A Review of Pectin-Based Material for Applications in Water Treatment
Abstract
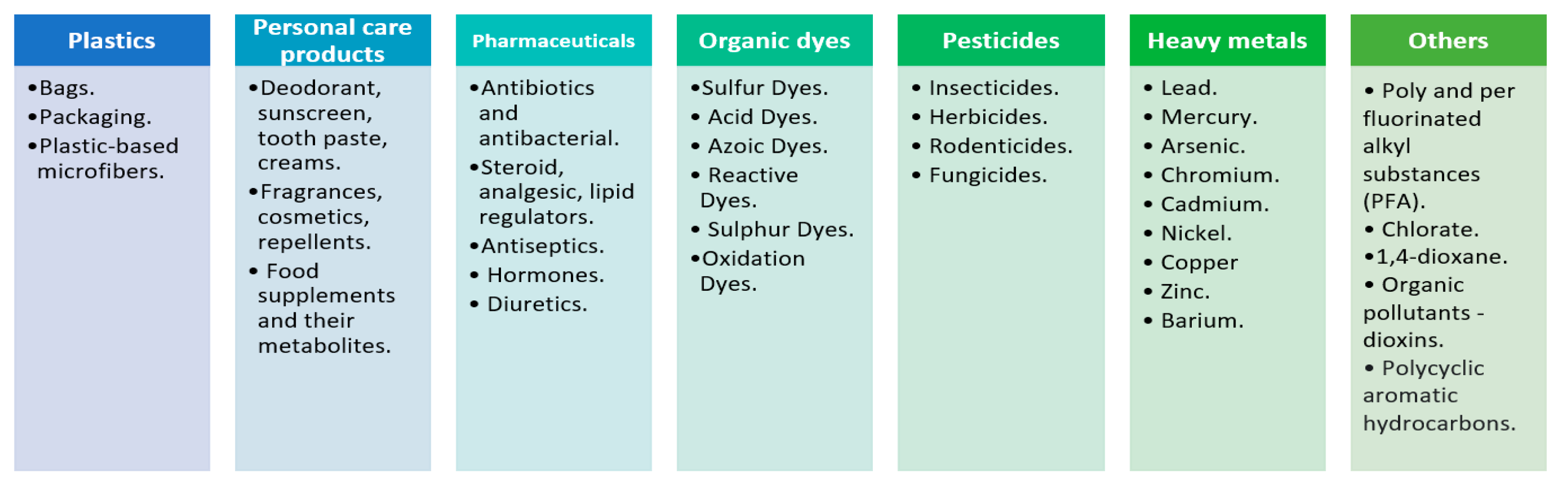
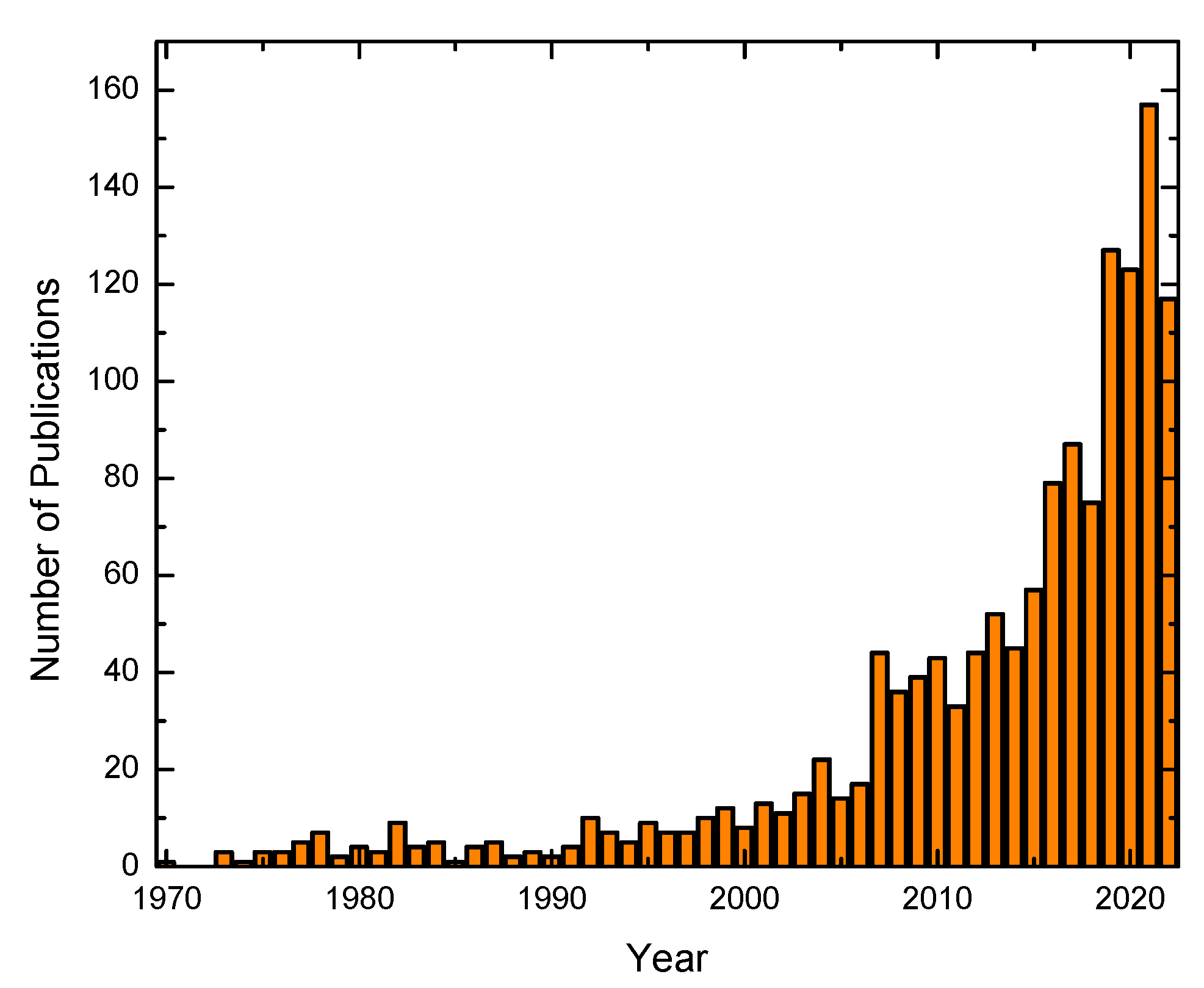
1. Introduction
2. Water Remediation Technologies
3. Pectin—Chemical Structure
4. Insoluble Pectin—Cross-Linking Agents That Generate Gelation
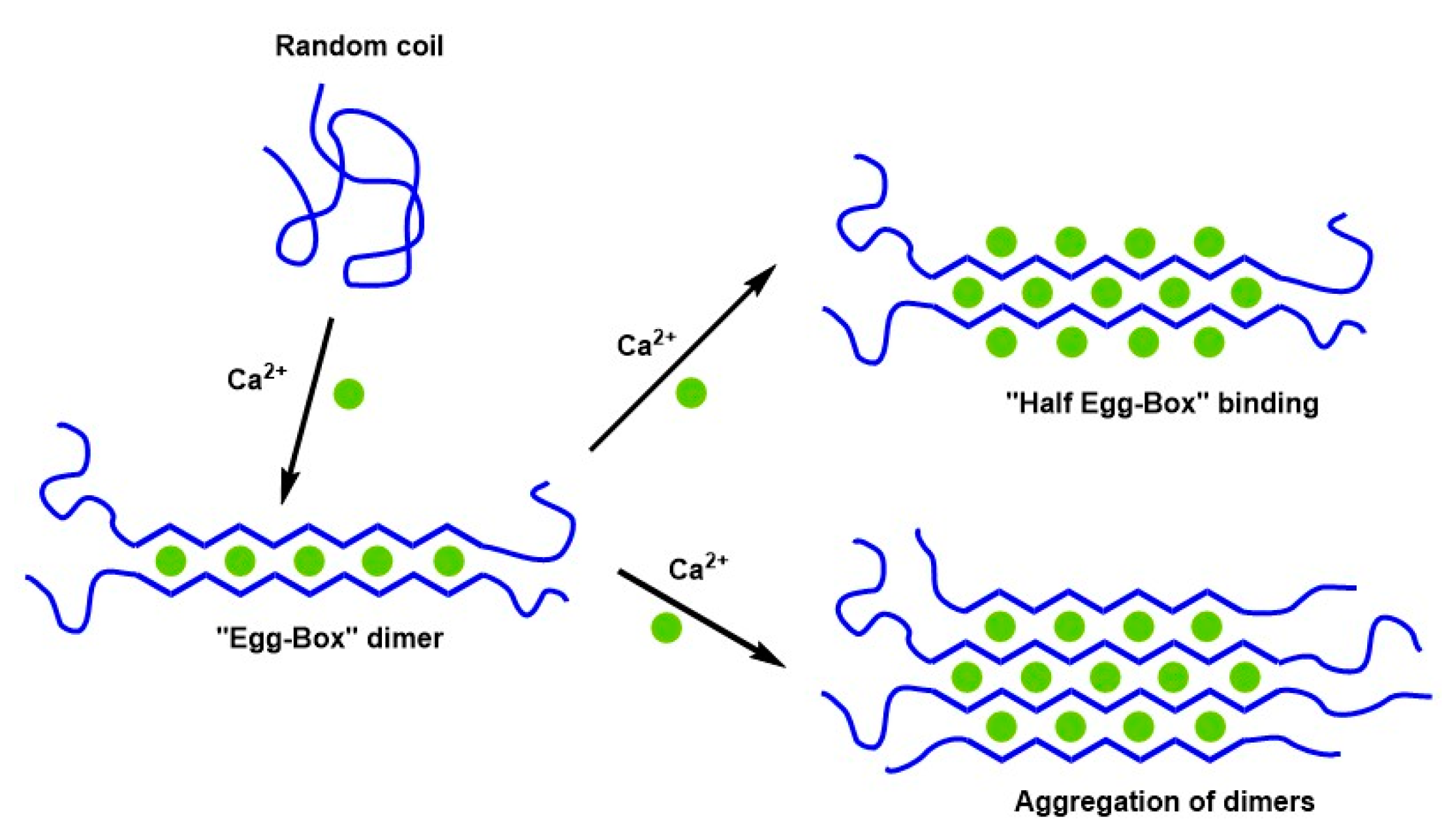
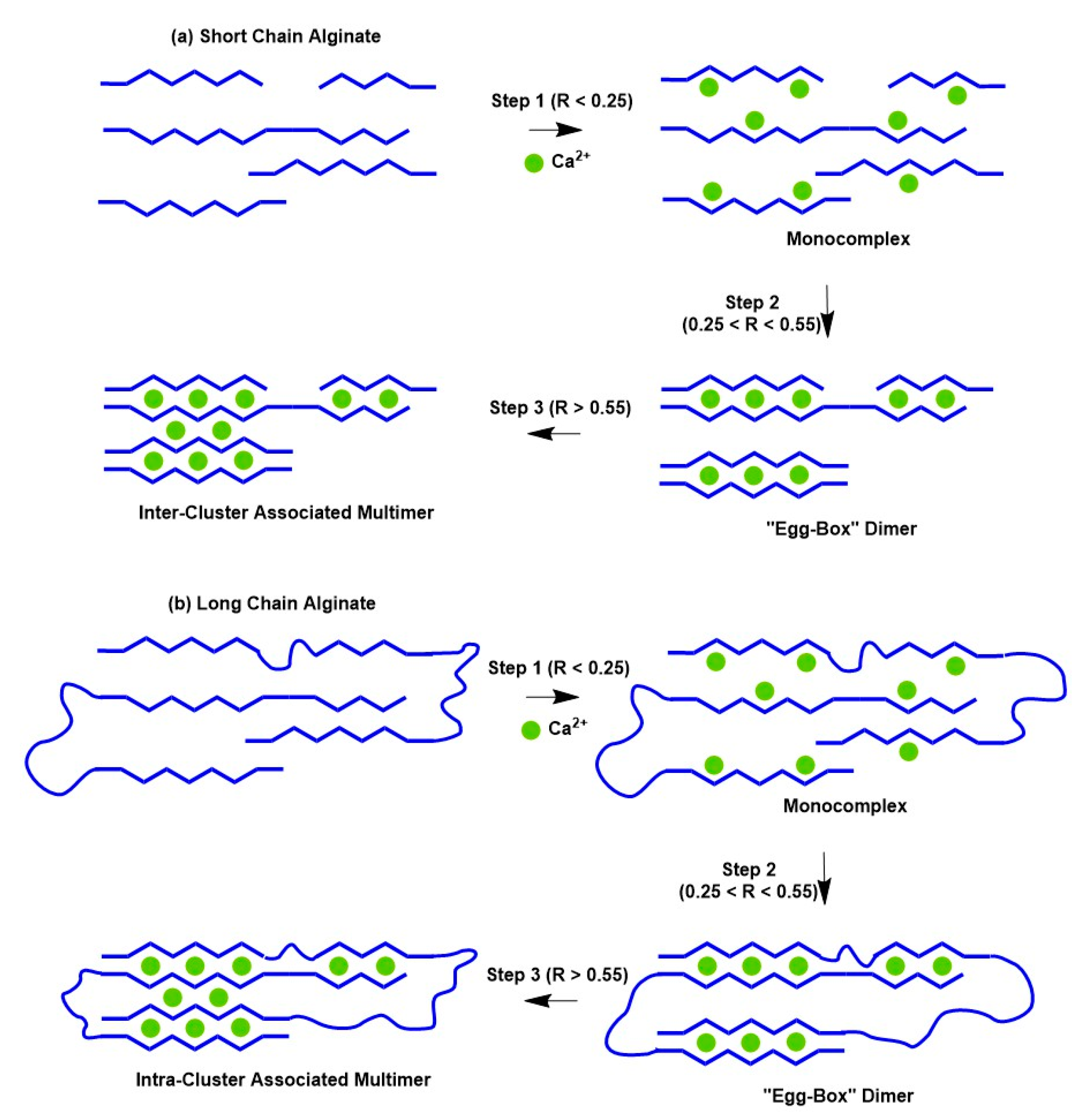
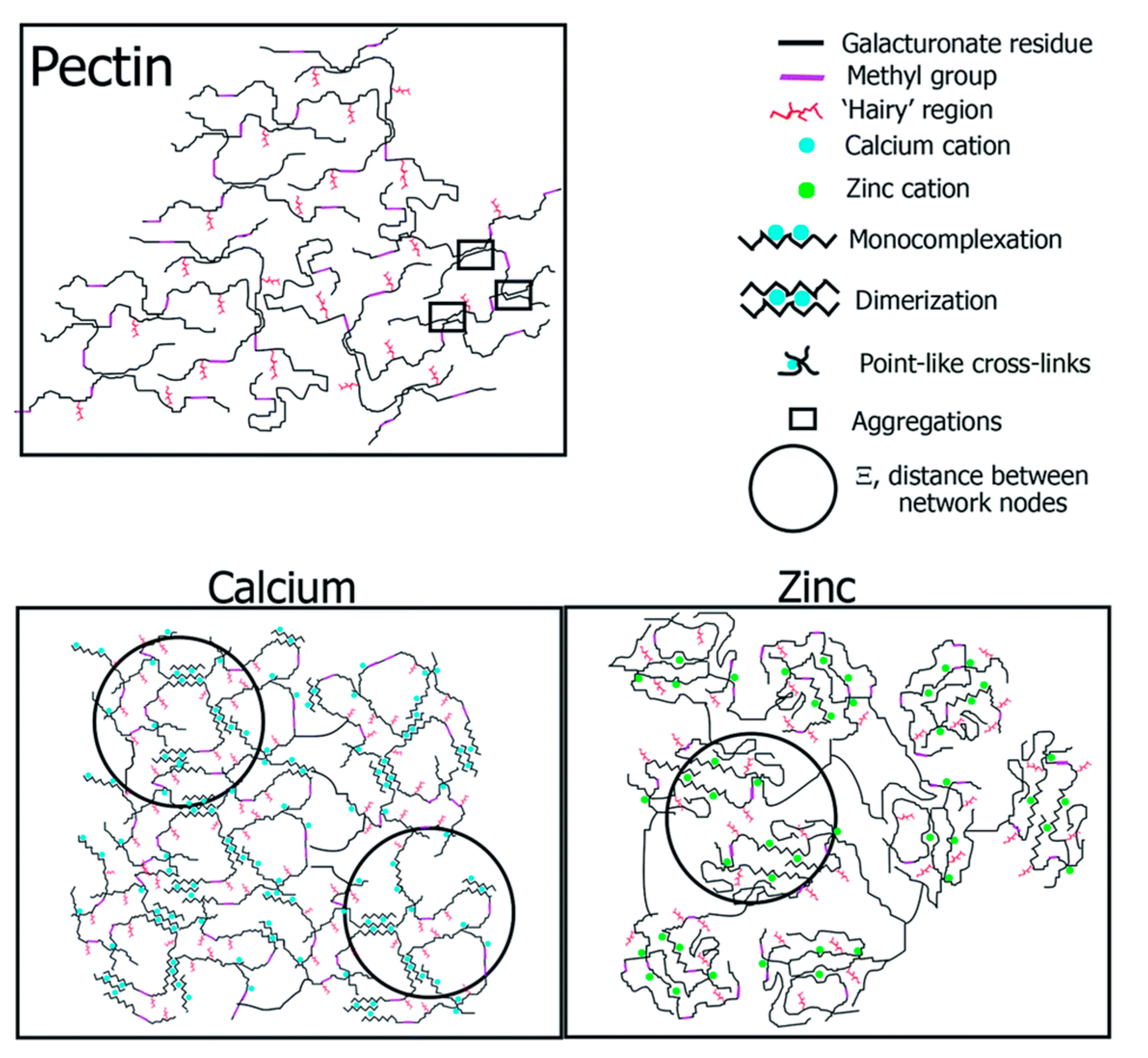
5. Structural Models of Cross-Linked LM Pectin and Alginates
5.1. Historical Perspective of Structural Models Induced by Calcium
5.2. Recent Evolution of Structural Models for Pectin Is Cross-Linked with Other Metals
6. Properties of Pectin That Influence the Absorption of Metal Ions
7. Future Perspectives
8. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hugonnet, R.; McNabb, R.; Berthier, E.; Menounos, B.; Nuth, C.; Girod, L.; Farinotti, D.; Huss, M.; Dussaillant, I.; Brun, F.; et al. Accelerated global glacier mass loss in the early twenty-first century. Nature 2021, 592, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Gan, J. Mitigation of Eutrophication and Hypoxia through Oyster Aquaculture: An Ecosystem Model Evaluation off the Pearl River Estuary. Environ. Sci. Technol. 2021, 55, 5506–5514. [Google Scholar] [CrossRef] [PubMed]
- Mihai, F.-C.; Gündoğdu, S.; Markley, L.A.; Olivelli, A.; Khan, F.R.; Gwinnett, C.; Gutberlet, J.; Reyna-Bensusan, N.; Llanquileo-Melgarejo, P.; Meidiana, C.; et al. Plastic Pollution, Waste Management Issues, and Circular Economy Opportunities in Rural Communities. Sustainability 2022, 14, 20. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; von Gunten, U.; Wehrli, B. The Challenge of Micropollutants in Aquatic Systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef]
- Jobling, S.; Williams, R.; Johnson, A.; Taylor, A.; Gross-Sorokin, M.; Nolan, M.; Tyler, C.R.; Van Aerle, R.; Santos, E.; Brighty, G. Predicted Exposures to Steroid Estrogens in U.K. Rivers Correlate with Widespread Sexual Disruption in Wild Fish Populations-1. Environ. Health Perspect. 2006, 114 (Suppl. 1), 32–39. [Google Scholar] [CrossRef]
- Vilela, C.L.S.; Bassin, J.P.; Peixoto, R.S. Water contamination by endocrine disruptors: Impacts, microbiological aspects and trends for environmental protection. Environ. Pollut. 2018, 235, 546–559. [Google Scholar] [CrossRef]
- Polverino, G.; Martin, J.M.; Bertram, M.G.; Soman, V.R.; Tan, H.; Brand, J.A.; Mason, R.T.; Wong, B.B.M. Psychoactive pollution suppresses individual differences in fish behaviour. Proc. R. Soc. B Boil. Sci. 2021, 288, 20202294. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- Tomczyk, A.; Kubaczyński, A.; Szewczuk-Karpisz, K. Assessment of agricultural waste biochars for remediation of degraded water-soil environment: Dissolved organic carbon release and immobilization of impurities in one- or two-adsorbate systems. Waste Manag. 2023, 155, 87–98. [Google Scholar] [CrossRef]
- Scaria, J.; Anupama, K.; Nidheesh, P. Tetracyclines in the environment: An overview on the occurrence, fate, toxicity, detection, removal methods, and sludge management. Sci. Total. Environ. 2021, 771, 145291. [Google Scholar] [CrossRef] [PubMed]
- WHO. 2012. Available online: https://apps.who.int/iris/bitstream/10665/44630/1/9789241502085_eng.pdf?ua=1 (accessed on 1 March 2023).
- Gerstrand, M.; Berg, C.; Björlenius, B.; Breitholtz, M.; Brunström, B.; Fick, J.; Gunnarsson, L.; Larsson, D.G.J.; Sumpter, J.P.; Tysklind, M.; et al. Improving Environmental Risk Assessment of Human Pharmaceuticals. Environ. Sci. Technol. 2015, 49, 5336–5345. [Google Scholar] [CrossRef] [PubMed]
- Agency, E.E. The European Pollutant Release and Transfer Register (E-PRTR). Available online: https://www.eea.europa.eu/data-and-maps/data/member-states-reporting-art-7-under-the-european-pollutant-release-and-transfer-register-e-prtr-regulation-18 (accessed on 16 October 2019).
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Hussain, S.; Habib-Ur-Rehman, M.; Khanam, T.; Sheer, A.; Kebin, Z.; Jianjun, Y. Health Risk Assessment of Different Heavy Metals Dissolved in Drinking Water. Int. J. Environ. Res. Public Health 2019, 16, 1737. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Kumar, V.; Thakur, V.K. Progress in pectin based hydrogels for water purification: Trends and challenges. J. Environ. Manag. 2019, 238, 210–223. [Google Scholar] [CrossRef]
- Wang, R.; Liang, R.; Dai, T.; Chen, J.; Shuai, X.; Liu, C. Pectin-based adsorbents for heavy metal ions: A review. Trends Food Sci. Technol. 2019, 91, 319–329. [Google Scholar] [CrossRef]
- World Health Organization. A Global Overview of National Regulations and Standards for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2018; ISBN 978-92-4-151376-0. [Google Scholar]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Alexa, E.T.; Bernal-Romero del Hombre Bueno, M.d.l.Á.; González, R.; Sánchez, A.V.; García, H.; Prats, D. Occurrence and Removal of Priority Substances and Contaminants of Emerging Concern at the WWTP of Benidorm (Spain). Water 2022, 14, 4129. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Hibzur Ali, M. Pharmaceutical wastewater as Emerging Contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.E.; Carter, L.J.; Kolpin, D.W.; Thomas-Oates, J.; Boxall, A.B. Temporal and spatial variation in pharmaceutical concentrations in an urban river system. Water Res. 2018, 137, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Backhaus, T.; Bowe, C.; Choi, K.; Connors, K.; Hickmann, S.; Hunter, W.; Kookana, R.; Marfil-Vega, R.; Verslycke, T. Pharmaceuticals in the environment: An introduction to the ET&C special issue. Environ. Toxicol. Chem. 2016, 35, 763–766. [Google Scholar] [PubMed]
