Influencing Factors of Sulfuric Acid Resistance of Ca-Rich Alkali-Activated Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.1.1. Precursors
2.1.2. Alkali Activator Solution (AS)
2.1.3. Chemical Admixtures
2.2. Fabrication of Test Specimens
2.3. Acid Resistance Experiments
2.4. XRD and SEM-EDS Analysis
3. Results and Discussion
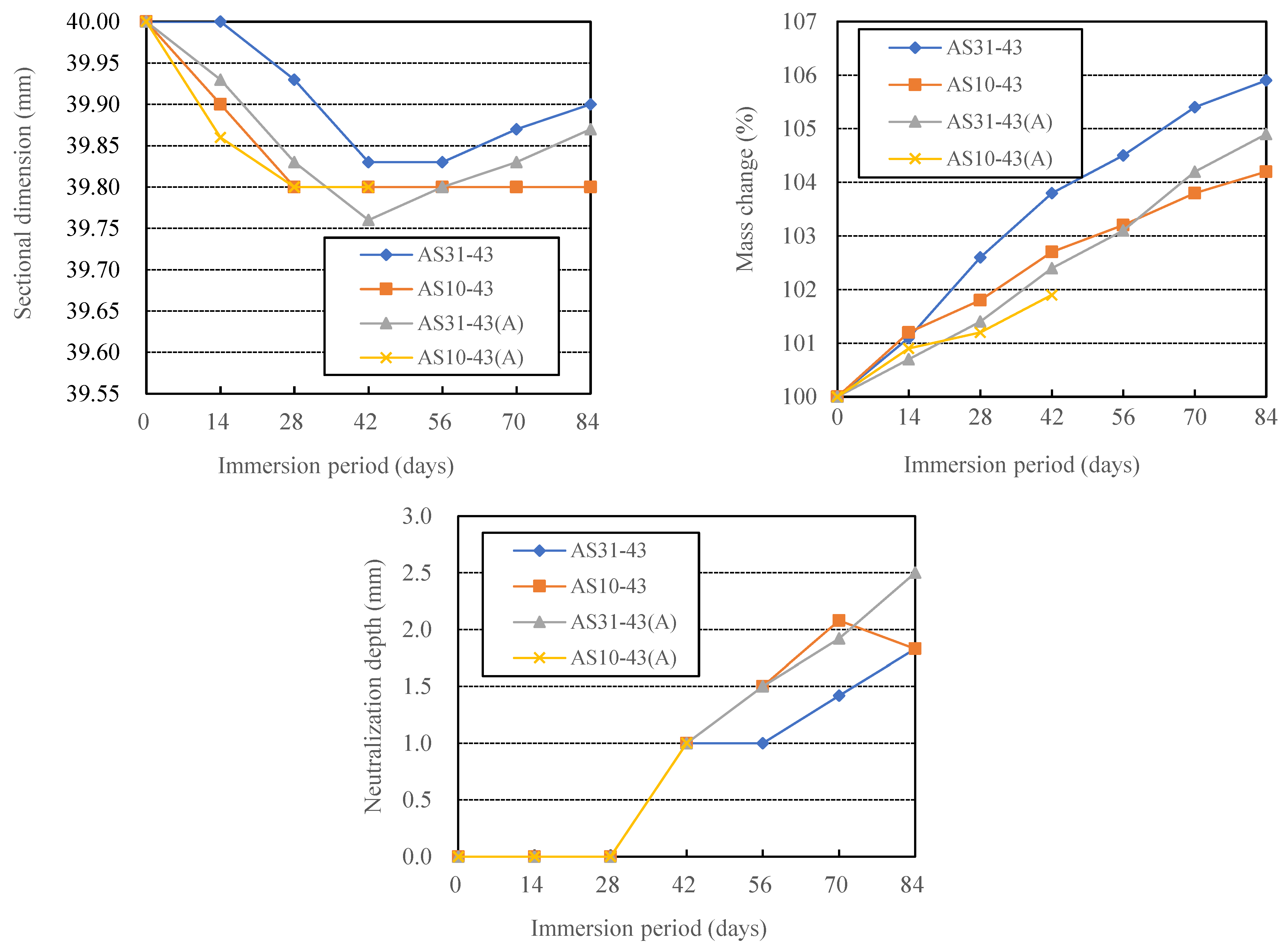
3.1. Effects of Curing Methods on the Acid Resistance of AAFS
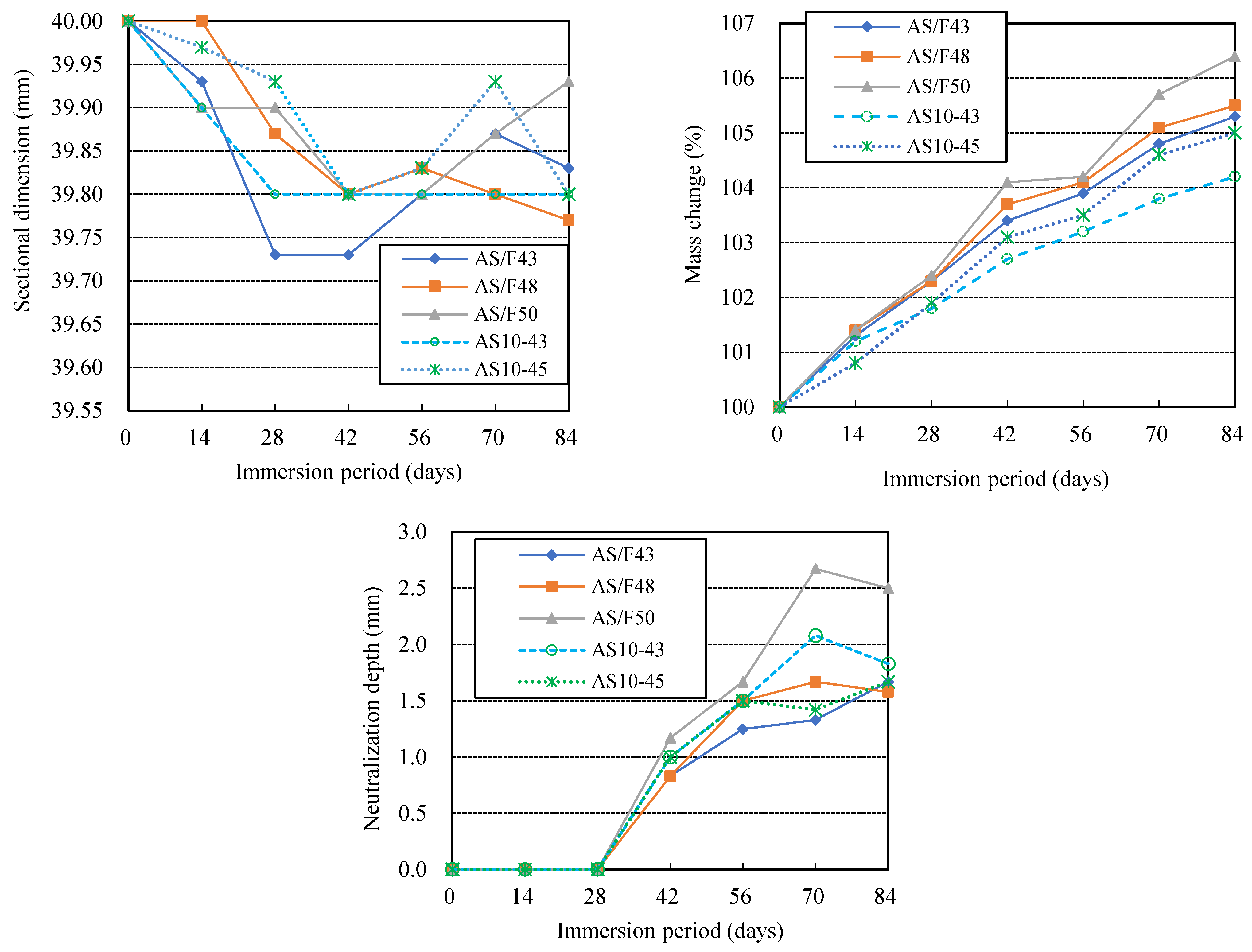
3.2. Effects of Alkali Activator Solution-Filler Ratio
