Microstructural Assessment, Mechanical and Corrosion Properties of a Mg-Sr Alloy Processed by Combined Severe Plastic Deformation
Abstract
1. Introduction
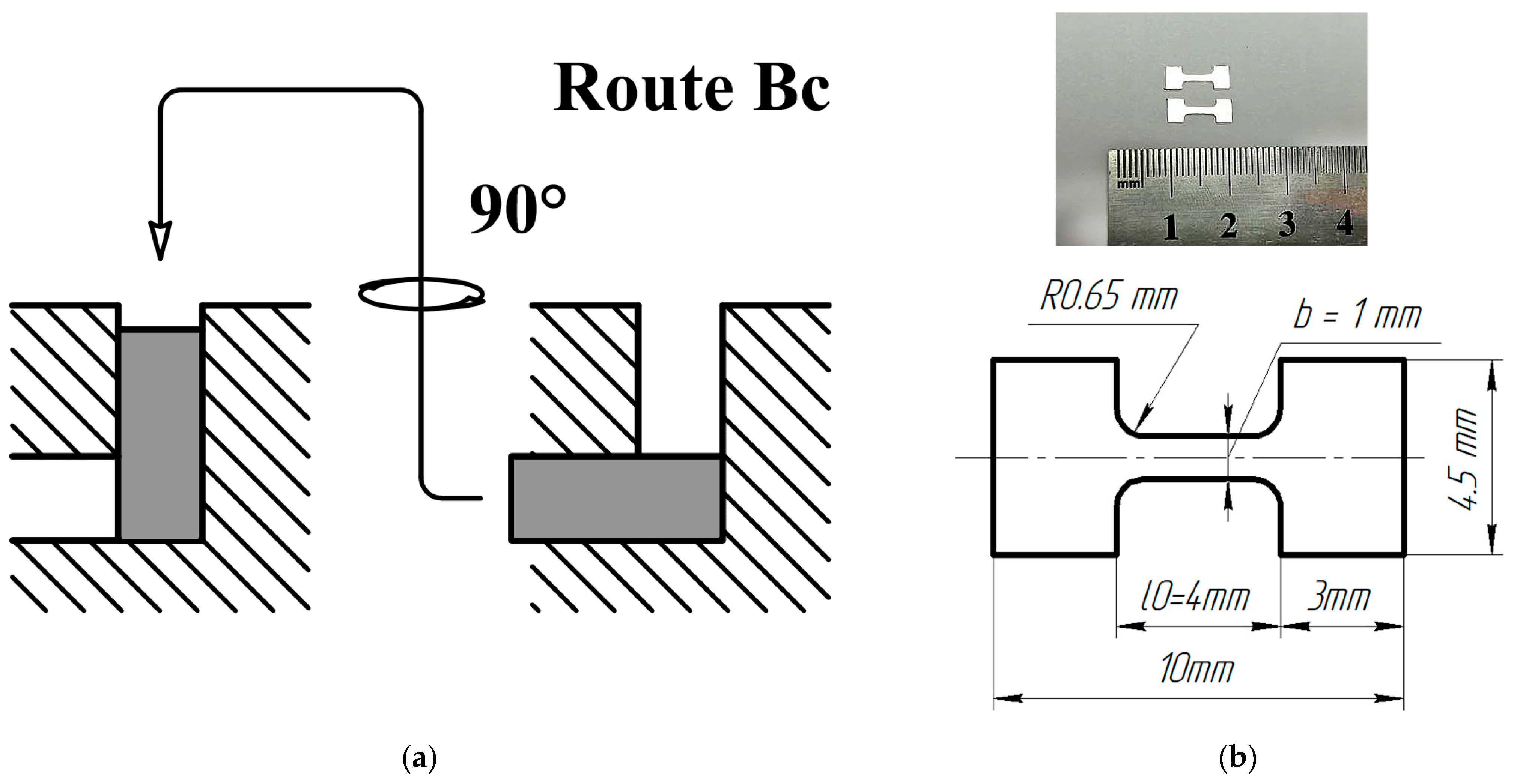
2. Materials and Methods
3. Results
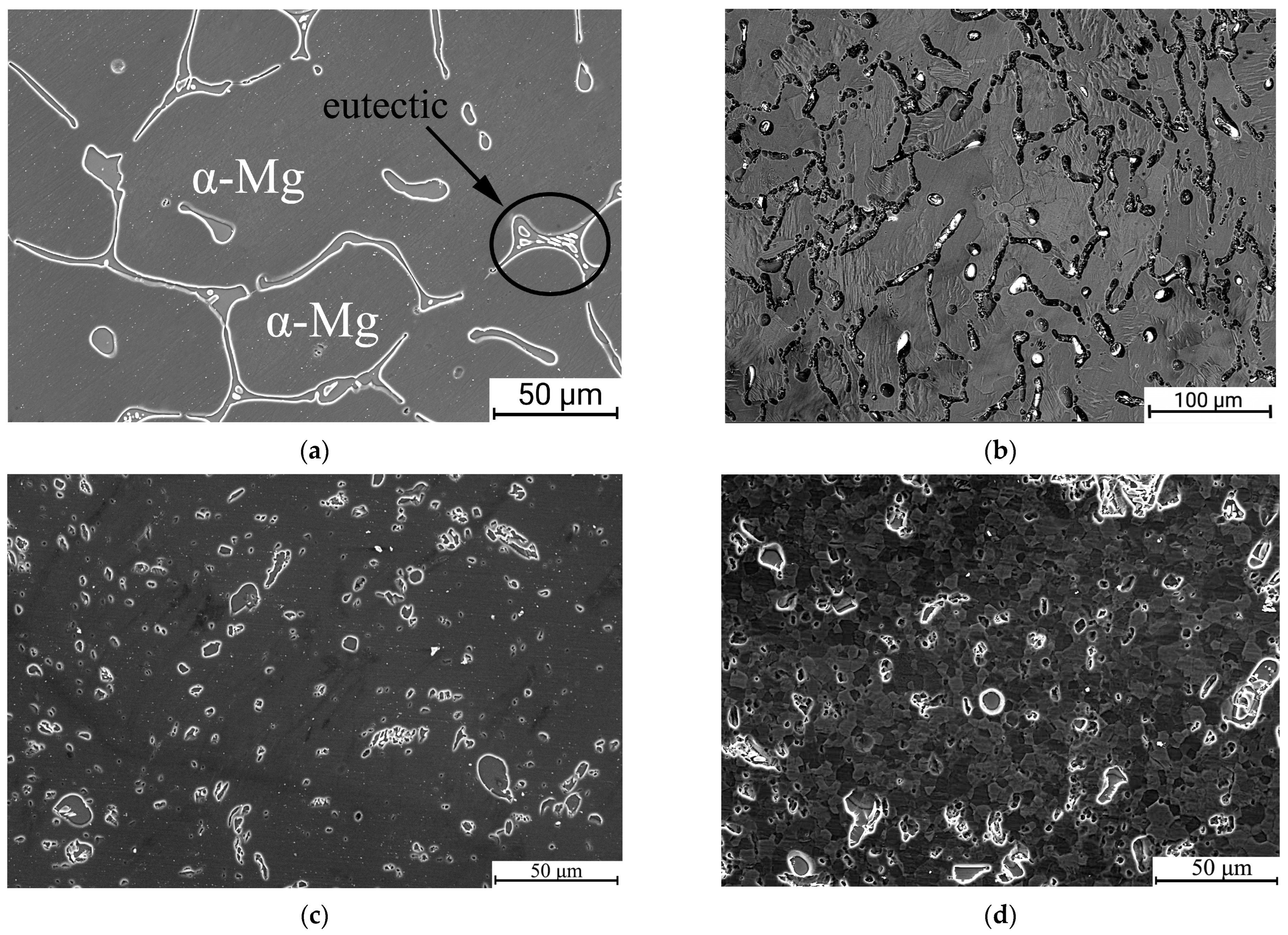
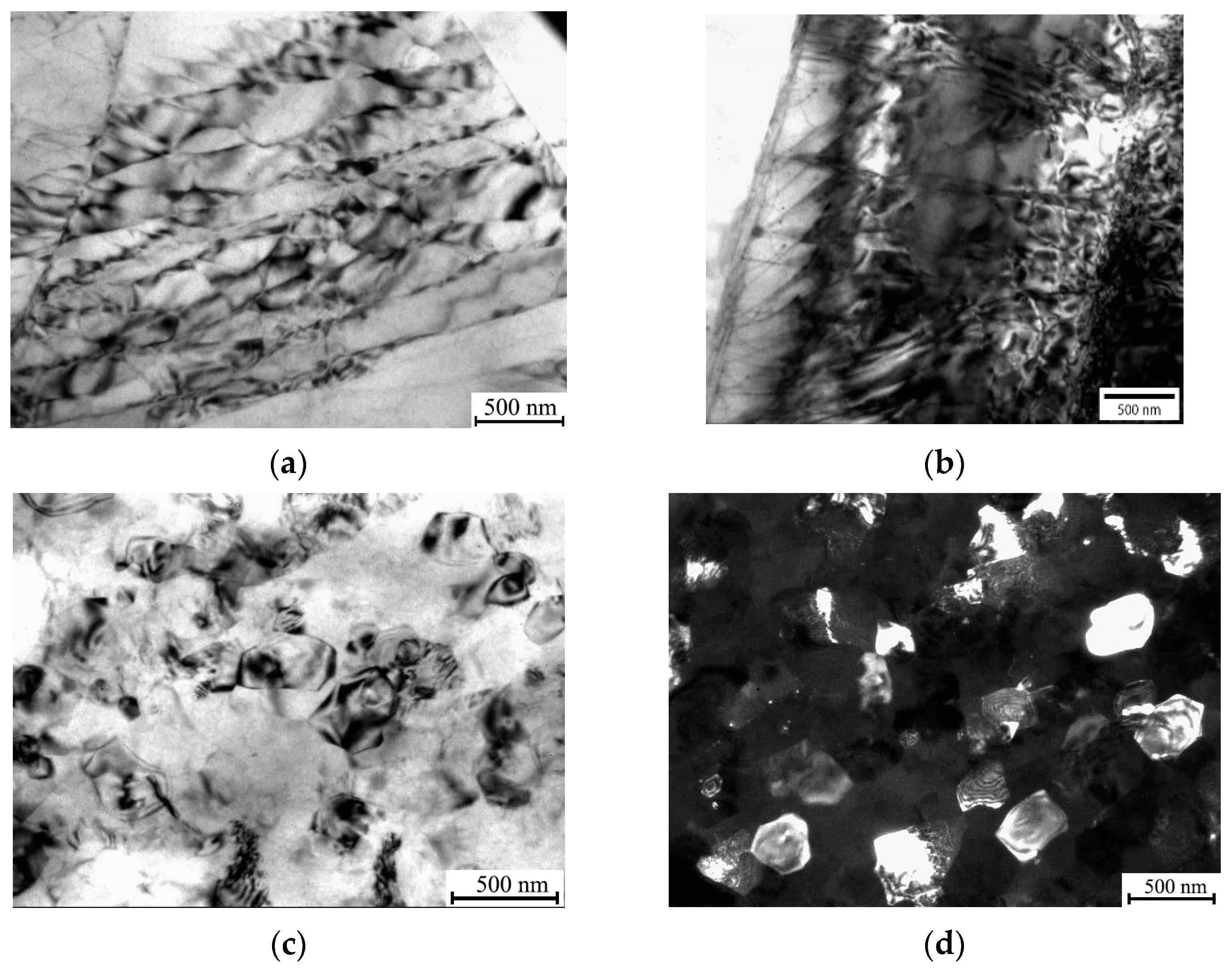
3.1. The Effect of ECAP, cSPD and Annealing on the Microstrcuture of Mg-2Sr Alloy
3.2. X-Ray Analysis of the Effect of ECAP, cSPD and Annealing on Microstrcuture of the Mg-Sr Alloy
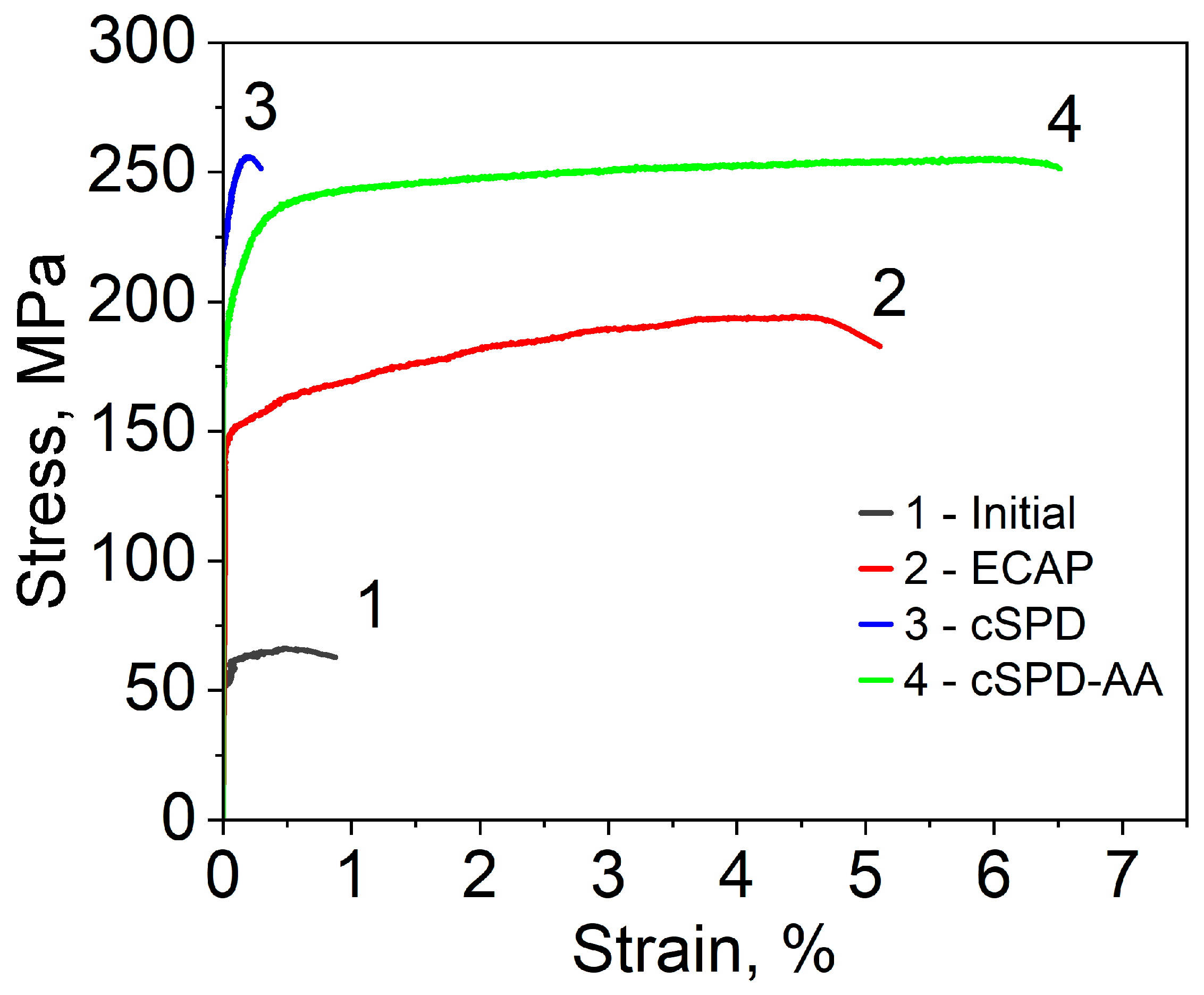
3.3. Hardness and Mechanical Properties
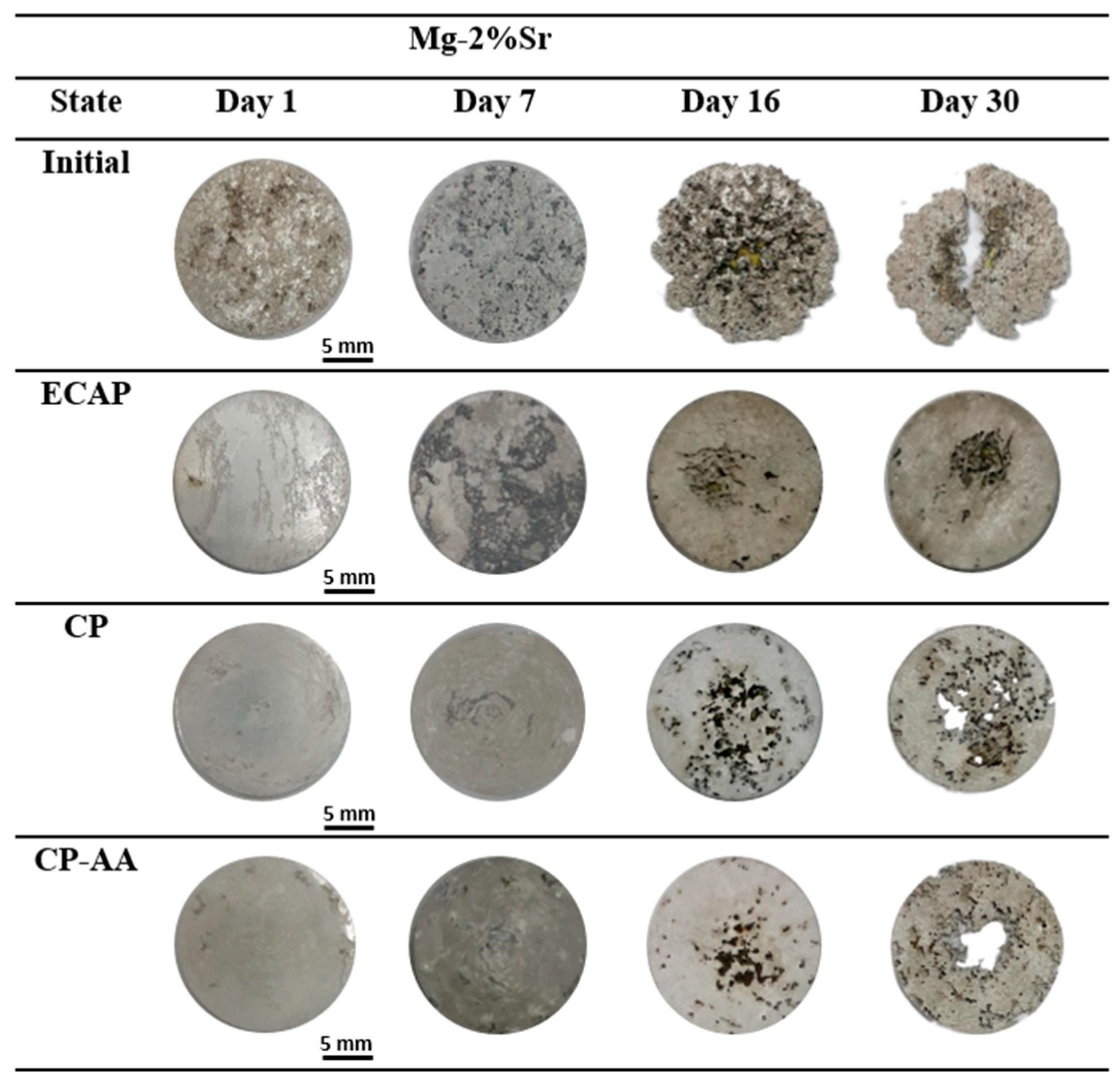
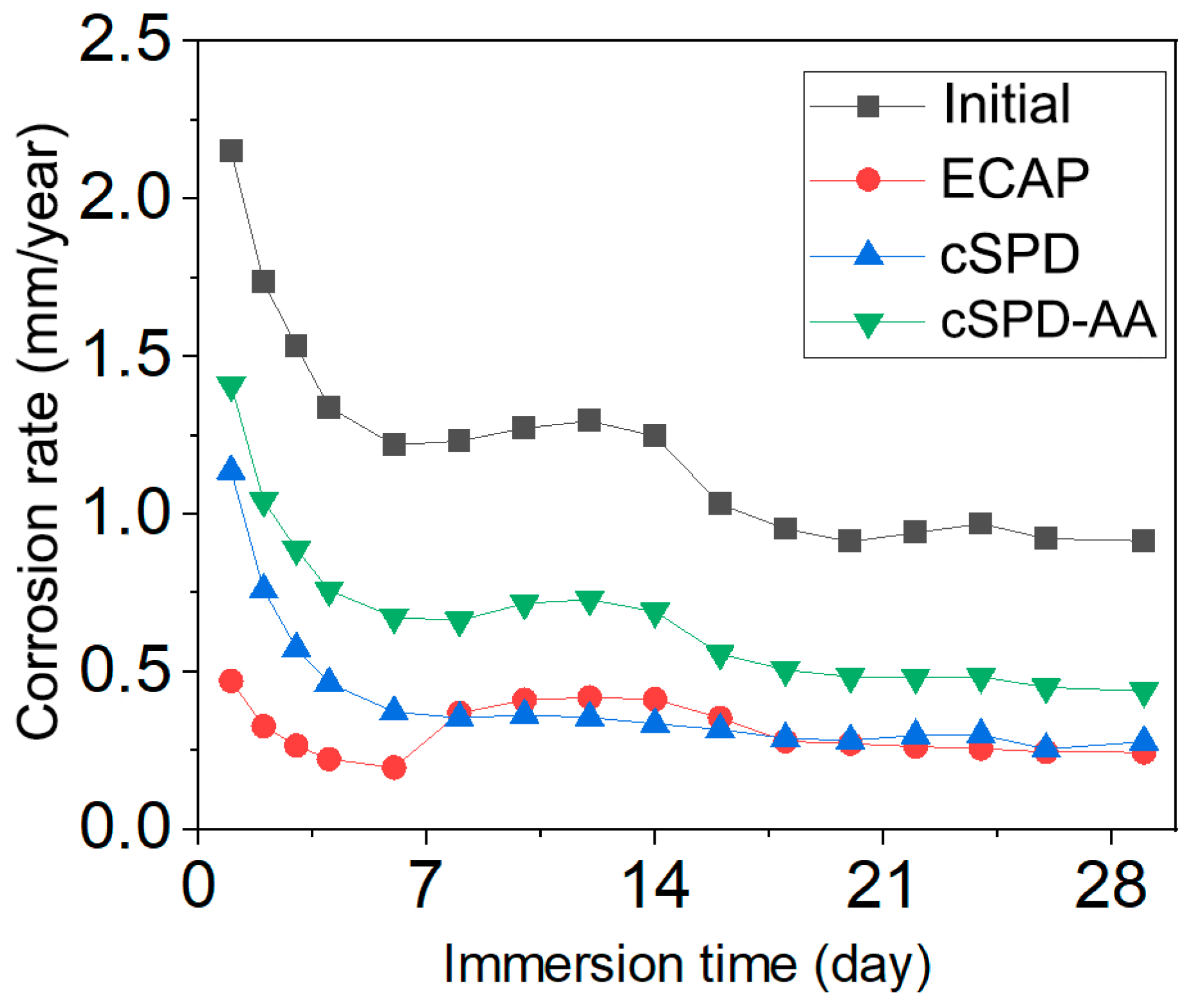
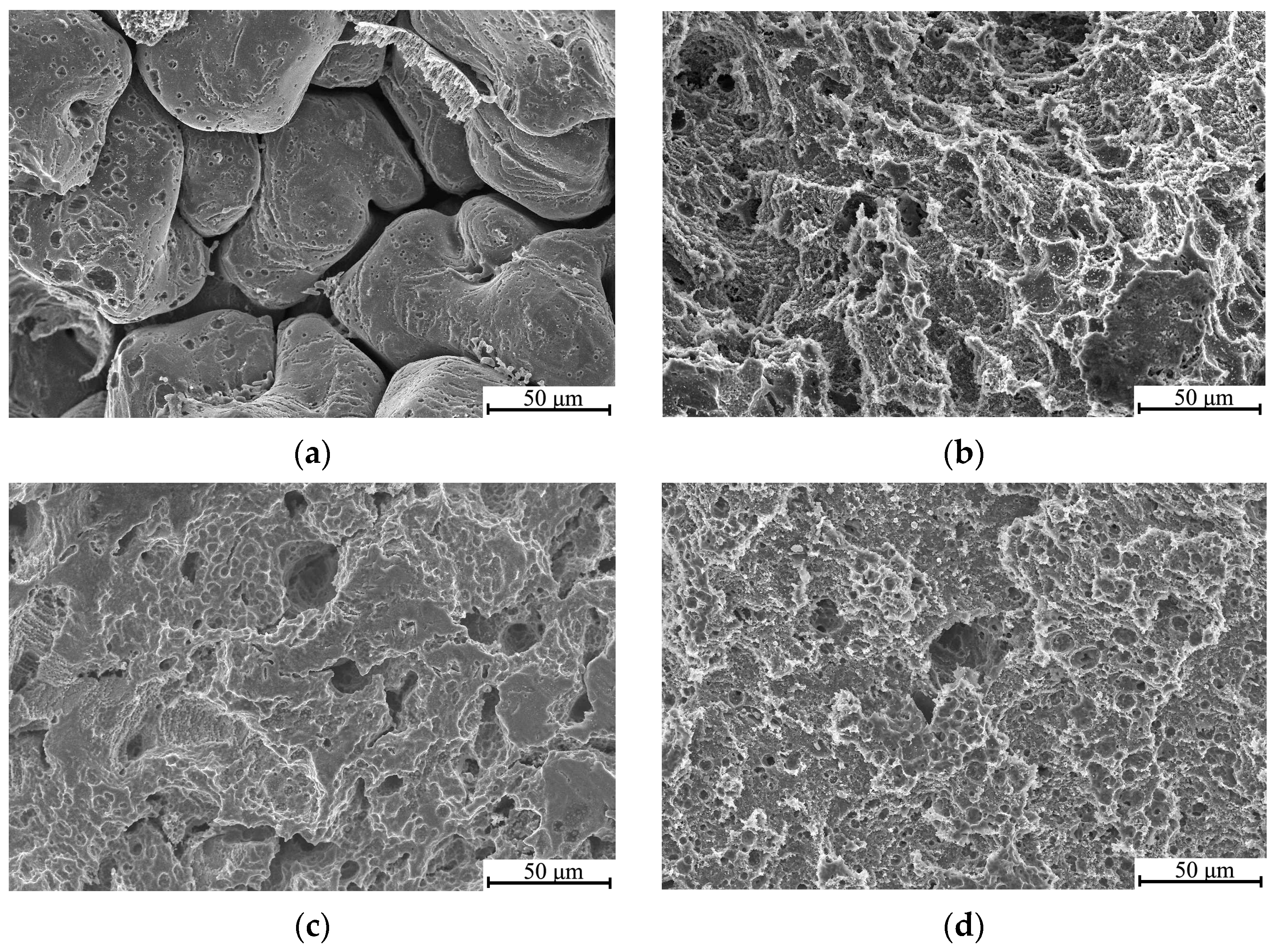
3.4. Corrosion Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.F.; Gu, X.N.; Witte, F.P. Biodegradable metals. Mater. Sci. Eng. R 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Gavish, U.; Amit, T.; Chauhan, A.; Bala, S. Magnesium based implants for functional bone tissue regeneration—A review. J. Magnes. Alloy. 2022, 10, 356–386. [Google Scholar] [CrossRef]
- Radha, R.; Sreekanth, D. Insight of magnesium alloys and composites for orthopedic implant applications—A review. J. Magnes. Alloy. 