Influence of the Duty Cycle of Pulse Electrodeposition-Coated Ni-Al2O3 Nanocomposites on Surface Roughness Properties
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Surface Morphology
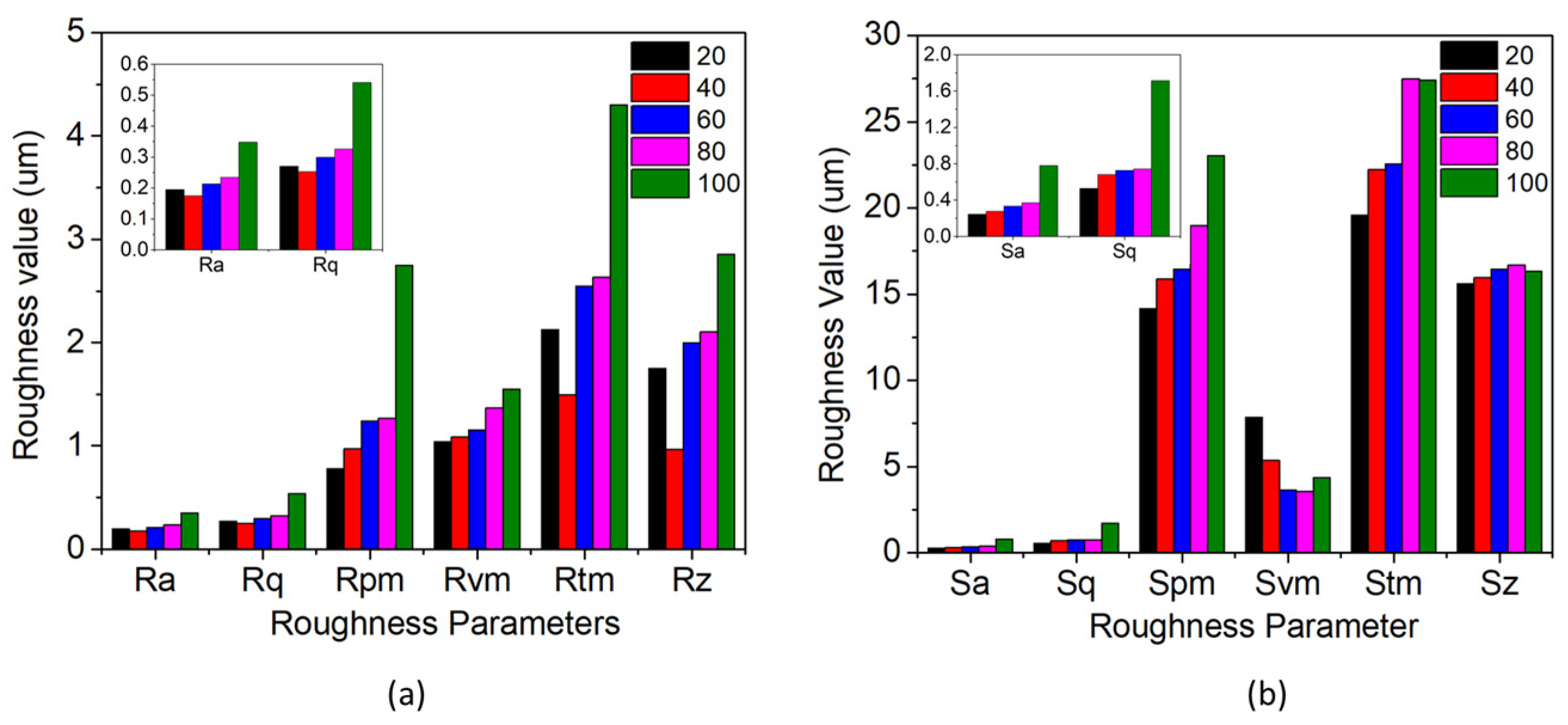
3.2. Roughness Analysis
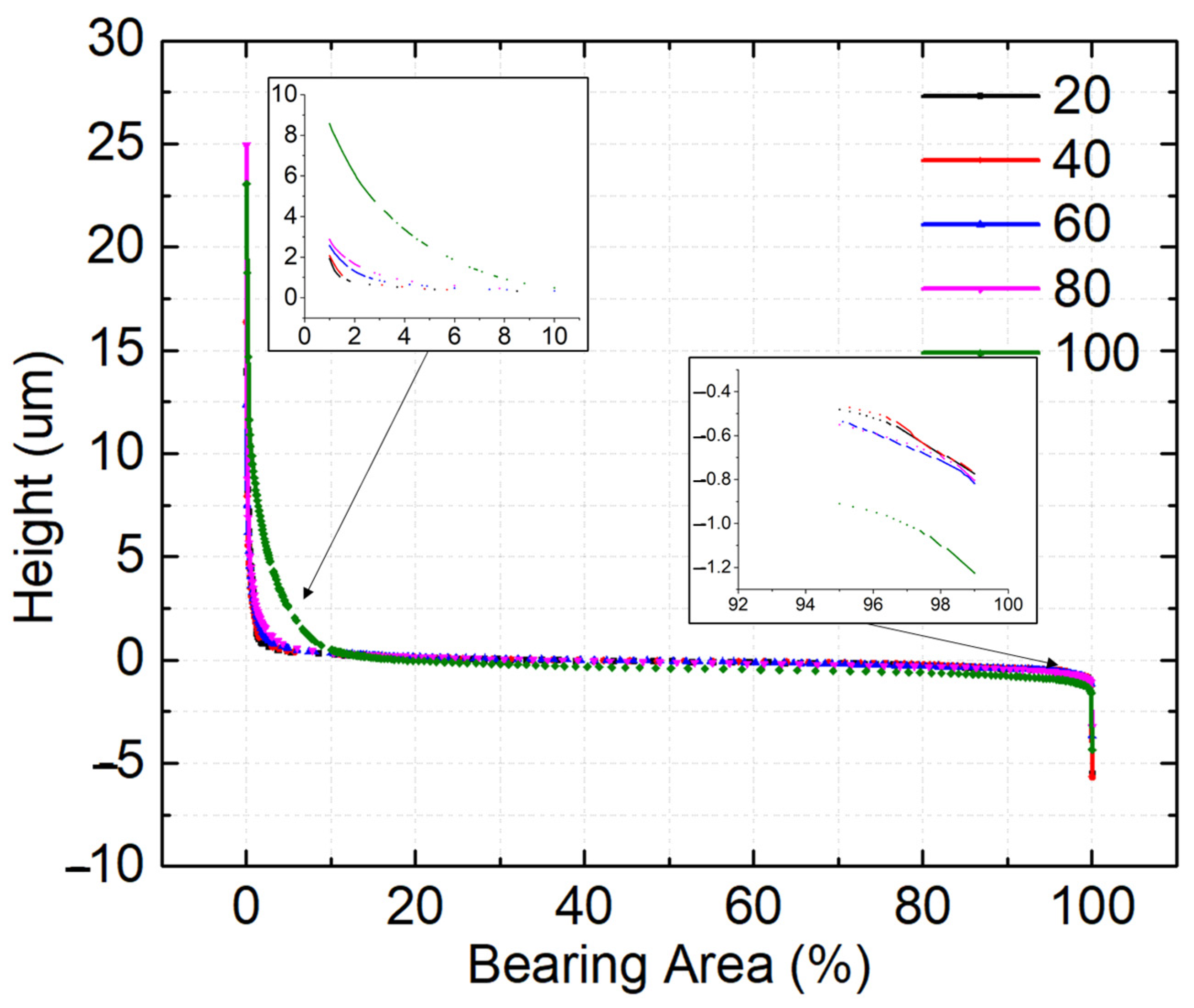
3.3. Bearing Area Curves
3.4. The Power Spectral Density
3.5. Nanohardness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Xue, Z.; Lei, W.; Wang, Y.; Qian, H.; Li, Q. Effect of pulse duty cycle on mechanical properties and microstructure of nickel-graphene composite coating produced by pulse electrodeposition under supercritical carbon dioxide. Surf. Coat. Technol. 2017, 325, 417–428. [Google Scholar] [CrossRef]
- Wang, W.; Hou, F.Y.; Wang, H.; Guo, H.T. Fabrication and characterization of Ni-ZrO2 composite nano-coatings by pulse electrodeposition. Scr. Mater. 2005, 53, 613–618. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; He, Y.; Li, H.; He, T.; Fan, Y.; Zhang, H. Mechanical properties and corrosion resistance of pulse electrodeposited Ni-B/B4C composite coatings. Surf. Coat. Technol. 2021, 421, 127458. [Google Scholar] [CrossRef]
- Vamsi, M.V.N.; Wasekar, N.P.; Sundararajan, G. Influence of heat treatment on microstructure and mechanical properties of pulse electrodeposited Ni-W alloy coatings. Surf. Coat. Technol. 2017, 319, 403–414. [Google Scholar] [CrossRef]