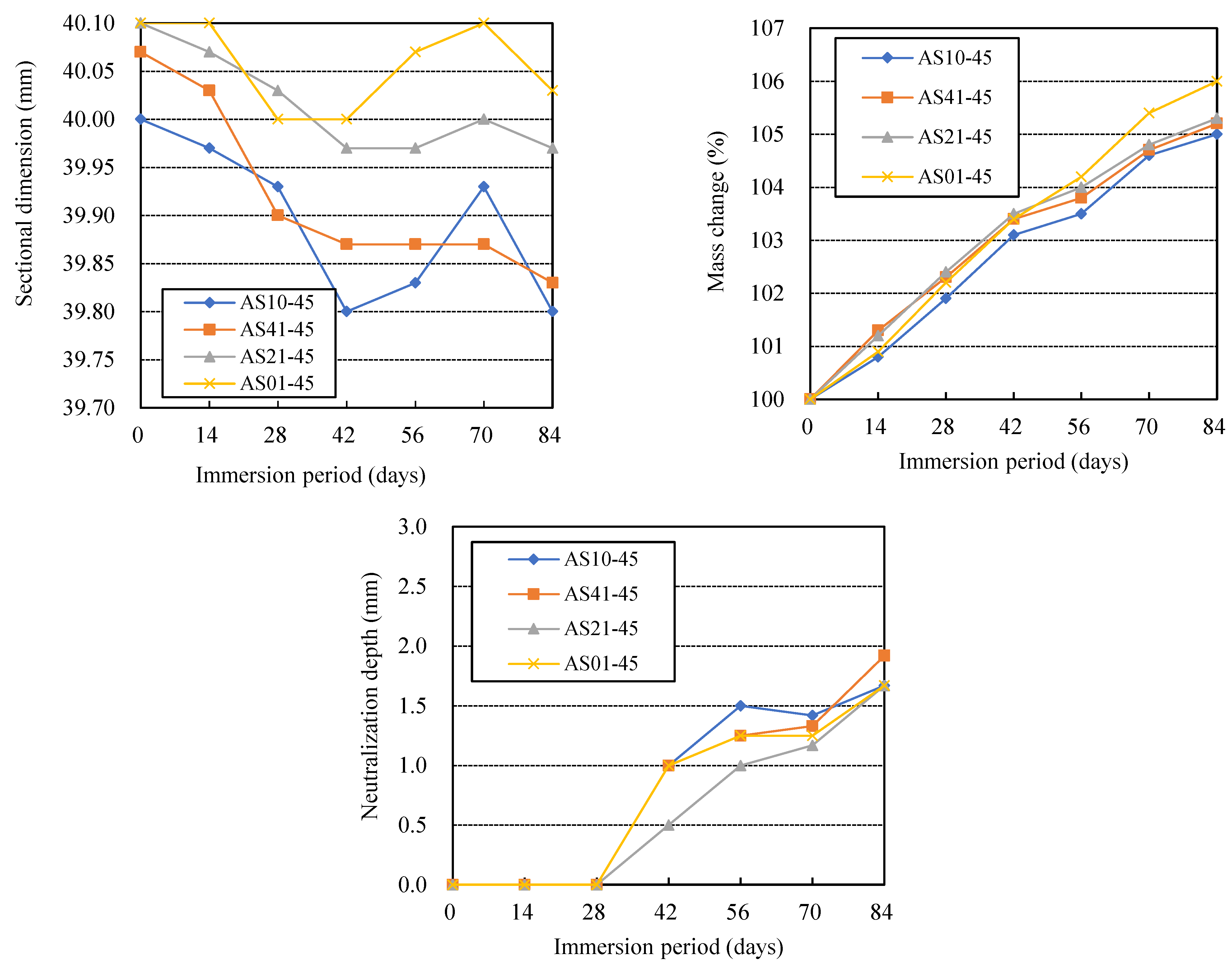
3.3. Effects of AS Components
3.4. Effects of Precursor
3.5. Effects of Chemical Admixture Addition
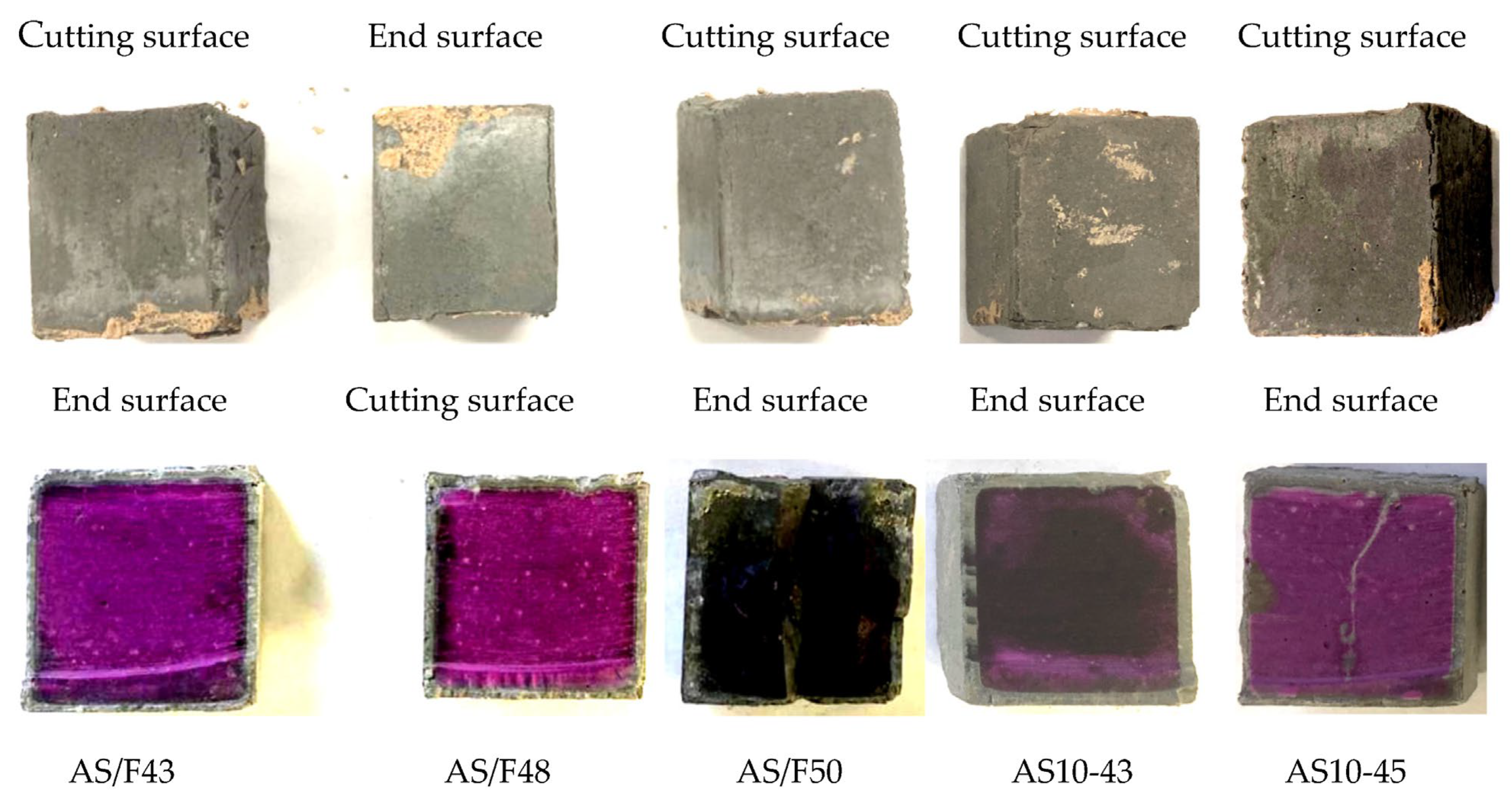
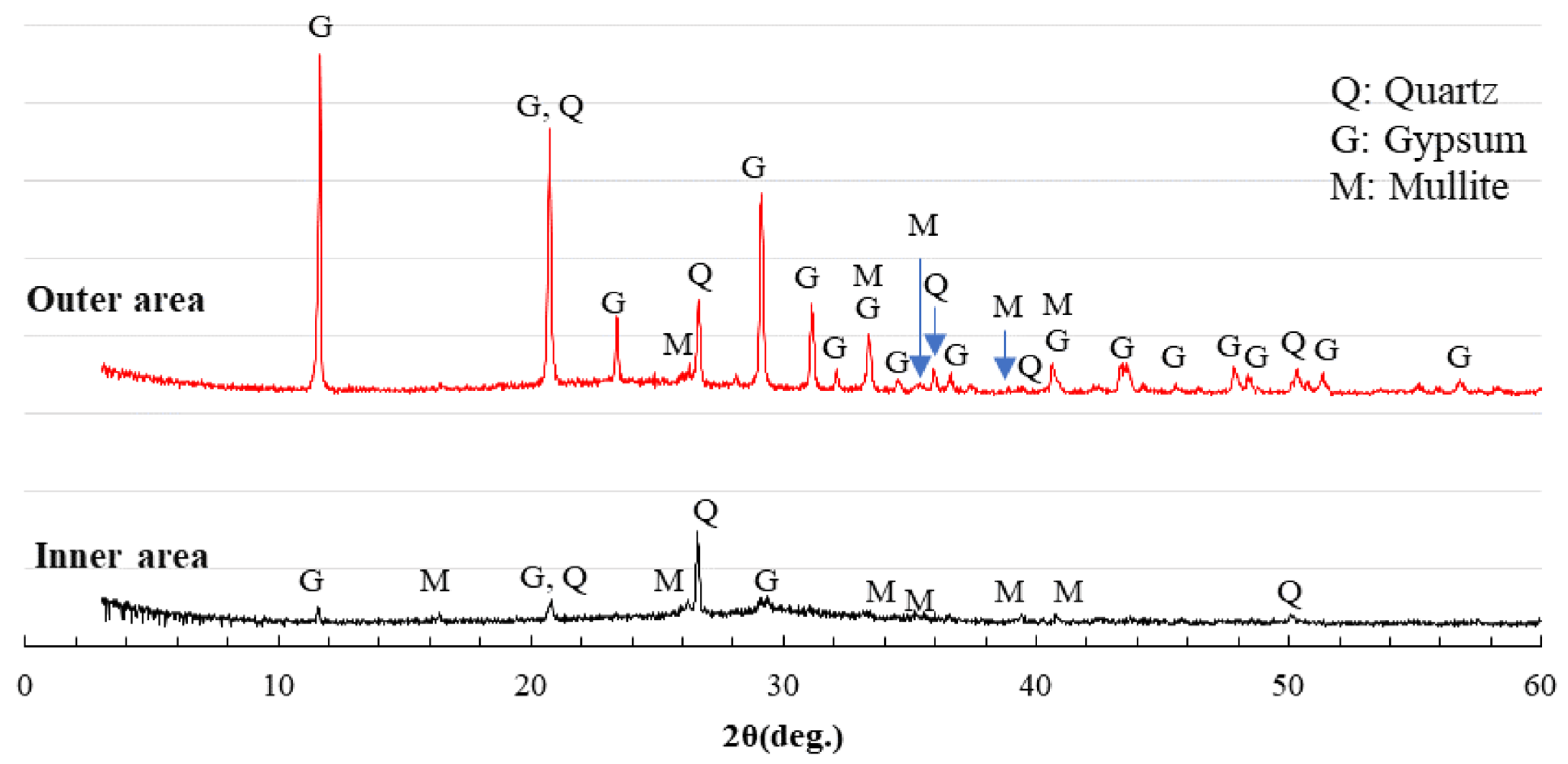
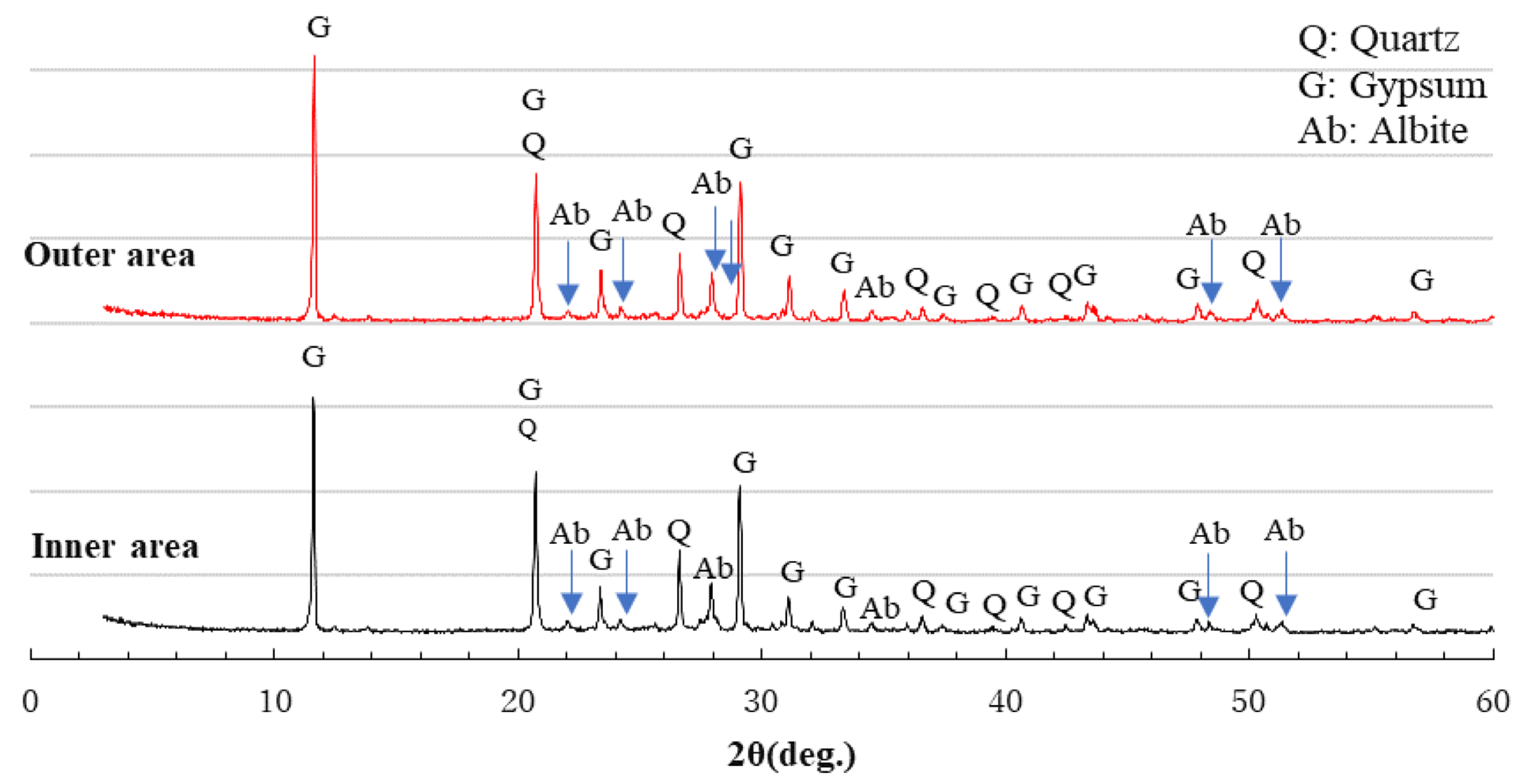