2017, 5, 286–312. [Google Scholar] [CrossRef]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants, A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Kamrani, S.; Fleck, C. Biodegradable magnesium alloys as temporary orthopaedic implants. Biometals 2019, 32, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, A.; Merson, E.; Myagkikh, P.; Linderov, M.; Brilevsky, A.; Merson, D. Attaining High Functional Performance in Biodegradable Mg-Alloys: An Overview of Challenges and Prospects for the Mg-Zn-Ca System. Materials 2023, 16, 1324. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.N.; Xie, X.H.; Li, N.; Zheng, Y.F.; Qin, L. In vitro and in vivo studies on a Mg-Sr binary alloy system developed as a new kind of biodegradable metal. Acta Biomater. 2012, 8, 2360–2374. [Google Scholar] [CrossRef]
- Canalis, E.; Hott, M.; Deloffre, P.; Tsouderos, Y.; Marie, P.J. The divalent strontium salt S12911 enhances bone cell replication and bone formation in vitro. Bone 1996, 18, 517–523. [Google Scholar] [CrossRef]
- Gorustovich, A.A.; Steimetz, T.; Cabrini, R.L.; Porto López, J.M. Osteoconductivity of strontium-doped bioactive glass particles: A histomorphometric study in rats. J. Biomed. Mater. Res. 2010, 92, 232–237. [Google Scholar] [CrossRef]
- Tie, D.; Guan, R.; Liu, H.; Cipriano, A.; Liu, Y.; Wang, Q.; Huang, Y.; Hort, N. An in vivo study on the metabolism and osteogenic activity of bioabsorbable Mg–1Sr alloy. Acta Biomater. 2016, 29, 455–467. [Google Scholar] [CrossRef]
