Fluorine-Modulated Electronic Structure and Atomic Bonding of the Titanium Surface
Abstract
1. Introduction
2. DFT Methods
3. Results
3.1. Adsorption Sites and Work Functions
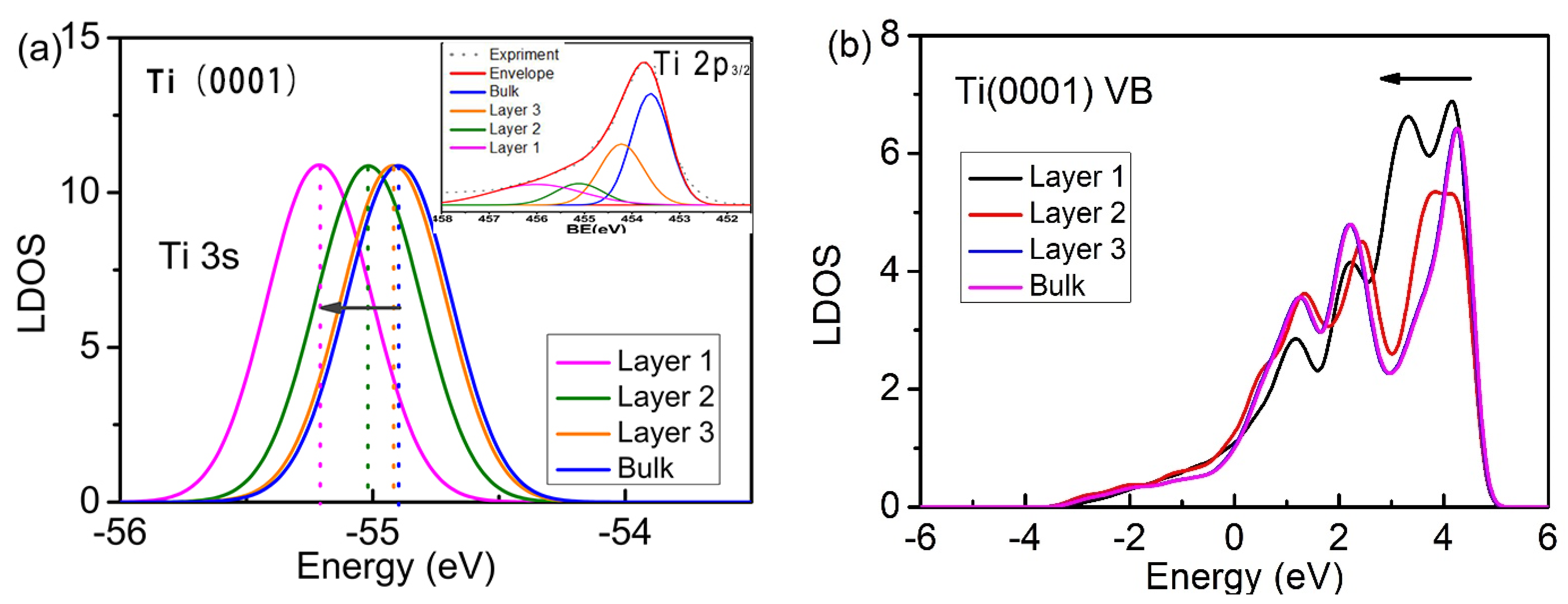
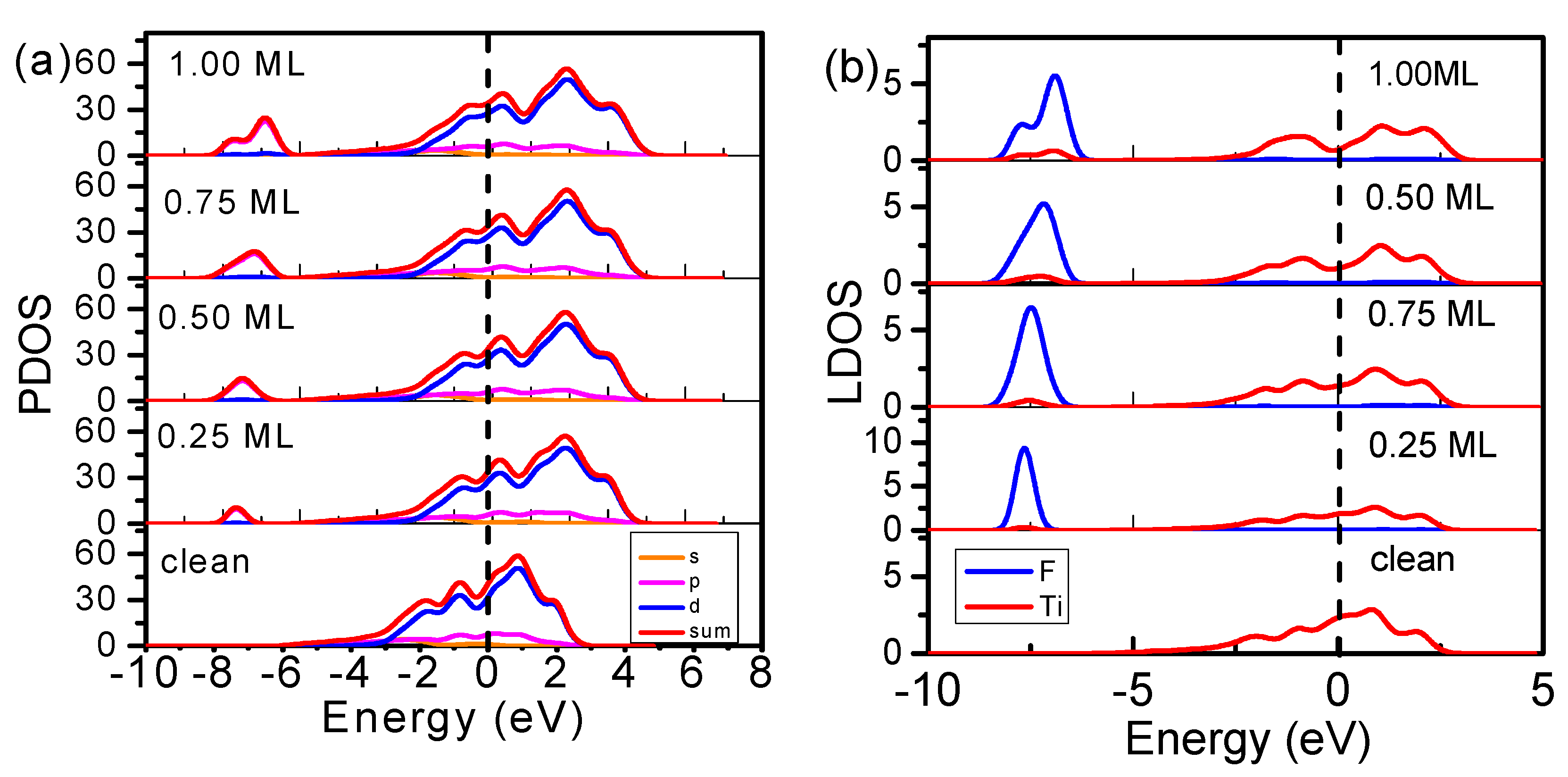
3.2. Electronic Structure
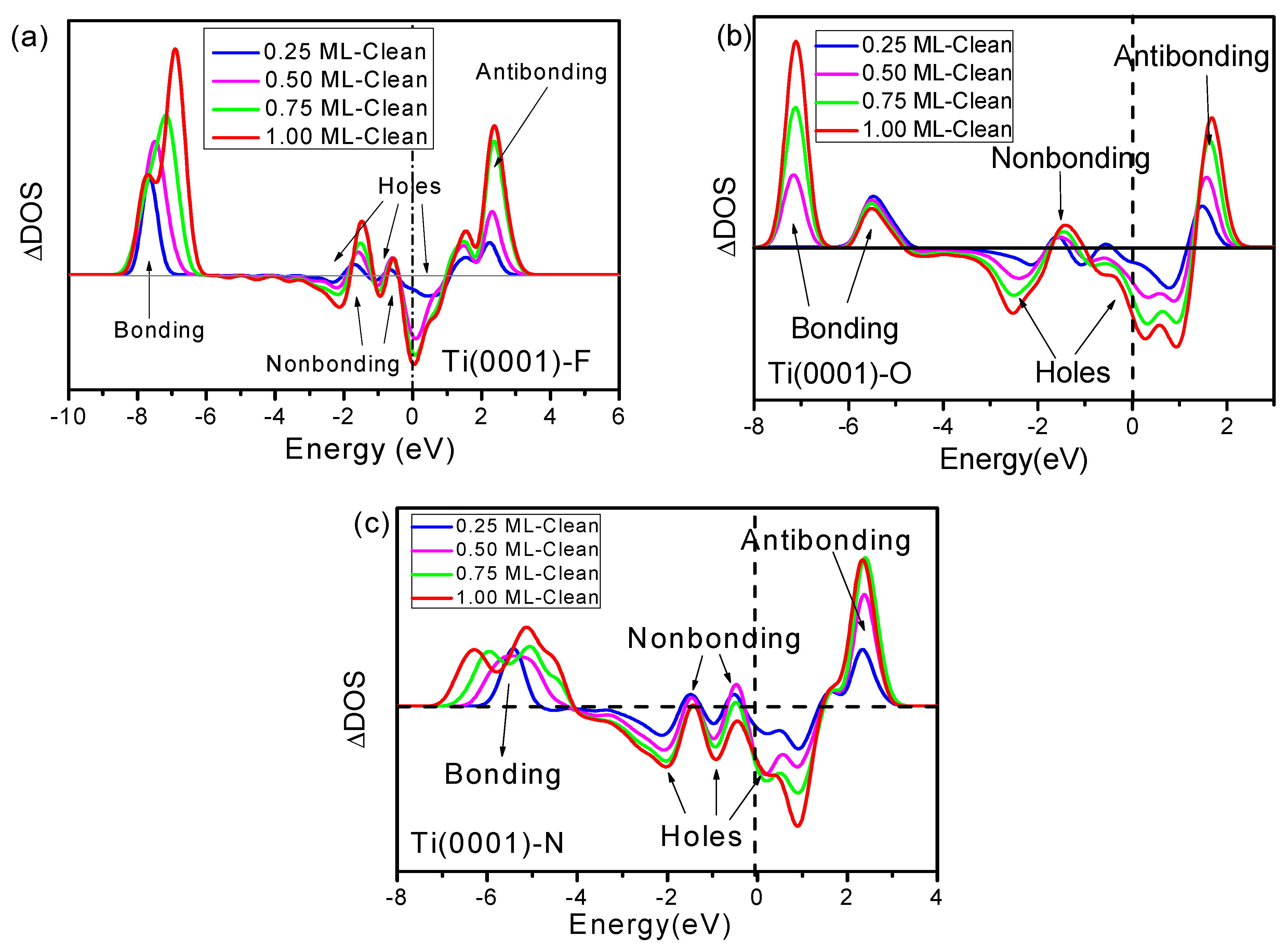
4. Discussion
5. Conclusions
- (1)
- In agreement with the predictions of BOLS theory and experimental XPS resolution spectra, the presence of undercoordinated atoms at the Ti(0001) surface reduces the local bond length and deepens the electronic energy. F adsorbed on the Ti(0001) surface only occupies the surface fcc sites.
- (2)
- Consistent with expectations based on 3B theory, DFT calculations demonstrated the necessity of the tetrahedral F–Ti structure formed through the adsorption of fluorine on the Ti(0001) surface, with four additional DOS features. These features correspond to F−Ti bonding, F electron lone pairs, Ti+ electron holes, and Ti antibonding dipoles, which determine the physical and chemical properties of the chemisorbed surface. The F–Ti bonding states lower the energy of the system and passivate the metal surface while the creation of holes can transform metallic Ti into a semiconductor upon the adsorption of fluoride. The antibonding dipoles and H-bond-like interactions modify the work function in a manner dependent on the fluorine adsorption coverage and the adsorption sites. These findings are consistent with the measurements of the adsorption of O and N on Ti(0001) skin.