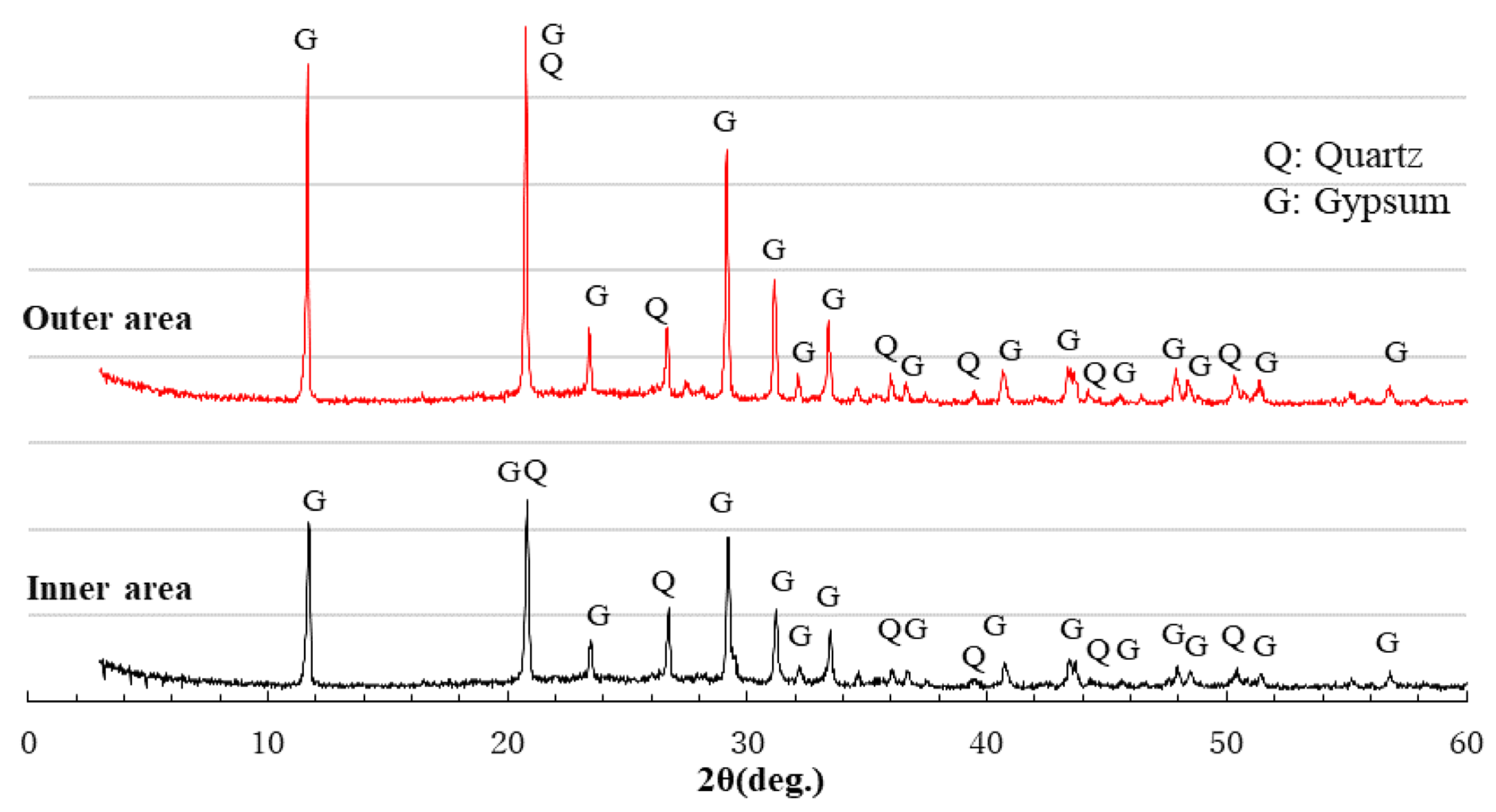
3.6. XRD Analysis
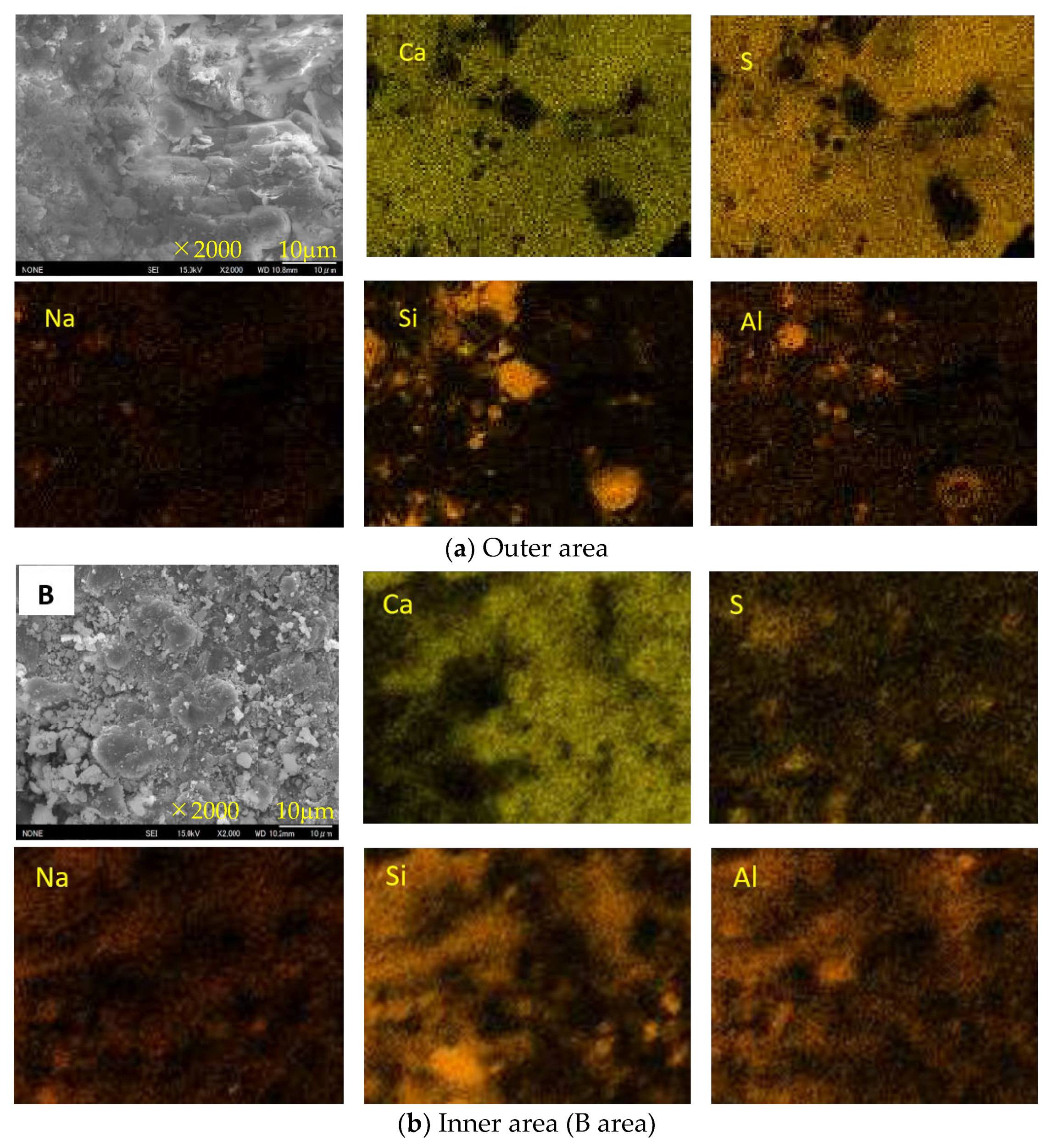
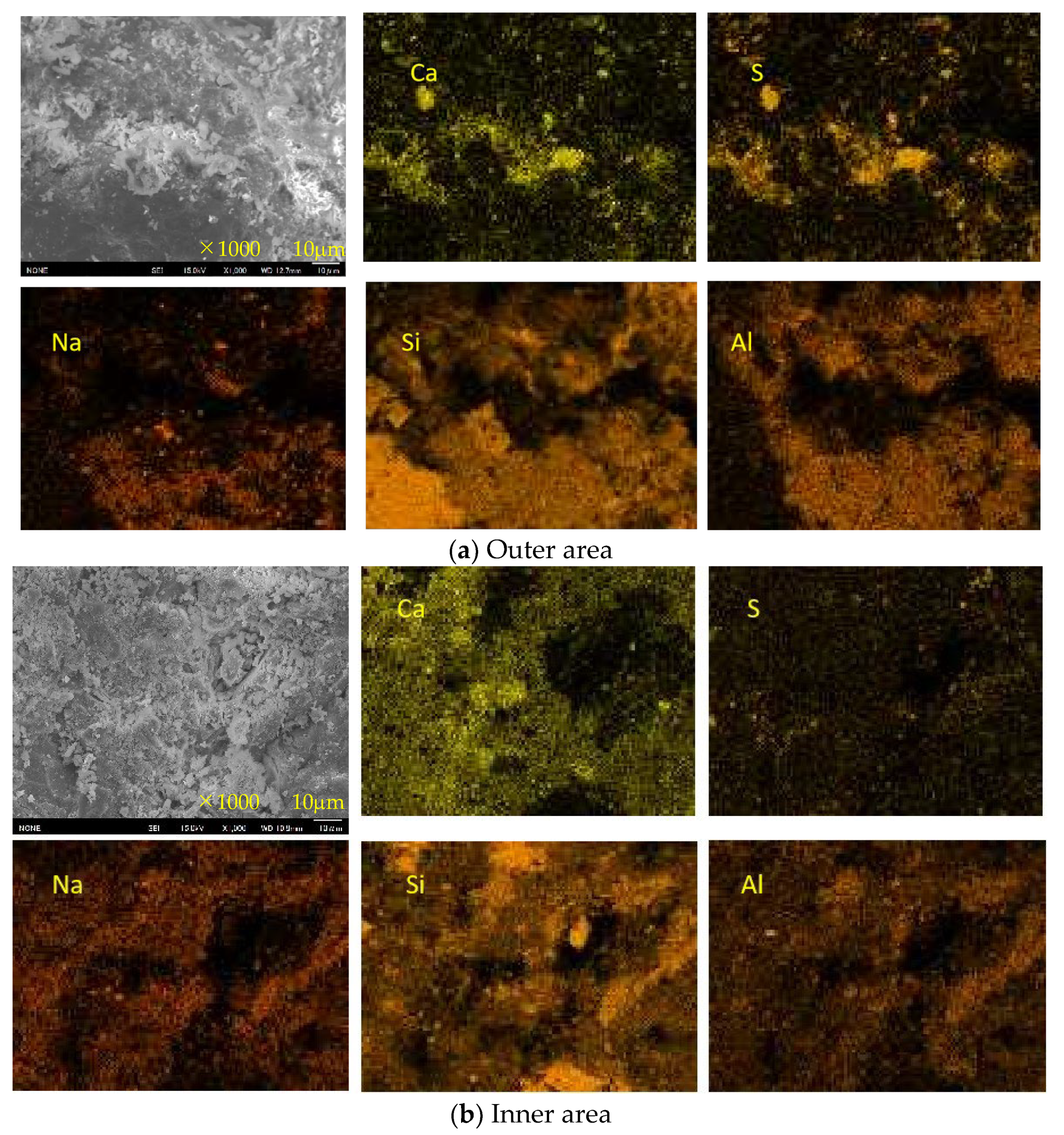
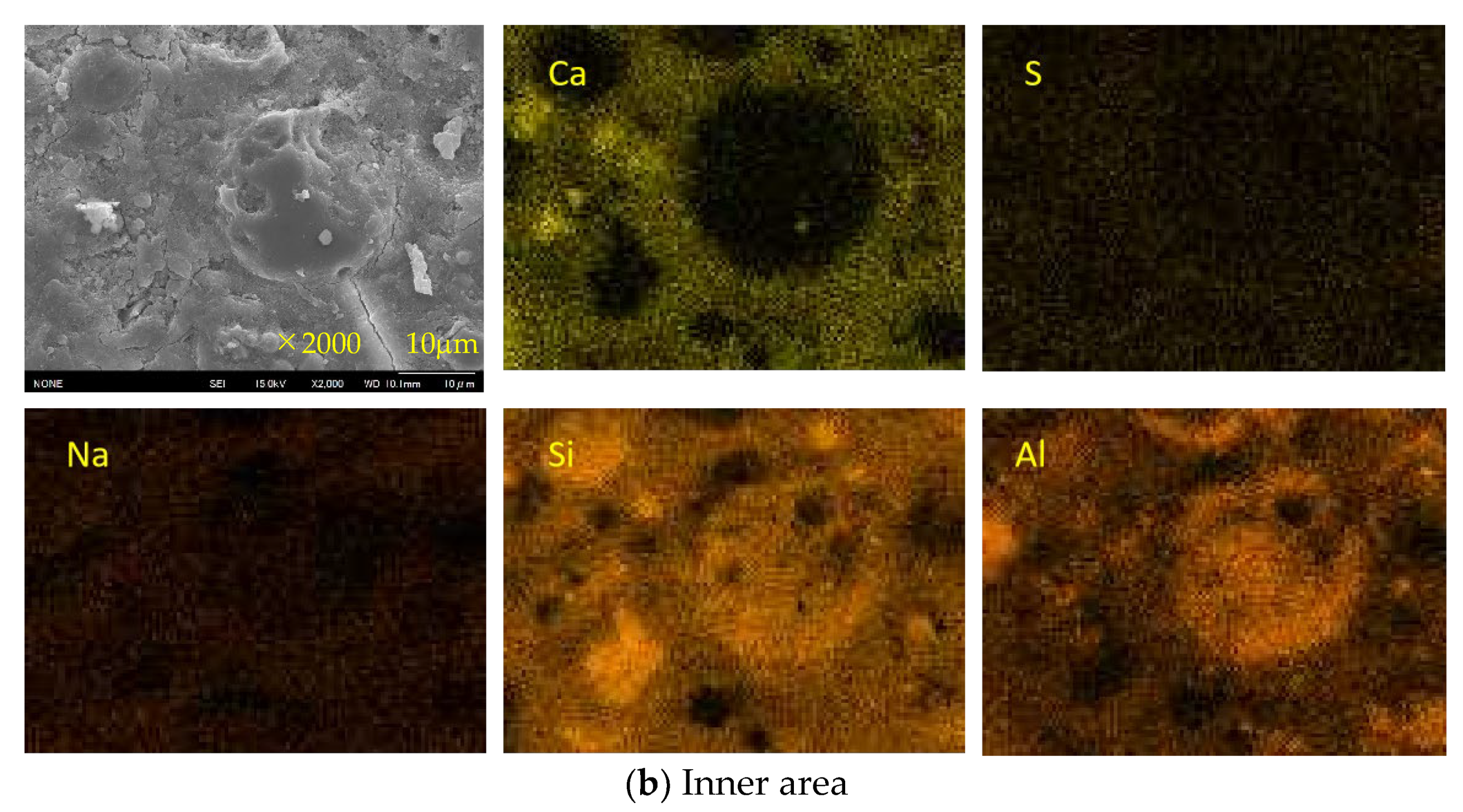
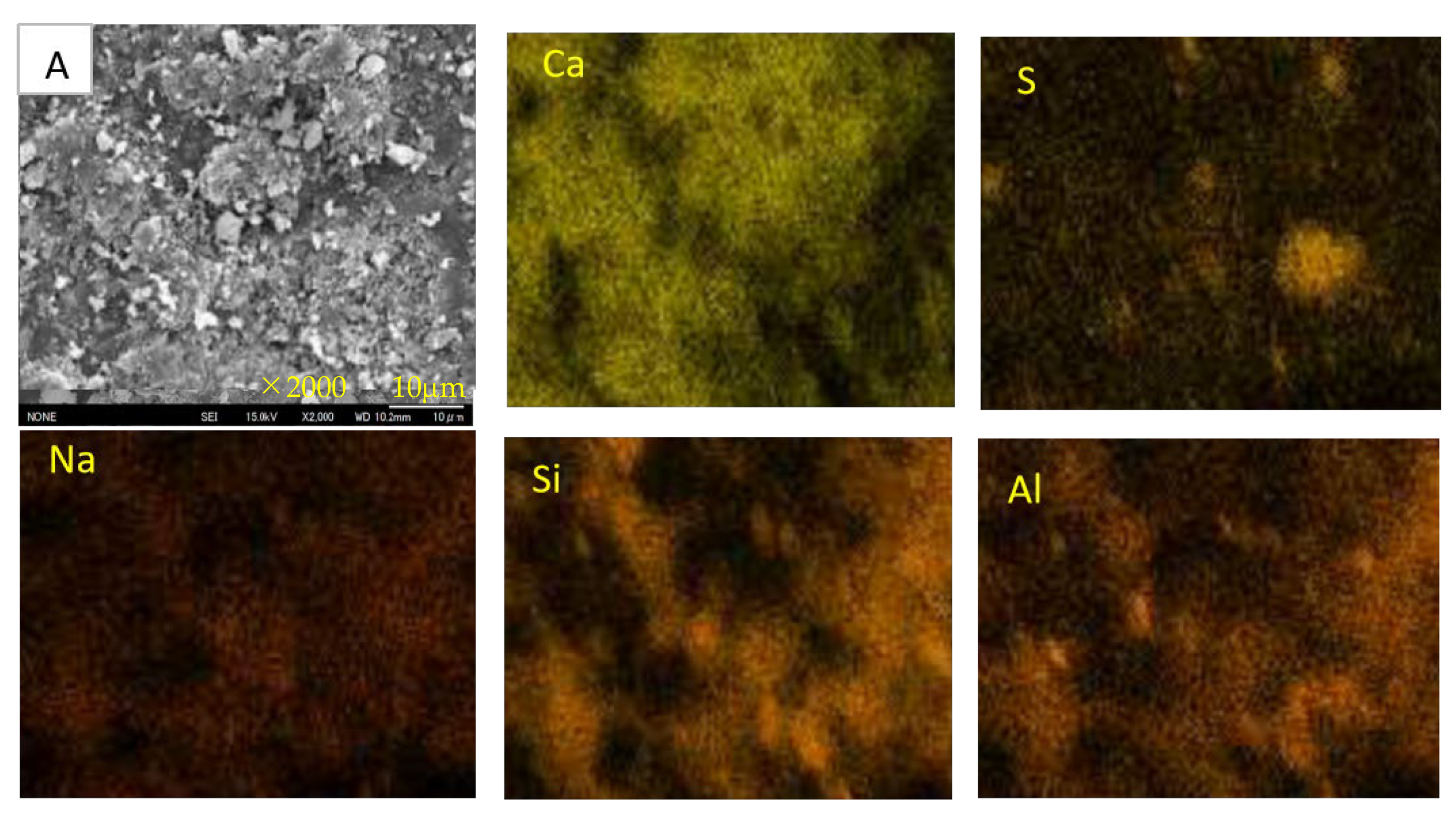
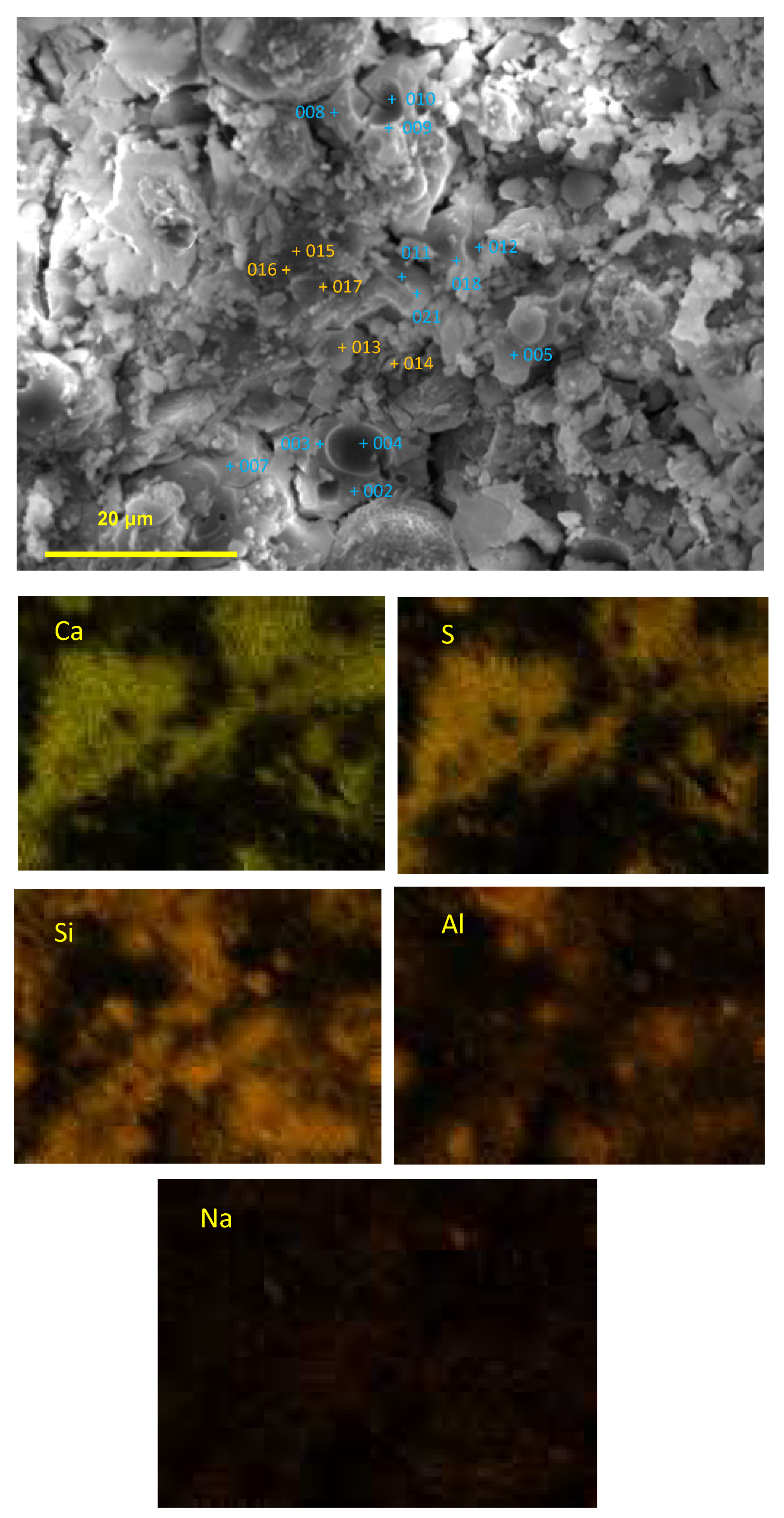