- Wasekar, N.P.; Latha, S.M.; Ramakrishna, M.; Rao, D.S.; Sundararajan, G. Pulsed electrodeposition and mechanical properties of Ni-W/SiC nano-composite coatings. Mater. Des. 2016, 112, 140–150. [Google Scholar] [CrossRef]
- Li, Q.; Yang, X.; Zhang, L.; Wang, J.; Chen, B. Corrosion resistance and mechanical properties of pulse electrodeposited Ni-TiO2 composite coating for sintered NdFeB magnet. J. Alloys Compd. 2009, 482, 339–344. [Google Scholar] [CrossRef]
- Bahadormanesh, B.; Dolati, A. The kinetics of Ni-Co/SiC composite coatings electrodeposition. J. Alloys Compd. 2010, 504, 514–518. [Google Scholar] [CrossRef]
- Alizadeh, M.; Mirak, M.; Salahinejad, E.; Ghaffari, M.; Amini, R.; Roosta, A. Structural characterization of electro-codeposited Ni-Al2O 3-SiC nanocomposite coatings. J. Alloys Compd. 2014, 611, 161–166. [Google Scholar] [CrossRef]
- Allahkaram, S.R.; Golroh, S.; Mohammadalipour, M. Properties of Al2O3 nano-particle reinforced copper matrix composite coatings prepared by pulse and direct current electroplating. Mater. Des. 2011, 32, 4478–4484. [Google Scholar] [CrossRef]
- Lodhi, Z.F.; Mol, J.M.C.; Hovestad, A.; Terryn, H.; de Wit, J.H.W. Electrodeposition of Zn-Co and Zn-Co-Fe alloys from acidic chloride electrolytes. Surf. Coat. Technol. 2007, 202, 84–90. [Google Scholar] [CrossRef]
- Li, H.; He, Y.; Luo, P.; Fan, Y.; Yu, H.; Wang, Y.; He, T.; Li, Z.; Zhang, H. Influence of pulse frequency on corrosion resistance and mechanical properties of Ni-W/B4C composite coatings. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127436. [Google Scholar] [CrossRef]
