Preparation and Application of a NaCl-KCl-CsCl-Cs2ZrCl6 Composite Electrolyte
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation Method of the Composite Electrolyte
2.3. Characterization Methods of Materials
3. Results and Discussion
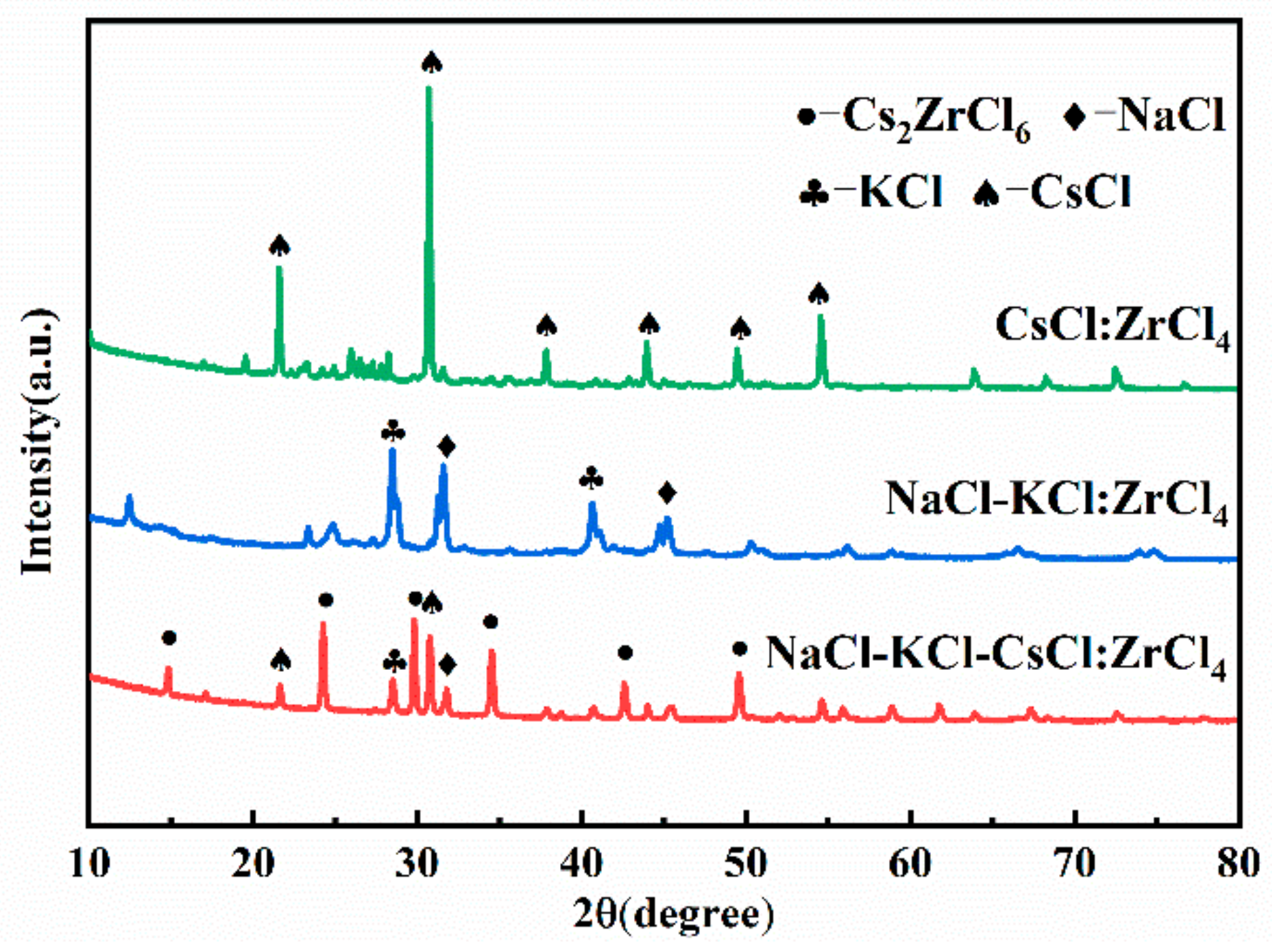
3.1. Feasibility of Adding ZrCl4 in Different Molten Salts
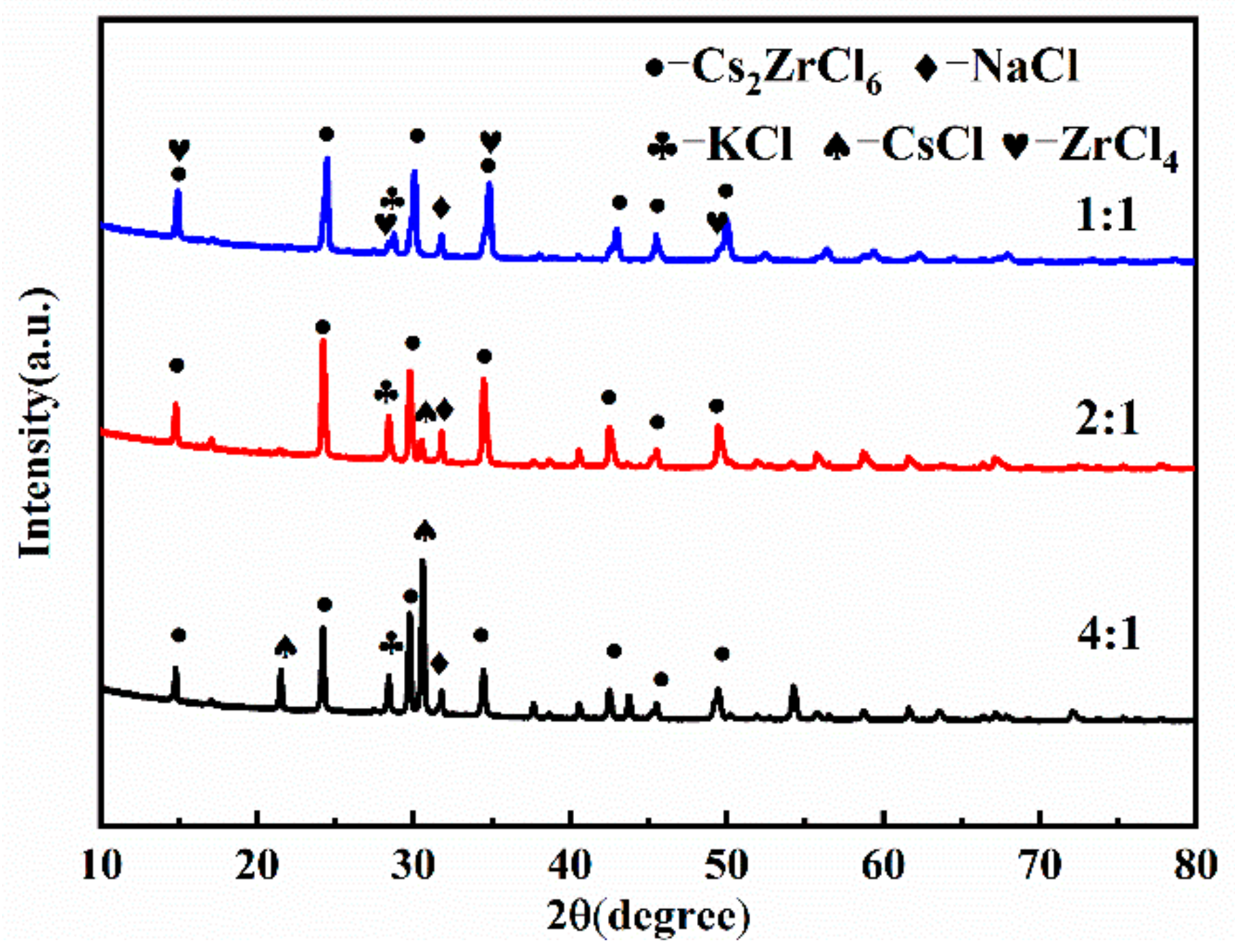
3.2. Effect of Raw Material Ratio on the Preparation of Composite Electrolyte
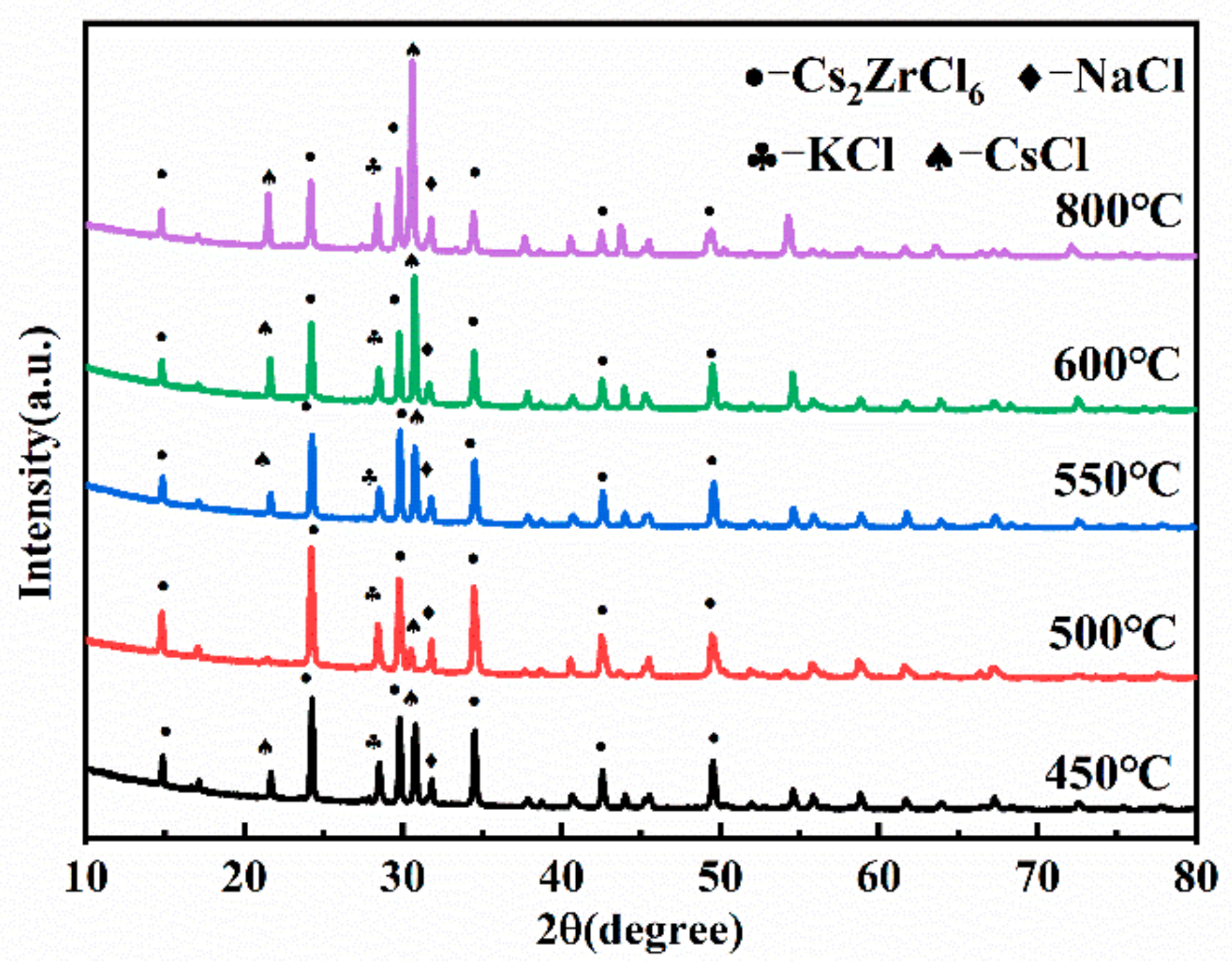
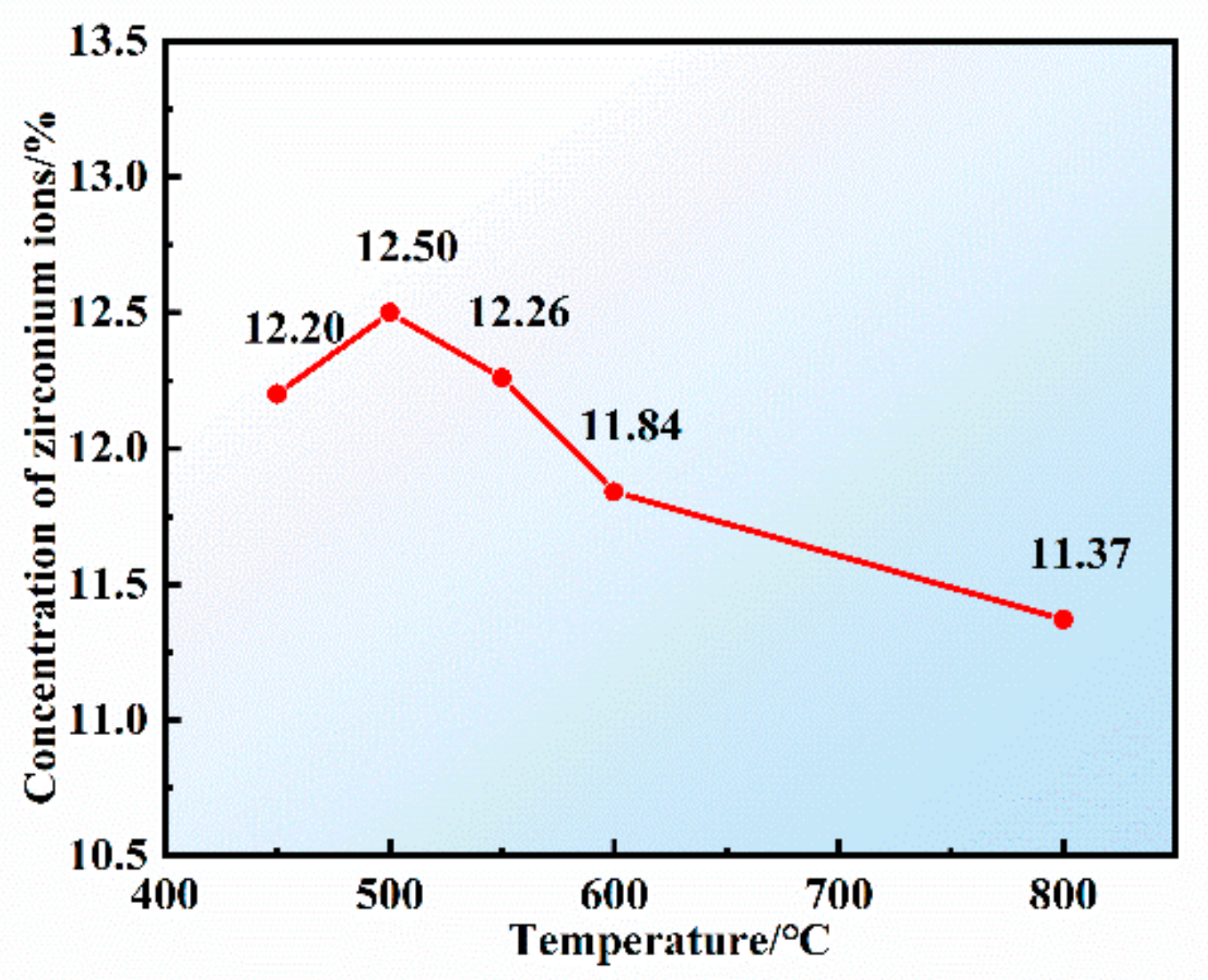
3.3. Effect of Reaction Temperature on the Preparation of Composite Electrolyte
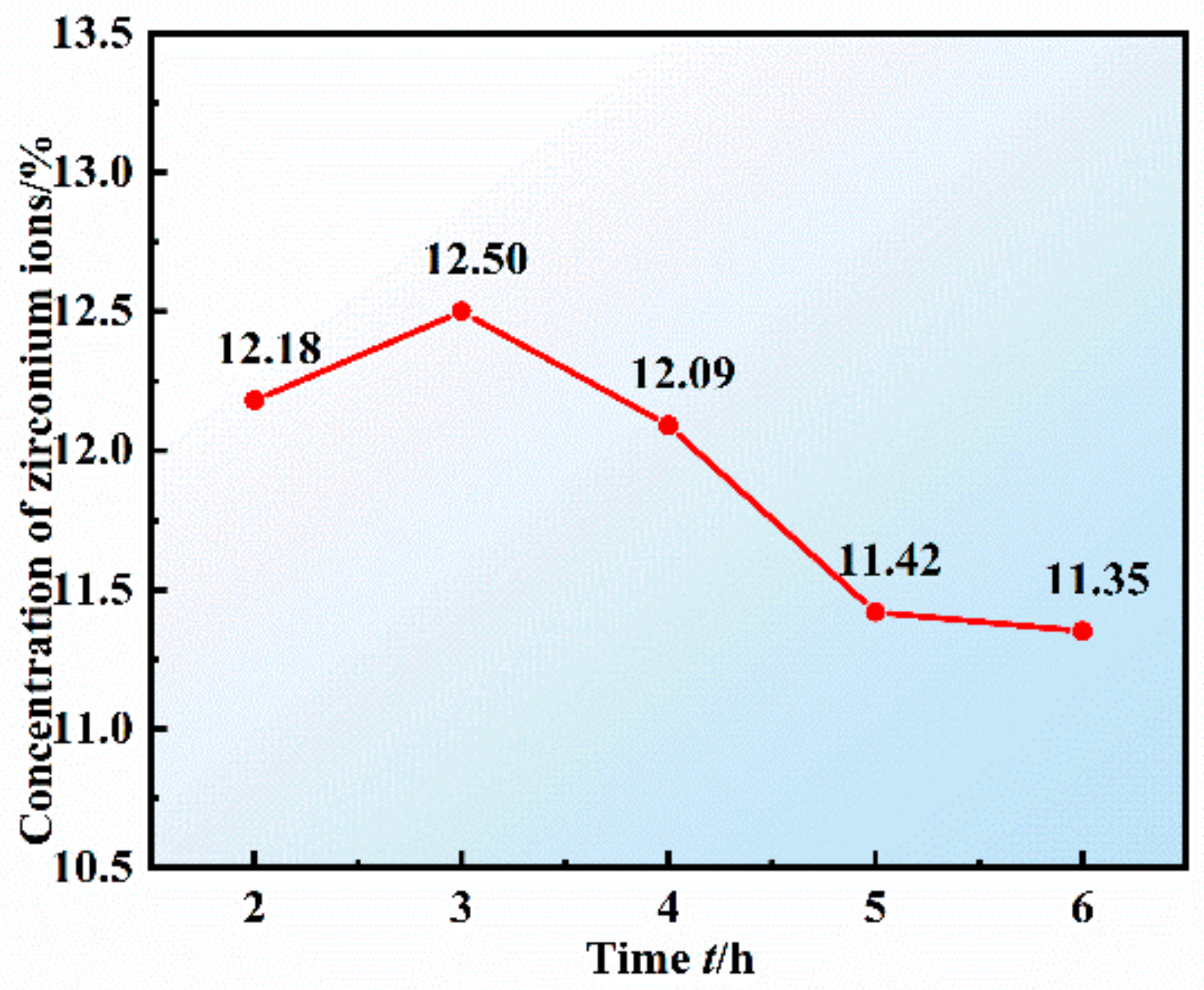
3.4. Effect of Reaction Time on the Preparation of the Composite Electrolyte
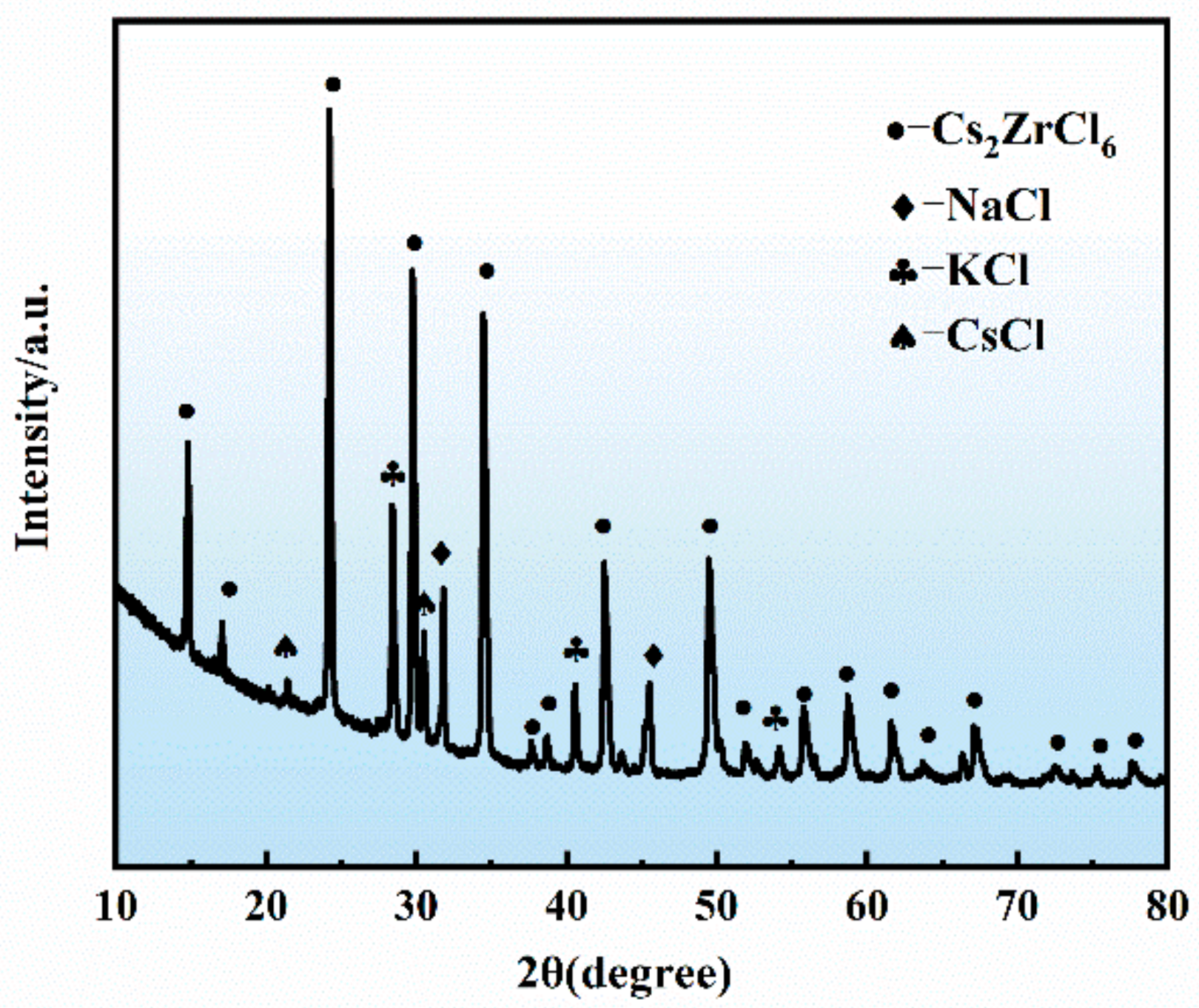
3.5. Preparation of the NaCl-KCl-CsCl-Cs2ZrCl6 Composite Electrolyte
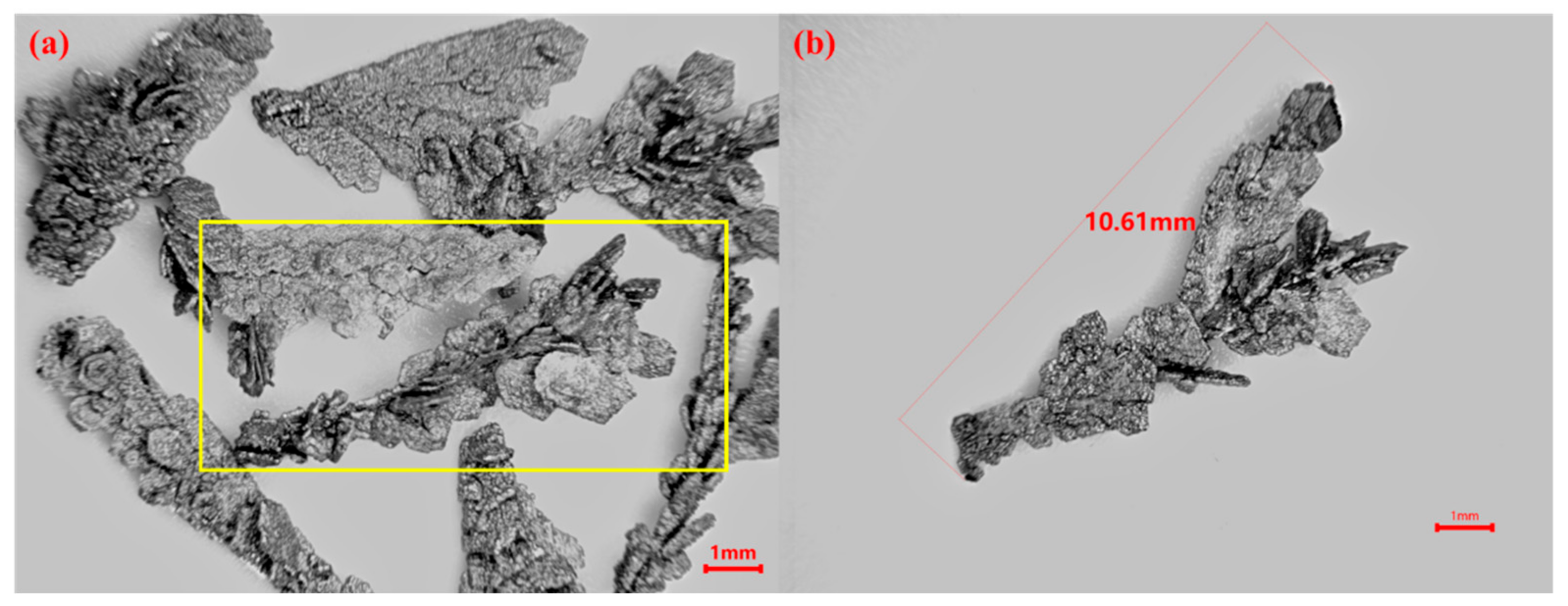
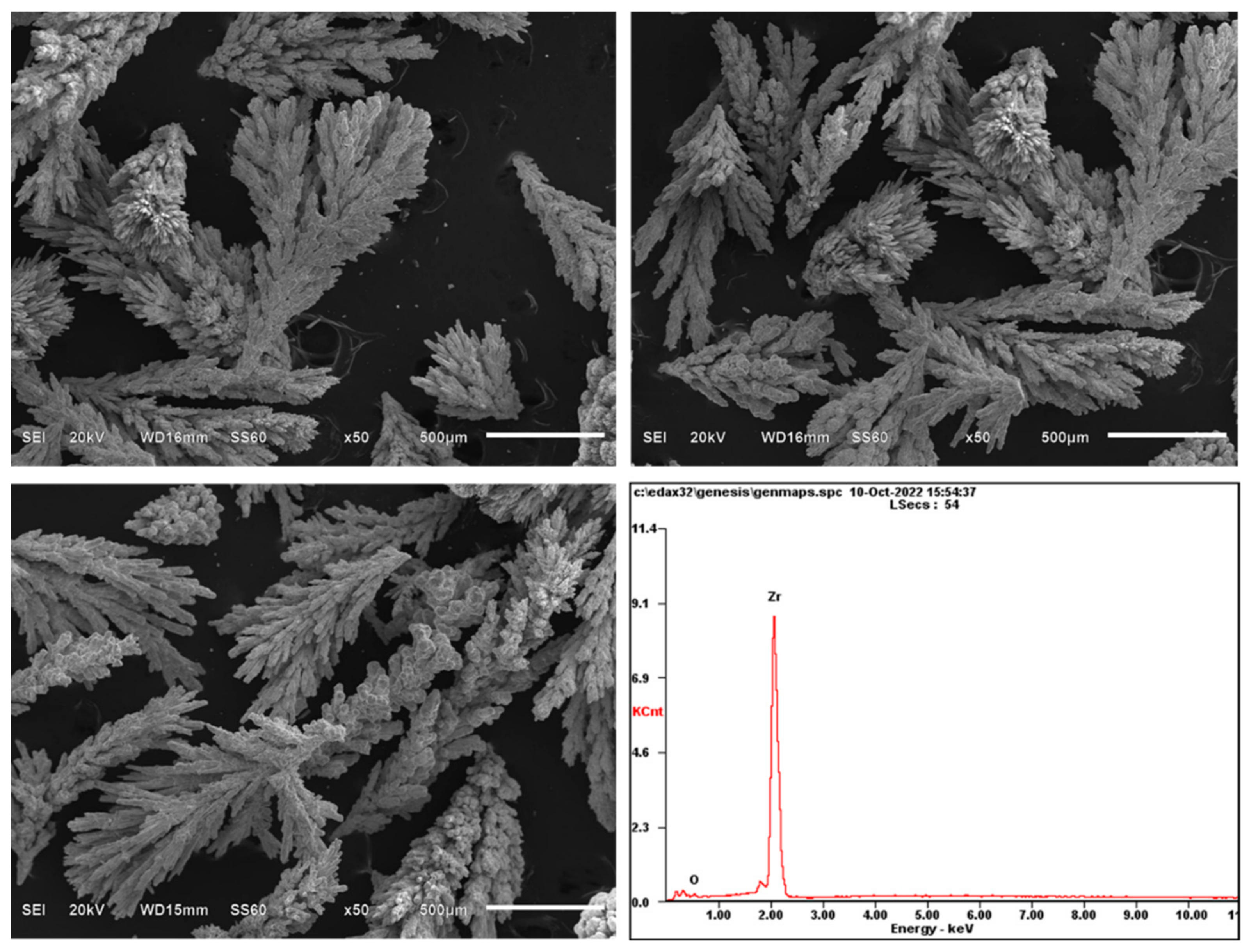
3.6. Application of the NaCl-KCl-CsCl-Cs2ZrCl6 Composite Electrolyte
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, C. Prospects in China for nuclear development up to 2050. Pro. Nucl. Energy 2018, 103, 81–90. [Google Scholar]
