Enhanced Electrorheological Polishing Efficiency of Alumina-Doped Titanium Dioxide Particles
Abstract
1. Introduction
2. Experimental Section
2.1. Preparation of Alumina-Doped Titanium Dioxide Particles
2.2. Preparation of ER Polishing Fluid
2.3. Characterization
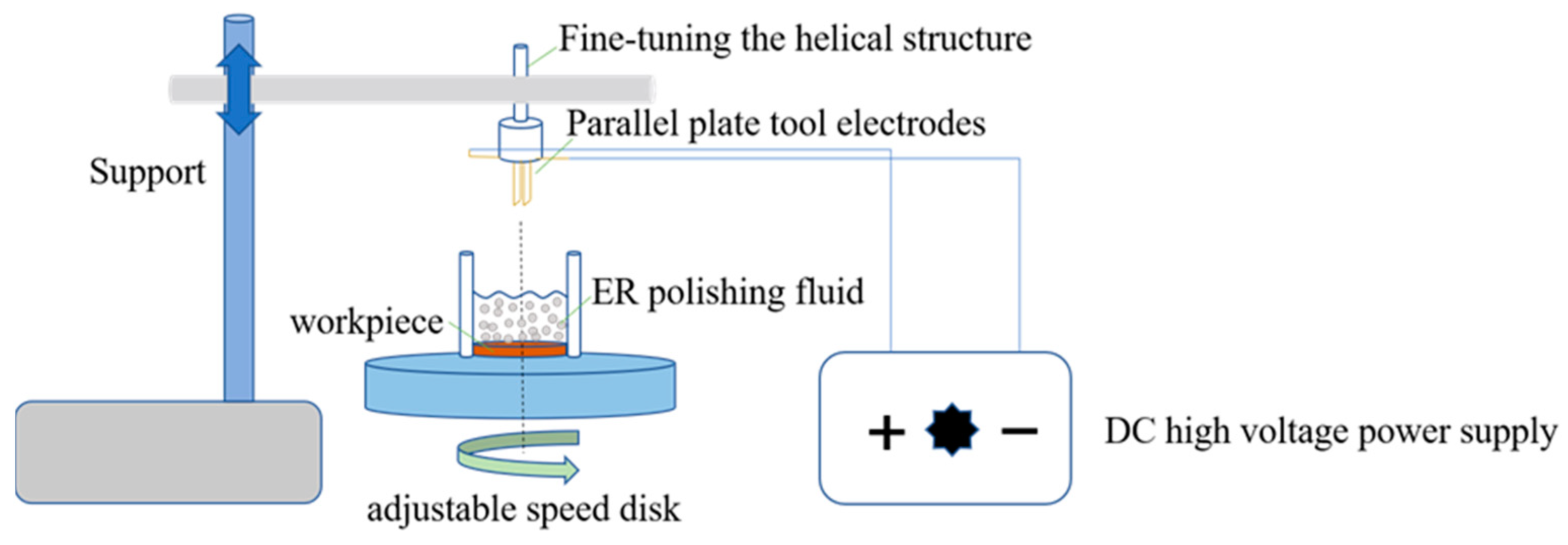
2.4. ER Properties and Polishing Properties Measurements
3. Results and Discussion
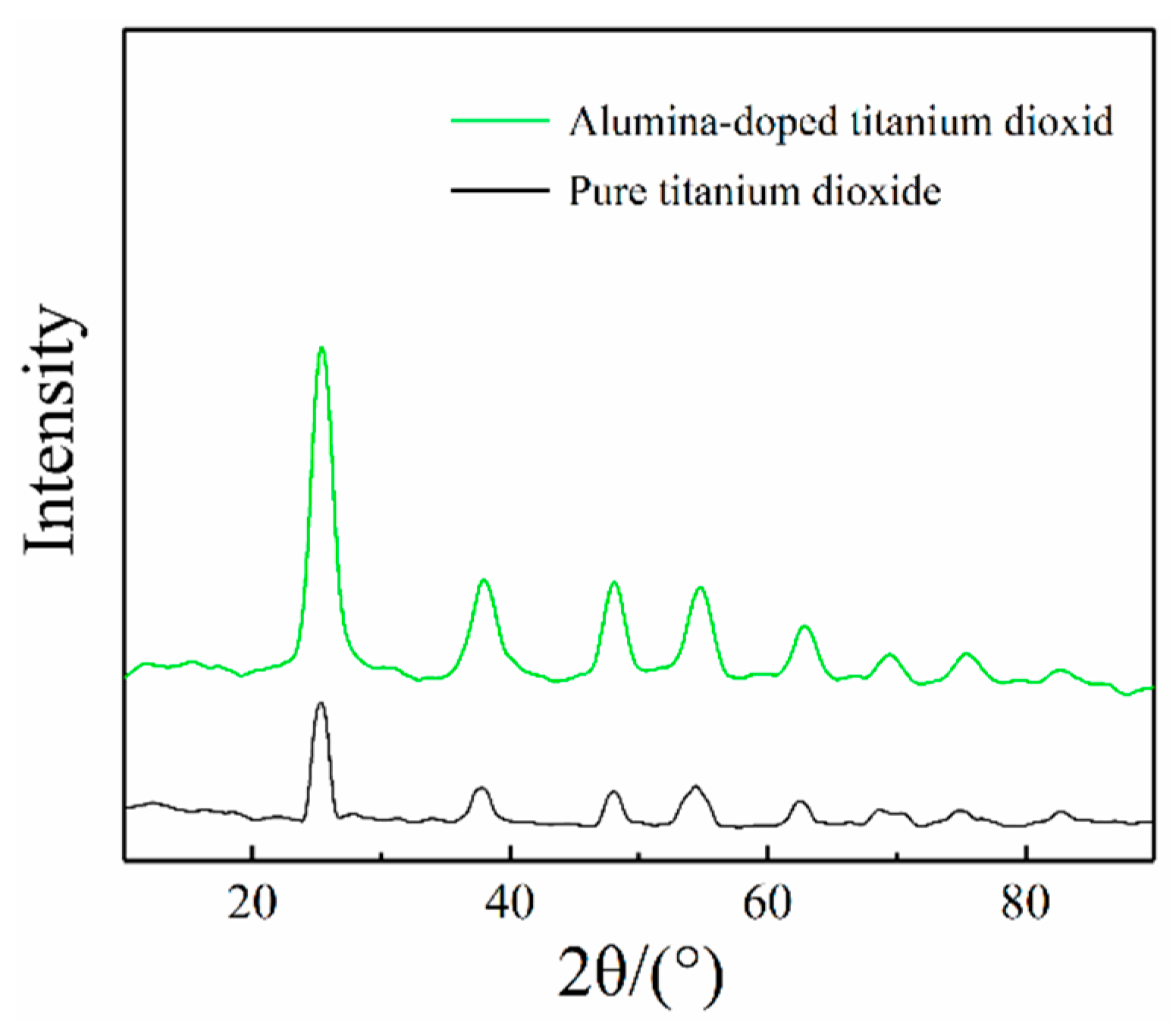
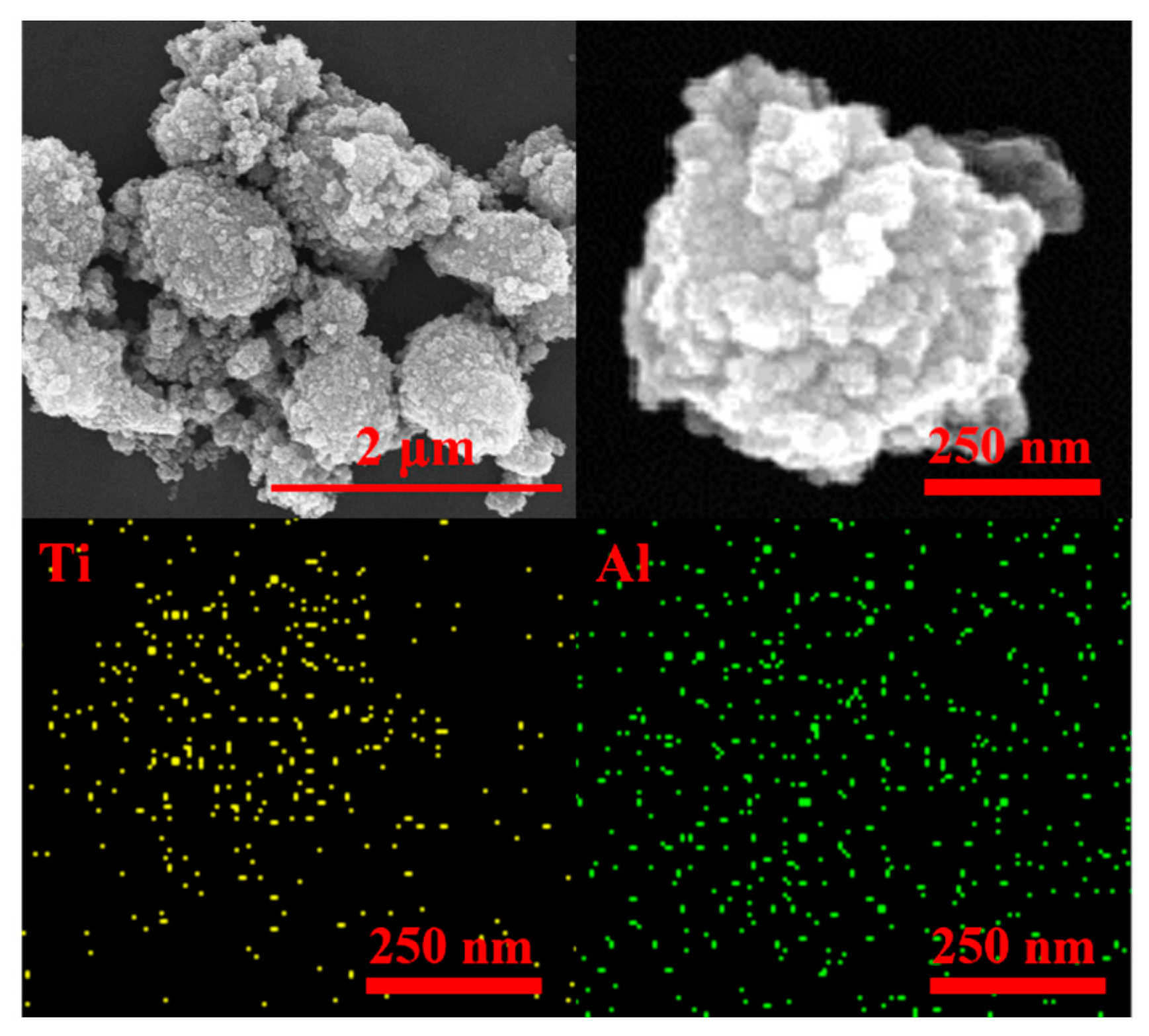
3.1. Material Structure
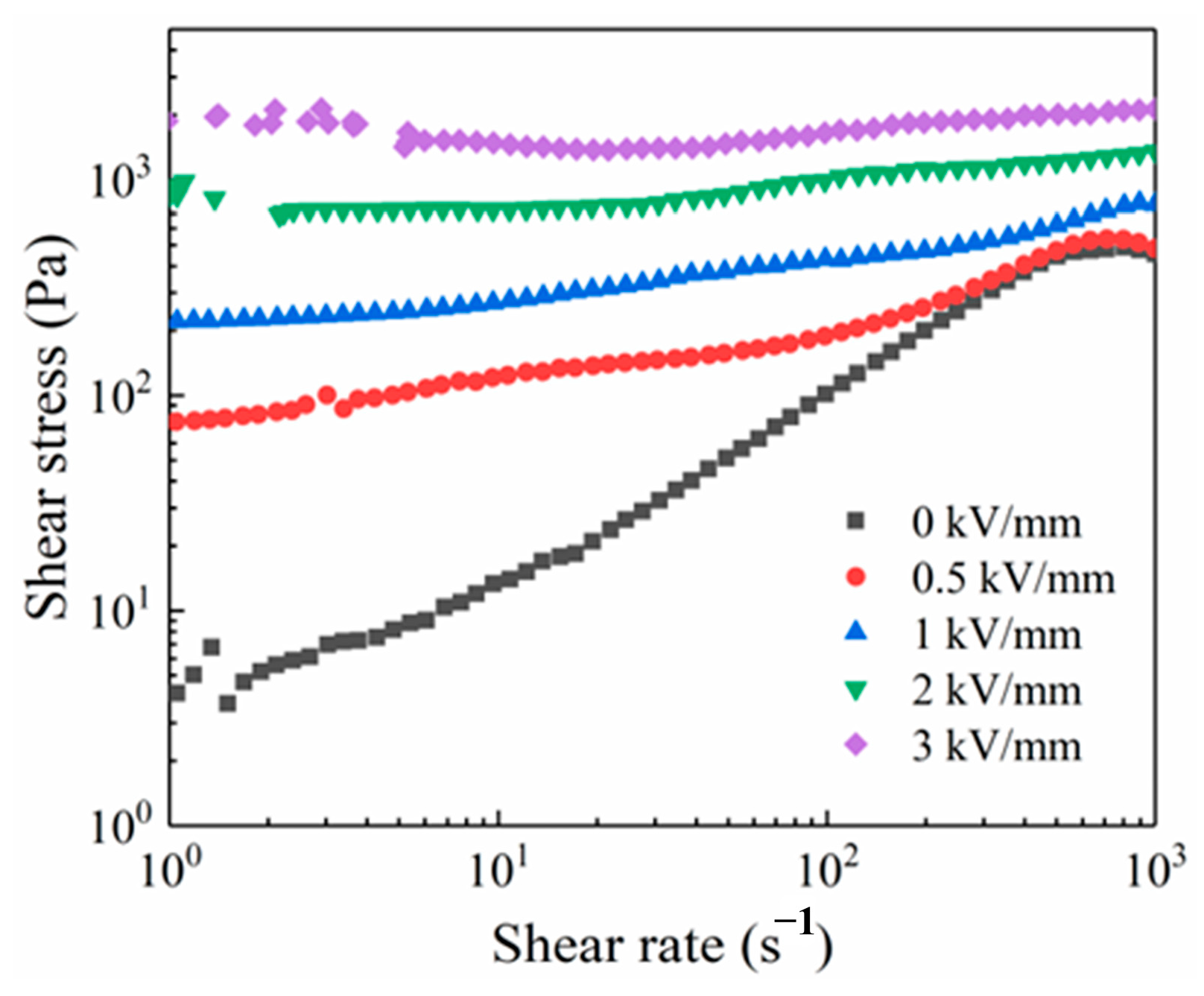
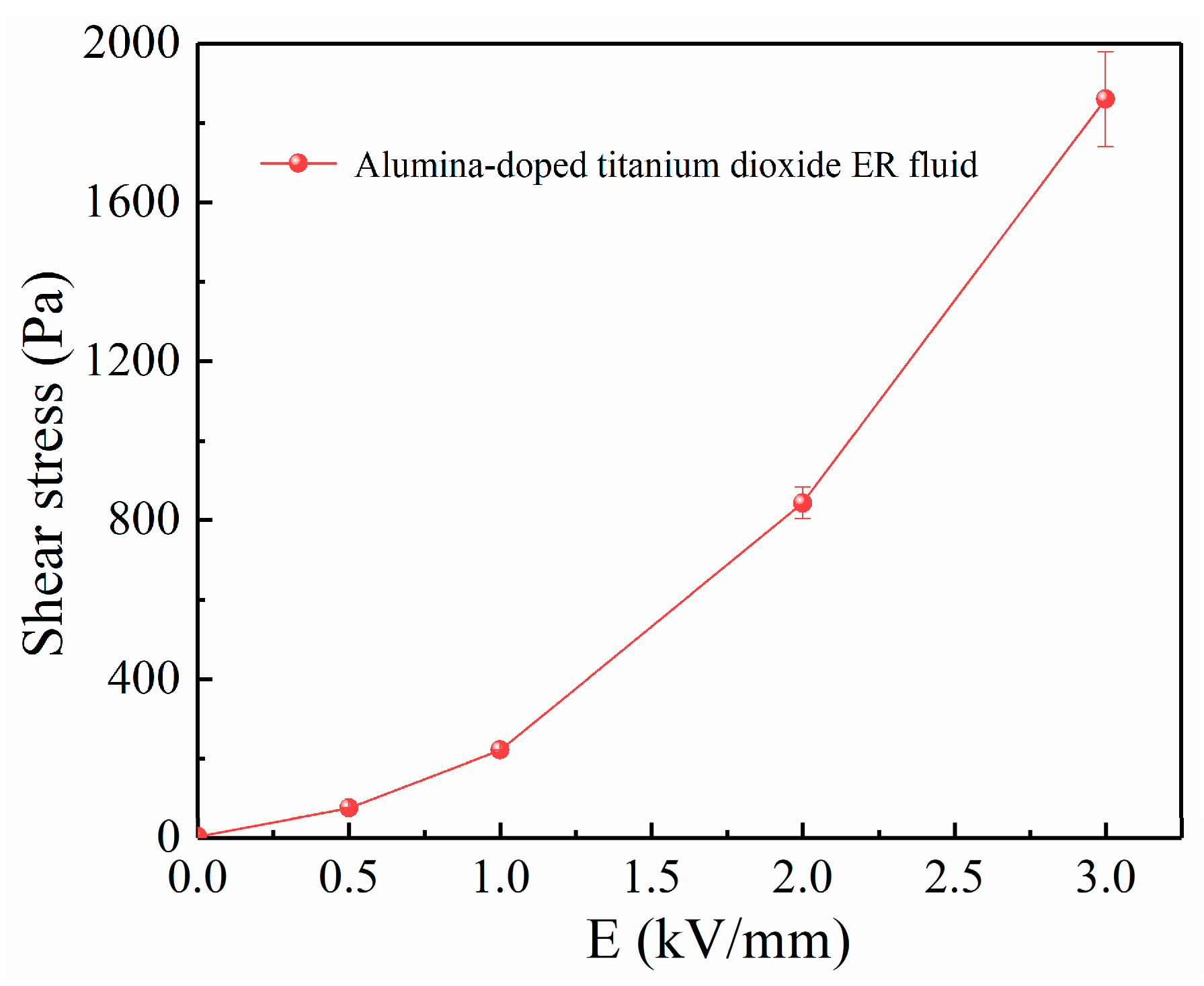
3.2. ER Property
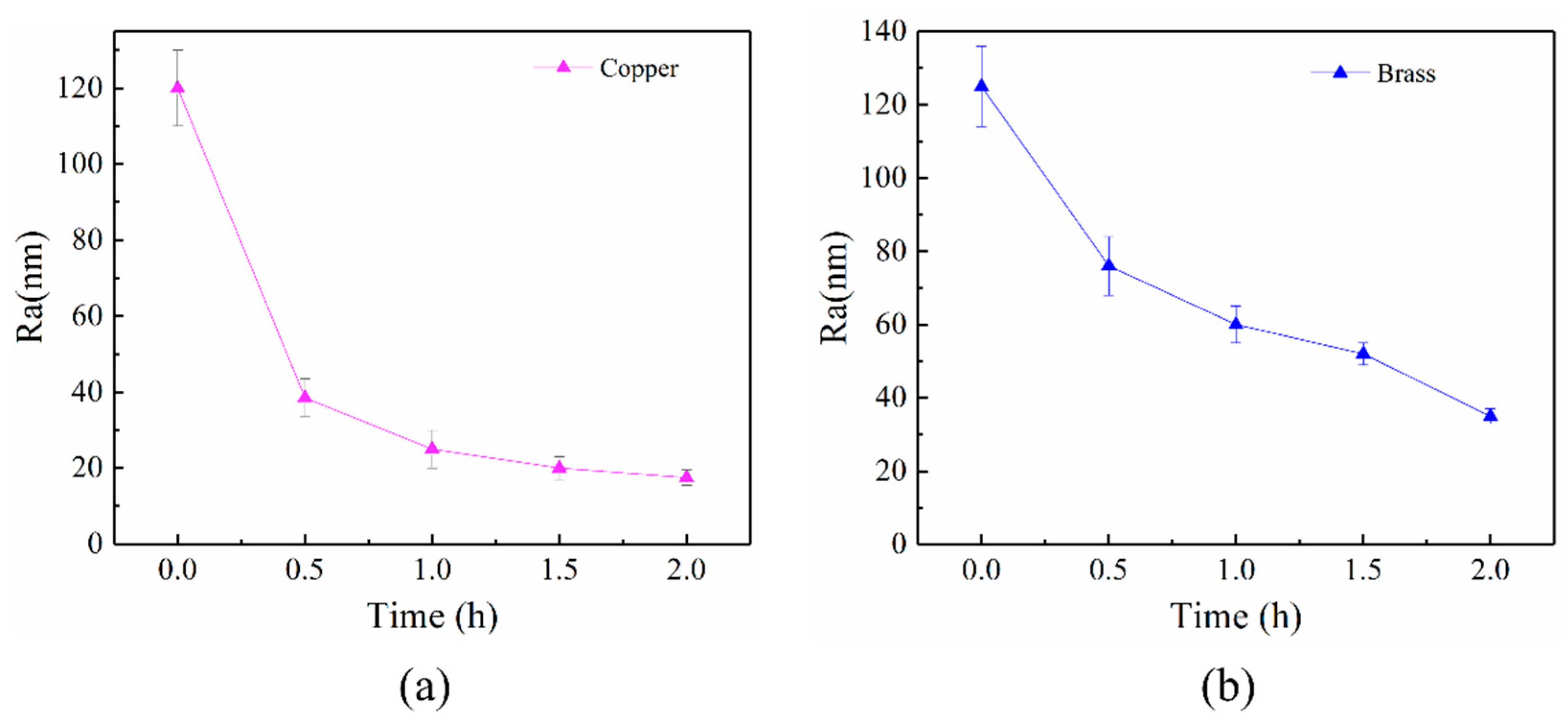
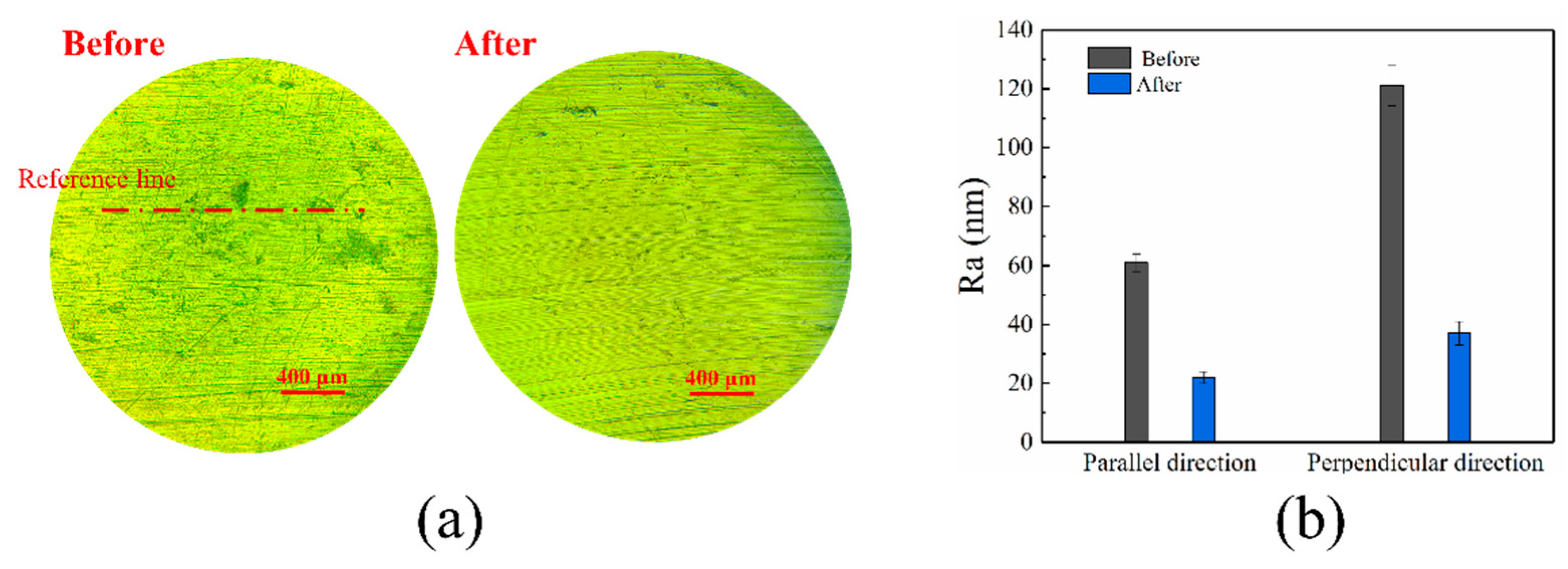
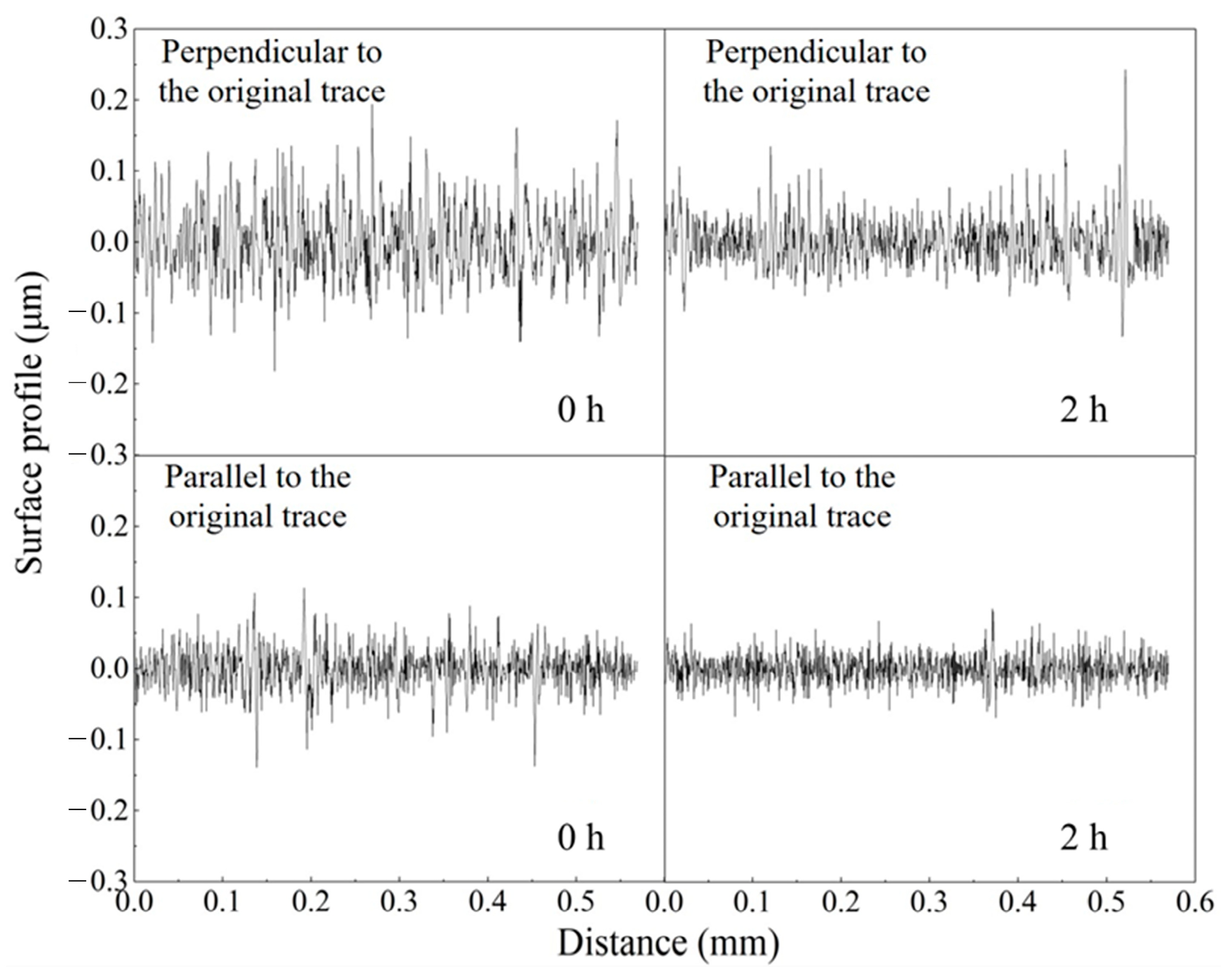
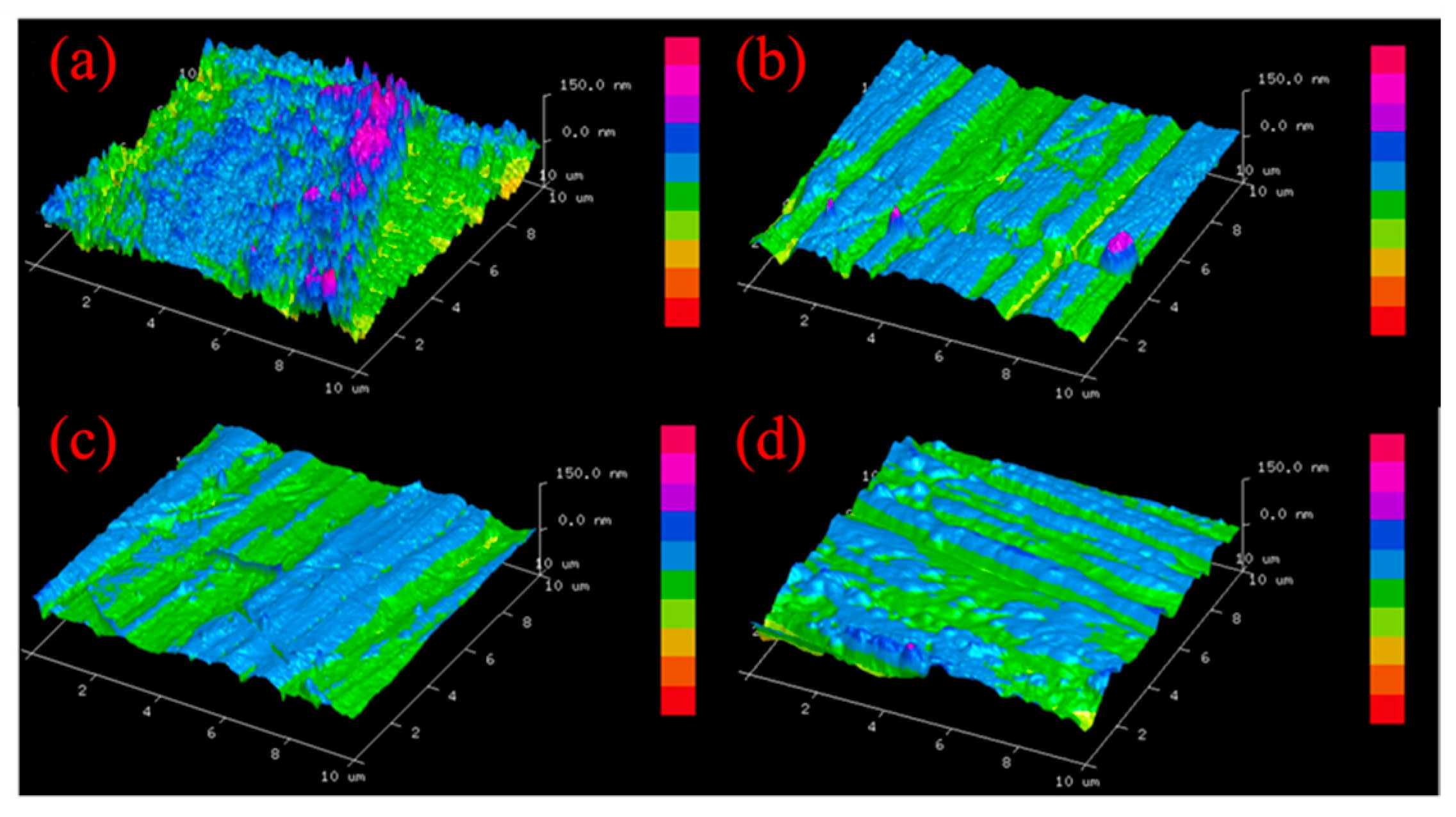
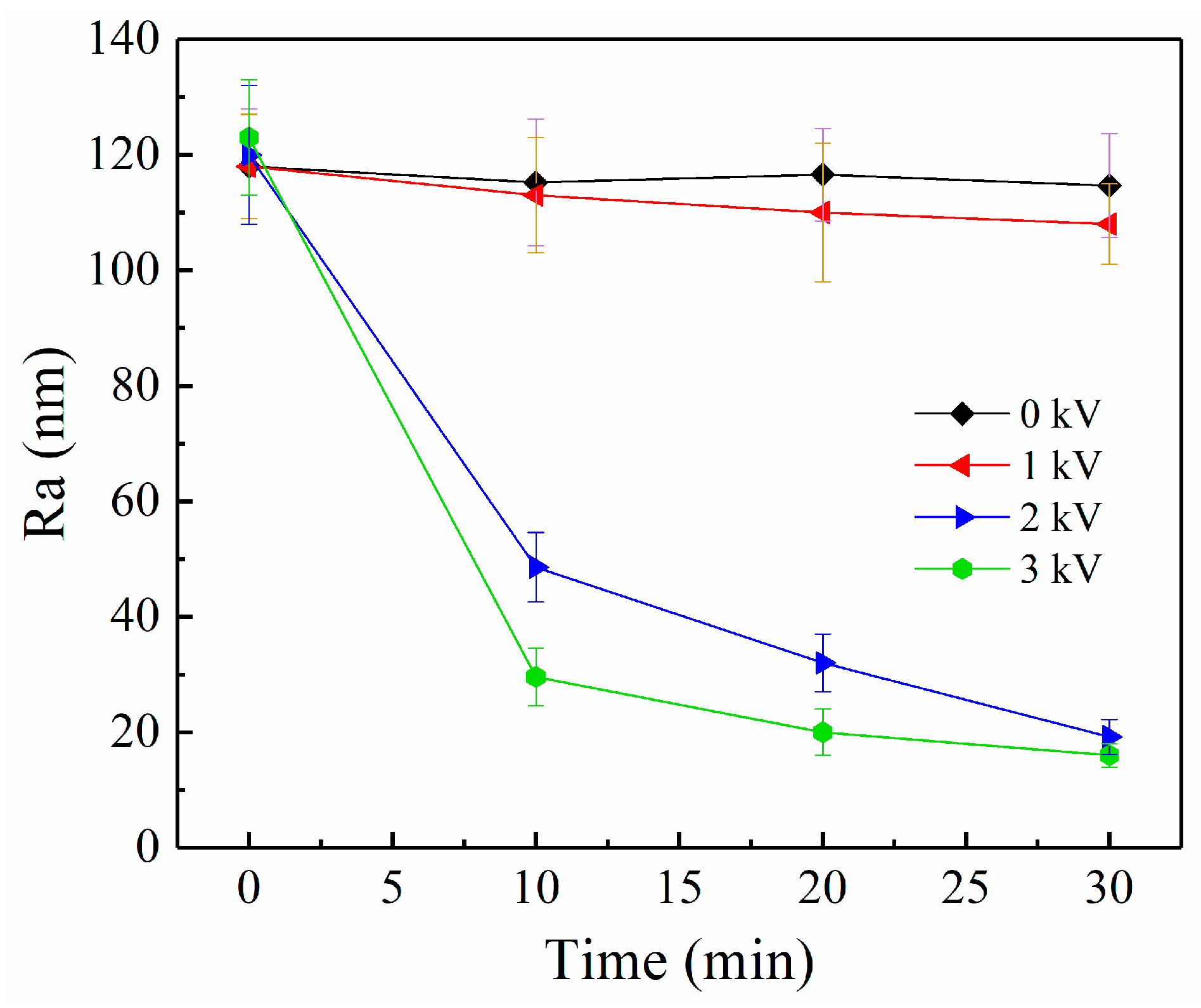