- aus der Beek, T.; Weber, F.-A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment—Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.L.; Boxall, A.B.A.; Kolpin, D.W.; Leung, K.M.Y.; Lai, R.W.S.; Galbán-Malagón, C.; Adell, A.D.; Mondon, J.; Metian, M.; Marchant, R.A.; et al. Pharmaceutical pollution of the world’s rivers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113947119. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, E.; Hernández, F.; Ibáñez, M.; Rico, A.; Pitarch, E.; Bijlsma, L. Occurrence and ecological risks of pharmaceuticals in a Mediterranean river in Eastern Spain. Environ. Int. 2020, 144, 106004. [Google Scholar] [CrossRef]
- Lindholm-Lehto, P.C.; Ahkola, H.S.J.; Knuutinen, J.S.; Herve, S.H. Widespread occurrence and seasonal variation of pharmaceuticals in surface waters and municipal wastewater treatment plants in central Finland. Environ. Sci. Pollut. Res. 2016, 23, 7985–7997. [Google Scholar] [CrossRef]
- Wang, H.; Ren, Z.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef]
- Gude, V.G. Integrating bioelectrochemical systems for sustainable wastewater treatment. Clean Technol. Environ. Policy 2018, 20, 911–924. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef]
- Kim, Y.; Logan, B.E. Series Assembly of Microbial Desalination Cells Containing Stacked Electrodialysis Cells for Partial or Complete Seawater Desalination. Environ. Sci. Technol. 2011, 45, 5840–5845. [Google Scholar] [CrossRef]
- Wang, H.; Ren, Z.J. Bioelectrochemical metal recovery from wastewater: A review. Water Res. 2014, 66, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; He, Z. Efficiently “pumping out” value-added resources from wastewater by bioelectrochemical systems: A review from energy perspectives. Water Res. 2018, 131, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Leicester, D.; Amezaga, J.; Heidrich, E. Is bioelectrochemical energy production from wastewater a reality? Identifying and standardising the progress made in scaling up microbial electrolysis cells. Renew. Sustain. Energy Rev. 2020, 133, 110279. [Google Scholar] [CrossRef]
- Xie, J.; Zou, X.; Chang, Y.; Liu, H.; Cui, M.-H.; Zhang, T.C.; Xi, J.; Chen, C. A feasibility investigation of a pilot-scale bioe-lectrochemical coupled anaerobic digestion system with centric electrode module for real membrane manufacturing wastewater treatment. Bioresour. Technol. 2023, 368, 128371. [Google Scholar] [CrossRef]
- Khan, M.; McDonald, M.; Mundada, K.; Willcox, M. Efficacy of Ultraviolet Radiations against Coronavirus, Bacteria, Fungi, Fungal Spores and Biofilm. Hygiene 2022, 2, 120–131. [Google Scholar] [CrossRef]
- Kheyrandish, A.; Taghipour, F.; Mohseni, M. UV-LED radiation modeling and its applications in UV dose determination for water treatment. J. Photochem. Photobiol. A Chem. 2018, 352, 113–121. [Google Scholar] [CrossRef]
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The Technology Horizon for Photocatalytic Water Treatment: Sunrise or Sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef]
- Serpone, N.; Terzian, R.; Lawless, D.; Pelletier, A.-M.; Minero, C.; Pelizzetti, E. Photocatalyzed Destruction of Water Contaminants. Environ. Sci. Technol. 1991, 25, 1522–1529. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Zularisam, A.; Ismail, A.; Salim, R. Behaviours of natural organic matter in membrane filtration for surface water treatment—A review. Desalination 2006, 194, 211–231. [Google Scholar] [CrossRef]
- Othman, N.H.; Alias, N.H.; Fuzil, N.S.; Marpani, F.; Shahruddin, M.Z.; Chew, C.M.; Ng, K.M.D.; Lau, W.J.; Ismail, A.F. A Review on the Use of Membrane Technology Systems in Developing Countries. Membranes 2022, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Vingerhoeds, M.H.; Vries, M.A.N.-D.; Ruepert, N.; van der Laan, H.; Bredie, W.L.; Kremer, S. Sensory quality of drinking water produced by reverse osmosis membrane filtration followed by remineralisation. Water Res. 2016, 94, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Fiksdal, L.; Leiknes, T. The effect of coagulation with MF/UF membrane filtration for the removal of virus in drinking water. J. Membr. Sci. 2006, 279, 364–371. [Google Scholar] [CrossRef]
- Schlichter, B.; Mavrov, V.; Chmiel, H. Study of a hybrid process combining ozonation and microfiltration/ultrafiltration for drinking water production from surface water. Desalination 2004, 168, 307–317. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.-H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Gupta, V.K. Suhas Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Kusrini, E.; Alhamid, M.I.; Widiantoro, A.B.; Daud, N.Z.A.; Usman, A. Simultaneous Adsorption of Multi-lanthanides from Aqueous Silica Sand Solution Using Pectin–Activated Carbon Composite. Arab. J. Sci. Eng. 2020, 45, 7219–7230. [Google Scholar] [CrossRef]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A.; et al. Recovery, regeneration and sustainable management of spent adsorbents from wastewater treatment streams: A review. Sci. Total Environ. 2022, 822, 153555. [Google Scholar] [CrossRef] [PubMed]
- Picón, D.; Torasso, N.; Baudrit, J.R.V.; Cerveny, S.; Goyanes, S. Bio-inspired membranes for adsorption of arsenic via immobilized L-Cysteine in highly hydrophilic electrospun nanofibers. Chem. Eng. Res. Des. 2022, 185, 108–118. [Google Scholar] [CrossRef]
- de Farias, A.B.V.; da Costa, T.B.; da Silva, M.G.C.; Vieira, M.G.A. Cerium recovery from aqueous solutions by bio/adsorption: A review in a circular economy context. J. Clean. Prod. 2021, 326, 129395. [Google Scholar] [CrossRef]