- Xu, Y.; Kang, J.J.; Yuan, J.H. The Prospective of Nuclear Power in China. Sustainability 2018, 10, 2086. [Google Scholar] [CrossRef]
- Zhao, C.K. Development status and prospect of nuclear power in China. Nucl. Power Eng. 2018, 39, 1–3. [Google Scholar]
- Liu, H.; Ma, Z.H.; Huang, J.C.; Zhang, J.D.; Yan, G.Q.; Wang, L.J. Preparation of Al-Hf master alloy by aluminothermic reduction of HfO2. Rare Met. 2021, 40, 3645–3650. [Google Scholar] [CrossRef]
- Takeda, O.; Suda, K.; Lu, X. Zirconium Metal Production by Electrorefining of Zr Oxycarbide. J. Sustain. Metall. 2018, 4, 506–515. [Google Scholar] [CrossRef]
- Xu, L.; Xiao, Y.; Van Sandwijk, A.; Xu, Q.; Yang, Y. Production of nuclear grade zirconium: A review. Nucl. Mater. 2015, 466, 21–28. [Google Scholar] [CrossRef]
- Wu, Y.K.; Li, Q.B.; Xu, Z.G.; Wang, L.J.; Xing, B.G. Preparation method of zirconium metal. Rare Met. 2009, 4, 462–466. [Google Scholar]
- Xu, L.; Xiao, Y.; Xu, Q.; van Sandwijk, A.; Zhao, Z.; Song, Q.; Cai, Y.; Yang, Y. Electrochemical studies on the redox behavior of zirconium in the LiF-NaF eutectic melt. J. Nucl. Mater. 2017, 488, 295–301. [Google Scholar] [CrossRef]