Author Contributions
Funding
Conflicts of Interest
References
- Muller, K.; Faeh, C.; Diederich, F. Fluorine in pharmaceuticals: Looking beyond intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Böhm, H.J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Müller, K.; Obst-Sander, U.; Stahl, M. Fluorine in medicinal chemistry. ChemBioChem 2004, 5, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P. The unique role of fluorine in the design of active ingredients for modern crop protection. ChemBioChem 2004, 5, 570–589. [Google Scholar] [CrossRef] [PubMed]
- Maienfisch, P.; Hall, R.G. The importance of fluorine in the life science industry. CHIMIA Int. J. Chem. 2004, 58, 93–99. [Google Scholar] [CrossRef]
- Magnussen, O.M. Ordered Anion Adlayers on Metal Electrode Surfaces. Chem. Rev. 2002, 102, 679–726. [Google Scholar] [CrossRef] [PubMed]
- Marković, N.M.; Ross, P.N. Surface science studies of model fuel cell electrocatalysts. Surf. Sci. Rep. 2002, 45, 117–229. [Google Scholar] [CrossRef]
- Babudri, F.; Farinola, G.M.; Naso, F.; Ragni, R. Fluorinated organic materials for electronic and optoelectronic applications: The role of the fluorine atom. Chem. Commun. 2007, 10, 1003–1022. [Google Scholar] [CrossRef]
- Cametti, M.; Crousse, B.; Metrangolo, P.; Milani, R.; Resnati, G. The fluorous effect in biomolecular applications. Chem. Soc. Rev. 2012, 41, 31–42. [Google Scholar] [CrossRef]
- Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Organic fluorine compounds: A great opportunity for enhanced materials properties. Chem. Soc. Rev. 2011, 40, 3496–3508. [Google Scholar] [CrossRef] [PubMed]
- Eastman, D.E. Photoemission energy level measurements of sorbed gases on titanium. Solid State Commun. 1972, 10, 933–935. [Google Scholar] [CrossRef]
- Shih, H.D.; Jona, F. Atomic underlayer formation during the reaction of Ti {0001} with nitrogen. Surf. Sci. 1976, 60, 445–465. [Google Scholar] [CrossRef]
- Shih, H.D.; Jona, F.; Jepsen, D.W.; Marcus, P.M. Low-Energy-Electron-Diffraction Determination of the Atomic Arrangement in a Monatomic Underlayer of Nitrogen on Ti (0001). Phys. Rev. Lett. 1976, 36, 798–801. [Google Scholar] [CrossRef]
