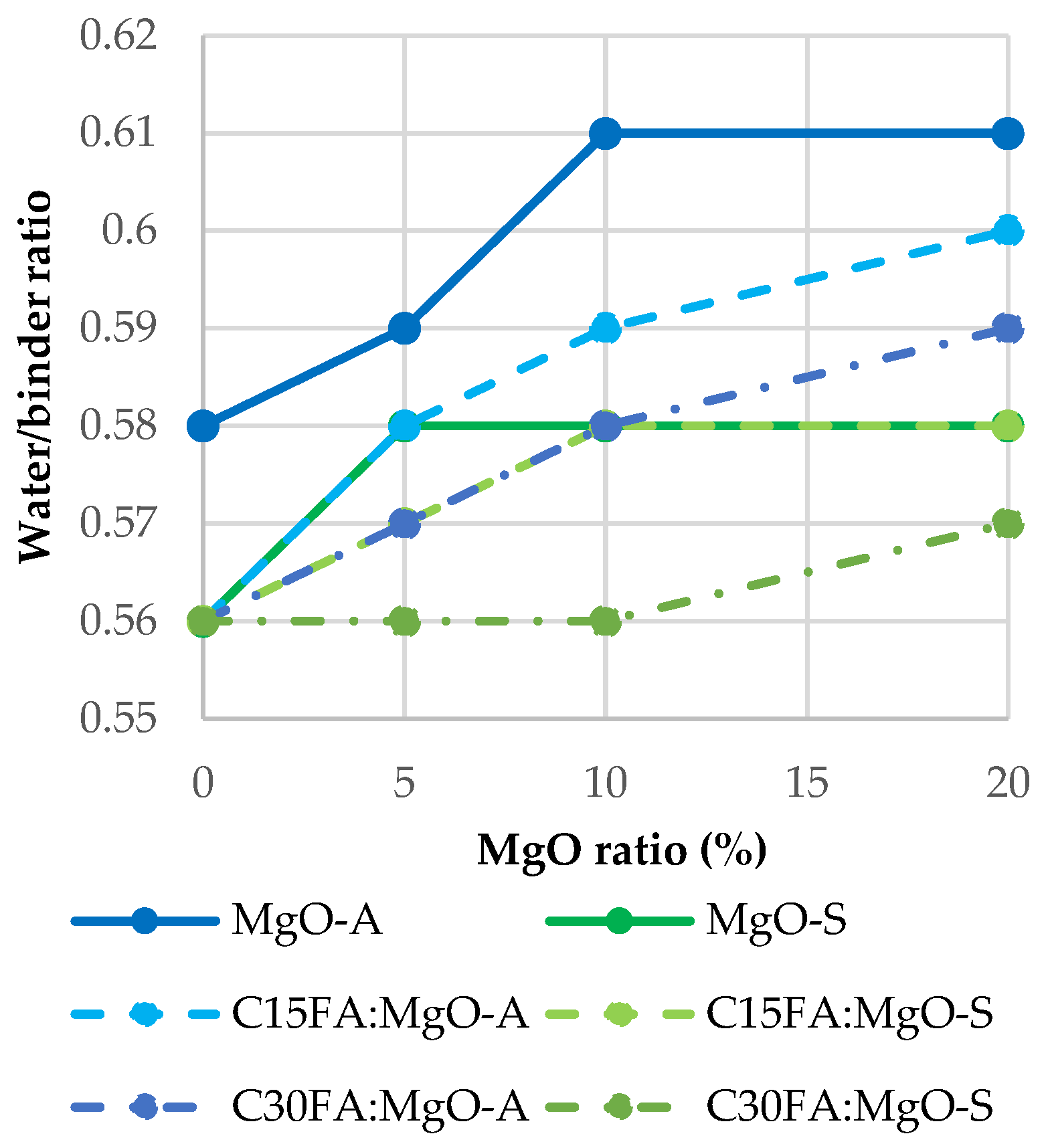
3.1. Consistency
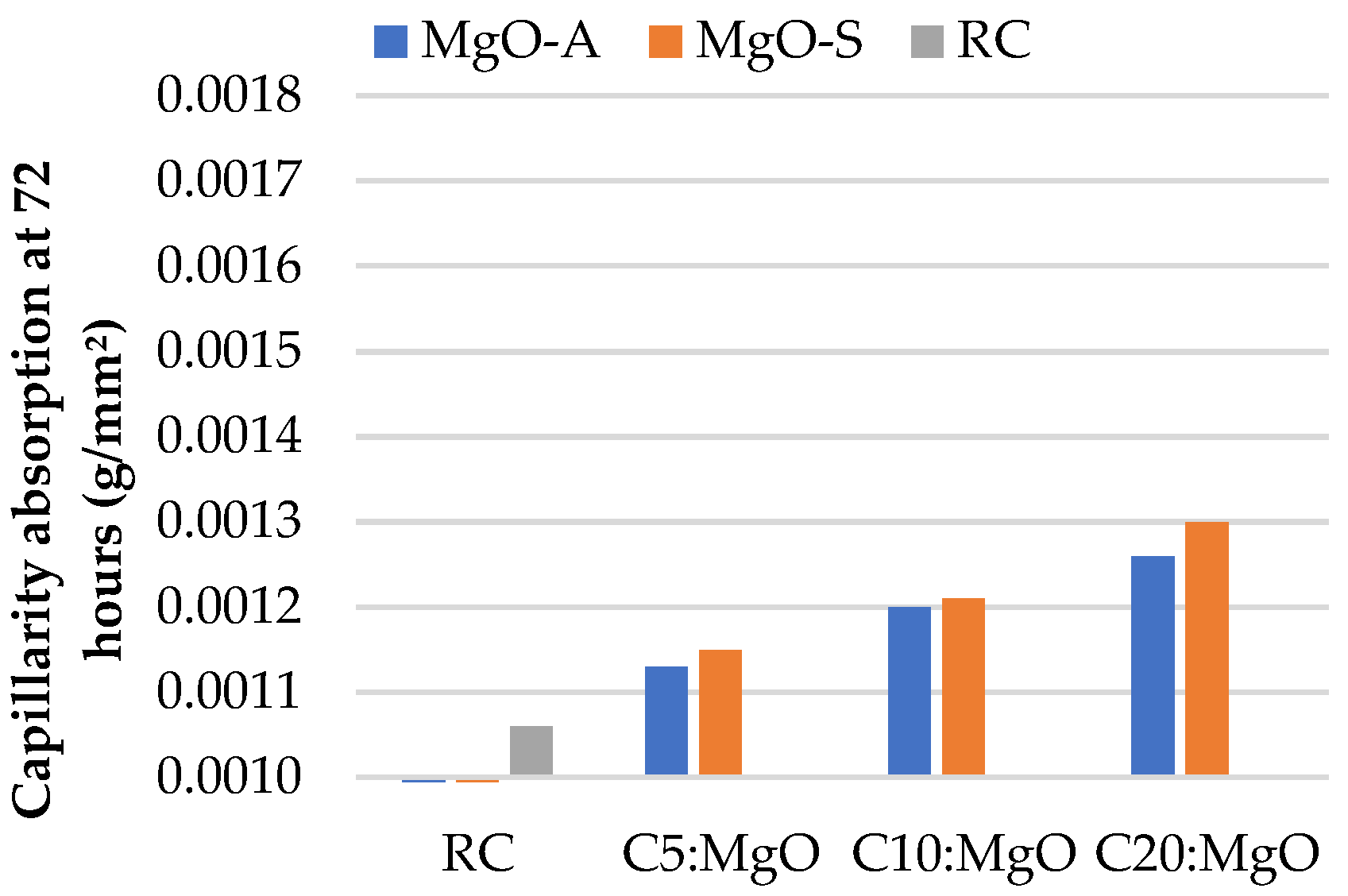
3.2. Water Absorption by Capillarity
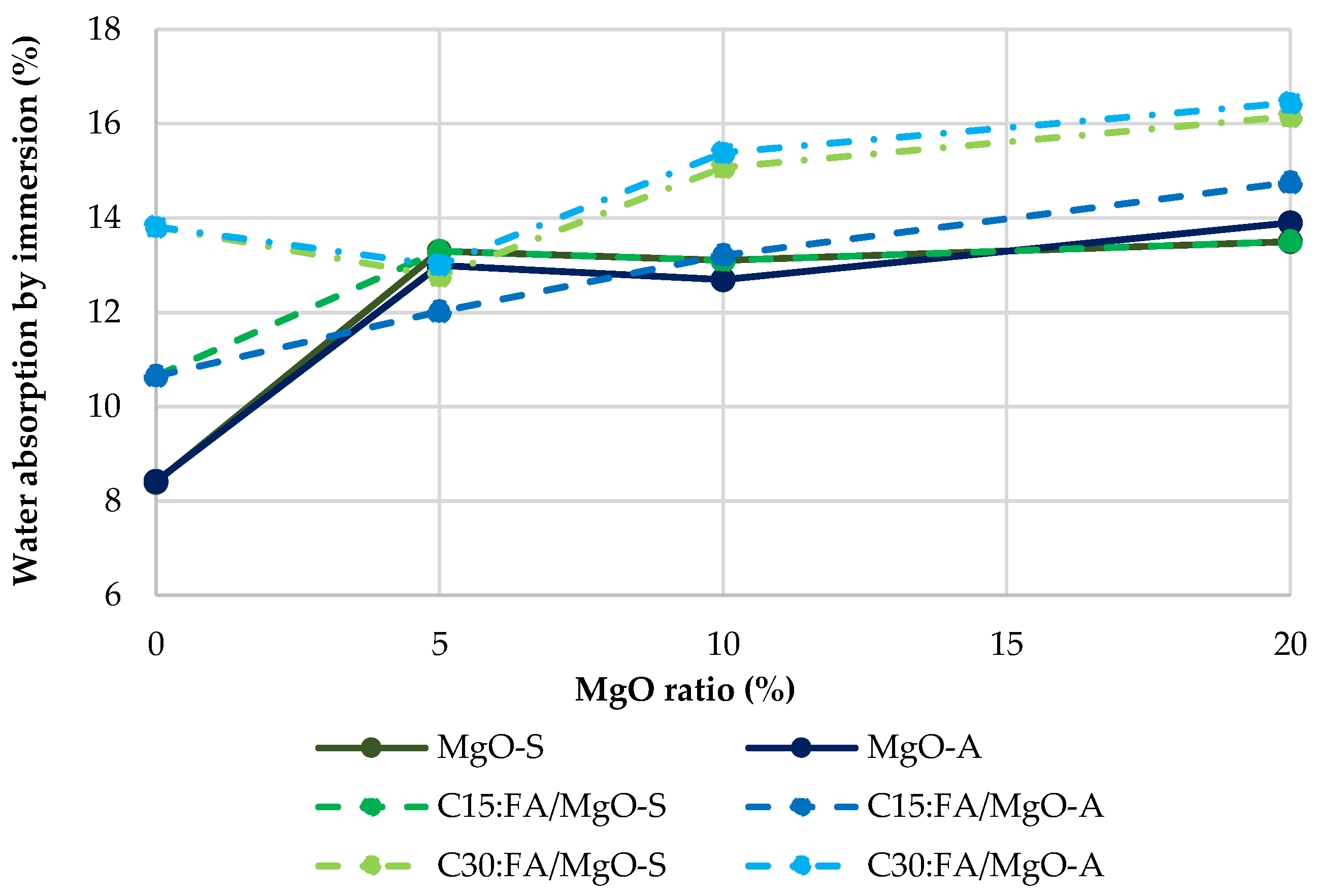
3.3. Water Absorption by Immersion
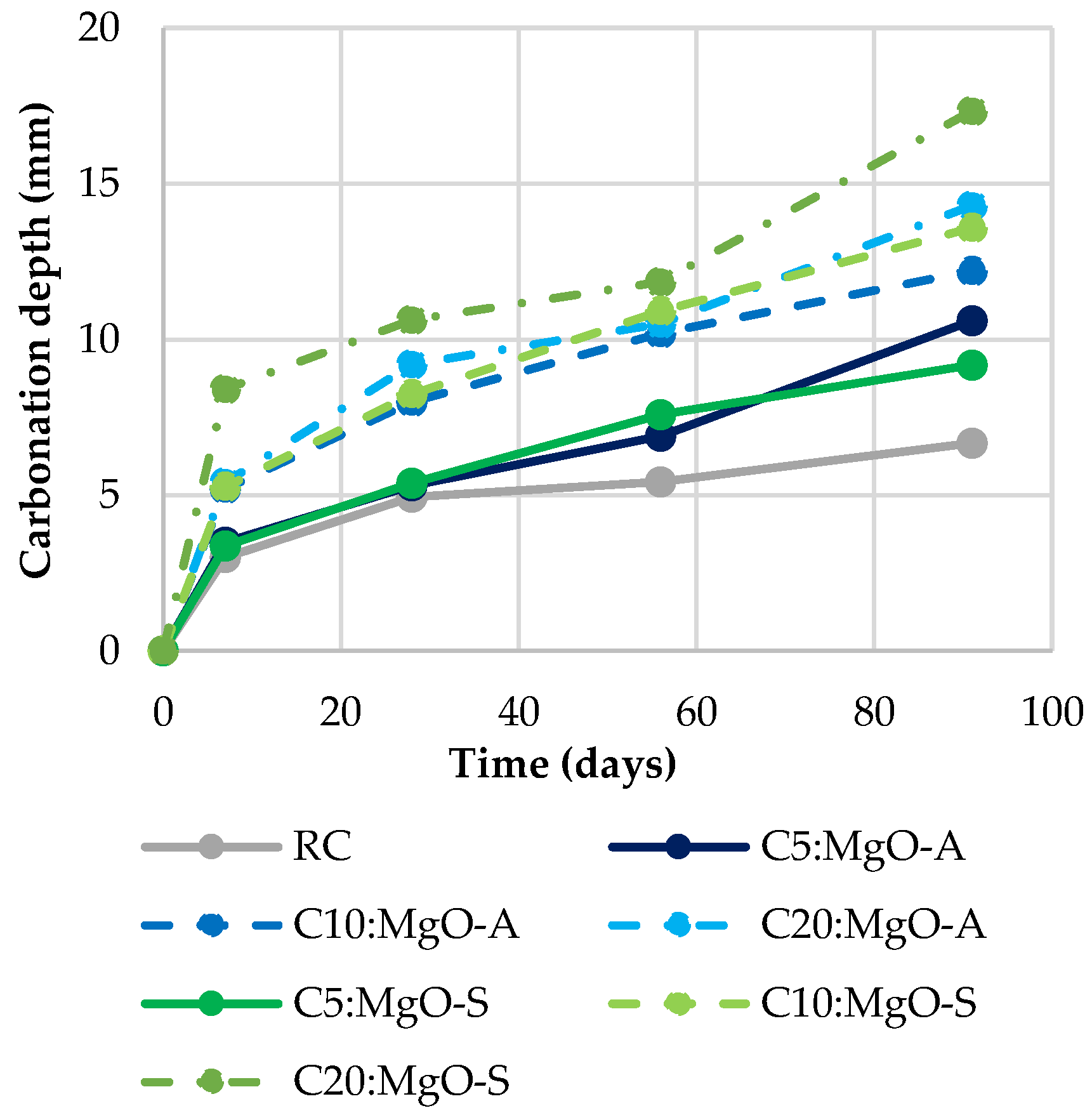
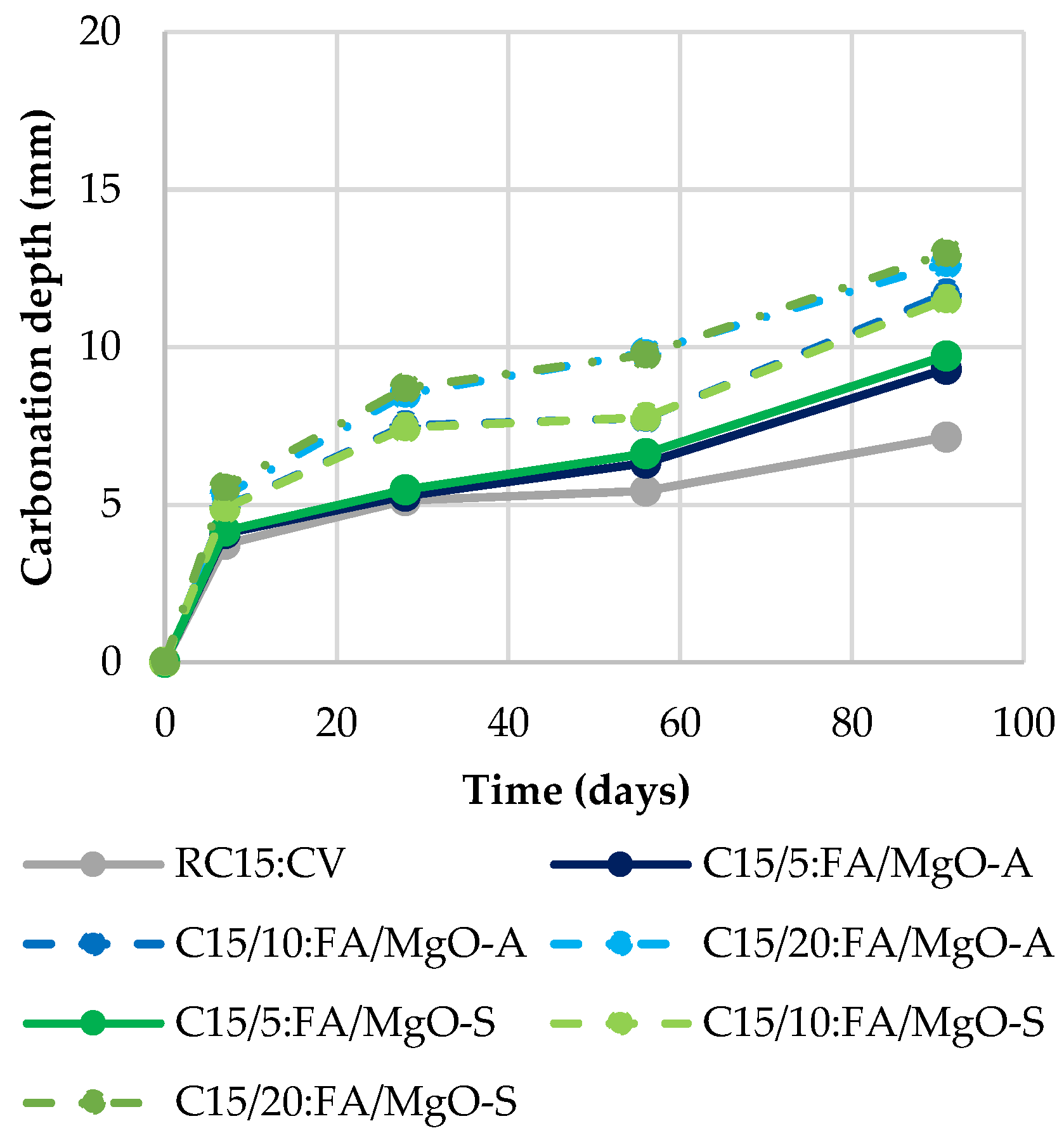
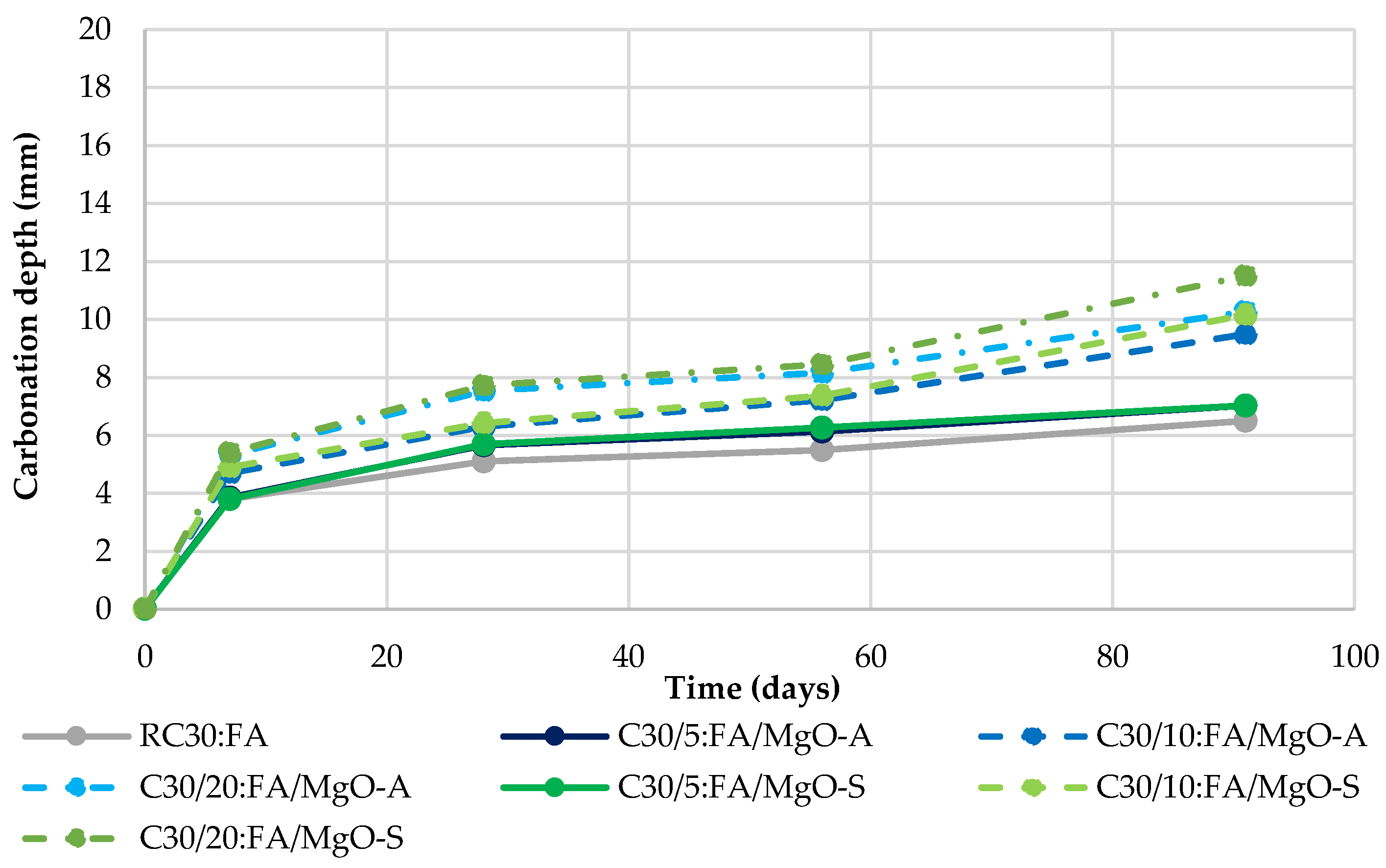
3.4. Carbonation
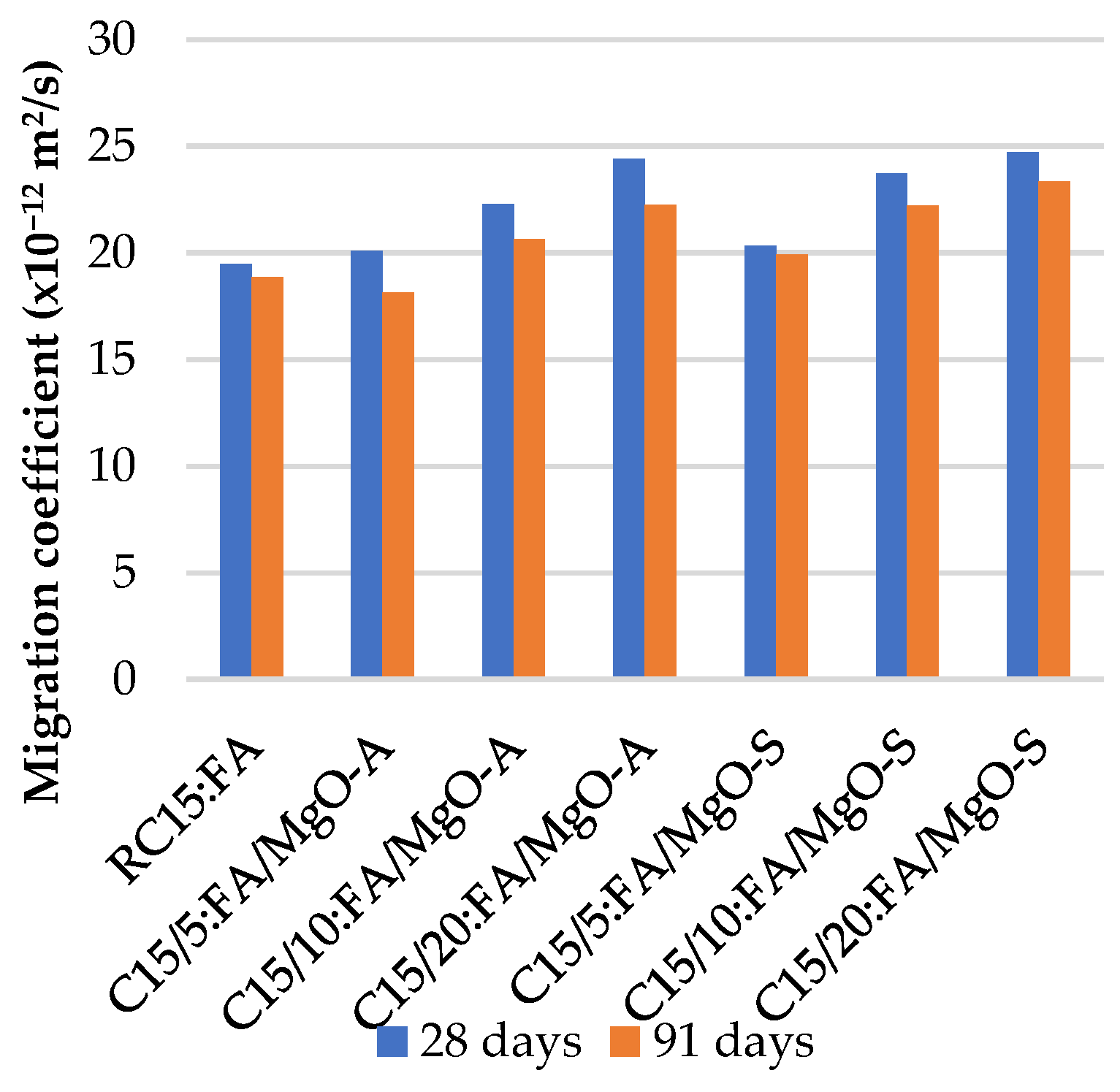
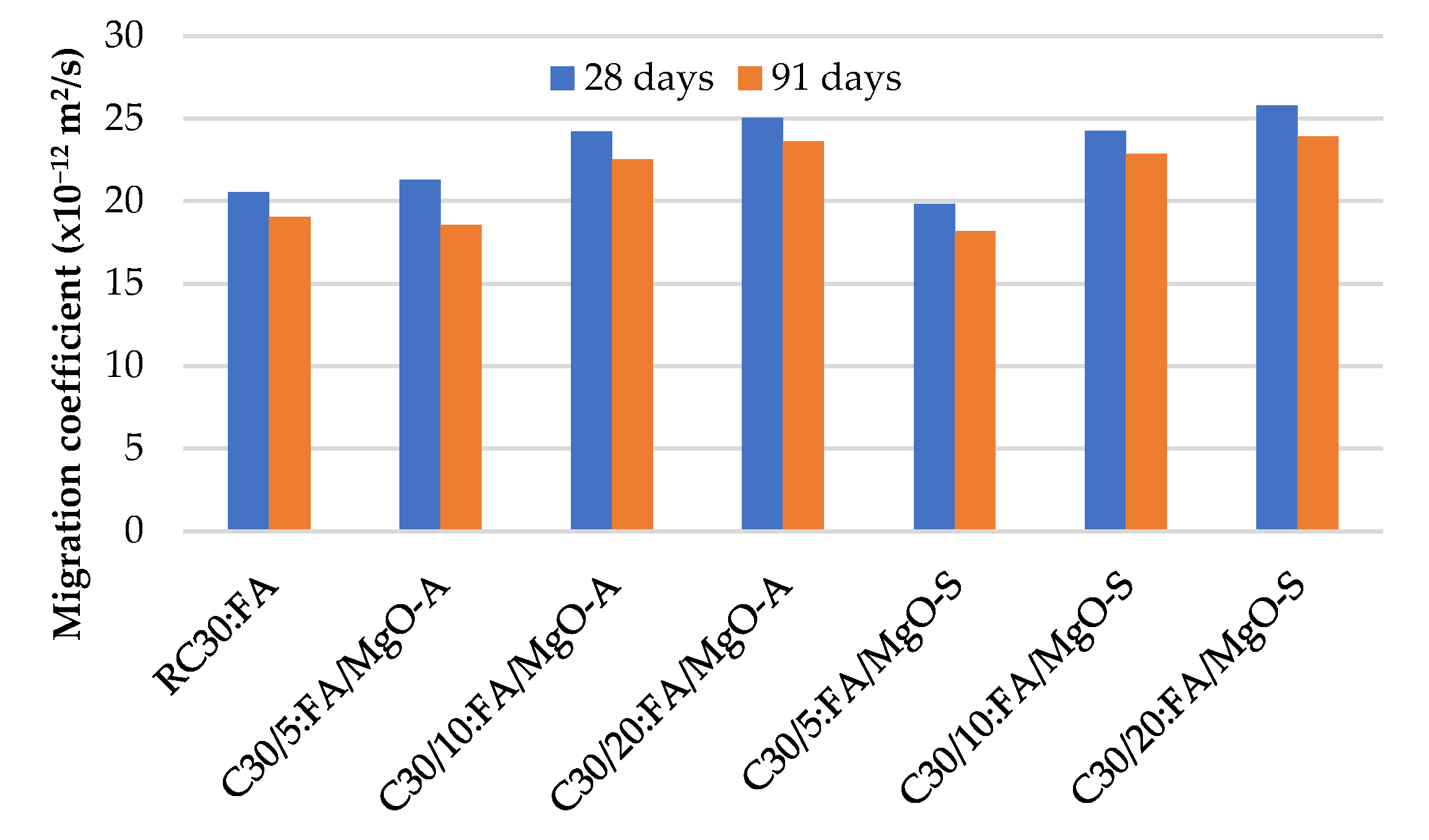
3.5. Resistance to Chloride Penetration
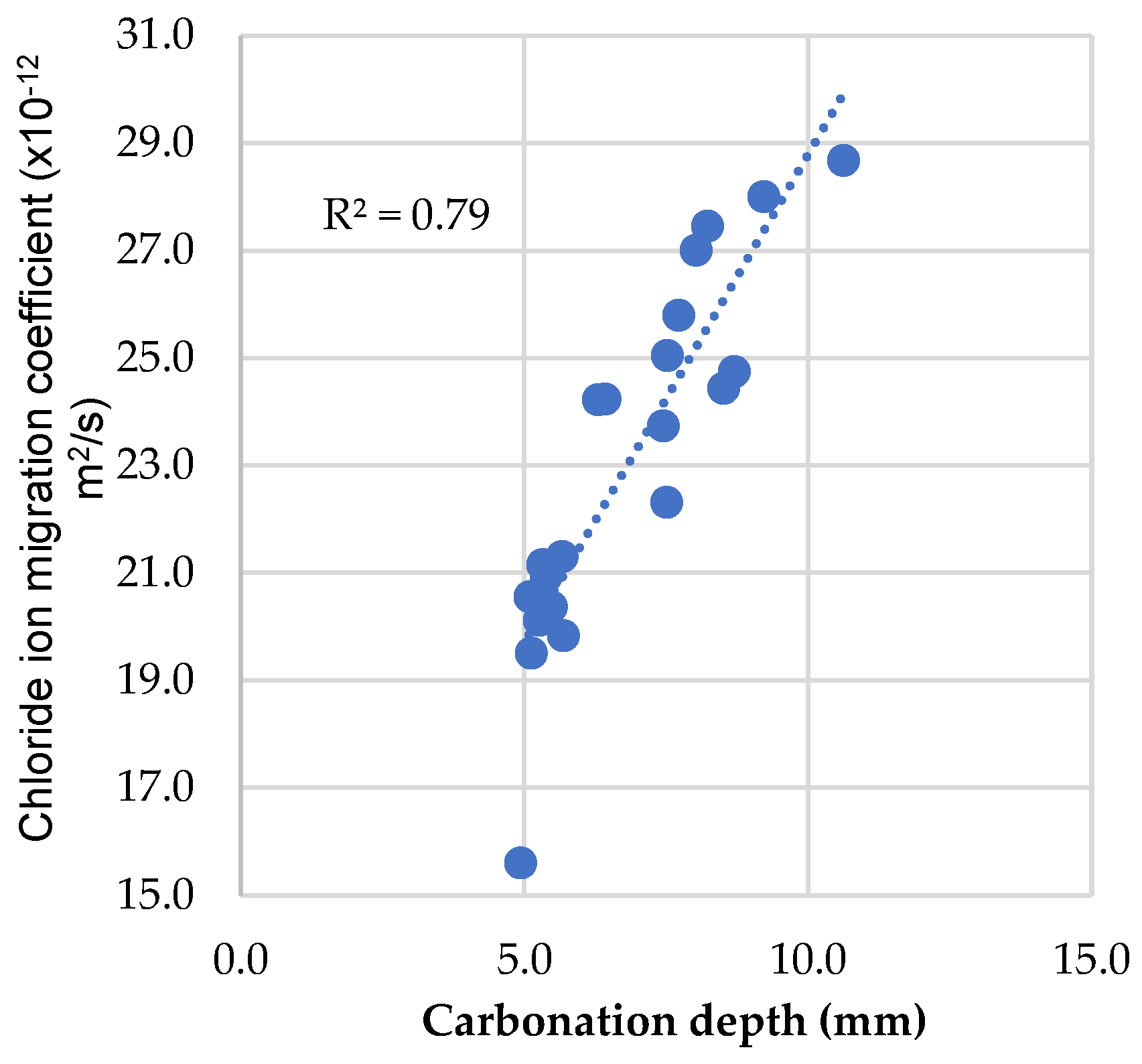
3.6. Comparative Analysis between Concrete Durability and Compressive Strength
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanjuán, M.Á.; Andrade, C.; Mora, P.; Zaragoza, A. Carbon Dioxide Uptake by Cement-Based Materials: A Spanish Case Study. Appl. Sci. 2020, 10, 339. [Google Scholar] [CrossRef]