- Emelyanov, V.S.; Bysttov, P.D.; Evstyukhin, A.I. A study of the iodide process of refining zirconium. Sov. At. Energy 1956, 1, 415–424. [Google Scholar] [CrossRef]
- Kipouros, G.; Flengas, S. Electrorefining of zirconium metal in alkali chloride and alkali fluoride fused electrolytes. J. Electrochem. Soc. 1985, 132, 1087–1098. [Google Scholar] [CrossRef]
- Groult, H.; Barhoun, A.; Elghallali, H.; Borensztjan, S.; Lantelme, F. Study of the electrochemical reduction of Zr4+ ions in molten alkali fluorides. J. Electrochem. Soc. 2008, 155, E19–E25. [Google Scholar] [CrossRef]
- Park, K.T.; Lee, T.H.; Jo, N.C.; Nersisyan, H.H.; Chun, B.S.; Lee, H.H. Purification of nuclear grade Zr scrap as the high purity dense Zr deposits from Zirlo scrap by electrorefining in LiF-KF-ZrF4 molten fluorides. Nucl. Mater. 2013, 436, 130–138. [Google Scholar] [CrossRef]
- Fujita, R.; Nakamura, H.; Mizuguchi, K.; Sato, M.; Shibano, T.; Ito, Y.; Goto, T.; Terai, T.; Ogawa, S. Zirconium recovery process for spent zircaloy components from Light Water Reactor (LWR) by electrorefining in molten salts. Electrochemistry 2005, 73, 751–753. [Google Scholar] [CrossRef]