- Wasekar, N.P.; Sundararajan, G. Sliding wear behavior of electrodeposited Ni-W alloy and hard chrome coatings. Wear 2015, 342–343, 340–348. [Google Scholar] [CrossRef]
- Jiang, W.; Shen, L.; Xu, M.; Wang, Z.; Tian, Z. Mechanical properties and corrosion resistance of Ni-Co-SiC composite coatings by magnetic field-induced jet electrodeposition. J. Alloys Compd. 2019, 791, 847–855. [Google Scholar] [CrossRef]
- Imaz, N.; García-Lecina, E.; Suárez, C.; Díez, J.A.; Rodríguez, J.; Molina, J.; García-Navas, V. Influence of additives and plating parameters on morphology and mechanical properties of copper coatings obtained by pulse electrodeposition. Trans. Inst. Met. Finish. 2009, 87, 64–71. [Google Scholar] [CrossRef]
- Lausmannl, G.A. Electrolytically Deposited Hardchrome. Surf. Coat. Technol. 1996, 86–87, 814–820. [Google Scholar] [CrossRef]
- Bahrololoom, M.E.; Sani, R. The influence of pulse plating parameters on the hardness and wear resistance of nickel-alumina composite coatings. Surf. Coat. Technol. 2005, 192, 154–163. [Google Scholar] [CrossRef]
- Jegan, A.; Venkatesan, R. Characterization and optimization of pulse electrodeposition of Ni/nano-Al2O3 composite coatings. Int. J. Miner. Metall. Mater. 2013, 20, 479–485. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Zeng, Z.; Xu, T. Influence of pulse frequency on the microstructure and wear resistance of electrodeposited Ni-Al2O3 composite coatings. Surf. Coat. Technol. 2006, 201, 599–605. [Google Scholar] [CrossRef]