- Elhami, V.; Antunes, E.C.; Temmink, H.; Schuur, B. Recovery Techniques Enabling Circular Chemistry from Wastewater. Molecules 2022, 27, 1389. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Ibrahim, M.H.; Abdullah, A.Z.; Salamatinia, B.; Gholami, Z. Oil Palm Biomass as an Adsorbent for Heavy Metals. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: Cham, Switzerland, 2014; Volume 232, pp. 61–88. [Google Scholar]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; El-Sheikh, A.H.; Walker, G.M. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigments 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Tee, G.T.; Gok, X.Y.; Yong, W.F. Adsorption of pollutants in wastewater via biosorbents, nanoparticles and magnetic biosorbents: A review. Environ. Res. 2022, 212, 113248. [Google Scholar] [CrossRef]
- Ceçen, F.; Aktaş, Ö. Water and Wastewater Treatment: Historical Perspective of Activated Carbon Adsorption and its Integration with Biological Processes. In Activated Carbon for Water and Wastewater Treatment; Wiley: Hoboken, NJ, USA, 2011; pp. 1–11. [Google Scholar]
- Fang, Z.; Gao, Y.; Bolan, N.; Shaheen, S.M.; Xu, S.; Wu, X.; Xu, X.; Hu, H.; Lin, J.; Zhang, F.; et al. Conversion of biological solid waste to graphene-containing biochar for water remediation: A critical review. Chem. Eng. J. 2020, 390, 124611. [Google Scholar] [CrossRef]
- Streit, A.F.; Côrtes, L.N.; Druzian, S.P.; Godinho, M.; Collazzo, G.C.; Perondi, D.; Dotto, G.L. Development of high quality activated carbon from biological sludge and its application for dyes removal from aqueous solutions. Sci. Total. Environ. 2019, 660, 277–287. [Google Scholar] [CrossRef]
- Azam, K.; Shezad, N.; Shafiq, I.; Akhter, P.; Akhtar, F.; Jamil, F.; Shafique, S.; Park, Y.-K.; Hussain, M. A review on activated carbon modifications for the treatment of wastewater containing anionic dyes. Chemosphere 2022, 306, 135566. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Liu, X.; Sheng, L. Preparation of straw activated carbon and its application in wastewater treatment: A review. J. Clean. Prod. 2020, 283, 124671. [Google Scholar] [CrossRef]
- Diagboya, P.N.E.; Dikio, E.D. Silica-based mesoporous materials; emerging designer adsorbents for aqueous pollutants re-moval and water treatment. Microporous Mesoporous Mater. 2018, 266, 252–267. [Google Scholar] [CrossRef]
- Fan, H.-T.; Wu, J.-B.; Fan, X.-L.; Zhang, D.-S.; Su, Z.-J.; Yan, F.; Sun, T. Removal of cadmium(II) and lead(II) from aqueous solution using sulfur-functionalized silica prepared by hydrothermal-assisted grafting method. Chem. Eng. J. 2012, 198-199, 355–363. [Google Scholar] [CrossRef]
- Sriram, G.; Bendre, A.; Altalhi, T.; Jung, H.-Y.; Hegde, G.; Kurkuri, M. Surface engineering of silica based materials with Ni–Fe layered double hydroxide for the efficient removal of methyl orange: Isotherms, kinetics, mechanism and high selectivity studies. Chemosphere 2022, 287, 131976. [Google Scholar] [CrossRef]
- Far, R.M.; Van der Bruggen, B.; Verliefde, A.; Cornelissen, E. A review of zeolite materials used in membranes for water purification: History, applications, challenges and future trends. J. Chem. Technol. Biotechnol. 2022, 97, 575–596. [Google Scholar] [CrossRef]
- Dessalegne, M.; Zewge, F.; Diaz, I. Aluminum hydroxide supported on zeolites for fluoride removal from drinking water. J. Chem. Technol. Biotechnol. 2016, 92, 605–613. [Google Scholar] [CrossRef]
- El Hanache, L.; Lebeau, B.; Nouali, H.; Toufaily, J.; Hamieh, T.; Daou, T.J. Performance of surfactant-modified *BEA-type zeolite nanosponges for the removal of nitrate in contaminated water: Effect of the external surface. J. Hazard. Mater. 2018, 364, 206–217. [Google Scholar] [CrossRef]
- ElBastamy, E.; Ibrahim, L.; Ghandour, A.; Zelenakova, M.; Vranayova, Z.; Abu-Hashim, M. Efficiency of Natural Clay Mineral Adsorbent Filtration Systems in Wastewater Treatment for Potential Irrigation Purposes. Sustainability 2021, 13, 5738. [Google Scholar] [CrossRef]
- Chang, P.-H.; Li, Z.; Jiang, W.-T.; Sarkar, B. Chapter 7—Clay minerals for pharmaceutical wastewater treatment. In Modified Clay and Zeolite Nanocomposite Materials; Mercurio, M., Sarkar, B., Langella, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 167–196. [Google Scholar]
- Kwok, K.C.M.; Koong, L.F.; Al Ansari, T.; McKay, G. Adsorption/desorption of arsenite and arsenate on chitosan and na-nochitosan. Environ. Sci. Pollut. Res. 2018, 25, 14734–14742. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano)materials for sustainable water treatment: A review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef]
- Carmalin Sophia, A.; Lima, E.C.; Allaudeen, N.; Rajan, S. Application of graphene based materials for adsorption of phar-maceutical traces from water and wastewater—A review. Desalination Water Treat. 2016, 57, 27573–27586. [Google Scholar]
- Perreault, F.; Fonseca de Faria, A.; Elimelech, M. Environmental applications of graphene-based nanomaterials. Chem. Soc. Rev. 2015, 44, 5861–5896. [Google Scholar] [CrossRef] [PubMed]
- Tareq, R.; Akter, N.; Azam, M.S. Chapter 10—Biochars and Biochar Composites: Low-Cost Adsorbents for Environmental Remediation. In Biochar from Biomass and Waste; Ok, Y.S., Tsang, D.C.W., Bolan, N., Novak, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 169–209. [Google Scholar]
- Zeghioud, H.; Fryda, L.; Djelal, H.; Assadi, A.; Kane, A. A comprehensive review of biochar in removal of organic pollutants from wastewater: Characterization, toxicity, activation/functionalization and influencing treatment factors. J. Water Process Eng. 2022, 47, 102801. [Google Scholar] [CrossRef]
- Khakimova, N.; Maravić, N.; Davidović, P.; Blagojević, D.; Bečelić-Tomin, M.; Simeunović, J.; Pešić, V.; Šereš, Z.; Mandić, A.; Pojić, M.; et al. Sugar Beet Processing Wastewater Treatment by Microalgae through Biosorption. Water 2022, 14, 860. [Google Scholar] [CrossRef]