- Goto, T.; Nohira, T.; Hagiwara, R.; Ito, Y. Selected topics of molten fluorides in the field of nuclear engineering. J. Fluorine Chem. 2009, 130, 102–107. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.J.; Li, S.J. Electrochemical behavior of zirconium in the LiCl-KCl molten salt at Mo electrode. J. Alloys Compd. 2011, 509, 5958–5961. [Google Scholar] [CrossRef]
- Wu, Y.K.; Xu, Z.; Chen, S.; Wang, L.J.; Li, G.X. Electrochemical behavior of zirconium in molten NaCl-KCl-K2ZrF6 system. Rare Met. 2011, 30, 8–13. [Google Scholar] [CrossRef]
- Inman, D.; Hills, G.J.; Young, L.; Bockris, J.O. Some thermodynamic aspects of molten salts: Halides of uranium, zirconium, thorium, and cerium in alkali halide eutectics. Ann. N. Y. Acad. Sci. 2010, 79, 803–829. [Google Scholar] [CrossRef]
- Baboian, R.; Hill, D.L.; Bailey, R.A. Electrochemical studies on zirconium and hafnium in molten LiCl-KCl eutectic. J. Electrochem. Soc. 1965, 112, 1221–1224. [Google Scholar] [CrossRef]
- Sakamura, Y. Zirconium behavior in molten LiCl-KCl eutectic. J. Electrochem. Soc. 2004, 151, C87–Cl93. [Google Scholar] [CrossRef]
- Ghosh, S.; Vandarkuzhali, S.; Venkatesh, P.; Seenivasan, G.; Subramanian, T.; Reddy, B.P.; Nagarajan, K. Electrochemical studies on the redox behaviour of zirconium in molten LiCl-KCl eutectic. J. Electrochem. Soc. 2009, 627, 15–27. [Google Scholar] [CrossRef]