- Edalati, K.; Bachmaier, A.; Beloshenko, V.A.; Beygelzimer, Y.; Blank, V.D.; Botta, W.J.; Bryła, K.; Čížek, J.; Divinski, S.; Enikeev, N.A.; et al. Nanomaterials by Severe Plastic Deformation: Review of Historical Developments and Recent Advances. Mater. Res. Lett. 2022, 10, 163–256. [Google Scholar] [CrossRef]
- Kasaeian-Naeini, M.; Sedighi, M.; Hashemi, R. Severe plastic deformation (SPD) of biodegradable magnesium alloys and composites: A review of developments and prospects. J. Magnes. Alloy. 2022, 10, 938–955. [Google Scholar] [CrossRef]
- Kulyasova, O.B.; Islamgaliev, R.K.; Parfenov, E.V.; Zheng, Y.F.; Valiev, R.Z. Microstructure, mechanical and corrosion properties of ultrafine-grained Mg-2%Sr alloy. IOP Conf. Series Mater. Sci. Eng. 2018, 380, 012014. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Zheng, Y.; Wang, W.; Qiao, W.; Yeung, K.W.K.; Cheung, K.M.C.; Guan, S.; Kulyasova, O.B.; Valiev, R.Z. In vitro andin vivo studies on ultrafine-grained biodegradable pure Mg, Mg-Ca alloy and Mg-Sr alloy processed by high-pressure torsion. Biomater. Sci. 2020, 8, 5071–5087. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shen, Y.; Shen, J.; Shen, D.; Liu, X.; Zheng, Y.; Yeung, K.W.K.; Guan, S.; Kulyasova, O.B.; Valiev, R.Z. In vitro and in vivostudies on pure Mg, Mg–1Ca and Mg–2Sr alloys processed by equal channel angular pressing. Nano Mater. Sci. 2020, 2, 96–108. [Google Scholar] [CrossRef]
