DFT Method Used for Prediction of Molecular and Electronic Structures of Mn(VI) Macrocyclic Complexes with Porhyrazine/Phthalocyanine and Two Oxo Ligands
Abstract
1. Introduction
2. Method
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mikhailov, O.V.; Chachkov, D.V. M(VI) Oxidation State Stabilization in Iron, Cobalt and Nickel Heteroligand Metal Chelates Containing 3,7,11,15-Tetraazaporphine and Two Axial Oxo Ligands: Quantum-Chemical Simulation. Int. J. Mol. Sci. 2020, 21, 1494. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Chachkov, D.V. Stabilization of dioxochromium(VI) in the complex with tetra[benzo]porphyrazine and two oxo ligands: DFT quantum-chemical consideration. Eur. Chem. Bull. 2020, 9, 416–419. [Google Scholar] [CrossRef]
- Chachkov, D.V.; Mikhailov, O.V. Heteroligand complexes of chromium, manganese, and iron with trans-dibenzoporphyrazine and two oxo ligands: DFT calculations. Russ. Chem. Bull. 2022, 71, 656–664. [Google Scholar] [CrossRef]
- Chachkov, D.V.; Mikhailov, O.V. DFT Quantum-chemical prediction of molecular structure of iron(VI) macrocyclic complex with phthalocyanine and two oxo ligands. J. Porph. Phthalocyanines 2022, 26, 367–375. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Chachkov, D.V. DFT Quantum-Chemical Modeling Molecular Structures of Cobalt Macrocyclic Complexes with Porphyrazine or Its Benzo-Derivatives and Two Oxygen Acido Ligands. Int. J. Mol. Sci. 2020, 21, 9085. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, O.V.; Chachkov, D.V. Copper macrocyclic complex with trans-di[benzo]porphyrazine and two oxo ligands: DFT quantum-chemical design. J. Porph. Phthalocyanines 2022, 26, 180–185. [Google Scholar] [CrossRef]
- Kasuda, K.; Tsutsui, M. Some new developments in the chemistry of metallophthalocyanines. Coord. Chem. Rev. 1980, 32, 67–95. [Google Scholar] [CrossRef]
- Thomas, A.L. Phthalocyanines. Research & Applications; CRC Press: London, UK, 1990. [Google Scholar]
- Sliva, W.; Mianovska, B. Metalloporphyrin arrays. Transit. Met. Chem. 2000, 25, 491–504. [Google Scholar] [CrossRef]