- Lee, C.H.; Kang, K.H.; Jeon, M.K.; Heo, C.M.; Lee, Y.L. Electrorefining of zirconium from zircaloy-4 cladding hulls in LiCl-KCl molten salts. J. Electrochem. Soc. 2012, 159, D463–D468. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, H.; Xu, Q.; Song, Q.; Xu, Q. Investigation on the reaction progress of zirconium and cuprous chloride in the LiCl-KCl melt. Electrochim 2015, 161, 177–185. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.i.n.; Peng, J.Q.; Wang, L.J.; Chen, S.; Wu, Y.K.; Chen, Y. Preparation and characterization of potassium chlorohafnium. Rare Met. Mat. Eng. 2014, 43, 450–454. [Google Scholar]
- Qiu, J.J.; Chen, S.; Wu, Y.K.; Wang, L.J. Study on process of molten salt electrolytic refining of zirconium. Rare Met. 2011, 35, 78–82. [Google Scholar]
- Basin, A.S.; Kaplun, A.B.; Meshalkin, A.B.; Uvarov, A.B. The LiCl-KCl binary system. Russ. J. Inorg. 2008, 53, 1509–1511. [Google Scholar] [CrossRef]
- Burton, B.P.; Walle, A. First-principles phase diagram calculations for the system NaCl-KCl: The role of excess vibrational entropy. Chem. Geol. 2006, 225, 222–229. [Google Scholar] [CrossRef]