- Dong, J.H.; Tan, L.L.; Ren, Y.B.; Yang, K. Effect of Microstructure on Corrosion Behavior of Mg–Sr Alloy in Hank’s Solution. Acta Metall. Sin. (Engl. Lett.) 2019, 32, 305–320. [Google Scholar] [CrossRef]
- Sabbaghianrad, S.; Langdon, T.G. A critical evaluation of the processing of an aluminum 7075 alloy using a combination of ECAP and HPT. Mater. Sci. Eng. A 2014, 596, 52–58. [Google Scholar] [CrossRef]
- Medvedev, A.E.; Murashkin, M.Y.; Enikeev, N.A.; Bikmukhametov, I.; Valiev, R.Z.; Hodgson, P.D.; Lapovok, R. Effect of the eutectic Al-(Ce, La) phase morphology on microstructure, mechanical properties, electrical conductivity and heat resistance of Al-4.5(Ce, La) alloy after SPD and subsequent annealing. J. Alloys Compd. 2019, 796, 321–330. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Langdon, T.G. Principles of equal-channel angular pressing as a processing tool for grain refinement. Prog. Mater. Sci. 2006, 51, 881–981. [Google Scholar] [CrossRef]
- Lutterotti, L.; Matthies, S.; Wenk, H.-R.; Schultz, A.; Richardson, J. Combined Texture and Structure Analysis of Deformed Limestone from Time-of-Flight Neutron Diffraction Spectra. J. Appl. Phys. 1997, 81, 594–600. [Google Scholar] [CrossRef]