- Steinbach, J.; Ferkel, H. Nanostructured Ni-Al2O3 films prepared by DC and pulsed DC electroplating. Scr. Mater. 2001, 44, 1813–1816. [Google Scholar] [CrossRef]
- Ma, C.Y.; Zhao, D.Q.; Xia, F.F.; Xia, H.; Williams, T.; Xing, H.Y. Ultrasonic-assisted electrodeposition of Ni-Al2O3 nanocomposites at various ultrasonic powers. Ceram. Int. 2020, 46, 6115–6123. [Google Scholar] [CrossRef]
- Gül, H.; Uysal, M.; Akbulut, H.; Alp, A. Effect of PC electrodeposition on the structure and tribological behavior of Ni-Al2O3 nanocomposite coatings. Surf. Coat. Technol. 2014, 258, 1202–1211. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, Y.F. Fabrication of Ni-Co-SiC composite coatings by pulse electrodeposition–Effects of duty cycle and pulse frequency. Surf. Coat. Technol. 2013, 216, 282–288. [Google Scholar] [CrossRef]
- Lajevardi, S.A.; Shahrabi, T. Effects of pulse electrodeposition parameters on the properties of Ni-TiO2 nanocomposite coatings. Appl. Surf. Sci. 2010, 256, 6775–6781. [Google Scholar] [CrossRef]
- Jackson, R.L. Lubrication. In Handbook of Lubrication and Tribology, Volume II: Theory and Design, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; Volume 123, pp. 14-1–14-14. [Google Scholar]
- Sedlaček, M.; Podgornik, B.; Vižintin, J. Influence of surface preparation on roughness parameters, friction and wear. Wear 2009, 266, 482–487. [Google Scholar] [CrossRef]