- Brearley, W.; Surplice, N.A. Changes in the work function of titanium films owing tothe chemisorption of N2, O2, CO and CO2. Surf. Sci. 1977, 64, 372–374. [Google Scholar] [CrossRef]
- Fukuda, Y.; Elam, W.T.; Park, R.L. Nitrogen, Oxygen, and Carbon Monoxide Chemisorption on Polycrystalline Titanium Surfaces. Appl. Surf. Sci. 1978, 1, 278–287. [Google Scholar] [CrossRef]
- Feibelman, P.; Himpsel, F. Spectroscopy of a surface of known geometry: Ti (0001)-N(1×1). Phys. Rev. B 1980, 21, 1394–1399. [Google Scholar] [CrossRef]
- Biwer, B.M.; Bernasek, S.L. A photoelectron and energy-loss spectroscopy study of Ti and its interaction with H2, O2, N2 and NH3. Surf. Sci. 1986, 167, 207–230. [Google Scholar] [CrossRef]
- Kuznetsov, M.V.; Shalaeva, E.V. Competing Adsorption of Nitrogen and Oxygen at the Ti (0001) Face: XPS Examination. Phys. Met. Metallogr. 2004, 97, 485–494. [Google Scholar]
- Li, L.; Meng, F.; Tian, H.; Hu, X.; Zheng, W.; Sun, C.Q. Oxygenation mediating the valence density-of-states and work function of Ti (0001) skin. Phys. Chem. Chem. Phys. 2015, 17, 9867–9872. [Google Scholar] [CrossRef]
- Li, L.; Meng, F.L.; Hu, X.Y.; Qiao, L.; Sun, C.Q.; Tian, H.W.; Zheng, W.T. Nitrogen mediated electronic structure of the Ti (0001) surface. RSC Adv. 2016, 6, 14651–14657. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Perdew, J.; Ziesche, P.; Eschrig, H. Chapter Unified Theory of Exchange and Correlation beyond the Local Density Approximation. In Electronic Structure of Solids; Ziesche, P., Eschrig, H., Eds.; Akademie: Berlin, Germany, 1991. [Google Scholar]
- Kittel, C. Introduction to Solid State Physics, 7th ed.; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Schneider, J.; Ciacchi, L.C. First principles and classical modeling of the oxidized titanium (0001) surface. Surf. Sci. 2010, 604, 1105–1115. [Google Scholar] [CrossRef]
- Bengtsson, L. Dipole correction for surface supercell calculations. Phys. Rev. B 1991, 59, 12301. [Google Scholar] [CrossRef]
- Shih, H.D.; Jona, F.; Jepsen, D.W.; Marcus, P.M. The structure of the clean Ti (0001) surface. J. Phys. C Solid State Phys. 1976, 9, 1405–1416. [Google Scholar] [CrossRef]
- Sun, C.Q. Relaxation of the Chemical Bond; Springer Series in Chemical Physics; Springer Press: Berlin/Heidelberg, Germany, 2014; Volume 108, ISBN 978-981-4585-20-0. [Google Scholar]