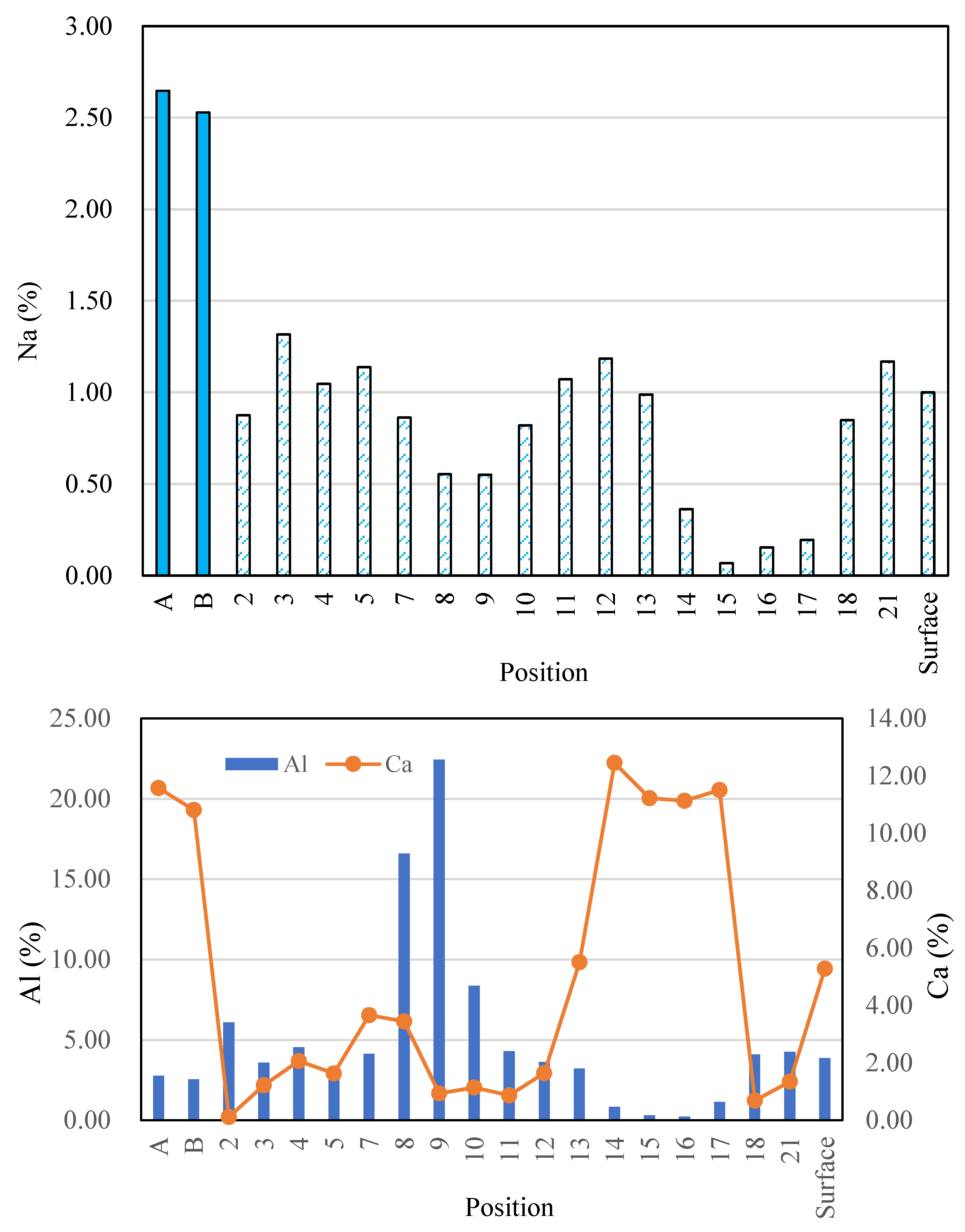
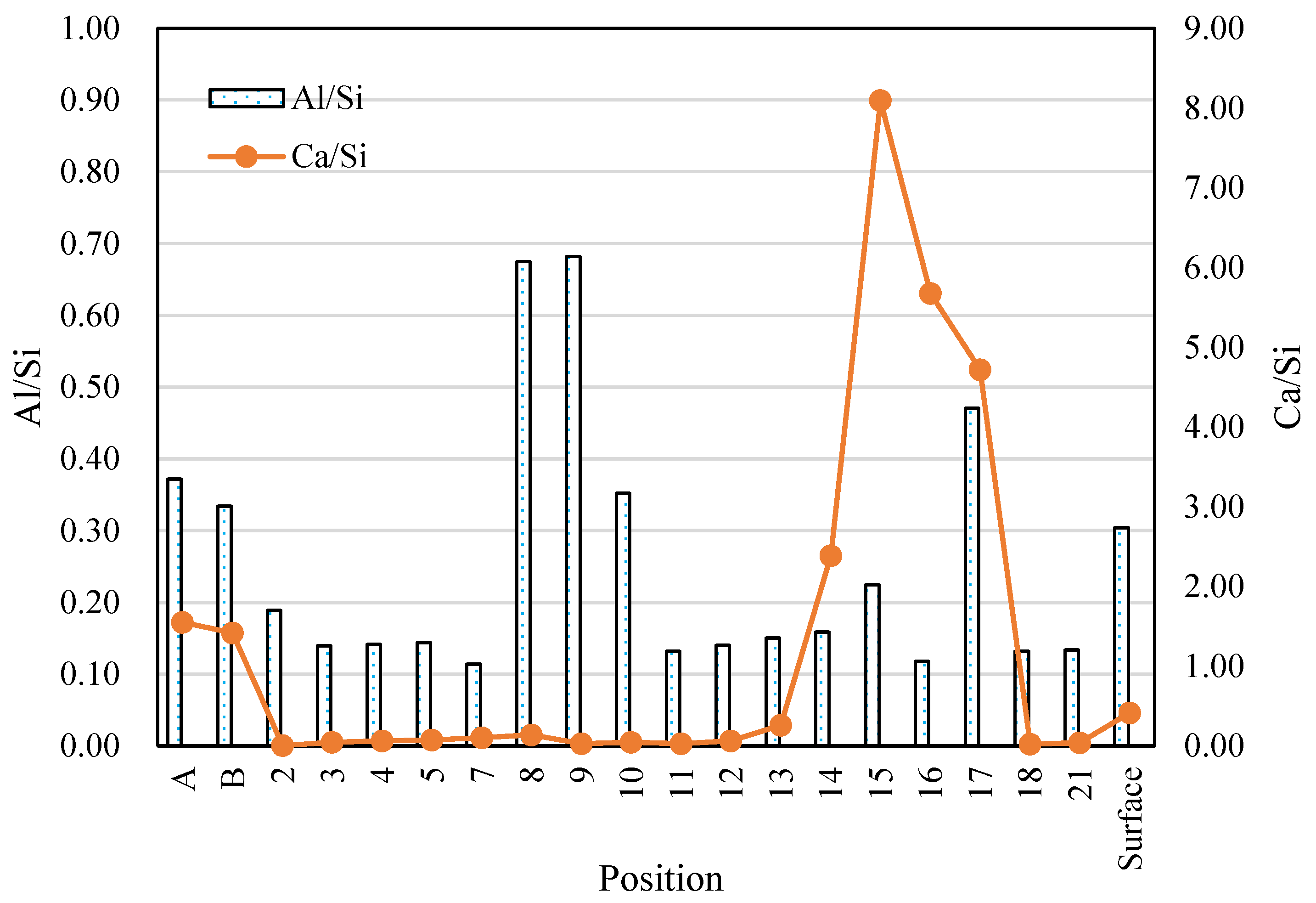
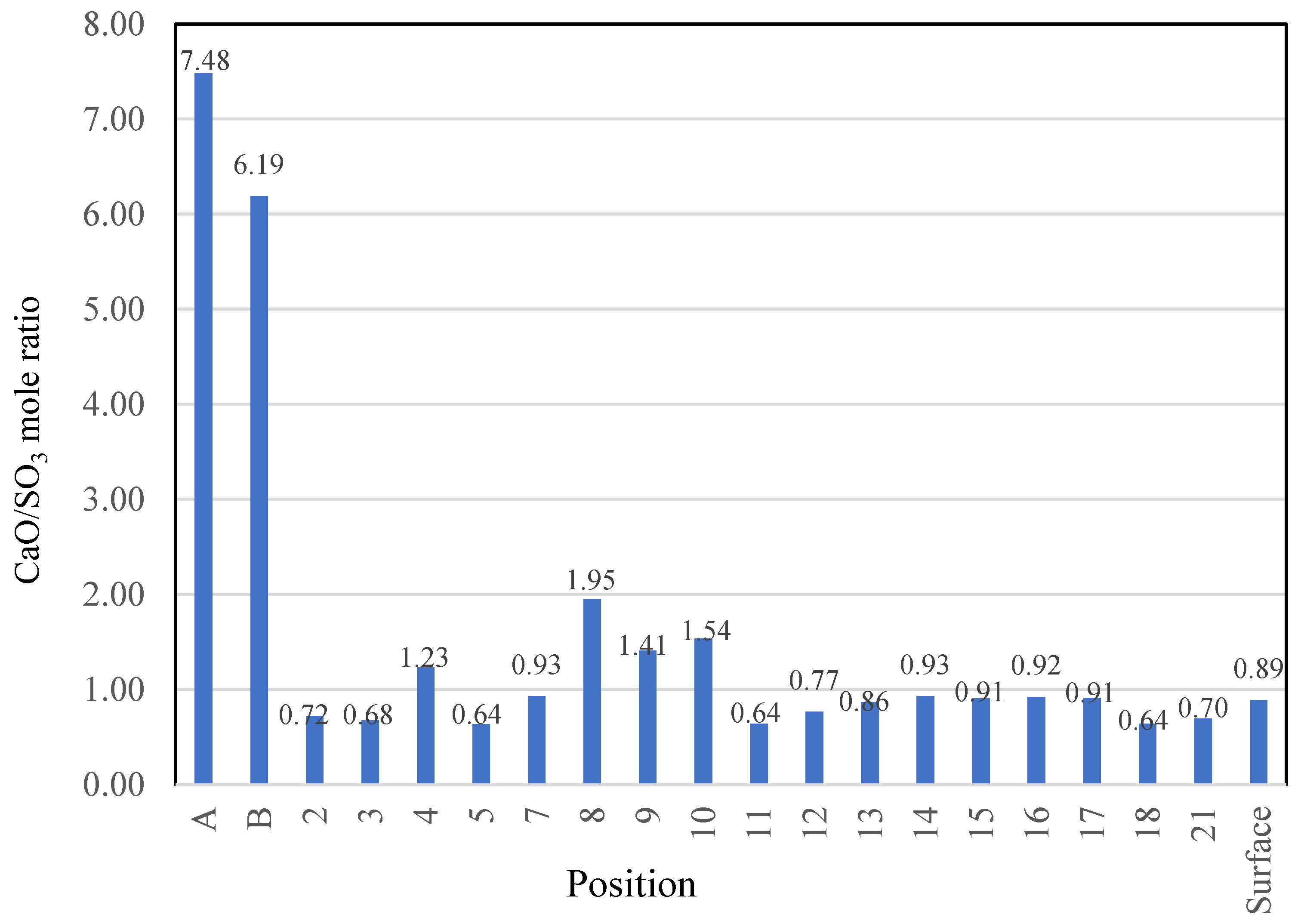
3.7. SEM-EDS Analysis
4. Conclusions
- (1)
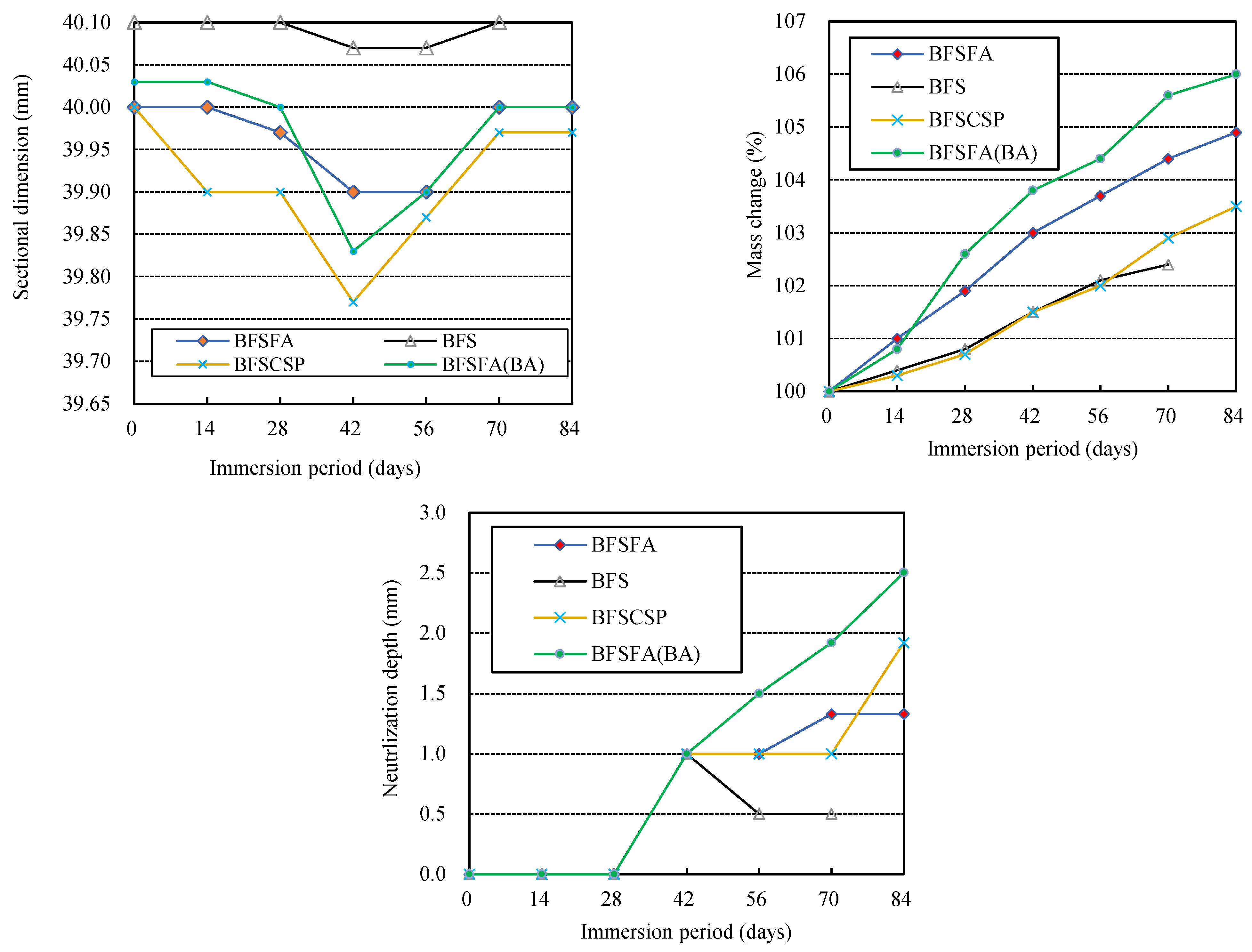
- During immersion in sulfuric acid, the Ca-rich alkali-activated binder without aggregate showed a decrease and then an increase in dimension, although the mass kept increasing. There was cracking and breakdown of the specimens, but no spalling, perhaps due to the absence of aggregate constraint.