- Bajwa, R.; Khan, Z.; Nazir, H.; Chacko, V.; Saeed, A. Wear and friction properties of electrodeposited Ni-based coatings subject to nano-enhanced lubricant and composite coating. Acta Metall. Sin. (Engl. Lett.) 2016, 29, 902–910. [Google Scholar] [CrossRef]
- Bhutta, M.U.; Khan, Z.A.; Garland, N. Wear performance analysis of Ni-Al2O3 nanocomposite coatings under nonconventional lubrication. Materials 2018, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, M.U.; Khan, Z.A. Wear and friction performance evaluation of nickel based nanocomposite coatings under refrigerant lubrication. Tribol. Int. 2020, 148, 106312. [Google Scholar] [CrossRef]
- Nazir, M.H.; Khan, Z.A.; Saeed, A.; Bakolas, V.; Braun, W.; Bajwa, R.; Rafique, S. Analyzing and modelling the corrosion behavior of Ni/Al2O3, Ni/SiC, Ni/ZrO2 and Ni/graphene nanocomposite coatings. Materials 2017, 10, 1225. [Google Scholar] [CrossRef]
- Bajwa, R.S.; Khan, Z.; Bakolas, V.; Braun, W. Effect of bath ionic strength on adhesion and tribological properties of pure nickel and Ni-based nanocomposite coatings. J. Adhes. Sci. Technol. 2016, 30, 653–665. [Google Scholar] [CrossRef]
- Bajwa, R.S.; Khan, Z.; Bakolas, V.; Braun, W. Water-lubricated Ni-based composite (Ni-Al2O3, Ni-SiC and Ni-ZrO2) thin film coatings for industrial applications. Acta Metall. Sin. (Engl. Lett.) 2016, 29, 8–16. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Gao, Y.; Xue, Q.; Hu, L.; Xu, T. Grain size effect in corrosion behavior of electrodeposited nanocrystalline Ni coatings in alkaline solution. Scr. Mater. 2006, 55, 657–660. [Google Scholar] [CrossRef]