- Spasojević, I.; Ines Batinić-Haberle, I. Manganese(III) complexes with porphyrins and related compounds as catalytic scavengers of superoxide. Inorg. Chim. Acta 2001, 317, 230–242. [Google Scholar] [CrossRef]
- Mamardashvili, G.M.; Mamardashvili, N.Z.; Koifman, O.I. Self-assembling systems based on porphyrins. Russ. Chem. Rev. 2008, 77, 59–75. [Google Scholar] [CrossRef]
- Donzello, M.P.; Ercolani, C.; Novakova, V.; Zimcik, P.; Stuzhin, P.A. Tetrapyrazinoporphyrazines and their metal derivatives. Part I: Synthesis and basic structural information. Coord. Chem. Rev. 2016, 309, 107–179. [Google Scholar] [CrossRef]
- Lomova, T.N. Axial Coordinated Metal Porphyrins in Science and Practice; URSS: Moscow, Russia, 2018; 700p. (In Russian) [Google Scholar]
- Khelevina, O.G.; Malyasova, A.S. 40 years with porphyrazines. J. Porph. Phthalocyanines 2019, 23, 1251–1264. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Chachkov, D.V. (H,H)-Isomerism of cis- and trans-di[benzo]porphyrazines: Quantum chemical modeling within the framework of the DFT method. J. Porph. Phthalocyanines 2021, 25, 858–865. [Google Scholar] [CrossRef]
- Chachkov, D.V.; Mikhailov, O.V. Nickel macrocyclic complexes with porphyrazine and some [benzo]substituted, oxo and fluoro ligands: DFT analysis. J. Porph. Phthalocyanines 2022, 26, 222–231. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Chachkov, D.V. New heteroligand complex of cobalt with phthalocyanine, oxo and fluoro ligands: DFT consideration. J. Porph. Phthalocyanines 2022, 26, 316–324. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Wang, Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 1996, 54, 16533–16539. [Google Scholar] [CrossRef]
- Schaefer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Schaefer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Medvedev, M.G.; Bushmarinov, I.S.; Sun, J.; Perdew, J.P.; Lyssenko, K.A. Density functional theory is straying from the path toward the exact functional. Science 2017, 355, 49–52. [Google Scholar] [CrossRef]
- Chachkov, D.V.; Mikhailov, O.V. Quantum-chemical calculation of molecular structures of (5656)macrotetracyclic 3d-metal complexes “self-assembled” in quaternary systems M(II) ion-ethanedithioamide-formaldehyde-ammonia by the density functional theory method. Russ. J. Inorg. Chem. 2014, 59, 218–223. [Google Scholar] [CrossRef]