- Gönder, Z.B.; Vergili, I.; Kaya, Y.; Barlas, H. Adsorption of cationic and anionic surfactants onto organic polymer resin Lewatit VPOC 1064 MD PH. Environ. Geochem. Health 2010, 32, 267–273. [Google Scholar] [CrossRef]
- Kassar, C.; Graham, C.; Boyer, T.H. Removal of perfluoroalkyl acids and common drinking water contaminants by weak-base anion exchange resins: Impacts of solution pH and resin properties. Water Res. X 2022, 17, 100159. [Google Scholar] [CrossRef]
- Torasso, N.; Vergara-Rubio, A.; Pereira, R.; Martinez-Sabando, J.; Baudrit, J.R.V.; Cerveny, S.; Goyanes, S. An in situ approach to entrap ultra-small iron oxide nanoparticles inside hydrophilic electrospun nanofibers with high arsenic adsorption. Chem. Eng. J. 2023, 454, 140168. [Google Scholar] [CrossRef]
- Torasso, N.; Vergara-Rubio, A.; Rivas-Rojas, P.; Huck-Iriart, C.; Larrañaga, A.; Fernández-Cirelli, A.; Cerveny, S.; Goyanes, S. Enhancing arsenic adsorption via excellent dispersion of iron oxide nanoparticles inside poly(vinyl alcohol) nanofibers. J. Environ. Chem. Eng. 2021, 9, 104664. [Google Scholar] [CrossRef]
- Picón, D.; Vergara-Rubio, A.; Estevez-Areco, S.; Cerveny, S.; Goyanes, S. Adsorption of Methylene Blue and Tetracycline by Zeolites Immobilized on a PBAT Electrospun Membrane. Molecules 2023, 28, 81. [Google Scholar] [CrossRef]
- Pereira, P.P.; Fernandez, M.; Cimadoro, J.; González, P.S.; Morales, G.M.; Goyanes, S.; Agostini, E. Biohybrid membranes for effective bacterial vehiculation and simultaneous removal of hexavalent chromium (CrVI) and phenol. Appl. Microbiol. Biotechnol. 2021, 105, 827–838. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Chavarría-Hernández, N.; Inés Rodríguez Hernández, A.; Pagliaro, M. Pectin: A new perspective from the biorefinery standpoint. Biofuels Bioprod. Biorefining 2015, 9, 368–377. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-Related Gelling of Pectins and Linking with Other Natural Compounds: A Review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Optimization of Pectin Enzymatic Extraction from Malus domestica ‘Fălticeni’ Apple Pomace with Celluclast 1.5L. Molecules 2019, 24, 2158. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, C.; Feng, L.; Han, Y.; Du, H.; Xiao, H.; Zheng, J. Pectins from fruits: Relationships between extraction methods, structural characteristics, and functional properties. Trends Food Sci. Technol. 2021, 110, 39–54. [Google Scholar] [CrossRef]
- Guo, X.; Han, D.; Xi, H.; Rao, L.; Liao, X.; Hu, X.; Wu, J. Extraction of pectin from navel orange peel assisted by ultra-high pressure, microwave or traditional heating: A comparison. Carbohydr. Polym. 2012, 88, 441–448. [Google Scholar] [CrossRef]
- Yeoh, S.; Shi, J.; Langrish, T.A.G. Comparisons between different techniques for water-based extraction of pectin from orange peels. Desalination 2008, 218, 229–237. [Google Scholar] [CrossRef]
- Ravn, H.C.; Meyer, A.S. Chelating agents improve enzymatic solubilization of pectinaceous co-processing streams. Process. Biochem. 2014, 49, 250–257. [Google Scholar] [CrossRef]
- Garna, H.; Mabon, N.; Robert, C.; Cornet, C.; Nott, K.; Legros, H.; Wathelet, B.; Paquot, M. Effect of Extraction Conditions on the Yield and Purity of Apple Pomace Pectin Precipitated but Not Washed by Alcohol. J. Food Sci. 2007, 72, C001–C009. [Google Scholar] [CrossRef] [PubMed]
- Gavahian, M.; Mathad, G.N.; Pandiselvam, R.; Lin, J.; Sun, D.-W. Emerging technologies to obtain pectin from food processing by-products: A strategy for enhancing resource efficiency. Trends Food Sci. Technol. 2021, 115, 42–54. [Google Scholar] [CrossRef]
- Hu, W.; Cassard, A.-M.; Ciocan, D. Pectin in Metabolic Liver Disease. Nutrients 2023, 15, 157. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-Q.; Li, J.; Dong, H.-L.; Li, X.; Zhang, J.-Q.; Ramaswamy, S.; Xu, F. Pectin in biomedical and drug delivery applications: A review. Int. J. Biol. Macromol. 2021, 185, 49–65. [Google Scholar] [CrossRef]
- Houron, C.; Ciocan, D.; Trainel, N.; Mercier-Nomé, F.; Hugot, C.; Spatz, M.; Perlemuter, G.; Cassard, A.-M. Gut Microbiota Reshaped by Pectin Treatment Improves Liver Steatosis in Obese Mice. Nutrients 2021, 13, 3725. [Google Scholar] [CrossRef]
- Munarin, F.; Guerreiro, S.G.; Grellier, M.A.; Tanzi, M.C.; Barbosa, M.A.; Petrini, P.; Granja, P.L. Pectin-Based Injectable Biomaterials for Bone Tissue Engineering. Biomacromolecules 2011, 12, 568–577. [Google Scholar] [CrossRef]
- Freitas, C.; Coimbra, J.; Souza, V.; Sousa, R. Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical Industry: A Review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Moslemi, M. Reviewing the recent advances in application of pectin for technical and health promotion purposes: From la-boratory to market. Carbohydr. Polym. 2021, 254, 117324. [Google Scholar] [CrossRef]
- Reichembach, L.H.; Petkowicz, C.L.D.O. Pectins from alternative sources and uses beyond sweets and jellies: An overview. Food Hydrocoll. 2021, 118, 106824. [Google Scholar] [CrossRef]
- Oliva-Moreno, E.E.; Encinas, A. Addition of Pine Rosin to Pectin bioplastic films for improved water resistance. Mater. Lett. 2021, 290, 129488. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Du, W.-X.; Avena-Bustillos, R.d.J.; Soares, N.d.F.F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties—A review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Huang, S.; Tu, Z.; Sha, X.; Hu, Y.; Chen, N.; Wang, H. Fabrication and performance evaluation of pectin–fish gelatin–resveratrol preservative films. Food Chem. 2021, 361, 129832. [Google Scholar] [CrossRef] [PubMed]