- Sanjuán, M.A.; Argiz, C.; Mora, P.; Zaragoza, A. Carbon Dioxide Uptake in the Roadmap 2050 of the Spanish Cement Industry. Energies 2020, 13, 3452. [Google Scholar] [CrossRef]
- Sanjuán, M.Á.; Estévez, E.; Argiz, C. Carbon Dioxide Absorption by Blast-Furnace Slag Mortars in Function of the Curing Intensity. Energies 2019, 12, 2346. [Google Scholar] [CrossRef]
- Walling, S.A.; Provis, J.L. Magnesia-based cements: A journey of 150 years, and cements for the future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Al-Tabbaa, A. Characterisation of different commercial reactive magnesia. Adv. Cem. Res. 2014, 26, 101–113. [Google Scholar] [CrossRef]
- Ruan, S.; Unluer, C. Comparative life cycle assessment of reactive MgO and Portland cement production. J. Clean. Prod. 2016, 137, 258–273. [Google Scholar] [CrossRef]
- Sinka, M.; Van den Heede, P.; De Belie, N.; Bajare, D.; Sahmenko, G.; Korjakins, A. Comparative life cycle assessment of magnesium binders as an alternative for hemp concrete. Resour. Conserv. Recycl. 2018, 133, 288–299. [Google Scholar] [CrossRef]
- Kramer, D. Magnesium, Its Alloys and Compounds. US Geological Survey 2001. Available online: https://pubs.usgs.gov/of/2001/of01-341/of01-341.pdf (accessed on 1 December 2022).
- Shand, M.A. The Chemistry and Technology of Magnesia; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Mo, L.; Deng, M.; Wang, A. Effects of MgO-based expansive additive on compensating the shrinkage of cement paste under non-wet curing conditions. Cem. Concr. Compos. 2012, 34, 377–383. [Google Scholar] [CrossRef]
- Du, C. A review of magnesium oxide in concrete. Concr. Int. 2005, 27, 45–50. [Google Scholar]
- Liska, M.; Al-Tabbaa, A. Ultra-green construction: Reactive magnesia masonry products. Proc. Inst. Civ. Eng.-Waste Resour. Manag. 2009, 162, 185–196. [Google Scholar] [CrossRef]