- Edachery, V.; John, A.; Rajendran, A.; Srinivasappa, V.; Mathiyalagan, S.; Kumar, S.; Kailas, S.V. Enhancing tribological properties of Inconel X-750 superalloy through surface topography modification by shot blasting. Mater. Perform. Charact. 2021, 10, 322–332. [Google Scholar] [CrossRef]
- Sedlaček, M.; Podgornik, B.; Vižintin, J. Correlation between standard roughness parameters skewness and kurtosis and tribological behaviour of contact surfaces. Tribol. Int. 2012, 48, 102–112. [Google Scholar] [CrossRef]
- Edachery, V.; Swamybabu, V.; Adarsh, D.; Kailas, S.V. Influence of surface roughness frequencies and roughness parameters on lubricant wettability transitions in micro-nano scale hierarchical surfaces. Tribol. Int. 2022, 165, 107316. [Google Scholar] [CrossRef]
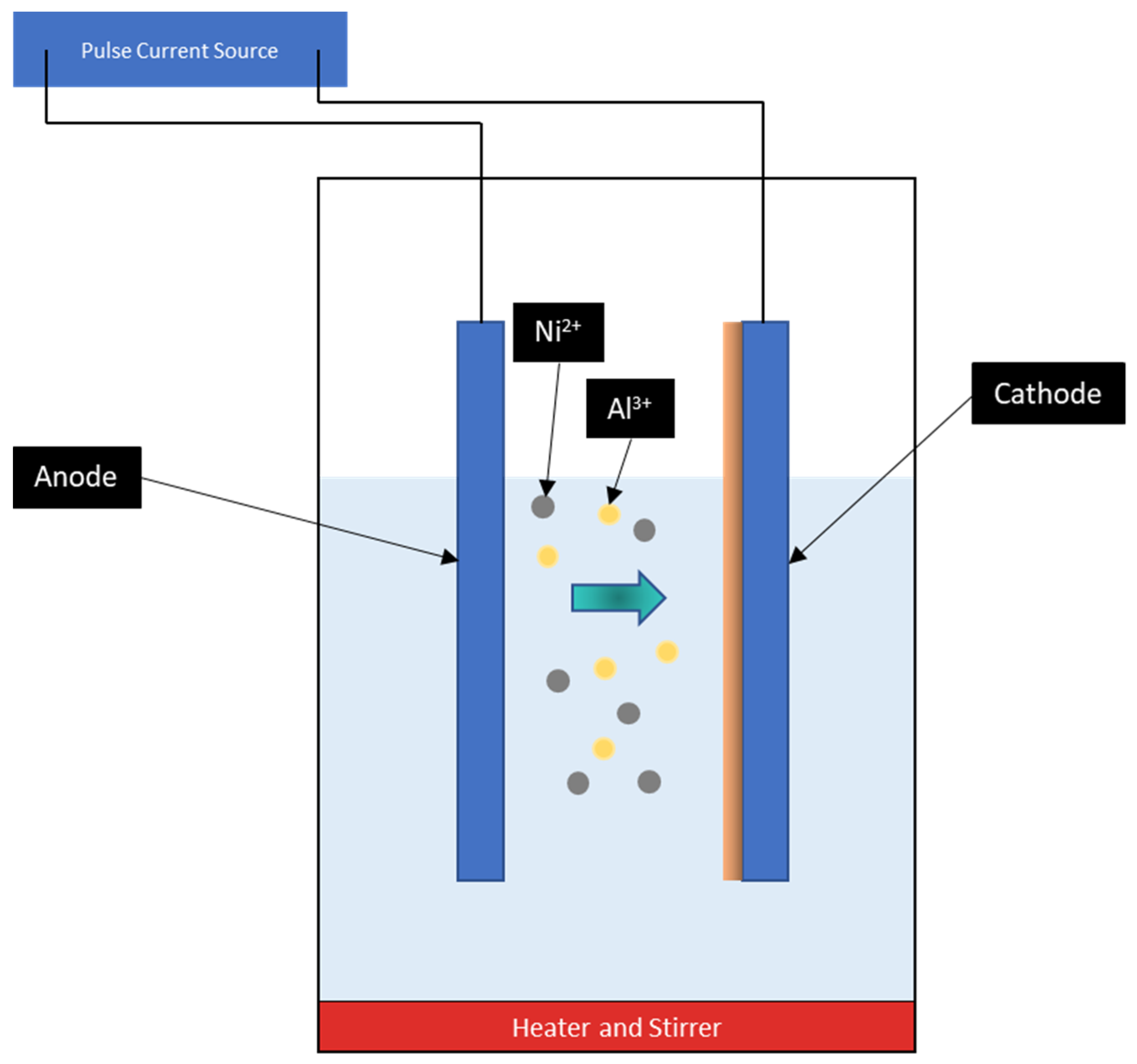
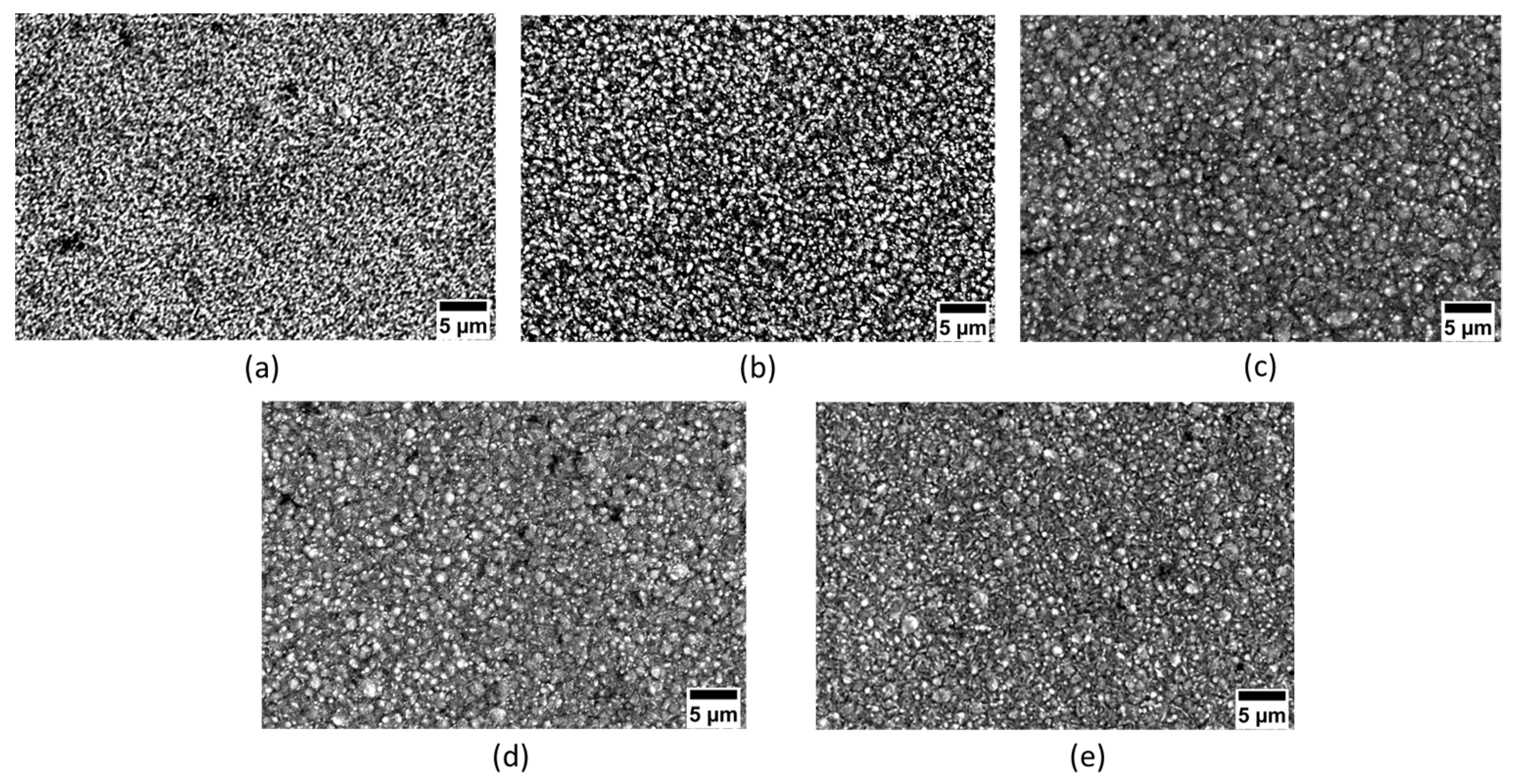
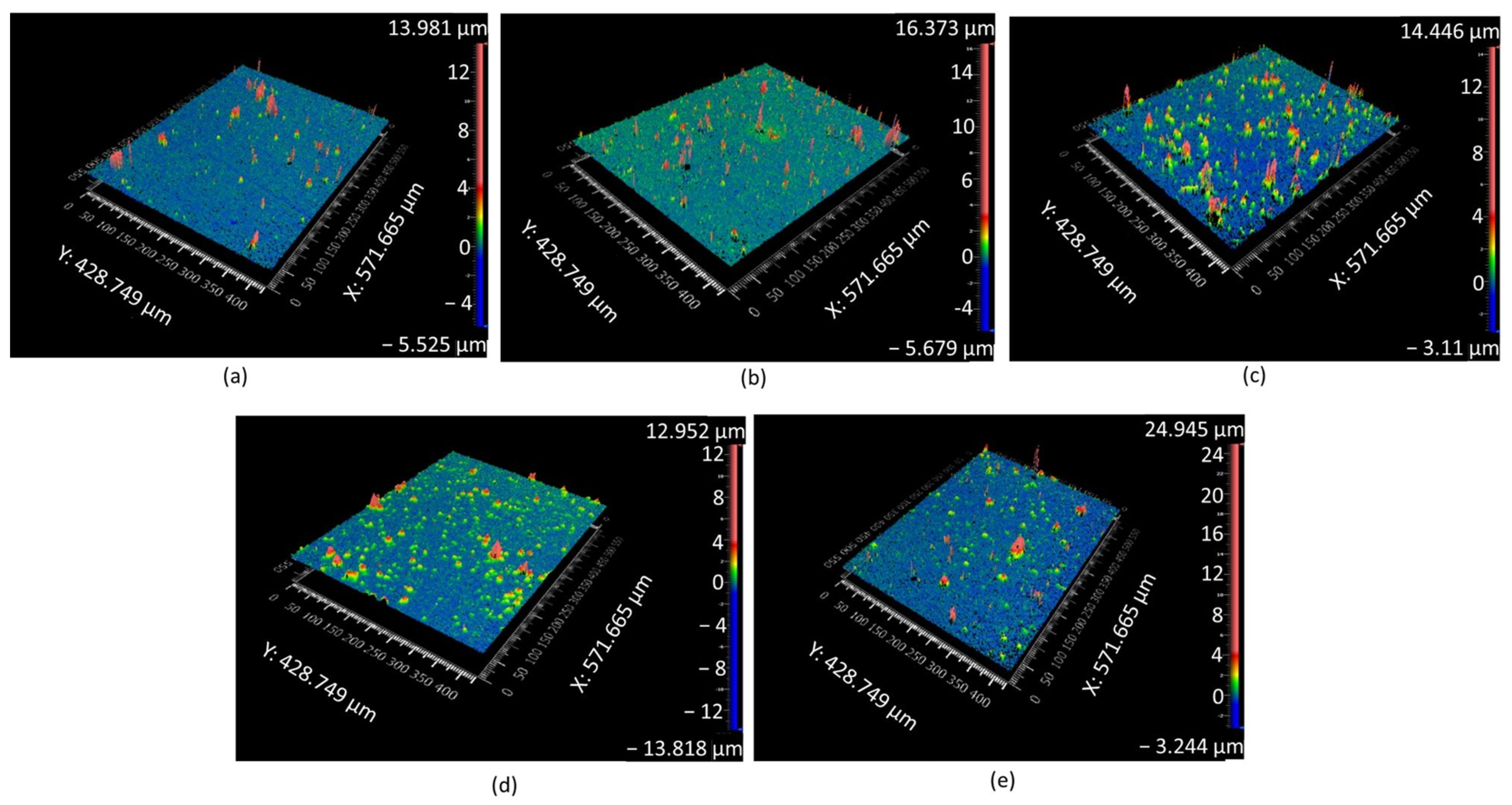


| Chemical Composition | Quantity (g/L) | Function |
|---|---|---|
| Nickel Sulphate Hexahydrate (NiSO4·6H2O) | 265 | Nickel Source |
| Nickel (II) Chloride (NiCl2·6H2O) | 48 | Conductivity and Nickel Source |
| Boric Acid (H3BO3) | 31 | Buffering agent and Stabilizer |
| Aluminium Oxide (Al2O3) | 10 | Reinforcing agent |
| Parameter 1 | Parameter 2 | Parameter 3 | Parameter 4 | Parameter 5 | |