- Makaremi, M.; Pasbakhsh, P.; Cavallaro, G.; Lazzara, G.; Aw, Y.K.; Lee, S.M.; Milioto, S. Effect of Morphology and Size of Halloysite Nanotubes on Functional Pectin Bionanocomposites for Food Packaging Applications. ACS Appl. Mater. Interfaces 2017, 9, 17476–17488. [Google Scholar] [CrossRef]
- Yoshimura, T.; Sengoku, K.; Fujioka, R. Pectin-based surperabsorbent hydrogels crosslinked by some chemicals: Synthesis and characterization. Polym. Bull. 2005, 55, 123–129. [Google Scholar] [CrossRef]
- Chen, B.; McClements, D.J.; Gray, D.A.; Decker, E.A. Stabilization of Soybean Oil Bodies by Enzyme (Laccase) Cross-Linking of Adsorbed Beet Pectin Coatings. J. Agric. Food Chem. 2010, 58, 9259–9265. [Google Scholar] [CrossRef] [PubMed]
- Groult, S.; Buwalda, S.; Budtova, T. Pectin hydrogels, aerogels, cryogels and xerogels: Influence of drying on structural and release properties. Eur. Polym. J. 2021, 149, 110386. [Google Scholar] [CrossRef]
- Allwyn, S.R.A. A Review on Pectin: Chemistry due to General Properties of Pectin and its Pharmaceutical Uses. Sci. Rep. 2012, 1, 550–553. [Google Scholar]
- Voragen, F.; Schols, H.; Visser, M. Advances in Pectin and Pectinase Research; Springer: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Chen, R.; Ratcliffe, I.; Williams, P.A.; Luo, S.; Chen, J.; Liu, C. The influence of pH and monovalent ions on the gelation of pectin from the fruit seeds of the creeping fig plant. Food Hydrocoll. 2020, 111, 106219. [Google Scholar] [CrossRef]
- Wang, H.; Wan, L.; Chen, D.; Guo, X.; Liu, F.; Pan, S. Unexpected gelation behavior of citrus pectin induced by monovalent cations under alkaline conditions. Carbohydr. Polym. 2019, 212, 51–58. [Google Scholar] [CrossRef]
- Jonassen, H.; Treves, A.; Kjøniksen, A.-L.; Smistad, G.; Hiorth, M. Preparation of Ionically Cross-Linked Pectin Nanoparticles in the Presence of Chlorides of Divalent and Monovalent Cations. Biomacromolecules 2013, 14, 3523–3531. [Google Scholar] [CrossRef]
- Ström, A.; Schuster, E.; Goh, S.M. Rheological characterization of acid pectin samples in the absence and presence of mon-ovalent ions. Carbohydr. Polym. 2014, 113, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-H.; Fishman, M.L.; Savary, B.J.; Hotchkiss, A.T. Monovalent Salt-Induced Gelation of Enzymatically Deesterified Pectin. J. Agric. Food Chem. 2003, 51, 7410–7417. [Google Scholar] [CrossRef]
- Wehr, J.B.; Blamey, F.P.C.; Hanna, J.V.; Kopittke, P.M.; Kerven, G.L.; Menzies, N.W. Hydrolysis and Speciation of Al Bound to Pectin and Plant Cell Wall Material and Its Reaction with the Dye Chrome Azurol S. J. Agric. Food Chem. 2010, 58, 5553–5560. [Google Scholar] [CrossRef] [PubMed]
- McKenna, B.A.; Nicholson, T.M.; Wehr, J.B.; Menzies, N. Effects of Ca, Cu, Al and La on pectin gel strength: Implications for plant cell walls. Carbohydr. Res. 2010, 345, 1174–1179. [Google Scholar] [CrossRef]
- Wu, X.; Sun, H.; Qin, Z.; Che, P.; Yi, X.; Yu, Q.; Zhang, H.; Sun, X.; Yao, F.; Li, J. Fully physically crosslinked pectin-based hydrogel with high stretchability and toughness for biomedical application. Int. J. Biol. Macromol. 2020, 149, 707–716. [Google Scholar] [CrossRef]
- Pellerin, P.; O’Neill, M.A. The interaction of the pectic polysaccharide Rhamnogalacturonan II with heavy metals and lan-thanides in wines and fruit juices. Analusis 1998, 26, 32–36. [Google Scholar] [CrossRef]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Chaichi, M.; Badii, F.; Mohammadi, A.; Hashemi, M. Water resistance and mechanical properties of low methoxy-pectin nanocomposite film responses to interactions of Ca2+ ions and glycerol concentrations as crosslinking agents. Food Chem. 2019, 293, 429–437. [Google Scholar] [CrossRef]
- Li, D.-Q.; Wang, J.; Guo, Z.-G.; Li, J.; Shuai, J. Pectin gels cross-linked by Ca 2+: An efficient material for methylene blue removal. J. Mol. Liq. 2017, 238, 36–42. [Google Scholar] [CrossRef]
- Huang, T.; Qin, Y.; Li, M.; Gao, S.; Shen, C. Preparation and characterization of deacetylated konjac glucomannan/pectin composite films crosslinked with calcium hydroxide. J. Polym. Res. 2022, 29, 238. [Google Scholar] [CrossRef]
- John, J.; Deshpande, A.P.; Varughese, S. Morphology control and ionic crosslinking of pectin domains to enhance the toughness of solvent cast PVA/pectin blends. J. Appl. Polym. Sci. 2021, 138, 50360. [Google Scholar] [CrossRef]
- Fraeye, I.; Colle, I.; Vandevenne, E.; Duvetter, T.; Van Buggenhout, S.; Moldenaers, P.; Van Loey, A.; Hendrickx, M. Influence of pectin structure on texture of pectin–calcium gels. Innov. Food Sci. Emerg. Technol. 2010, 11, 401–409. [Google Scholar] [CrossRef]
- Ngouémazong, D.E.; Kabuye, G.; Fraeye, I.; Cardinaels, R.; Van Loey, A.; Moldenaers, P.; Hendrickx, M. Effect of debranching on the rheological properties of Ca2+–pectin gels. Food Hydrocoll. 2012, 26, 44–53. [Google Scholar] [CrossRef]
- Yang, X.; Nisar, T.; Liang, D.; Hou, Y.; Sun, L.; Guo, Y. Low methoxyl pectin gelation under alkaline conditions and its rhe-ological properties: Using NaOH as a pH regulator. Food Hydrocoll. 2018, 79, 560–571. [Google Scholar] [CrossRef]
- Capel, F.; Nicolai, T.; Durand, D.; Boulenguer, P.; Langendorff, V. Calcium and acid induced gelation of (amidated) low methoxyl pectin. Food Hydrocoll. 2006, 20, 901–907. [Google Scholar] [CrossRef]
- Yuliarti, O.; Hoon, A.L.S.; Chong, S.Y. Influence of pH, pectin and Ca concentration on gelation properties of low-methoxyl pectin extracted from Cyclea barbata Miers. Food Struct. 2017, 11, 16–23. [Google Scholar] [CrossRef]
- Gołębiowski, A.; Kowalkowski, T.; Buszewski, B. Molecular parameters of low methoxylated pectin affected by gelation with copper and cadmium cations. Bioact. Carbohydrates Diet. Fibre 2020, 21, 100211. [Google Scholar] [CrossRef]