- Chachkov, D.V.; Mikhailov, O.V. Structure of (5656)macrotetracyclic chelates in the ternary systems M(II)-ethanedithioamide-acetone (M = Mn, Fe, Co, Ni, Cu, Zn) according to DFT calculations. Russ. J. Inorg. Chem. 2013, 58, 1073–1078. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Chachkov, D.V. Copper(IV) Stabilization in Macrocyclic Complexes with 3,7,11,15-Tetraazaporphine, Its Di[benzo]- or Tetra[benzo] Derivatives and Oxide Anion: Quantum-Chemical Research. Materials 2020, 13, 3162. [Google Scholar] [CrossRef] [PubMed]
- Hoe, W.M.; Cohen, A.; Handy, N.C. Assessment of a new local exchange functional OPTX. Chem. Phys. Lett. 2001, 341, 319–328. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
- Paulsen, H.; Duelund, L.; Winkler, H.; Toftlund, H.; Trautwein, A.X. Free Energy of Spin-Crossover Complexes Calculated with Density Functional Methods. Inorg. Chem. 2001, 40, 2201–2203. [Google Scholar] [CrossRef] [PubMed]
- Swart, M.; Groenhof, A.R.; Ehlers, A.W.; Lammertsma, K. Validation of Exchange−Correlation Functionals for Spin States of Iron Complexes. J. Phys. Chem. A 2004, 108, 5479–5483. [Google Scholar] [CrossRef]
- Swart, M.; Ehlers, A.W.; Lammertsma, K. Performance of the OPBE exchange-correlation functional. Mol. Phys. 2004, 102, 2467–2474. [Google Scholar] [CrossRef]
- Swart, M. Metal–ligand bonding in metallocenes: Differentiation between spin state, electrostatic and covalent bonding. Inorg. Chim. Acta 2007, 360, 179–189. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Weinhold, F.; Landis, C.R.; Glendening, E.D. What is NBO analysis and how is it useful? Int. Rev. Phys. Chem. 2016, 35, 399–440. [Google Scholar] [CrossRef]
- Ochterski, J.W. Thermochemistry in Gaussian; Gaussian, Inc.: Wallingford, CT, USA, 2000. [Google Scholar]
- Mikhailov, O.V.; Chachkov, D.V. About possibility of stabilization of unusual copper(IV) oxidation state in complexes with porphyrazine and two fluorine ligands: Quantum-chemical design. Inorg. Chem. Commun. 2019, 106, 224–227. [Google Scholar] [CrossRef]