3.3. ER Polishing Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, Y.Z.; Seo, Y.; Choi, H.J. Recent development of electro-responsive smart electrorheological fluids. Soft Matter 2019, 15, 3473–3486. [Google Scholar] [CrossRef] [PubMed]
- Hao, T. Electrorheological fluids. Adv. Mater. 2001, 13, 1847–1857. [Google Scholar] [CrossRef]
- Sheng, P.; Wen, W. Electrorheological fluids: Mechanisms, dynamics, and microfluidics applications. Annu. Rev. Fluid Mech. 2012, 44, 143–174. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, G.; Yin, Y.; Dong, C.; Liu, Y.D. The electric field responses of inorganic ionogels and poly(ionic liquid)s. Molecules 2020, 25, 4547. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.J.; Ma, J.B.; Xi, Z.Y.; Lin, Y.S.; Wang, B.X.; Hao, C.C. Titanium oxide-coated titanium-loaded metal organic framework (MOF-Ti) nanoparticles show improved electrorheological performance. Soft Matter 2020, 16, 9292–9305. [Google Scholar] [CrossRef]
- Zhang, W.L.; Tian, J.; Zeng, H.; Liu, J.; Tian, Y. Promoted electro-responsive performances in an interface-confined oxidized niobium carbide MXene. Chem. Eng. J. 2019, 366, 321–329. [Google Scholar] [CrossRef]
- Weiss, K.D.; Carlson, J.D.; Coulter, J.P. Review: Material aspects of electrorheological systems. J. Intell. Mater. Syst. Struct. 1993, 4, 13–34. [Google Scholar] [CrossRef]
- Sadeghi, A.; Beccai, L.; Mazzolai, B. Innovative soft robots based on electro-rheological fluids. In Proceedings of the International Conference on Intelligent Robots and Systems, Vilamoura-Algarve, Portugal, 7–12 October 2012; pp. 4237–4242. [Google Scholar]