- Mo, L.; Panesar, D.K. Effects of accelerated carbonation on the microstructure of Portland cement pastes containing reactive MgO. Cem. Concr. Res. 2012, 42, 769–777. [Google Scholar] [CrossRef]
- Papadakis, V.; Vayenas, C.; Fardis, M. Physical and chemical characteristics affecting the durability of concrete. ACI Mater. J. 1991, 88, 186–196. [Google Scholar]
- Liu, Z.; Cui, X.; Tang, M. MgO-type delayed expansive cement. Cem. Concr. Res. 1991, 21, 1049–1057. [Google Scholar]
- Bravo, M.; Forero, J.A.; Nobre, J.; de Brito, J.; Evangelista, L. Performance of Mortars with commercially-available reactive magnesium oxide as alternative binder. Materials 2021, 14, 938. [Google Scholar] [CrossRef] [PubMed]
- Mavroulidou, M.; Morrison, T.; Unsworth, C.; Gunn, M. Properties of concrete made of multicomponent mixes of low-energy demanding binders. Constr. Build. Mater. 2015, 101, 1122–1141. [Google Scholar] [CrossRef]
- Pu, L.; Unluer, C. Investigation of carbonation depth and its influence on the performance and microstructure of MgO cement and PC mixes. Constr. Build. Mater. 2016, 120, 349–363. [Google Scholar] [CrossRef]
- Gonçalves, T.; Silva, R.V.; de Brito, J.; Fernández, J.M.; Esquinas, A.R. Mechanical and durability performance of mortars with fine recycled concrete aggregates and reactive magnesium oxide as partial cement replacement. Cem. Concr. Compos. 2020, 105, 103420. [Google Scholar] [CrossRef]
- ASTM C618. Standard Specification for Coal Fly Ash and Raw Calcined Natural Pozzolan for Use as a Mineral Admixture in Concrete. Annual Book of ASTM Standards. 1998. Available online: https://www.osti.gov/biblio/305700 (accessed on 1 December 2022).