- Popa, N.C. The (hkl) Dependence of diffraction-line broadening caused by strain and size for all Laue groups in Rietveld refinement. J. Appl. Cryst. 1998, 31, 176–180. [Google Scholar] [CrossRef]
- Griffiths, M.; Winegar, J.E.; Mecke, J.E.; Holt, R.A. Determination of dislocation densities in hexagonal closed-packed metals using X-ray diffraction and transmission electron microscopy. Adv. X-Ray Anal. 1991, 35, 593–599. [Google Scholar] [CrossRef]
- Dyakonov, G.S.; Mironov, S.; Enikeev, N.; Semenova, I.P.; Valiev, R.Z.; Semiatin, S.L. Annealing behavior of severely-deformed titanium Grade 4. Mater. Sci. Eng. A 2019, 742, 89–101. [Google Scholar] [CrossRef]
- Lomakin, I.V.; Arutyunyan, A.R.; Valiev, R.R.; Gadzhiev, F.A.; Murashkin, M.Y. Design and Evaluation of an Experimental Technique for Mechanical and Fatigue Testing of Sub-Sized Samples. Exp. Tech. 2018, 42, 261–270. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Wu, X.L. Ductility and plasticity of nanostructured metals: Differences and issues. Mater. Today Nano 2018, 2, 15–20. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Guo, Y.Z.; Wei, Q.; Dangelewiez, A.M.; Zhu, Y.T.; Langdon, T.G.; Zhou, Y.Z.; Lavernia, E.J.; Xu, C. Influence of specimen dimensions on the tensile behavior of ultrafine-grained Cu. Scripta Mater. 2008, 59, 627–630. [Google Scholar] [CrossRef]