- Chachkov, D.V.; Mikhailov, O.V. Molecular structures of heteroligand ScIII complexes with porphyrazine, its dibenzo and tetrabenzo derivatives, and fluoride anion, as determined from DFT calculations. Russ. Chem. Bull. 2021, 70, 276–282. [Google Scholar] [CrossRef]
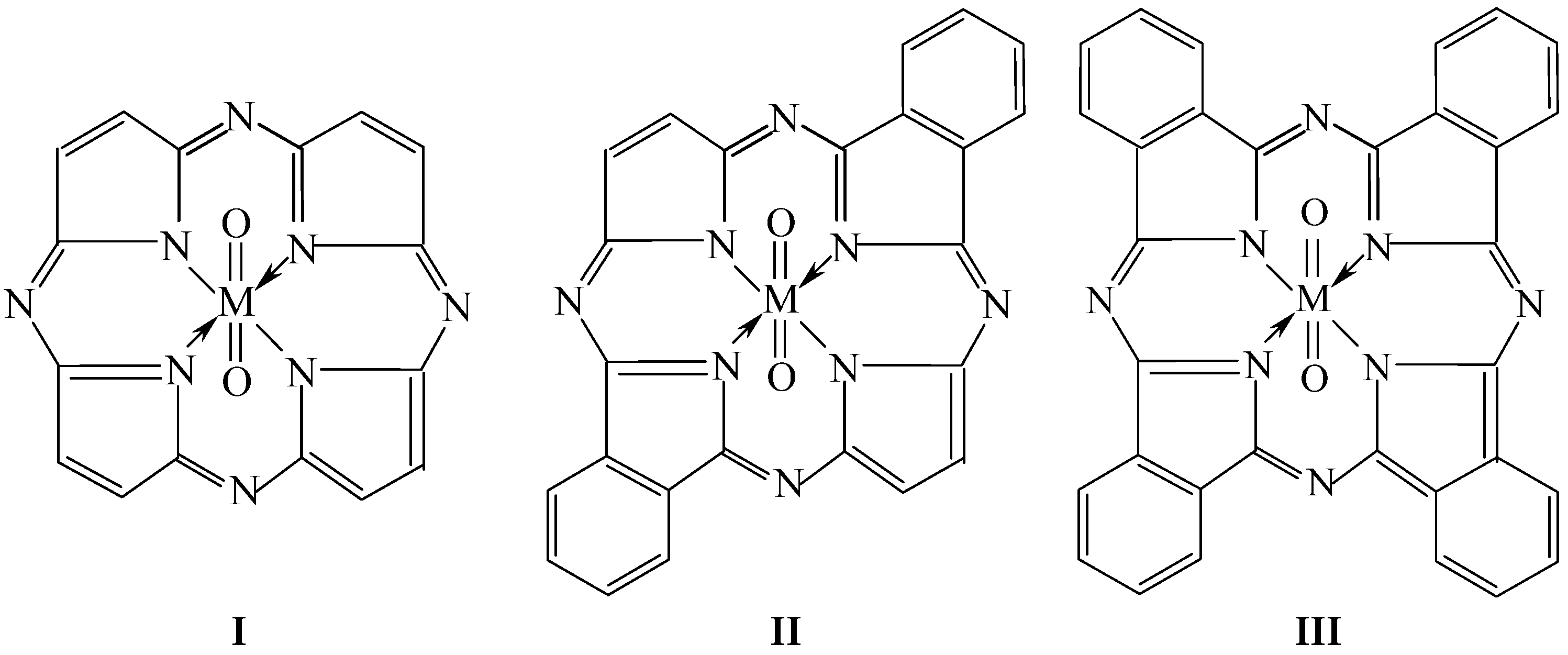

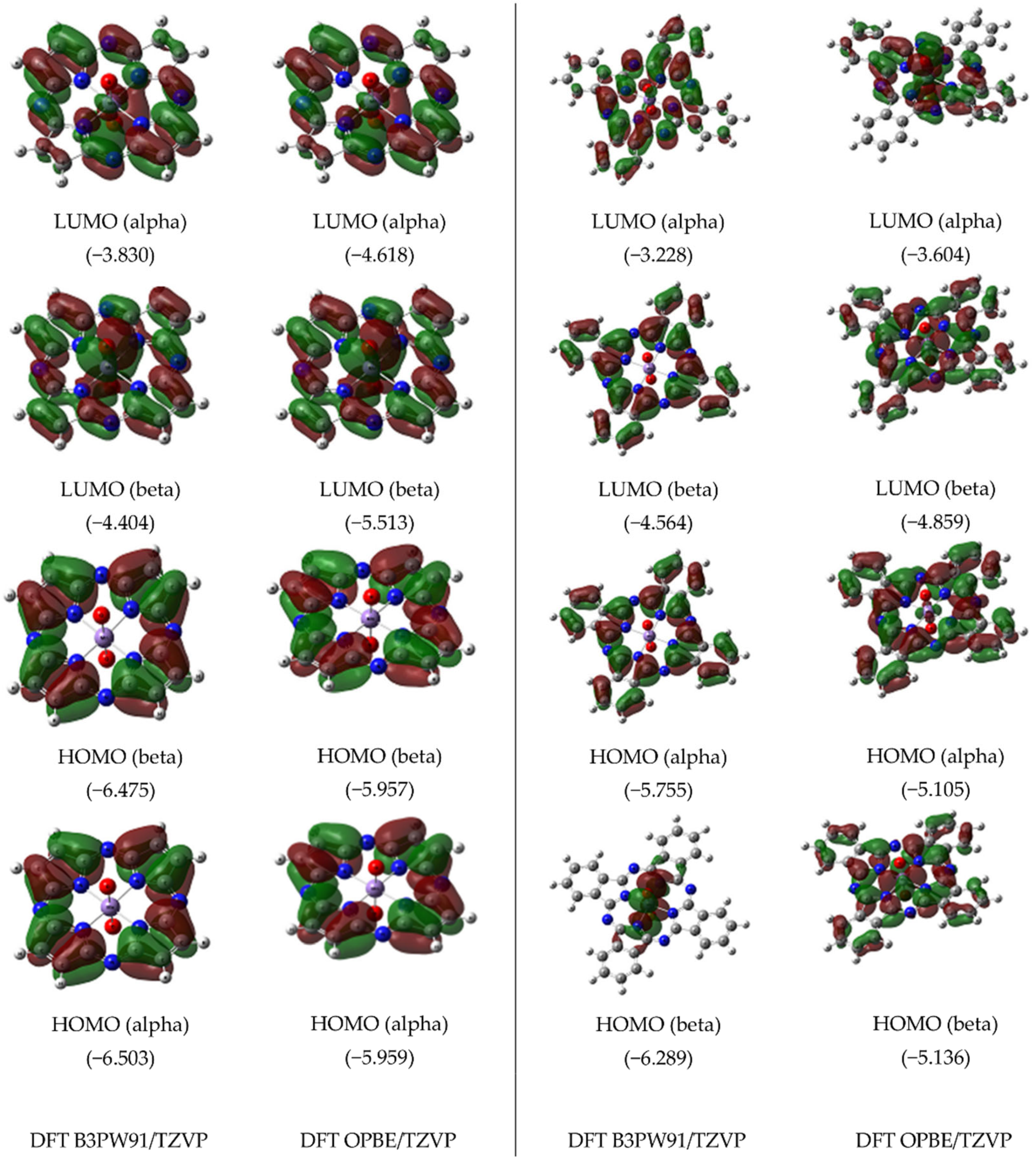

| Macrocyclic Complex | [Mn(P)(O)2] | [Mn(Pc)(O)2] | ||
|---|---|---|---|---|
| Parameter of Molecular Structure | Calculated Using DFT B3PW91/TZVP | Calculated Using DFT OPBE/TZVP | Calculated Using DFT B3PW91/TZVP | Calculated Using DFT OPBE/TZVP |
| Mn-N bond lengths in the MnN4 chelate node, pm | ||||
| Mn1N1 | 193.2 | 195.0 | 198.2 | 198.0 |
| Mn1N2 | 196.1 | 195.0 | 198.2 | 198.0 |
| Mn1N3 | 193.2 | 196.5 | 198.2 | 198.0 |
| Mn1N4 | 196.1 | 196.5 | 198.2 | 198.0 |
| Axial Mn-O bond lengths, pm | ||||
| Mn1O1 | 187.9 | 165.1 | 164.0 | 164.9 |
| Mn1O2 | 177.7 | 165.3 | 164.0 | 164.9 |
| C-N bond lengths in 6-numbered chelate rings, pm | ||||
| N1C3 | 137.0 | 136.5 | 135.3 | 136.2 |
| N1C4 | 137.0 | 137.0 | 135.3 | 136.2 |
| N2C1 | 135.9 | 137.0 | 135.3 | 136.2 |
| N2C2 | 135.9 | 136.5 | 135.3 | 136.2 |
| N3C7 | 137.0 | 136.5 | 135.3 | 136.2 |
| N3C8 | 137.0 | 135.8 | 135.3 | 136.2 |
| N4C5 | 135.9 | 135.8 | 135.3 | 136.2 |
| N4C6 | 135.9 | 136.5 | 135.3 | 136.2 |
| N5C2 | 132.0 | 132.5 | 132.2 | 132.3 |
| N5C3 | 132.0 | 132.5 | 132.2 | 132.3 |
| N6C6 | 132.0 | 132.5 | 132.2 | 132.3 |
| N6C7 | 132.0 | 132.5 | 132.2 | 132.3 |
| N7C4 | 132.0 | 132.5 | 132.2 | 132.3 |
| N7C5 | 132.0 | 132.6 | 132.2 | 132.3 |
| N8C1 | 132.0 | 132.5 | 132.2 | 132.3 |
| N8C8 | 132.0 | 132.6 | 132.2 | 132.3 |
| C-C bond lengths in 5-numbered chelate ring (N1C4C9C10C3), pm | ||||
| C4C9 | 144.4 | 145.4 | 146.8 | 146.3 |
| C9C10 | 135.5 | 135.8 | 139.9 | 140.5 |
| C10C3 | 144.4 | 145.3 | 146.8 | 146.3 |
| Bond angles in the MnN4 chelate node, deg | ||||
| (N1Mn1N2) | 89.9 | 89.4 | 90.0 | 90.0 |
| (N2Mn1N3) | 89.9 | 89.8 | 90.0 | 90.0 |
| (N3Mn1N4) | 89.9 | 91.1 | 90.0 | 90.0 |
| (N4Fe1N1) | 89.9 | 89.8 | 90.0 | 90.0 |
| Bond angles sum (BAS), deg | 359.6 | 360.1 | 360.0 | 360.0 |