- Tkalec, G.; Knez, Ž.; Novak, Z. PH sensitive mesoporous materials for immediate or controlled release of NSAID. Microporous Mesoporous Mater. 2016, 224, 190–200. [Google Scholar] [CrossRef]
- Kohila rani, K.; Liu, Y.-X.; Devasenathipathy, R.; Yang, C.; Wang, S.-F. Simple preparation of gold nanoparticle-decorated copper cross-linked pectin for the sensitive determination of hydrogen peroxide. Ionics 2019, 25, 309–317. [Google Scholar] [CrossRef]
- Nešić, A.; Onjia, A.; Davidović, S.; Dimitrijević, S.; Errico, M.E.; Santagata, G.; Malinconico, M. Design of pectin-sodium alginate based films for potential healthcare application: Study of chemico-physical interactions between the components of films and assessment of their antimicrobial activity. Carbohydr. Polym. 2017, 157, 981–990. [Google Scholar] [CrossRef]
- Das, S.; Ng, K.-Y.; Ho, P.C. Design of a pectin-based microparticle formulation using zinc ions as the cross-linking agent and glutaraldehyde as the hardening agent for colonic-specific delivery of resveratrol: In vitro and in vivo evaluations. J. Drug Target. 2011, 19, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Sohail, M.; Buabeid, M.A.; Murtaza, G.; Ullah, A.; Rashid, H.; Khan, M.A.; Khan, S.A. Pectin-based (LA-co-MAA) semi-IPNS as a potential biomaterial for colonic delivery of oxaliplatin. Int. J. Pharm. 2019, 569, 118557. [Google Scholar] [CrossRef] [PubMed]
- Li, F.T.; Yang, H.; Zhao, Y.; Xu, R. Novel modified pectin for heavy metal adsorption. Chin. Chem. Lett. 2007, 18, 325–328. [Google Scholar] [CrossRef]
- Chen, S.; Cui, S.; Zhang, H.; Pei, X.; Hu, J.; Zhou, Y.; Liu, Y. Cross-Linked Pectin Nanofibers with Enhanced Cell Adhesion. Biomacromolecules 2018, 19, 490–498. [Google Scholar] [CrossRef]
- Jung, J.; Wicker, L. Laccase mediated conjugation of sugar beet pectin and the effect on emulsion stability. Food Hydrocoll. 2012, 28, 168–173. [Google Scholar] [CrossRef]
- McCune, D.; Guo, X.; Shi, T.; Stealey, S.; Antrobus, R.; Kaltchev, M.; Chen, J.; Kumpaty, S.; Hua, X.; Ren, W.; et al. Electrospinning pectin-based nanofibers: A parametric and cross-linker study. Appl. Nanosci. 2018, 8, 33–40. [Google Scholar] [CrossRef]
- Mongkolkitikul, S.; Paradee, N.; Sirivat, A. Electrically controlled release of ibuprofen from conductive poly(3-methoxydiphenylamine)/crosslinked pectin hydrogel. Eur. J. Pharm. Sci. 2018, 112, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Morris, E.R.; Rees, D.A.; Thom, D.; Boyd, J. Chiroptical and stoichiometric evidence of a specific, primary dimerisation process in alginate gelation. Carbohydr. Res. 1978, 66, 145–154. [Google Scholar] [CrossRef]
- Walkinshaw, M.; Arnott, S. Conformations and interactions of pectins: II. Models for junction zones in pectinic acid and calcium pectate gels. J. Mol. Biol. 1981, 153, 1075–1085. [Google Scholar] [CrossRef]
- Powell, D.A.; Morris, E.R.; Gidley, M.J.; Rees, D.A. Conformations and interactions of pectins: II. Influence of residue se-quence on chain association in calcium pectate gels. J. Mol. Biol. 1982, 155, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Kohn, R. Binding of divalent cations to oligomeric fragments of pectin. Carbohydr. Res. 1987, 160, 343–353. [Google Scholar] [CrossRef]
- Walter, R.H. The Chemistry and Technology of Pectin; Academic Press: London, UK, 1991. [Google Scholar]
- Braccini, I.; Pérez, S. Molecular Basis of Ca2+-Induced Gelation in Alginates and Pectins: The Egg-Box Model Revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fang, Y.; Vreeker, R.; Appelqvist, I.; Mendes, E. Reexamining the Egg-Box Model in Calcium−Alginate Gels with X-ray Diffraction. Biomacromolecules 2007, 8, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Mackie, W. Conformations of crystalline alginic acids and their salts. Biochem. J. 1971, 125, 89P. [Google Scholar] [CrossRef]
- Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Nishinari, K.; Funami, T.; Williams, P.A.; Li, L. Multiple Steps and Critical Behaviors of the Binding of Calcium to Alginate. J. Phys. Chem. B 2007, 111, 2456–2462. [Google Scholar] [CrossRef]
- Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Nishinari, K.; Funami, T.; Williams, P.A. Binding behavior of calcium to polyuronates: Comparison of pectin with alginate. Carbohydr. Polym. 2008, 72, 334–341. [Google Scholar] [CrossRef]
- Gohil, R.M. Synergistic blends of natural polymers, pectin and sodium alginate. J. Appl. Polym. Sci. 2011, 120, 2324–2336. [Google Scholar] [CrossRef]
- Ventura, I.; Jammal, J.; Bianco-Peled, H. Insights into the nanostructure of low-methoxyl pectin–calcium gels. Carbohydr. Polym. 2013, 97, 650–658. [Google Scholar] [CrossRef]
- Wang, H.; Fei, S.; Wang, Y.; Zan, L.; Zhu, J. Comparative study on the self-assembly of pectin and alginate molecules regulated by calcium ions investigated by atomic force microscopy. Carbohydr. Polym. 2019, 231, 115673. [Google Scholar] [CrossRef] [PubMed]
- Assifaoui, A.; Lerbret, A.; Uyen, H.T.D.; Neiers, F.; Chambin, O.; Loupiac, C.; Cousin, F. Structural behaviour differences in low methoxy pectin solutions in the presence of divalent cations (Ca2+ and Zn2+): A process driven by the binding mechanism of the cation with the galacturonate unit. Soft Matter 2014, 11, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Huynh, U.T.D.; Lerbret, A.; Neiers, F.; Chambin, O.; Assifaoui, A. Binding of Divalent Cations to Polygalacturonate: A Mechanism Driven by the Hydration Water. J. Phys. Chem. B 2016, 120, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Huynh, U.T.D.; Chambin, O.; du Poset, A.M.; Assifaoui, A. Insights into gelation kinetics and gel front migration in cation-induced polysaccharide hydrogels by viscoelastic and turbidity measurements: Effect of the nature of divalent cations. Carbohydr. Polym. 2018, 190, 121–128. [Google Scholar] [CrossRef]