|---|---|---|---|---|---|
| Current Density (A/dm2) | 3 | ||||
| Duty Cycle (%) | 20 | 40 | 60 | 80 | 100 |
| Frequency (kHz) | 10 | ||||
| Time (min) | 60 | ||||
| pH | 4.2 ± 0.2 | ||||
| Temperature (°C) | 60 ± 5 | ||||
| Anode | Nickel Plate | ||||
| Cathode | EN1A | ||||
| Areal Roughness Parameters (µm) | ||||||
|---|---|---|---|---|---|---|
| Duty Cycle | Ra | Rq | Rpm | Rvm | Rtm | Rz |
| 20 | 0.195 | 0.271 | 0.781 | 1.043 | 2.125 | 0.752 |
| 40 | 0.175 | 0.253 | 0.972 | 1.084 | 1.495 | 0.967 |
| 60 | 0.214 | 0.299 | 1.244 | 1.155 | 2.549 | 1.998 |
| 80 | 0.234 | 0.326 | 1.267 | 1.368 | 2.635 | 2.107 |
| 100 | 0.348 | 0.541 | 2.748 | 1.550 | 4.299 | 2.857 |
| Surface Roughness Parameters (µm) | ||||||
|---|---|---|---|---|---|---|
| Duty Cycle | Sa | Sq | Spm | Svm | Stm | Sz |
| 20 | 0.245 | 0.529 | 14.161 | 7.830 | 19.560 | 15.612 |
| 40 | 0.277 | 0.682 | 15.865 | 5.345 | 22.212 | 15.974 |
| 60 | 0.334 | 0.728 | 16.427 | 3.631 | 22.559 | 16.436 |
| 80 | 0.371 | 0.742 | 18.948 | 3.531 | 27.479 | 16.687 |
| 100 | 0.779 | 1.714 | 23.046 | 4.350 | 27.396 | 16.339 |
| Skewness and Kurtosis | ||
|---|---|---|
| Duty Cycle | Ssk | Sku |
| 20 | 8.404 | 124.368 |
| 40 | 8.566 | 118.789 |
| 60 | 7.171 | 82.631 |
| 80 | 7.365 | 51.362 |
| 100 | 5.698 | 46.365 |
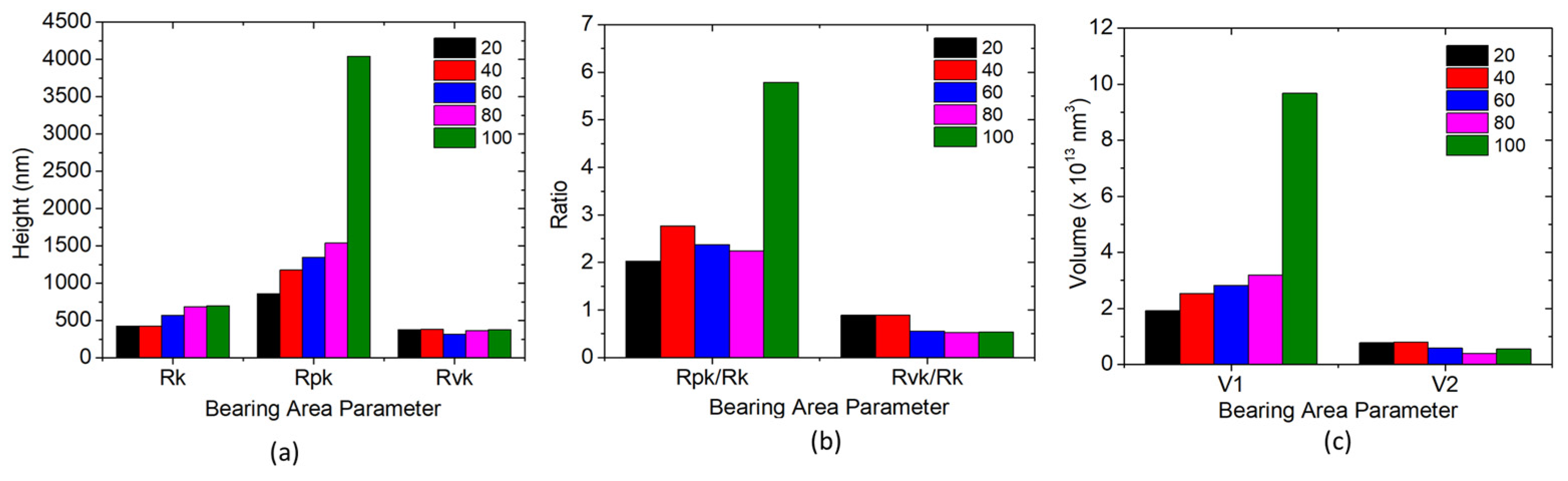
| Bearing Area Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Duty Cycle | Rk (nm) | Rpk (nm) | Rvk (nm) | Rpk/Rk | Rvk/Rk | Mr1 (%) | Mr2 (%) | V1 (×1013 nm3) | V2 (×1013 nm3) |