| Non-bond angles between N atoms in N4 grouping, deg | ||||
| (N1N2N3) | 89.1 | 90.7 | 90.0 | 90.0 |
| (N2N3N4) | 90.9 | 89.3 | 90.0 | 90.0 |
| (N3N4N1) | 89.1 | 89.3 | 90.0 | 90.0 |
| (N4N1N2) | 90.9 | 90.7 | 90.0 | 90.0 |
| Non-bond angles sum (NBAS), deg | 360.0 | 360.0 | 360.0 | 360.0 |
| Bond angles in 6-numbered chelate ring (Mn1N1C4N7C5N4), deg | ||||
| (Mn1N1C4) | 125.8 | 125.4 | 125.0 | 124.8 |
| (N1C4N7) | 127.9 | 128.2 | 128.2 | 127.7 |
| (C4N7C5) | 122.9 | 123.2 | 123.7 | 123.5 |
| (N7C5N4) | 127.4 | 126.7 | 128.1 | 127.7 |
| (C5N4Mn1) | 125.9 | 126.7 | 125.0 | 124.8 |
| (N4Mn1N1) | 89.9 | 89.8 | 90.0 | 90.0 |
| Bond angles sum (BAS61), deg | 719.8 | 720.0 | 720.0 | 718.5 |
| Bond angles in 6-numbered chelate ring (Mn1N4C6N6C7N3), deg | ||||
| (Mn1N4C6) | 125.9 | 124.7 | 125.0 | 124.8 |
| (N4C6N6) | 127.4 | 127.8 | 128.2 | 127.7 |
| (C6N6C7) | 122.9 | 124.1 | 123.7 | 123.5 |
| (N6C7N3) | 127.9 | 127.8 | 128.1 | 127.7 |
| (C7N3Mn1) | 125.8 | 124.7 | 125.0 | 124.8 |
| (N3Mn1N4) | 89.9 | 91.1 | 90.0 | 90.0 |
| Bond angles sum (BAS62), deg | 719.8 | 720.0 | 720.0 | 718.5 |
| Bond angles in 5-numbered ring (C3N1C4C9C10), deg | ||||
| (C3N1C4) | 108.2 | 108.0 | 110.0 | 109.8 |
| (N1C4C9) | 108.5 | 108.7 | 108.8 | 108.8 |
| (C4C9C10) | 107.4 | 107.2 | 106.2 | 106.3 |
| (C9C10C3) | 107.4 | 107.0 | 106.2 | 106.3 |
| (C10C3N1) | 108.5 | 109.1 | 108.8 | 108.8 |
| Bond angles sum (BAS51), deg | 540.0 | 540.0 | 540.0 | 540.0 |
| Bond angles in 5-numbered ring (C1N2C2C12C11), deg | ||||
| (C1N2C2) | 108.2 | 108.0 | 110.0 | 109.8 |
| (N2C2C12) | 108.9 | 109.1 | 108.8 | 108.8 |
| (C2C12C11) | 107.0 | 107.0 | 106.2 | 106.3 |
| (C12C11C1) | 107.0 | 107.2 | 106.2 | 106.3 |
| (C11C1N2) | 108.9 | 108.7 | 108.8 | 108.8 |
| Bond angles sum (BAS51), deg | 540.0 | 540.0 | 540.0 | 540.0 |
| Bond angles between O, Fe and N atoms, deg | ||||
| (O1Mn1N1) | 86.2 | 93.8 | 90.0 | 90.0 |
| (O1Mn1N2) | 88.9 | 93.8 | 90.0 | 90.0 |
| (O1Mn1N3) | 86.2 | 86.2 | 90.0 | 90.0 |
| (O1Mn1N4) | 88.9 | 86.2 | 90.0 | 90.4 |
| (O2Mn1N1) | 93.8 | 93.8 | 90.0 | 90.0 |
| (O2Mn1N2) | 91.1 | 93.8 | 90.0 | 89.6 |