- Cataldo, S.; Gianguzza, A.; Pettignano, A.; Villaescusa, I. Mercury(II) removal from aqueous solution by sorption onto alginate, pectate and polygalacturonate calcium gel beads. A kinetic and speciation based equilibrium study. React. Funct. Polym. 2013, 73, 207–217. [Google Scholar] [CrossRef]
- Celus, M.; Kyomugasho, C.; Kermani, Z.J.; Roggen, K.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Fe2+ adsorption on citrus pectin is influenced by the degree and pattern of methylesterification. Food Hydrocoll. 2017, 73, 101–109. [Google Scholar] [CrossRef]
- Yu, L.-L.; Jiang, L.-N.; Wang, S.-Y.; Sun, M.-M.; Li, D.-Q.; Du, G.-M. Pectin microgel particles as high adsorption rate material for methylene blue: Performance, equilibrium, kinetic, mechanism and regeneration studies. Int. J. Biol. Macromol. 2018, 112, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-H.; Zhu, X.-F.; Qian, W.; Yu, Y.-C.; Xu, R.-K. Effect of pectin on adsorption of Cu(II) by two variable-charge soils from southern China. Environ. Sci. Pollut. Res. 2015, 22, 19687–19694. [Google Scholar] [CrossRef]
- Arachchige, M.P.M.; Mu, T.; Ma, M. Effect of high hydrostatic pressure-assisted pectinase modification on the Pb2+ adsorption capacity of pectin isolated from sweet potato residue. Chemosphere 2021, 262, 128102. [Google Scholar] [CrossRef]
- Shao, Z.; Lu, J.; Ding, J.; Fan, F.; Sun, X.; Li, P.; Fang, Y.; Hu, Q. Novel green chitosan-pectin gel beads for the removal of Cu(II), Cd(II), Hg(II) and Pb(II) from aqueous solution. Int. J. Biol. Macromol. 2021, 176, 217–225. [Google Scholar] [CrossRef]
- Mata, Y.; Blázquez, M.; Ballester, A.; González, F.; Muñoz, J. Optimization of the continuous biosorption of copper with sugar-beet pectin gels. J. Environ. Manag. 2009, 90, 1737–1743. [Google Scholar] [CrossRef]
- Li, F.; Xu, Z.; Wen, X.; Li, X.; Bai, Y.; Li, J. Preparation and characterization of Ca(II) cross-linking modified pectin microspheres for Pb(II) adsorption. Water Sci. Technol. 2019, 79, 1484–1493. [Google Scholar] [CrossRef]
- Subroto, N.N.P.; Tarmidzi, F.M.; Wati, I.; Armans, V. Lead Ion Removal in Water Using Low Methoxy Pectin-Guar Gum Beads Hybrid Adsorbent. Indo. J. Chem. Res. 2022, 10, 53–57. [Google Scholar] [CrossRef]
- Khozhaenko, E.; Kovalev, V.; Podkorytova, E.; Khotimchenko, M. Removal of the metal ions from aqueous solutions by na-noscaled low molecular pectin isolated from seagrass Phyllospadix iwatensis. Sci. Total Environ. 2016, 565, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Song, J.; He, Q.; Wang, H.; Lyu, W.; Feng, H.; Xiong, W.; Guo, W.; Wu, J.; Chen, L. Novel pectin based composite hydrogel derived from grapefruit peel for enhanced Cu(II) removal. J. Hazard. Mater. 2020, 384, 121445. [Google Scholar] [CrossRef] [PubMed]
- Lessa, E.F.; Medina, A.L.; Ribeiro, A.S.; Fajardo, A.R. Removal of multi-metals from water using reusable pectin/cellulose microfibers composite beads. Arab. J. Chem. 2020, 13, 709–720. [Google Scholar] [CrossRef]
- Kartel, M.; Kupchik, L.A.; Veisov, B.K. Evaluation of pectin binding of heavy metal ions in aqueous solutions. Chemosphere 1999, 38, 2591–2596. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Lü, X. Pectin extracted from apple pomace and citrus peel by subcritical water. Food Hydrocoll. 2014, 38, 129–137. [Google Scholar] [CrossRef]
- Ralet, M.-C.; Crépeau, M.-J.; Buchholt, H.-C.; Thibault, J.-F. Polyelectrolyte behaviour and calcium binding properties of sugar beet pectins differing in their degrees of methylation and acetylation. Biochem. Eng. J. 2003, 16, 191–201. [Google Scholar] [CrossRef]
- Löfgren, C.; Guillotin, S.; Evenbratt, H.; Schols, H.; Hermansson, A.-M. Effects of Calcium, pH, and Blockiness on Kinetic Rheological Behavior and Microstructure of HM Pectin Gels. Biomacromolecules 2005, 6, 646–652. [Google Scholar] [CrossRef]
- Khotimchenko, M.; Kovalev, V.; Khotimchenko, Y. Equilibrium studies of sorption of lead(II) ions by different pectin com-pounds. J. Hazard. Mater. 2007, 149, 693–699. [Google Scholar] [CrossRef]
- Kyomugasho, C.; Gwala, S.; Christiaens, S.; Jamsazzadeh Kermani, Z.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Pectin nanostructure influences pectin-cation interactions and in vitro-bioaccessibility of Ca2+, Zn2+, Fe2+ and Mg2+-ions in model systems. Food Hydrocoll. 2017, 62, 299–310. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef] [PubMed]


| Adsorbent Type | Contaminant | Reference |
|---|---|---|
| Activated carbon | Dyes, heavy metals | [64,65,66] |
| Graphene oxide | Pharmaceuticals, Organic compounds, metal ions | [77,78] |
| Silica-based materials | inorganic and organic pollutants | [67,68,69] |
| Zeolites | Petroleum, fluoride, nitrate, dyes, heavy metals, cesium | [70,71,72] |
| Biochar | Heavy metals | [10,79,80] |
| GO-biochar | Persulfate, metal ions, dyes, pharmaceuticals | [63] |
| Mineral Clays (Montmorillonite, Bentonite, Kaolinite, clinoptilolite, etc.) | Nuclear waste, pharmaceuticals | [73,74] |
| Sugar beet pulp | Nitrites and nitrates | [81] |
| Organic polymer resin | Cationic and anionic surfactants, perfluoroalkyl acids | [82,83] |
| Poly(saccharide)-based materials | heavy metals (arsenic) | [75,76] |
| Poly (vinyl alcohol) nanofibers + Iron NPs | Arsenic | [84,85] |
| Poly (vinyl alcohol) nanofibers + L-cysteine | Arsenic | [56] |
| Polybutylene adipate terephthalate (PBAT) nanofibrous | Dyes, pharmaceuticals | [86] |
| Biohybrid membrane of polymeric nanofibers and free-living bacteria | Chromium (Cr-VI) | [87] |