- Şevik, H.; Kurnaz, C.S. The effect of strontium on the microstructure and mechanical properties of Mg–6Al–0.3Mn–0.3Ti–1Sn. J. Magnes. Alloy. 2014, 2, 214–219. [Google Scholar] [CrossRef]
- Kulyasova, O.B.; Khudododova, G.D.; Dyakonov, G.S.; Zheng, Y.; Valiev, R.Z. Effect of Microstructure Refinement on the Corrosion Behavior of the Bioresorbable Mg-1Zn-0.2Ca and Mg-1Ca Alloys. Materials 2022, 15, 6749. [Google Scholar] [CrossRef]
- Argadea, G.R.; Panigrahia, S.K.; Mishra, R.S. Corrosion behavior of a friction stir processed rare-earth added magnesium alloy. Corros. Sci. 2012, 58, 321–326. [Google Scholar] [CrossRef]
- Saha, P.; Roy, M.; Datta, M.K.; Lee, B.; Kumta, P.N. Effects of grain refinement on the biocorrosion and in vitro bioactivity of magnesium. Mat. Sci. Eng. C 2015, 57, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.; Murashkin, M.; Enikeev, N.; Medvedev, E.; Sauvage, X. Influence of morphology of intermetallic particles on the microstructure and properties evolution in severely deformed Al-Fe alloys. Metals 2021, 11, 815. [Google Scholar] [CrossRef]