- Miyoshi, T.; Yoshida, K.; Kim, J.; Eom, S.; Yokota, S. An MEMS-based multiple electro-rheological bending actuator system with an alternating pressure source. Sens. Actuators A Phys. 2016, 245, 68–75. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.; Chen, Y.; Majidi, C.; Fumiya, F.; Askouiis, E. Controllable and reversible tuning of material rigidity for robot applications. Mater. Today 2018, 21, 563–576. [Google Scholar] [CrossRef]
- Milecki, A.; Hauke, M. Application of magnetorheological fluid in industrial shock absorbers. Mech. Syst. Signal Process. 2012, 28, 528–541. [Google Scholar] [CrossRef]
- Ahamed, R.; Ferdaus, M.M.; Li, Y. Advancement in energy harvesting magneto-rheological fluid damper: A review. Korea-Aust. Rheol. J. 2016, 28, 355–379. [Google Scholar] [CrossRef]
- Liang, Y.; Huang, D.; Zhou, X.; Wang, Z.; Shi, Q.; Hong, Y. Efficient electrorheological technology for materials, energy, and mechanical engineering: From mechanisms to applications. Engineering 2022, 14, 2095–8099. [Google Scholar] [CrossRef]
- Akagami, Y.; Asari, K.; Jeyadevan, B.; Fujita, T. Characterization of particle motion for polishing and texturing under AC field by using particle dispersion type ER fluid. J. Intell. Mater. Syst. Struct. 1998, 9, 672–675. [Google Scholar] [CrossRef]
- Hung, M.C.; Tsai, Y.Y.; Wang, L. Using electro-rheological chain structure to improve SKD11 surface. Adv. Mater. Res. 2010, 126, 527–532. [Google Scholar] [CrossRef]
- Zhang, L.; He, X.S.; Yang, H.R.; Zhang, Y. An integrated tool for five-axis electrorheological fluid-assisted polishing. Int. J. Mach. Tools Manuf. 2010, 50, 737–740. [Google Scholar] [CrossRef]
- Chen, B.; Cheng, H.; Tam, H.Y.; Li, H. Design of integrated-electrode tool for electrorheological finishing of optical glasses. Front. Optoelectron. China 2011, 4, 467–471. [Google Scholar] [CrossRef]