| Intrinsic Parameters | |
|---|---|
| Methoxylation degree (DM) | Decreasing DM: the number of sequences of non-methoxylated GalA residues long enough for the egg-box formation to increase. This results in a substantial increase in the calcium ion binding capacity. |
| Pattern of methoxylation | Pectin with a block-wise distribution of non-methoxylated carboxyl groups can associate with egg-box formation at a higher DM. Pectin with carboxyl groups randomly distributed does not form egg-box patterns. |
| Chain length | The lower the molecular mass of pectin, the lower the gel strength. |
| Branching | Large side chains are likely to cause steric hindrance, which may hamper pectin-pectin interactions. |
| Amidation | Amidated pectin can form stronger gels, especially at a low pH, because of the formation of hydrogen bonds between amide groups. |
| Acetylation | Acetyl groups drastically decrease the binding strength of pectin with calcium ions. |
| Extrinsic Parameters (Environment) | |
| Calcium content | The influence of calcium ion concentration on the gelation of LM pectin is mostly described in terms of a stoichiometric ratio: R = 2[Ca2+]/[COO−]. R = 0.5, theoretically, all calcium ions are bound, forming the “egg-box” structure. |
| Pectin content | When R is kept constant, gel strength increases with the polymer concentration. |
| pH | To form ionic cross-links between the pectin carboxyl groups and calcium ions, pectin needs to be charged, i.e., the carboxyl groups need to be dissociated. Above pH = 4.5, the gel properties are relatively independent of pH, but when the pH of a pectin-calcium gel decreases below 4.5, the charge density of pectin decreases and, consequently, its affinity for calcium ions decreases. However, this effect is partly compensated by forming hydrogen bonds between protonated carboxyl groups. |
| Temperature | At high temperatures, a chain scission is promoted, and, as a consequence, dimers are formed. These junction zones are stabilized upon cooling through hydrogen bonding, which is accompanied by cooperative calcium immobilization. |
| Year | Event | Reference |
|---|---|---|
| 1973 | First postulation of egg-box model for pectin | [125] |
| 1978 | Dimers formation | [147] |
| 1981 | Junction zones model | [148] |
| 1982 | Junction zone distribution | [149] |
| 1987 | Divalent cations coordination | [150] |
| 1991 | First book in pectin chemistry and technology | [151] |
| 2001 | Shifted egg box model for pectin gels | [152] |
| 2007 | Multi-step binding ions behavior | [153] |
| [155] | ||
| 2008 | Alginate and pectin comparison | [156] |
| 2010 | Structural reorganization of pectin and alginate after calcium binding | [157] |
| 2013 | Egg-box model with semi-flexible chains | [158] |
| 2015 | Cross-link differences between different multivalent cations | [160] |
| 2016 | [161] | |
| 2018 | [162] | |
| 2020 | Different ways in pectin dimers aggregation | [159] |
| Origin | Adsorbent | CA | DM [%] | Pollutant | qmax [mg/g] | Ref. |
|---|---|---|---|---|---|---|
| Citrus fruit | Pectin | Ca2+ | <50 | Hg2+ | 1.7 mmol/g | [163] |
| Commercial pectin | Demethylated pectin | Ca2+/Mg2+ | 13 | Fe2+ | 0.523 mol Fe2+/mol GalA | [164] |
| Commercial pectin | Pectin microgel particles | Ca2+ | 16.4 | MB * | 284 mg/g | [165] |
| Citrus peel | Pectin with Oxisol | Nr. | 6.7 | Cu2+ | 33.8 mmol/kg | [166] |
| Pectin with Utisol | Cu2+ | 37.0 mmol/kg | ||||
| Sweet potato residue | HHP-AP ** modified pectin | Nr. | 16.11 | Pb2+ | 263.15 mg/g | [167] |
| Pectin | 28.01 | 163.93 mg/g | ||||
| Commercial pectin | Chitosan-pectin gel beads | Alkaline solution | 6.68 | Cu2+ | 169 mg/g | [168] |
| Cd2+ | 177.6 mg/g | |||||
| Hg2+ | 208.5 mg/g | |||||
| Pb2+ | 266.5 mg/g | |||||
| Sugar beet pectin | Pectin xerogel beds | Ca2+ | <50 | Cd2+ | 0.151 mmol/g | [169] |
| Pb2+ | 0.290 mmol/g | |||||
| Cu2+ | 0.343 mmol/g | |||||
| Citrus pectin | Pectin microspheres | Ca2+ | 47.9 | Pb2+ | 127 mg/g | [170] |
| LM pectin microspheres | 18.04 | 292 mg/g | ||||
| Pectic acid microspheres | 0.9 | 325 mg/g | ||||
| Citrus peel | Pectin and guar gum beds | Ca2+ | 20 | Pb2+ | 104.8 mg/g | [171] |
| Citrus peel | Pectin-alginate beds | Ca2+ | <50 | Cu2+ | 2.79 µmol/bead | [163] |
| Cd2+ | 3.4 µmol/bead | |||||
| Phyllospadix iwatensis | Native Pectin | Nr. | 6.91 | Pb2+ | 2.447 mM/g | [172] |
| Hydrolized Pectin | 2.54 | Cd2+ | 1.643 mM/g | |||
| Pb2+ | 2.818 mM/g | |||||
| Cd2+ | 2.396 mM/g | |||||
| Grapefruit peel | Biochar-pectin-alginate beads | Ca2+ | 17.5 | Cu2+ | 80.6 mg/g | [173] |
| Commercial pectin | Pectin | Ca2+ | <50 | MB ** | 354.6 mg/g | [127] |
| Orange Waste | Pectin/cellulose microfibers beds | Ca2+ | <50 | Fe2+ | 98.0 mg/g | [174] |
| Cu2+ | 88.5 mg/g | |||||
| Cd2+ | 192.3 mg/g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Sabando, J.; Coin, F.; Melillo, J.H.; Goyanes, S.; Cerveny, S. A Review of Pectin-Based Material for Applications in Water Treatment. Materials 2023, 16, 2207. https://doi.org/10.3390/ma16062207
Martínez-Sabando J, Coin F, Melillo JH, Goyanes S, Cerveny S. A Review of Pectin-Based Material for Applications in Water Treatment. Materials. 2023; 16(6):2207. https://doi.org/10.3390/ma16062207
Chicago/Turabian StyleMartínez-Sabando, Javier, Francesco Coin, Jorge H. Melillo, Silvia Goyanes, and Silvina Cerveny. 2023. "A Review of Pectin-Based Material for Applications in Water Treatment" Materials 16, no. 6: 2207. https://doi.org/10.3390/ma16062207
APA StyleMartínez-Sabando, J., Coin, F., Melillo, J. H., Goyanes, S., & Cerveny, S. (2023). A Review of Pectin-Based Material for Applications in Water Treatment. Materials, 16(6), 2207. https://doi.org/10.3390/ma16062207