| 20 | 422.495 | 858.300 | 376.515 | 2.032 | 0.891 | 16.74 | 82.885 | 1.920 | 0.769 |
| 40 | 423.675 | 1174.255 | 380.045 | 2.772 | 0.897 | 17.97 | 82.910 | 2.525 | 0.7865 |
| 60 | 567.250 | 1348.270 | 314.545 | 2.377 | 0.555 | 18.49 | 84.415 | 2.820 | 0.576 |
| 80 | 685.720 | 1535.510 | 364.475 | 2.239 | 0.532 | 19.215 | 90.980 | 3.185 | 0.393 |
| 100 | 697.960 | 4040.490 | 376.69 | 5.789 | 0.540 | 19.73 | 92.080 | 9.670 | 0.545 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
John, A.; Saeed, A.; Khan, Z.A. Influence of the Duty Cycle of Pulse Electrodeposition-Coated Ni-Al2O3 Nanocomposites on Surface Roughness Properties. Materials 2023, 16, 2192. https://doi.org/10.3390/ma16062192
John A, Saeed A, Khan ZA. Influence of the Duty Cycle of Pulse Electrodeposition-Coated Ni-Al2O3 Nanocomposites on Surface Roughness Properties. Materials. 2023; 16(6):2192. https://doi.org/10.3390/ma16062192
Chicago/Turabian StyleJohn, Aashish, Adil Saeed, and Zulfiqar Ahmad Khan. 2023. "Influence of the Duty Cycle of Pulse Electrodeposition-Coated Ni-Al2O3 Nanocomposites on Surface Roughness Properties" Materials 16, no. 6: 2192. https://doi.org/10.3390/ma16062192
APA StyleJohn, A., Saeed, A., & Khan, Z. A. (2023). Influence of the Duty Cycle of Pulse Electrodeposition-Coated Ni-Al2O3 Nanocomposites on Surface Roughness Properties. Materials, 16(6), 2192. https://doi.org/10.3390/ma16062192