- Fu, X.; Wang, Z.; Tao, W.; Yang, C.; Hou, W.; Dong, Y.; Wu, X. Studies on blended cement with a large amount of fly ash. Cem. Concr. Res. 2002, 32, 1153–1159. [Google Scholar] [CrossRef]
- Malek, R.I.; Khalil, Z.H.; Imbaby, S.S.; Roy, D.M. The contribution of class-F fly ash to the strength of cementitious mixtures. Cem. Concr. Res. 2005, 35, 1152–1154. [Google Scholar] [CrossRef]
- Hanehara, S.; Tomosawa, F.; Kobayakawa, M.; Hwang, K.J.C.; Research, C. Effects of water/powder ratio, mixing ratio of fly ash, and curing temperature on pozzolanic reaction of fly ash in cement paste. Cem. Concr. Res. 2001, 31, 31–39. [Google Scholar] [CrossRef]
- Saha, A.K. Effect of class F fly ash on the durability properties of concrete. Sustain. Environ. Res. 2018, 28, 25–31. [Google Scholar] [CrossRef]
- Nayak, D.K.; Abhilash, P.P.; Singh, R.; Kumar, R.; Kumar, V. Fly ash for sustainable construction: A review of fly ash concrete and its beneficial use case studies. Clean. Mater. 2022, 6, 100143. [Google Scholar] [CrossRef]
- Islam, M.M.; Alam, M.T.; Islam, M.S. Effect of fly ash on freeze–thaw durability of concrete in marine environment. Aust. J. Struct. Eng. 2018, 19, 146–161. [Google Scholar] [CrossRef]
- Shehata, M.H.; Thomas, M.D.A.; Bleszynski, R.F.J.C.; Research, C. The effects of fly ash composition on the chemistry of pore solution in hydrated cement pastes. Cem. Concr. Res. 1999, 29, 1915–1920. [Google Scholar] [CrossRef]
- Sadrmomtazi, A.; Tahmouresi, B.; Kohani Khoshkbijari, R. Effect of fly ash and silica fume on transition zone, pore structure and permeability of concrete. Mag. Concr. Res. 2017, 70, 519–532. [Google Scholar] [CrossRef]
- Turk, K.; Karatas, M.; Gonen, T. Effect of fly ash and silica fume on compressive strength, sorptivity and carbonation of SCC. KSCE J. Civ. Eng. 2013, 17, 202–209. [Google Scholar] [CrossRef]
- Khunthongkeaw, J.; Tangtermsirikul, S.; Leelawat, T. A study on carbonation depth prediction for fly ash concrete. J. Constr. Build. Mater. Res. 2009, 20, 744–753. [Google Scholar] [CrossRef]
- Papadakis, V.G. Effect of supplementary cementing materials on concrete resistance against carbonation and chloride ingress. Cem. Concr. Res. 2000, 30, 291–299. [Google Scholar] [CrossRef]
- Brew, D.R.M.; Glasser, F.P. Synthesis and characterisation of magnesium silicate hydrate gels. Cem. Concr. Res. 2005, 35, 85–98. [Google Scholar] [CrossRef]
- Choi, S.-W.; Jang, B.-S.; Kim, J.-H.; Lee, K.-M. Durability characteristics of fly ash concrete containing lightly-burnt MgO. Constr. Build. Mater. 2014, 58, 77–84. [Google Scholar] [CrossRef]
- BS EN 12620; Aggregates for Concrete. British Standard: London, UK, 2013.
- Nepomuceno, M.; Oliveira, L.; Lopes, S.M.R. Methodology for mix design of the mortar phase of self-compacting concrete using different mineral additions in binary blends of powders. Constr. Build. Mater. 2012, 26, 317–326. [Google Scholar] [CrossRef]