- (2)
- The physical degradation (scaling, spalling, cracking, breaking, etc.) and the chemical degradation (alkalinity decrease) of calcium-rich AAMs are not affected by the used raw materials and mix proportions in exactly the same way, so the physical and chemical degradation need to be considered separately when discussing the influencing factors of acid resistance of AAMs.
- (3)
- For AAFS, heat curing and the use of a blend type of alkali activator solution (AS) of sodium silicate (SS) and NaOH can reduce both physical and chemical degradation, compared with ambient curing and the use of a sodium silicate (SS) or NaOH solution. The higher the blending ratio of SS in a blend type AS of SS and NaOH, the more likely the AAFS was to undergo physical degradation and chemical degradation. The AAFS has a higher resistance to chemical degradation when the blended AS has a higher NaOH content.
- (4)
- The larger the liquid-filler ratio, the more severe the physical and chemical degradation of AAM. The higher the blending ratio of GGBFS in the fillers, the more severe the physical degradation of AAFS exposed to sulfuric acid, but the lesser the chemical degradation. Replacing FA in AAFS with CSP or adding BA to AAFS will increase AAM’s physical and chemical degradation.
- (5)
- The use of retarder of sodium tartrate increases the physical and chemical degradation, but the AE only increases the chemical degradation. Using a drying shrinkage reducer composed of polyether derivatives has almost no effect on the sulfuric acid resistance of AAFS.
- (6)
- After sulfuric acid immersion, XRD analysis can identify whether gypsum is formed in Ca-rich AAMs. The more severe the physical degradation of the Ca-rich AAMs, the higher the gypsum peak in the XRD pattern of the corroded area.
- (7)
- For Ca-rich AAMs exposed to sulfuric acid, the dealkalization, decalcification, dealumination of C-A-S-H gels can be found from the distribution patterns of Ca, S, Al, and Na elements obtained by the SEM-EDS analysis. The average Ca/Si atomic ratio and the average Na atomic percentage in the acid corrosion area are smaller than in the unaffected area. The CaO/SO3 molar ratio at the location of gypsum generation ranges from 0.8 to 1.0. However, the Al atomic percentage and Al/Si atomic ratio do not demonstrate if the dealumination of the C-A-S-H gel occurs in the acid corrosion area.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, B.; Cohen, M.D. Does Gypsum Formation during Sulfate Attack on Concrete Lead to Expansion? Cem. Concr. Res. 2000, 30, 117–123. [Google Scholar] [CrossRef]
- Goyal, S.; Kumar, M.; Sidhu, D.S.; Bhattacharjee, B. Resistance of Mineral Admixture Concrete to Acid Attack. J. Adv. Concr. Technol. 2009, 7, 273–283. [Google Scholar] [CrossRef]
- Khitab, A.; Arshad, M.T.; Awan, F.M.; Khan, I. Development of an Acid Resistant Concrete: A Review. Int. J. Sustain. Constr. Eng. Technol. 2013, 4, 33–38. [Google Scholar]
- Rathinam, K.; Kanagarajan, V.; Banu, S. Evaluation of Protective Coatings for Geopolymer Mortar under Aggressive Environment. Adv. Mater. Res. 2020, 9, 219–231. [Google Scholar] [CrossRef]
- Yuan, H.; Dangla, P.; Chatellier, P.; Chaussadent, T. Degradation Modelling of Concrete Submitted to Sulfuric Acid Attack. Cem. Concr. Res. 2013, 53, 267–277. [Google Scholar] [CrossRef]
- Ministry of Land, Infrastructure, Transport and Tourism. Transport and Tourism, Japan, Report of Committee for Sewerage Management Method toward Sustainable Sewerage Business Management in the Era of Reduced Population; Ministry of Land, Infrastructure, Transport and Tourism: Tokyo, Japan, 2020. [Google Scholar]
- Davidovits, J. A Review on GP Cement; Geopolymer Institute: Saint-Quentin, France, 2003; pp. 1–10. Available online: http://www.geopolymer.org/ (accessed on 22 February 2023).
- Nath, P.; Sarker, P.K. Effect of GGBFS on Setting, Workability and Early Strength Properties of Fly Ash Geopolymer Concrete Cured in Ambient Condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Rafeet, A.; Vinai, R.; Soutsos, M.; Sha, W. Effects of Slag Substitution on Physical and Mechanical Properties of Fly Ash-Based Alkali Activated Binders (AABs). Cem. Concr. Res. 2019, 122, 118–135. [Google Scholar] [CrossRef]
- Xue, G.; Yilmaz, E.; Song, W.; Cao, S. Compressive Strength Characteristics of Cemented Tailings Backfill with Alkali-Activated Slag. Appl. Sci. 2018, 8, 1537. [Google Scholar] [CrossRef]
- Ajay, A.; Ramaswamy, K.P.; Thomas, A.V. A Critical Review on the Durability of Geopolymer Composites in Acidic Environment. IOP Conf. Ser. Earth Environ. Sci. 2020, 491, 012044. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Mohammed, A.A.; Mohammed, A.S. The Role of Nanomaterials in Geopolymer Concrete Composites: A State-of-the-Art Review. J. Build. Eng. 2022, 49, 104062. [Google Scholar] [CrossRef]
- Gevaudan, J.P.; Caicedo-Ramirez, A.; Hernandez, M.T.; Srubar, W.V. Copper and Cobalt Improve the Acid Resistance of Alkali-Activated Cements. Cem. Concr. Res. 2019, 115, 327–338. [Google Scholar] [CrossRef]
- Aliques-Granero, J.; Tognonvi, T.M.; Tagnit-Hamou, A. Durability Test Methods and Their Application to AAMs: Case of Sulfuric-Acid Resistance. Mater. Struct. Constr. 2017, 50, 36. [Google Scholar] [CrossRef]
- Aiken, T.A.; Kwasny, J.; Sha, W. Resistance of Alkali-Activated Binders to Organic Acids Found in Agri-Food Effluents. J. Mater. Civ. Eng. 2021, 33, 04021024. [Google Scholar] [CrossRef]
- Jia, Z.; Cao, R.; Chen, C.; Zhang, Y. Using In-Situ Observation to Understand the Leaching Behavior of Portland Cement and Alkali-Activated Slag Pastes. Compos. Part B Eng. 2019, 177, 107366. [Google Scholar] [CrossRef]
- Aiken, T.A.; Kwasny, J.; Sha, W.; Soutsos, M.N. Effect of Slag Content and Activator Dosage on the Resistance of Fly Ash Geopolymer Binders to Sulfuric Acid Attack. Cem. Concr. Res. 2018, 111, 23–40. [Google Scholar] [CrossRef]
- Lloyd, R.R.; Provis, J.L.; Deventer, J.S.J. Acid Resistance of Inorganic Polymer Binders. 1. Corrosion Rate. Mater. Struct. 2012, 45, 1–14. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, H.K. Influence of the Slag Content on the Chloride and Sulfuric Acid Resistances of Alkali-Activated Fly Ash/Slag Paste. Cem. Concr. Compos. 2016, 72, 168–179. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A. Alkali-Aggregate Reaction in Activated Fly Ash Systems. Cem. Concr. Res. 2007, 37, 175–183. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of Geopolymer Materials to Acid Attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Thokchom, S.; Ghosh, P.; Ghosh, S. Resistance of Fly Ash Based Geopolymer Mortars in Sulfuric Acid. ARPN J. Eng. Appl. Sci. 2009, 4, 65–70. [Google Scholar]