- Dzidziguri, E.L.; Salangina, E.A.; Sidorova, E.N. Hafnium powder production processes. Metally 2010, 9, 768–771. [Google Scholar] [CrossRef]
- Murakami, T.; Nohira, T.; Ogata, Y.H.; Ogata, Y.H.; Ito, Y. Electrochemical Window of a LiCl-KCl-CsCl Melt. ECS Solid State Lett. 2005, 8, E1–E3. [Google Scholar] [CrossRef]
- Liu, K.; Tang, H.B.; Pang, J.W.; Liu, L.Y.; Feng, Y.X.; Chai, Z.F.; Shi, W.Q. Electrochemical Properties of Uranium on the Liquid Gallium Electrode in LiCl-KCl Eutectic. J. Electrochem. Soc. 2016, 163, D554–D561. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, H.; Ye, Y. Research Progress on Preparation of Iridium and Iridium Alloy Coatings by Molten Salt Electrodeposition. Rare Met. Mat. Eng. 2015, 44, 1815–1820. [Google Scholar]
- Zhu, F.X.; Qiu, K.H.; Sun, Z.H. Preparations of titanium dichloride and trichloride in NaCl-KCl melt. Nonferrous Met. 2017, 3, 15–19. [Google Scholar]
- Ning, X.; Åsheim, H.; Ren, H. Preparation of Titanium Deposit in Chloride Melts. Met. Mater. Trans. B 2011, 42, 1181–1187. [Google Scholar] [CrossRef]