- LNEC E-394; Concrete. Determination of Water Absorption by Immersion. Test at Atmospheric Pressure. National Laboratory of Civil Engineering (LNEC): Lisbon, Portugal, 1993. (In Portuguese)
- LNEC E-391; Concrete. Determination of Carbonation Resistance. National Laboratory of Civil Engineering (LNEC): Lisbon, Portugal, 1993. (In Portuguese)
- NORDTEST. Chloride Migration Coefficient from Non Steady-State Migration Experiments. NT BUILD 492; 1999; pp. 1–11. Available online: https://salmanco.com/wp-content/uploads/2018/10/NT-Build-492.pdf (accessed on 1 December 2022).
- LNEC E-393; Concrete. Determination of Water Absorption by Capillarity. National Laboratory of Civil Engineering (LNEC): Lisbon, Portugal, 1993. (In Portuguese)
- Ferreira, R. Evaluation of Concrete Durability Tests; Final Course Work in Civil Engineering; Minho University: Guimarães, Portugal, 2000; 248p. (In Portuguese) [Google Scholar]
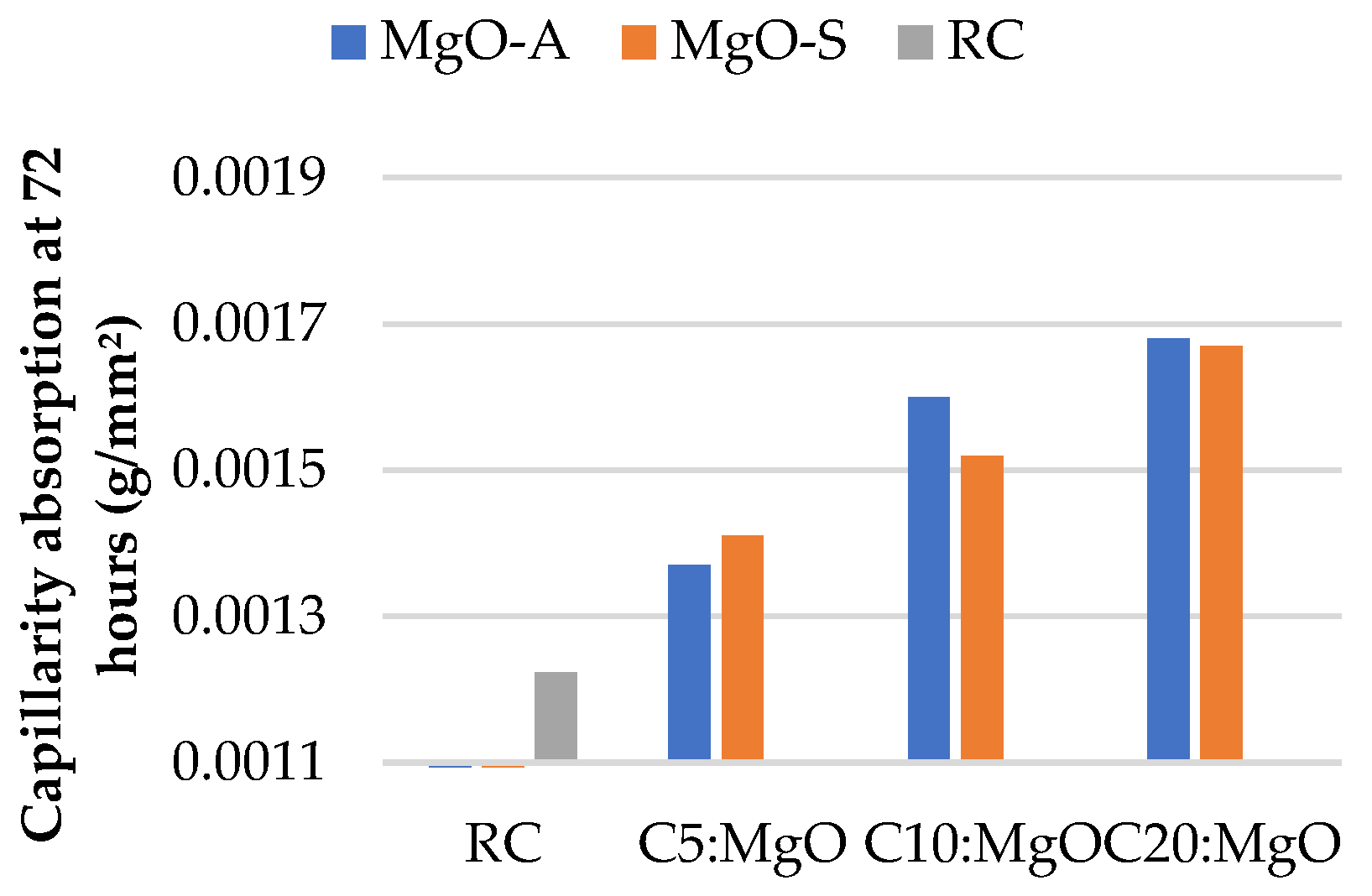
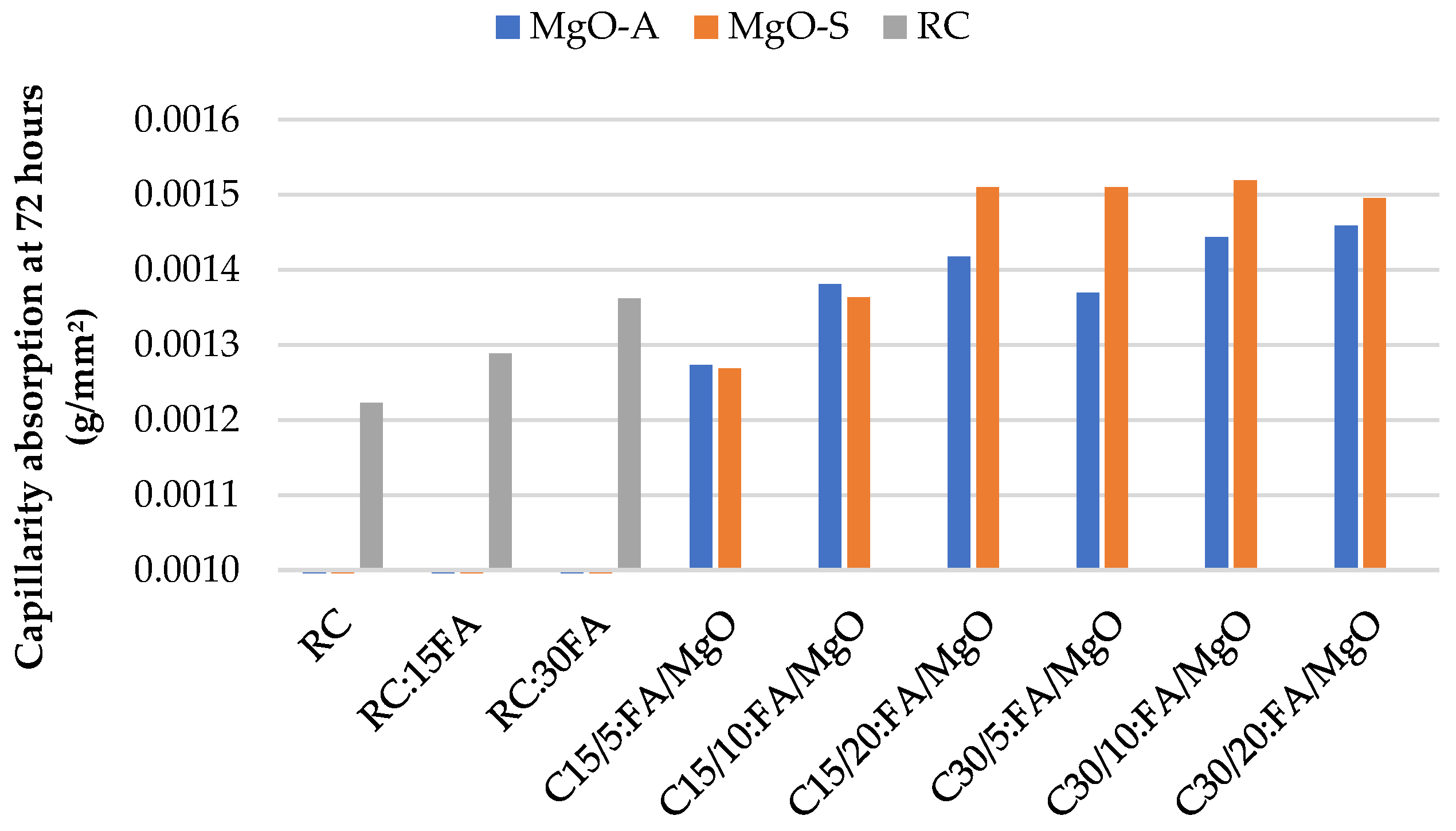

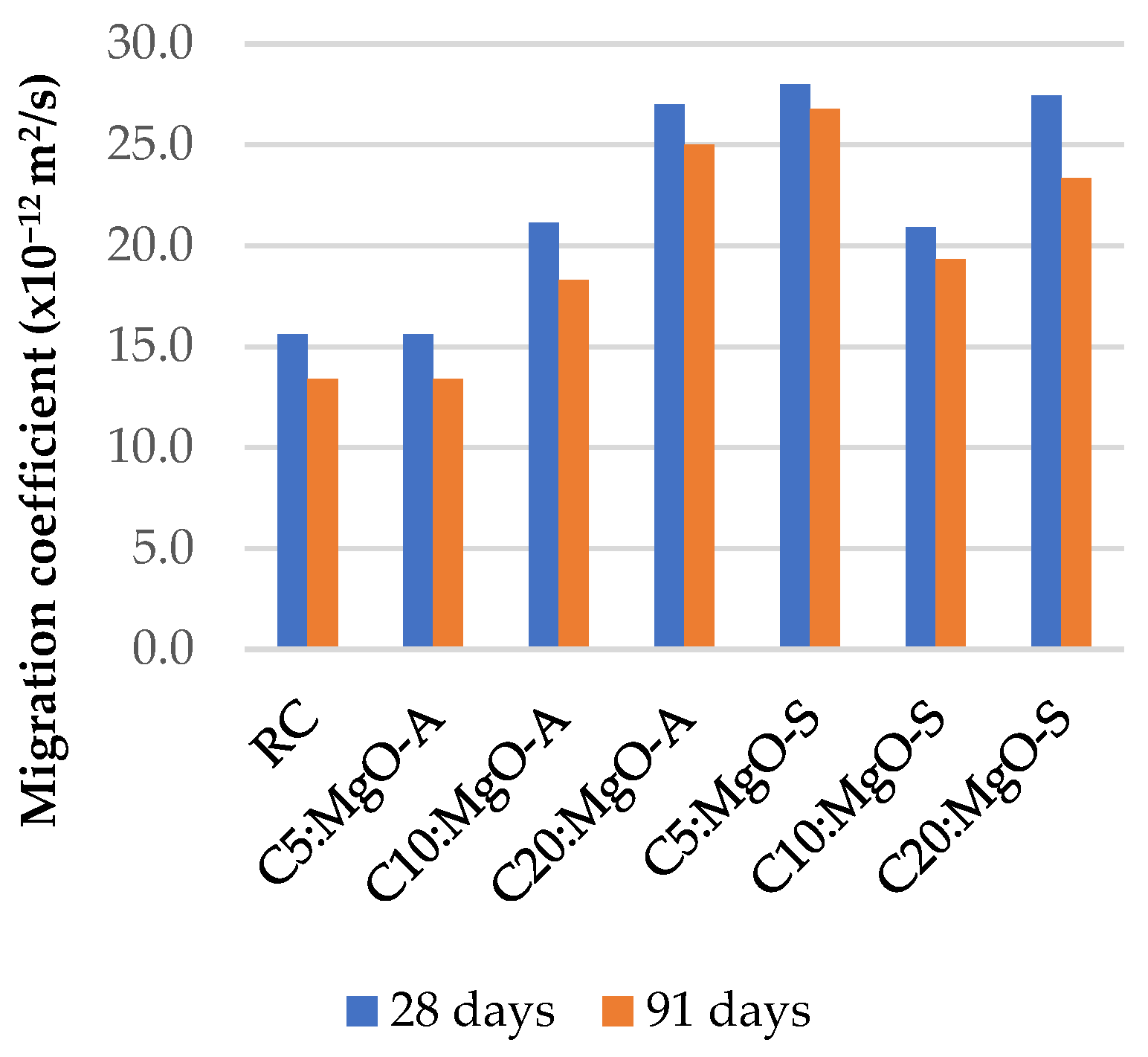

| Material | <3 μm (%) | Between 3 and 32 μm (%) | >32 μm (%) |
|---|---|---|---|
| OPC | 25.9 | 56.2 | 17.9 |
| FA | 21.3 | 55.9 | 22.8 |
| MgO-S | 12.1 | 15.3 | 72.6 |
| MgO-A | 40.1 | 56.0 | 3.9 |
| Binder | 0.098 | |
| Fine aggregates | 0–0.063 | 0.001 |
| 0.063–0.125 | 0.004 | |
| 0.125–0.25 | 0.030 | |
| 0.25–0.5 | 0.079 | |
| 0.5–1 | 0.097 | |
| 1–2 | 0.085 | |
| Coarse aggregates | 2–4 | 0.036 |
| 4–5.6 | 0.012 | |
| 5.6–8 | 0.050 | |
| 8–11.2 | 0.072 | |
| 11.2–16 | 0.153 | |
| 16–22.4 | 0.092 | |
| Water | 0.174 | |
| Voids | 0.017 | |
| Total | 1.000 | |
| Mix | 28 Days | 91 Days | ||
|---|---|---|---|---|
| Δ Real (%) | Δ Expected Theoretical (%) | Δ Real (%) | Δ Expected Theoretical (%) | |
| C15/5:FA/MgO-A | +4.1 | +17.4 | +4.4 | +8.9 |
| C15/5:FA/MgO-S | +3.7 | +20.7 | +3.4 | +10.8 |
| C15/10:FA/MgO-A | +13.0 | +36.2 | +5.7 | +15.5 |
| C15/10:FA/MgO-S | +11.5 | +29.7 | +6.5 | +17.4 |
| C15/20:FA/MgO-A | +16.0 | +42.8 | +9.0 | +21.2 |
| C15/20:FA/MgO-S | +23.5 | +41.9 | +3.4 | +24.9 |
| C30/5:FA/MgO-A | +12.0 | +23.4 | +7.3 | +13.6 |
| C30/5:FA/MgO-S | +23.5 | +26.7 | +3.3 | +15.5 |
| C30/10:FA/MgO-A | +18.0 | +42.2 | +12.8 | +20.2 |
| C30/10:FA/MgO-S | +24.3 | +35.7 | +16.4 | +22.1 |
| C30/20:FA/MgO-A | +19.3 | +48.8 | +17.1 | +25.8 |
| C30/20:FA/MgO-S | +22.3 | +47.9 | +20.1 | +29.6 |
| Mix | 91 Days | |
|---|---|---|
| Δ Real (%) | Δ Expected Theoretical (%) | |
| C15/5:FA/MgO-A | 39.6 | 65.5 |
| C15/5:FA/MgO-S | 46.0 | 44.9 |
| C15/10:FA/MgO-A | 75.5 | 90.4 |
| C15/10:FA/MgO-S | 72.7 | 110.9 |
| C15/20:FA/MgO-A | 90.1 | 121.6 |
| C15/20:FA/MgO-S | 94.9 | 167.2 |
| C30/5:FA/MgO-A | 5.4 | 37.1 |
| C30/5:FA/MgO-S | 5.4 | 35.2 |