- Hanson, D.; Stockbauer, R.; Madey, T. Photon-stimulated desorption and other spectroscopic studies of the interaction of oxygen with a titanium (001) surface. Phys. Rev. B 1981, 24, 5513–5521. [Google Scholar] [CrossRef]
- Huda, M.; Kleinman, L. Density functional calculations of the influence of hydrogen adsorption on the surface relaxation of Ti (0001). Phys. Rev. B 2005, 71, 241406. [Google Scholar] [CrossRef]
- Li, L.; Tian, H.W.; Meng, F.L.; Hu, X.Y.; Zheng, W.T.; Sun, C.Q. Defects improved photocatalytic ability of TiO2. Appl. Surf. Sci. 2014, 317, 568–572. [Google Scholar] [CrossRef]
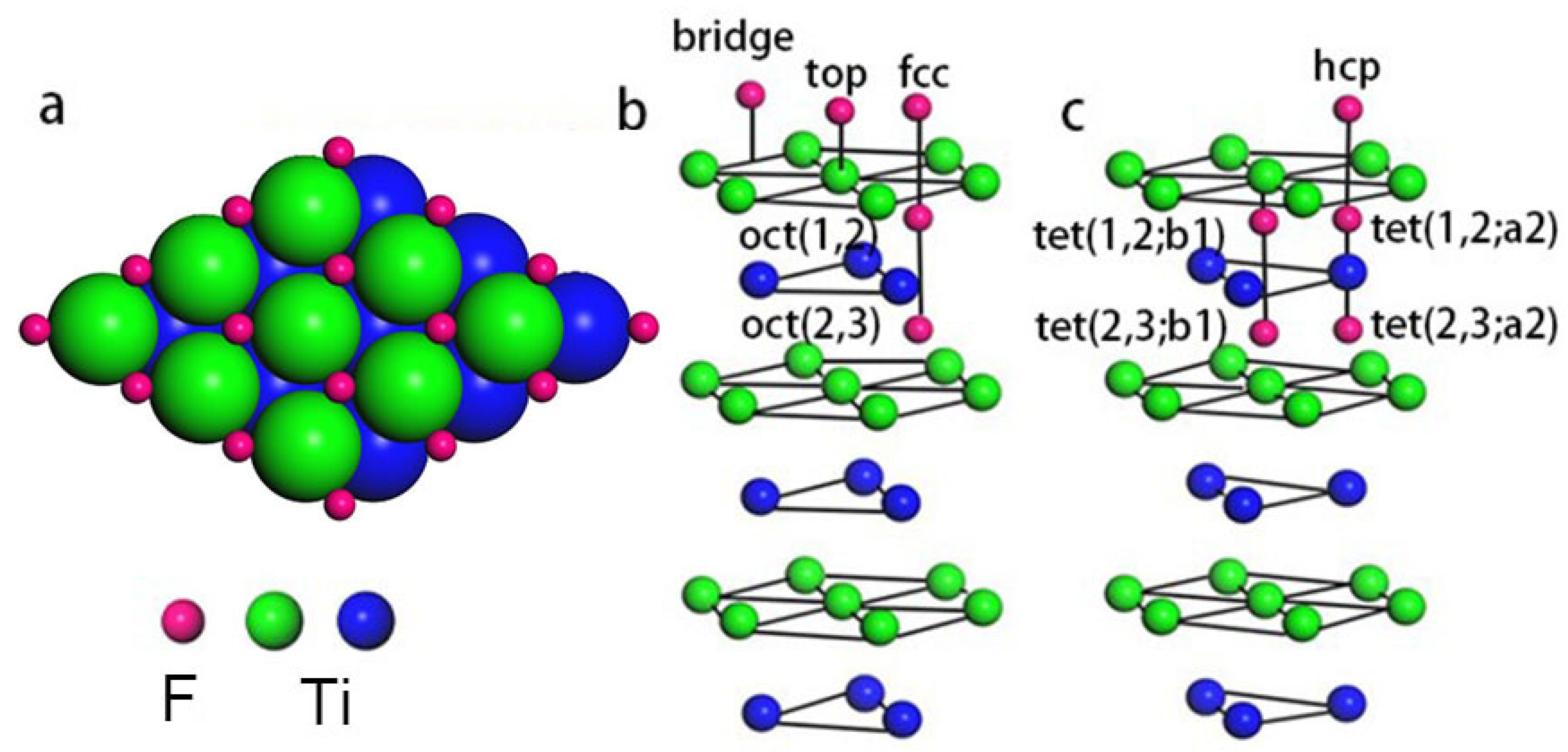


| Adsorption Coverage | Adsorption Site | Adsorption Energy (eV) | Work Function (eV) | Charge Transfer (e) (F → Ti) | |
|---|---|---|---|---|---|
| 0.25 | fcc | 2.93 | 4.198 | 2.098 | 0.46 |
| hcp | 2.72 | 4.19 | |||
| bridge | - | - | |||
| top | 1.92 | 5.21 | |||
| oct (1,2) | 0.35 | 3.84 | |||
| tet(1,2; b1) | −0.29 | 4.385 | |||
| tet(1,2; a2) | - | - | |||
| oct(2,3) | 1.09 | 4.371 | |||
| 0.50 | fcc | 2.91 | 4.206 | 2.112 | 0.46 |
| oct(1,2) | 0.54 | 4.20 | |||
| fcc + oct(1,2) | 1.64 | 4.22 | |||
| hcp + oct(1,2) | 1.61 | 4.21 | |||
| fcc + tet(1,2; b1) | - | - | |||
| fcc + oct(2,3) | 2.04 | 4.215 | |||
| 0.75 | fcc | 2.86 | 4.218 | 2.131 | 0.46 |
| oct(1,2) | 0.52 | 4.197 | |||
| fcc + oct(1,2) | 1.99 | 4.207 | |||
| 1.00 | fcc | 2.81 | 4.518 | 2.109 | 0.46 |
| fcc + oct(1,2) | 1.60 | ||||
| fcc + oct(2,3) | 1.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Huang, H. Fluorine-Modulated Electronic Structure and Atomic Bonding of the Titanium Surface. Materials 2022, 15, 8492. https://doi.org/10.3390/ma15238492
Li L, Huang H. Fluorine-Modulated Electronic Structure and Atomic Bonding of the Titanium Surface. Materials. 2022; 15(23):8492. https://doi.org/10.3390/ma15238492
Chicago/Turabian StyleLi, Lei, and Haihua Huang. 2022. "Fluorine-Modulated Electronic Structure and Atomic Bonding of the Titanium Surface" Materials 15, no. 23: 8492. https://doi.org/10.3390/ma15238492
APA StyleLi, L., & Huang, H. (2022). Fluorine-Modulated Electronic Structure and Atomic Bonding of the Titanium Surface. Materials, 15(23), 8492. https://doi.org/10.3390/ma15238492