- Su, J.; Cheng, H.; Wen, Y.; Feng, Y.; Tam, H.Y. Investigation into the mechanism for ultra smooth electrorheological finishing using wheel-like finishing tool. J. Mater. Process. Technol. 2016, 238, 124–131. [Google Scholar] [CrossRef]
- Yang, H.; Cheng, H.; Feng, Y. Improvement of high-power laser performance for super-smooth optical surfaces using electrorheological finishing technology. Appl. Opt. 2017, 56, 9822–9829. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Teng, D.; Liew, P.J.; Huang, C. Electrorheological fluid–assisted ultrasonic polishing for IN625 additively manufactured surfaces. Int. J. Adv. Manuf. Technol. 2022, 120, 891–905. [Google Scholar] [CrossRef]
- Chen, Z.; Pan, J.; Yan, Q.; Huang, Z.; Zhang, F.; Chen, S. Study on the rheological and polishing properties of electromagnetic two-phase composite particles with abrasive characteristics. Smart Mater. Struct. 2022, 31, 45012. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Yang, Z.; Wang, Y.; Zhao, X.; Yin, J. Improved electrorheological polishing property of poly (ionic fluid)/Al2O3 composite particles prepared via Pickering emulsion polymerization. ACS Appl. Polym. Mater. 2021, 3, 5778–5787. [Google Scholar] [CrossRef]
- Hu, X.F.; Sun, H.; Yang, Z.Y.; Zhao, X.; Yin, J. Electrorheological polishing performance of cerium-doped titanium dioxide particles. Front. Mater. 2022, 9, 1053847. [Google Scholar] [CrossRef]
- Liu, Q.P.; Huang, H.J.; Zhou, Y.; Duan, Y.D.; Sun, Q.W.; Lin, Y. Photovoltaic performance of dye-sensitized solar cells based on Al-doped TiO2 thin films. Acta Phys. Chim. Sin. 2012, 28, 591–595. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Sun, H.; Zhao, X.; Yin, J. Enhanced Electrorheological Polishing Efficiency of Alumina-Doped Titanium Dioxide Particles. Materials 2023, 16, 2347. https://doi.org/10.3390/ma16062347
Hu X, Sun H, Zhao X, Yin J. Enhanced Electrorheological Polishing Efficiency of Alumina-Doped Titanium Dioxide Particles. Materials. 2023; 16(6):2347. https://doi.org/10.3390/ma16062347
Chicago/Turabian StyleHu, Xufeng, Han Sun, Xiaopeng Zhao, and Jianbo Yin. 2023. "Enhanced Electrorheological Polishing Efficiency of Alumina-Doped Titanium Dioxide Particles" Materials 16, no. 6: 2347. https://doi.org/10.3390/ma16062347
APA StyleHu, X., Sun, H., Zhao, X., & Yin, J. (2023). Enhanced Electrorheological Polishing Efficiency of Alumina-Doped Titanium Dioxide Particles. Materials, 16(6), 2347. https://doi.org/10.3390/ma16062347