- Aiken, T.A.; Gu, L.; Kwasny, J.; Huseien, G.F.; McPolin, D.; Sha, W. Acid Resistance of Alkali-Activated Binders: A Review of Performance, Mechanisms of Deterioration and Testing Procedures. Constr. Build. Mater. 2022, 342, 128057. [Google Scholar] [CrossRef]
- Takagaito, H.; Li, Z.; Nagai, T.; Okada, T. Experimental Study on Sulfuric Acid Resistance of FA/GGBFS Blend-Based Geopolymer Concrete. Proc. Japan Concr. Inst. 2017, 39, 80–86. [Google Scholar] [CrossRef]
- Li, Z. Study on Geopolymers Using Crushed Stone Powder, a by-Product of Crushed Stone Production by Wet Process. Proc. Japan Concr. Inst. 2022, 44, 1078–1083. (In Japanese) [Google Scholar] [CrossRef]
- Li, Z.; Kondo, R.; Ikeda, K. Recycling of Waste Incineration Bottom Ash and Heavy Metal Immobilization by Geopolymer Production. J. Adv. Concr. Technol. 2021, 19, 259–279. [Google Scholar] [CrossRef]
- Okada, T.; Suga, A.; Hashitume, S.; Li, Z. Study on Retarder of Geopolymer. Proc. Japan Concr. Inst. 2015, 37, 1975–1980. [Google Scholar] [CrossRef]
- Okada, T.; Li, Z.; Hashizume, S.; Nagai, T. Effect of Shrinkage Reducing Agents on the Performances of Geopolymer Concrete with Fly Ash and GGBFS. Proc. Japan Concr. Inst. 2017, 39, 2034. (In Japanese) [Google Scholar]
- Ren, J.; Zhang, L.; Walkley, B.; Black, J.R.; San Nicolas, R. Degradation Resistance of Different Cementitious Materials to Phosphoric Acid Attack at Early Stage. Cem. Concr. Res. 2022, 151, 106606. [Google Scholar] [CrossRef]
- Gu, L.; Bennett, T.; Visintin, P. Sulphuric Acid Exposure of Conventional Concrete and Alkali-Activated Concrete: Assessment of Test Methodologies. Constr. Build. Mater. 2019, 197, 681–692. [Google Scholar] [CrossRef]
- Allahverdi, A.; Škvára, F. Sulfuric Acid Attack on Hardened Paste of Geopolymer Cements Part 1. Mechanism of Corrosion at Relatively High Concentrations. Ceram. Silikaty 2005, 49, 225–229. [Google Scholar]
- Allahverdi, A.; Škvára, F. Sulfuric Acid Attack on Hardened Paste of Geopolymer Cements Part 2. Corrosion Mechanism at Mild and Relatively Low Concentrations. Ceram. Silikaty 2006, 50, 1–4. [Google Scholar]
- Gu, L.; Visintin, P.; Bennett, T. Evaluation of Accelerated Degradation Test Methods for Cementitious Composites Subject to Sulfuric Acid Attack; Application to Conventional and Alkali-Activated Concretes. Cem. Concr. Compos. 2018, 87, 187–204. [Google Scholar] [CrossRef]
- Sata, V.; Sathonsaowaphak, A.; Chindaprasirt, P. Resistance of Lignite Bottom Ash Geopolymer Mortar to Sulfate and Sulfuric Acid Attack. Cem. Concr. Compos. 2012, 34, 700–708. [Google Scholar] [CrossRef]
- Izquierdo, M.; Querol, X.; Davidovits, J.; Antenucci, D.; Nugteren, H.; Fernández-Pereira, C. Coal Fly Ash-Slag-Based Geopolymers: Microstructure and Metal Leaching. J. Hazard. Mater. 2009, 166, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Tahri, W.; Abdollahnejad, Z.; Mendes, J.; Pacheco-Torgal, F.; de Aguiar, J.B. Cost Efficiency and Resistance to Chemical Attack of a Fly Ash Geopolymeric Mortar versus Epoxy Resin and Acrylic Paint Coatings. Eur. J. Environ. Civ. Eng. 2017, 21, 555–571. [Google Scholar] [CrossRef]
- Li, Z.; Takagaito, H.; Okada, T.; Hashizume, S. Factors Affecting Mechanical Performance of Fly Ash/Slag-Based Geopolymer Concrete. Proc. Japan Concr. Inst. 2019, 4, 1955–1960. (In Japanese) [Google Scholar]
- Li, Z.; Li, S. Carbonation Resistance of Fly Ash and Blast Furnace Slag Based Geopolymer Concrete. Constr. Build. Mater. 2018, 163, 668–680. [Google Scholar] [CrossRef]
- Okada, T.; Li, Z.; Hashitume, S.; Nagai, T. Experimental Study on the Performances of Geopolymer Concrete with Retarder. Proc. Japan Concr. Inst. 2016, 38, 2295–2300. Available online: https://data.jci-net.or.jp/data_pdf/38/038-01-1378.pdf (accessed on 22 February 2023).
- Komljenović, M.M.; Baščarević, Z.; Marjanović, N.; Nikolić, V. Decalcification Resistance of Alkali-Activated Slag. J. Hazard. Mater. 2012, 233–234, 112–121. [Google Scholar] [CrossRef]







| Precursors | Chemical Compositions (%, by Mass) | Specific Gravity | Blaine Value (cm2/g) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | CaO | Al2O3 | FexOy | Na2O | K2O | MgO | P2O5 | TiO2 | Cl | Others | |||
| GGBFS | 33.9 | 42.7 | 15.3 | 0.3 | 0.3 | 0.3 | 5.8 | 0.0 | 0.6 | - | 0.8 | 2.90 | 4080 |
| FA | 58.6 | 3.6 | 24.6 | 6.1 | 1.1 | 1.6 | 1.2 | 0.9 | 1.2 | - | 0.6 | 2.28 | 4392 |
| CSP | 58.8 | 9.2 | 14.7 | 7.8 | 3.1 | 2.4 | 2.7 | 0.2 | 0.7 | - | 0.4 | 2.73 | 2500 |
| BA | 40.1 | 24.0 | 16.0 | 4.8 | 4.2 | 2.2 | 1.9 | 2.8 | 1.6 | 0.7 | 1.2 | 2.12 | 3.58 * |
| Designation of AS | Components (Volume Ratio) | Specific Gravity | Solid Concentration by Mass (%) | Na2O Concentration by Mass (%) | Mole Ratio | |
|---|---|---|---|---|---|---|
| SiO2/Na2O | Na/H2O | |||||
| AS10 | Diluted Na2SiO3 solution | 1.352 | 63.0 | 11.4 | 2.10 | 0.101 |
| AS01 | 10 mol/L NaOH solution | 1.334 | 30.0 | 23.2 | 0.00 | 0.193 |
| AS41 | 4 AS10 + 1 AS01 | 1.348 | 56.5 | 13.7 | 1.40 | 0.120 |
| AS31 | 3 AS10 + 1 AS01 | 1.347 | 54.8 | 14.3 | 1.26 | 0.125 |
| AS21 | 2 AS10 + 1 AS01 | 1.346 | 52.1 | 15.3 | 1.05 | 0.132 |
| Series | AS | F | GGBFS:FA | AS/F | Admixture | Curing Method, Age |
|---|---|---|---|---|---|---|
| AS31-43 | AS31 | GGBFS + FA | 4:6 | 0.43 | None | Heat, 28 days |
| AS31-43(A) | Ambient, 28 days | |||||
| AS10-43 | AS10 | Heat, 28 days | ||||
| AS10-43(A) | 0.43 | Ambient, 28 days | ||||
| AS/F43 | AS31 | GGBFS + FA | 4:6 | 0.43 | None | Heat, 7days |
| AS/F48 | 0.48 | |||||
| AS/F50 | 0.50 | |||||
| AS10-45 | AS10 | GGBFS + FA | 4:6 | 0.45 | None | Heat, 7days |
| AS41-45 | AS41 | |||||
| AS21-45 | AS21 | |||||
| AS01-45 | AS01 | |||||
| BFSFA | AS31 | GGBFS + FA | 5:5 | 0.40 | None | Heat, 7days |
| BFS | GGBFS | 10:0 | ||||
| BFSCSP | GGBFS + CSP | 4:6 | ||||
| BFSFA(BA) | GGBFS + FA | 4:6 | 0.60 | BA = F × 150% | ||
| R | AS31 | GGBFS + FA | 4:6 | 0.40 | R = F × 5% | Heat, 7days |
| SRA | SRA = F × 3% | |||||
| AE | 4:6 | AE = F × 0.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Ikeda, K. Influencing Factors of Sulfuric Acid Resistance of Ca-Rich Alkali-Activated Materials. Materials 2023, 16, 2473. https://doi.org/10.3390/ma16062473
Li Z, Ikeda K. Influencing Factors of Sulfuric Acid Resistance of Ca-Rich Alkali-Activated Materials. Materials. 2023; 16(6):2473. https://doi.org/10.3390/ma16062473
Chicago/Turabian StyleLi, Zhuguo, and Ko Ikeda. 2023. "Influencing Factors of Sulfuric Acid Resistance of Ca-Rich Alkali-Activated Materials" Materials 16, no. 6: 2473. https://doi.org/10.3390/ma16062473
APA StyleLi, Z., & Ikeda, K. (2023). Influencing Factors of Sulfuric Acid Resistance of Ca-Rich Alkali-Activated Materials. Materials, 16(6), 2473. https://doi.org/10.3390/ma16062473