- Kipouros, G.J.; Flengas, S.N. Equilibrium decomposition pressures of the compounds Cs2ZrCl6 and Cs2HfCl6 and X-ray identification of Na2HfCl6, K2HfCl6 and Cs2HfCl6. Can. J. Chem. 1983, 61, 2183–2188. [Google Scholar] [CrossRef]


| Raw Material | Purity | Source |
|---|---|---|
| NaCl | Analytical reagent | Guangxi Xilong Chemical Co., Ltd. |
| KCl | Analytical reagent | Guangxi Xilong Chemical Co., Ltd. |
| CsCl | Analytical reagent | Guangxi Xilong Chemical Co., Ltd. |
| ZrCl4 | Analytical reagent | Guangdong Oriental Zirconium Co., Ltd. |
| Zirconium sponge | Industry grade | Guangdong Oriental Zirconium Co., Ltd. |
| No. | Molten Salt System | Ratio | Temperature | Time |
|---|---|---|---|---|
| 1 | NaCl-KCl:ZrCl4 | 2:1 | 550 °C | 3 h |
| 2 | CsCl:ZrCl4 | 2:1 | 550 °C | 3 h |
| 3 | NaCl-KCl-CsCl:ZrCl4 | 2:1 | 550 °C | 3 h |
| 4 | NaCl-KCl-CsCl:ZrCl4 | 4:1 | 550 °C | 3 h |
| 5 | NaCl-KCl-CsCl:ZrCl4 | 1:1 | 550 °C | 3 h |
| 6 | NaCl-KCl-CsCl:ZrCl4 | 2:1 | 550 °C | 3 h |
| 7 | NaCl-KCl-CsCl:ZrCl4 | 2:1 | 450 °C | 3 h |
| 8 | NaCl-KCl-CsCl:ZrCl4 | 2:1 | 500 °C | 3 h |
| 9 | NaCl-KCl-CsCl:ZrCl4 | 2:1 | 600 °C | 3 h |
| 10 | NaCl-KCl-CsCl:ZrCl4 | 2:1 | 800 °C | 3 h |
| 11 | NaCl-KCl-CsCl:ZrCl4 | 2:1 | 500 °C | 2 h |
| 12 | NaCl-KCl-CsCl:ZrCl4 | 2:1 | 500 °C | 4 h |