| (O2Mn1N3) | 93.8 | 86.2 | 90.0 | 90.0 |
| (O2Mn1N4) | 91.1 | 86.2 | 90.0 | 90.0 |
| Bond angles between O and Mn atoms, deg | ||||
| (O1Mn1O2) | 180.0 | 169.2 | 180.0 | 180.0 |
| Complex | Calculated Using DFT | Charges on the Atoms in Electron Charge Units (ē) | <S**2> a | ||||
|---|---|---|---|---|---|---|---|
| Mn1 | N1 (N3) | N2 (N4) | O1 | O2 | |||
| [Mn(P)(O)2] | B3PW91/TZVP | 0.2002 | −0.3617 (−0.3617) | −0.3561 (−0.3561) | −0.3698 | −0.4155 | 3.9600 |
| OPBE/TZVP | −0.0715 | −0.3051 (−0.3024) | −0.3051 (−0.3024) | −0.0999 | −0.0996 | 0.8218 | |
| [Mn(Pc)(O)2] | B3PW91/TZVP | −0.1560 | −0.3180 (−0.3180) | −0.3180 (−0.3180) | −0.2483 | −0.2483 | 0.7803 |
| OPBE/TZVP | −0.1752 | −0.2816 (−0.2816) | −0.2817 (−0.2817) | −0.1493 | −0.1500 | 0.7668 | |
| Complex | DFT Version | ΔH0f, 298, kJ/mol | S0f, 298, J/mol ∙ K | ΔG0f, 298, kJ/mol |
|---|---|---|---|---|
| [Mn(P)(O)2] | OPBE/TZVP | 264.9 | 769.8 | 488.3 |
| B3PW91/TZVP | 689.7 | 759.4 | 916.2 | |
| [Mn(Pc)(O)2] | OPBE/TZVP | 151.1 | 1129.2 | 422.8 |
| B3PW91/TZVP | 756.8 | 1123.2 | 1030.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chachkov, D.V.; Mikhailov, O.V. DFT Method Used for Prediction of Molecular and Electronic Structures of Mn(VI) Macrocyclic Complexes with Porhyrazine/Phthalocyanine and Two Oxo Ligands. Materials 2023, 16, 2394. https://doi.org/10.3390/ma16062394
Chachkov DV, Mikhailov OV. DFT Method Used for Prediction of Molecular and Electronic Structures of Mn(VI) Macrocyclic Complexes with Porhyrazine/Phthalocyanine and Two Oxo Ligands. Materials. 2023; 16(6):2394. https://doi.org/10.3390/ma16062394
Chicago/Turabian StyleChachkov, Denis V., and Oleg V. Mikhailov. 2023. "DFT Method Used for Prediction of Molecular and Electronic Structures of Mn(VI) Macrocyclic Complexes with Porhyrazine/Phthalocyanine and Two Oxo Ligands" Materials 16, no. 6: 2394. https://doi.org/10.3390/ma16062394
APA StyleChachkov, D. V., & Mikhailov, O. V. (2023). DFT Method Used for Prediction of Molecular and Electronic Structures of Mn(VI) Macrocyclic Complexes with Porhyrazine/Phthalocyanine and Two Oxo Ligands. Materials, 16(6), 2394. https://doi.org/10.3390/ma16062394