- Pei, R.; Zou, Y.; Wei, D.; Al-Samman, T. Grain boundary co-segregation in magnesium alloys with multiple substitutional elements. Acta Mater. 2021, 208, 116749. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Y.; Wen, C. Effects of Mg17Sr2 phase on the bio-corrosion behavior of Mg–Zr–Sr alloys. Adv. Eng. Mater. 2016, 2, 18. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]

| State | Lattice Parameters, Å | <ε2>1/2, % | ρa, m−2 | dxrd, nm | D, μm |
|---|---|---|---|---|---|
| Initial | a = 3.2112 ± 0.0008 c = 5.2115 ± 0.00007 | - | - | - | 227 ± 21 |
| ECAP | a = 3.2105 ± 0.0005 c = 5.2121 ± 0.0003 | 0.0385 ± 0.0005 | 1.7 × 1015 | 100 ± 15 | 10 ± 2 |
| CP | a = 3.2081 ± 0.0003 c = 5.2090 ± 0.0001 | 0.0438 ± 0.0004 | 2.4 × 1015 | 76.1 ± 5 | 0.25 ± 0.05 |
| CP-AA | a = 3.2098 ± 0.0002 c = 5.2115 ± 0.0003 | 0.0016 ± 0.0002 | 0.2 × 1015 | 245.8 ± 13 | 6.7 ± 1.7 |
| State of the Mg-2%Sr Alloy | Microhardness, HV | YS, MPa | UTS, MPa | Elongation, % |
|---|---|---|---|---|
| Initial alloy | 38 ± 5 | 63 ± 3 | 65 ± 4 | 0.7 ± 0.3 |
| ECAP | 55 ± 5 | 151 ± 15 | 193 ± 9 | 4.3 ± 0.5 |
| CP | 65 ± 6 | 225 ± 24 | 253 ± 25 | 0.2 ± 0.1 |
| CP-AA | 60 ± 6 | 180 ± 18 | 250 ± 23 | 6.5 ± 0.5 |
| State | Corrosion Rate, mm/y | |||
|---|---|---|---|---|
| Day 1 | Day 3 | Day 7 | Day 30 | |
| Initial | 2.15 ± 0.21 | 1.53 ± 0.12 | 1.27 ± 0.10 | 0.91 ± 0.13 |