| 13 | NaCl-KCl-CsCl:ZrCl4 | 2:1 | 500 °C | 5 h |
| 14 | NaCl-KCl-CsCl:ZrCl4 | 2:1 | 500 °C | 6 h |
| No. | CsCl:ZrCl4 | Added Concentration (%) | Post-Reaction Concentration (%) |
|---|---|---|---|
| 4 | 4:1 | 7.47 | 7.32 |
| 6 | 2:1 | 12.54 | 12.50 |
| 5 | 1:1 | 19.00 | 17.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, W.; Wu, Y.; Wang, L.; Yan, G.; Ma, Z.; Zhang, J. Preparation and Application of a NaCl-KCl-CsCl-Cs2ZrCl6 Composite Electrolyte. Materials 2023, 16, 2270. https://doi.org/10.3390/ma16062270
Zou W, Wu Y, Wang L, Yan G, Ma Z, Zhang J. Preparation and Application of a NaCl-KCl-CsCl-Cs2ZrCl6 Composite Electrolyte. Materials. 2023; 16(6):2270. https://doi.org/10.3390/ma16062270
Chicago/Turabian StyleZou, Wenzhen, Yanke Wu, Lijun Wang, Guoqing Yan, Zhaohui Ma, and Jiandong Zhang. 2023. "Preparation and Application of a NaCl-KCl-CsCl-Cs2ZrCl6 Composite Electrolyte" Materials 16, no. 6: 2270. https://doi.org/10.3390/ma16062270
APA StyleZou, W., Wu, Y., Wang, L., Yan, G., Ma, Z., & Zhang, J. (2023). Preparation and Application of a NaCl-KCl-CsCl-Cs2ZrCl6 Composite Electrolyte. Materials, 16(6), 2270. https://doi.org/10.3390/ma16062270