| C30/10:FA/MgO-A | 42.4 | 80.8 |
| C30/10:FA/MgO-S | 52.4 | 101.3 |
| C30/20:FA/MgO-A | 54.0 | 111.9 |
| C30/20:FA/MgO-S | 72.5 | 157.6 |
| Mix | 91 Days | |
|---|---|---|
| Δ Real (%) | Δ Expected Theoretical (%) | |
| C15/5:FA/MgO-A | 35.4 | 77.2 |
| C15/5:FA/MgO-S | 48.6 | 84.8 |
| C15/10:FA/MgO-A | 53.9 | 127.2 |
| C15/10:FA/MgO-S | 65.7 | 114.6 |
| C15/20:FA/MgO-A | 66.0 | 140.4 |
| C15/20:FA/MgO-S | 74.1 | 139.8 |
| C30/5:FA/MgO-A | 38.1 | 78.3 |
| C30/5:FA/MgO-S | 35.4 | 85.9 |
| C30/10:FA/MgO-A | 67.7 | 128.3 |
| C30/10:FA/MgO-S | 70.5 | 115.8 |
| C30/20:FA/MgO-A | 76.0 | 141.5 |
| C30/20:FA/MgO-S | 78.2 | 140.9 |
| Variation Relative to RC (%) | ||||||
|---|---|---|---|---|---|---|
| Mix | Compressive Strength | Water Absorption by Capillarity | Water Absorption by Immersion | Carbonation Depth | Chloride Penetration | |
| 28 Days | 91 Days | 91 Days | 28 Days | 91 Days | 91 Days | |
| C15/5:FA/MgO-A | −26.9 | −15.5 | +4.40 | +42.7 | +39.6 | +35.4 |
| C15/5:FA/MgO-S | −23.1 | −13.7 | +3.43 | +39.5 | +46.0 | +48.6 |
| C15/10:FA/MgO-A | −29.3 | −15.5 | +5.65 | +57.0 | +75.5 | +53.9 |
| C15/10:FA/MgO-S | −30.5 | −17.3 | +6.54 | +65.4 | +72.7 | +65.7 |
| C15/20:FA/MgO-A | −41.1 | −34.5 | +9.03 | +75.3 | +90.1 | +66.0 |
| C15/20:FA/MgO-S | −44.8 | −36.8 | +3.38 | +82.1 | +94.9 | +74.1 |
| C30/5:FA/MgO-A | −34.6 | −15.0 | +7.30 | +54.4 | +5.4 | +38.1 |
| C30/5:FA/MgO-S | −34.1 | −18.9 | +3.34 | +51.7 | +5.4 | +35.4 |
| C30/10:FA/MgO-A | −46.9 | −29.1 | +12.77 | +82.8 | +42.4 | +67.7 |
| C30/10:FA/MgO-S | −42.6 | −26.2 | +16.41 | +79.9 | +52.4 | +70.5 |
| C30/20:FA/MgO-A | −58.2 | −39.5 | +17.13 | +95.3 | +54.0 | +76.0 |
| C30/20:FA/MgO-S | −59.1 | −38.5 | +20.11 | +91.9 | +72.5 | +78.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sequeira, L.; Forero, J.; Bravo, M.; Evangelista, L.; de Brito, J. Durability of Concrete with Partial Replacement of Portland Cement by Incorporating Reactive Magnesium Oxide and Fly Ash. Materials 2023, 16, 2670. https://doi.org/10.3390/ma16072670
Sequeira L, Forero J, Bravo M, Evangelista L, de Brito J. Durability of Concrete with Partial Replacement of Portland Cement by Incorporating Reactive Magnesium Oxide and Fly Ash. Materials. 2023; 16(7):2670. https://doi.org/10.3390/ma16072670
Chicago/Turabian StyleSequeira, Lucas, Javier Forero, Miguel Bravo, Luís Evangelista, and Jorge de Brito. 2023. "Durability of Concrete with Partial Replacement of Portland Cement by Incorporating Reactive Magnesium Oxide and Fly Ash" Materials 16, no. 7: 2670. https://doi.org/10.3390/ma16072670
APA StyleSequeira, L., Forero, J., Bravo, M., Evangelista, L., & de Brito, J. (2023). Durability of Concrete with Partial Replacement of Portland Cement by Incorporating Reactive Magnesium Oxide and Fly Ash. Materials, 16(7), 2670. https://doi.org/10.3390/ma16072670