| ECAP | 0.47 ± 0.04 | 0.26 ± 0.03 | 0.36 ± 0.02 | 0.24 ± 0.02 |
| cSPD | 1.14 ± 0.05 | 0.57 ± 0.02 | 0.35 ± 0.05 | 0.27 ± 0.05 |
| cSPD-AA | 1.41 ± 0.10 | 0.89 ± 0.08 | 0.66 ± 0.10 | 0.44 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nafikov, R.K.; Kulyasova, O.B.; Khudododova, G.D.; Enikeev, N.A. Microstructural Assessment, Mechanical and Corrosion Properties of a Mg-Sr Alloy Processed by Combined Severe Plastic Deformation. Materials 2023, 16, 2279. https://doi.org/10.3390/ma16062279
Nafikov RK, Kulyasova OB, Khudododova GD, Enikeev NA. Microstructural Assessment, Mechanical and Corrosion Properties of a Mg-Sr Alloy Processed by Combined Severe Plastic Deformation. Materials. 2023; 16(6):2279. https://doi.org/10.3390/ma16062279
Chicago/Turabian StyleNafikov, Ruslan K., Olga B. Kulyasova, Ganjina D. Khudododova, and Nariman A. Enikeev. 2023. "Microstructural Assessment, Mechanical and Corrosion Properties of a Mg-Sr Alloy Processed by Combined Severe Plastic Deformation" Materials 16, no. 6: 2279. https://doi.org/10.3390/ma16062279
APA StyleNafikov, R. K., Kulyasova, O. B., Khudododova, G. D., & Enikeev, N. A. (2023). Microstructural Assessment, Mechanical and Corrosion Properties of a Mg-Sr Alloy Processed by Combined Severe Plastic Deformation. Materials, 16(6), 2279. https://doi.org/10.3390/ma16062279







