Experiment Study on the Effect of Aluminum Sulfate-Based Alkali-Free Accelerator and the w/c on Cement Hydration and Leaching
Abstract
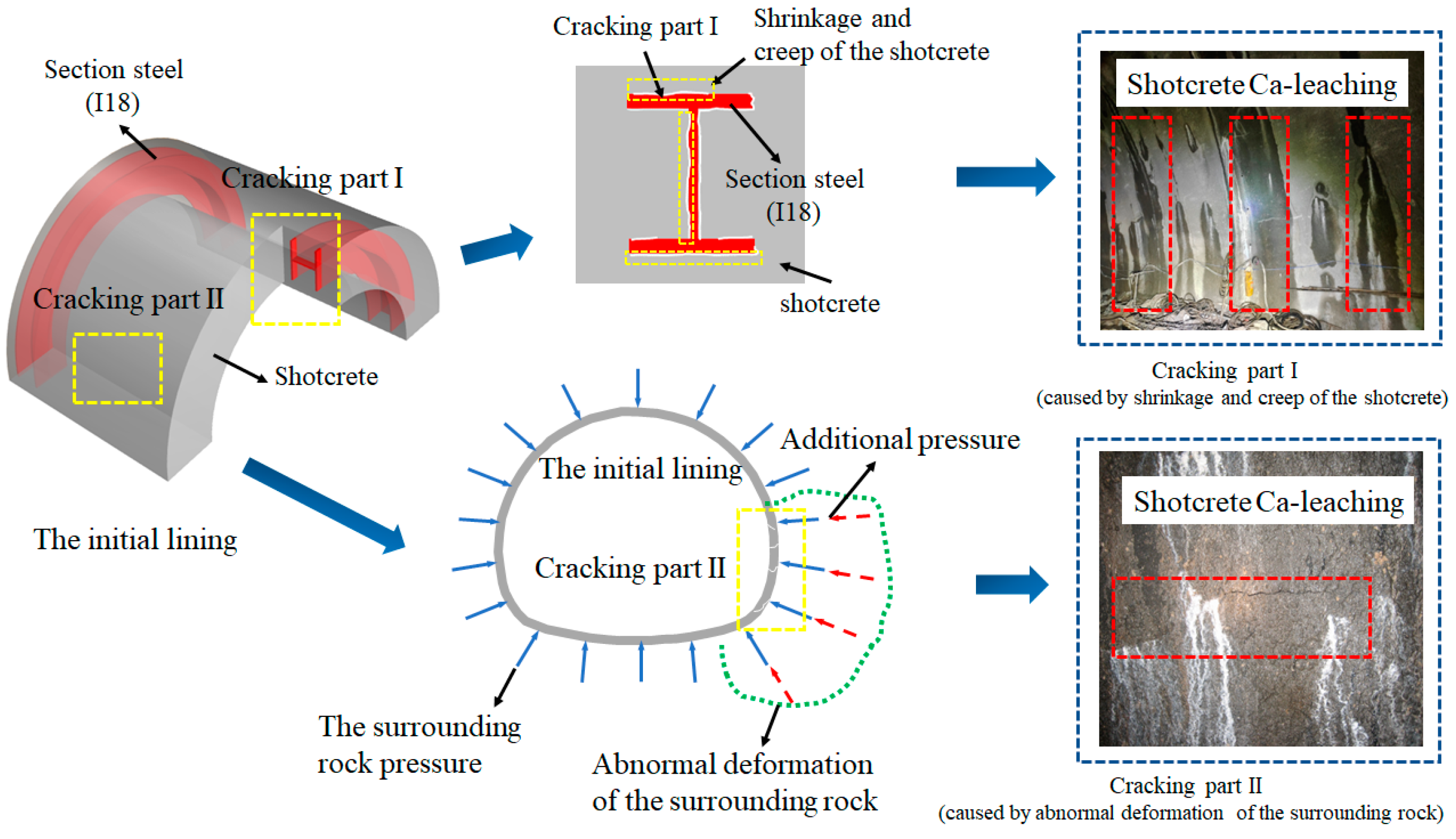
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
2.2.1. The RNW Test
2.2.2. Test Methods
- Isothermal calorimetry
- 2.
- X-ray diffraction
- 3.
- Scanning electron microscopy
- 4.
- Porosity
- 5.
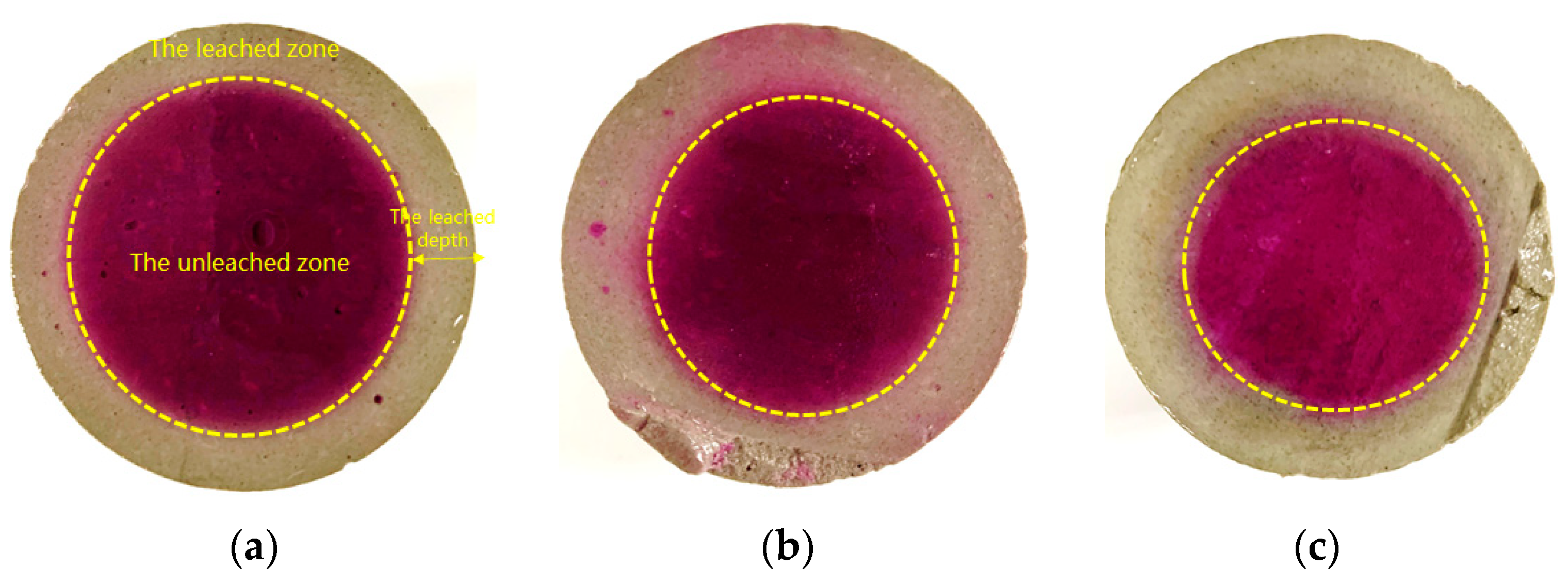
- Leaching length
3. Results
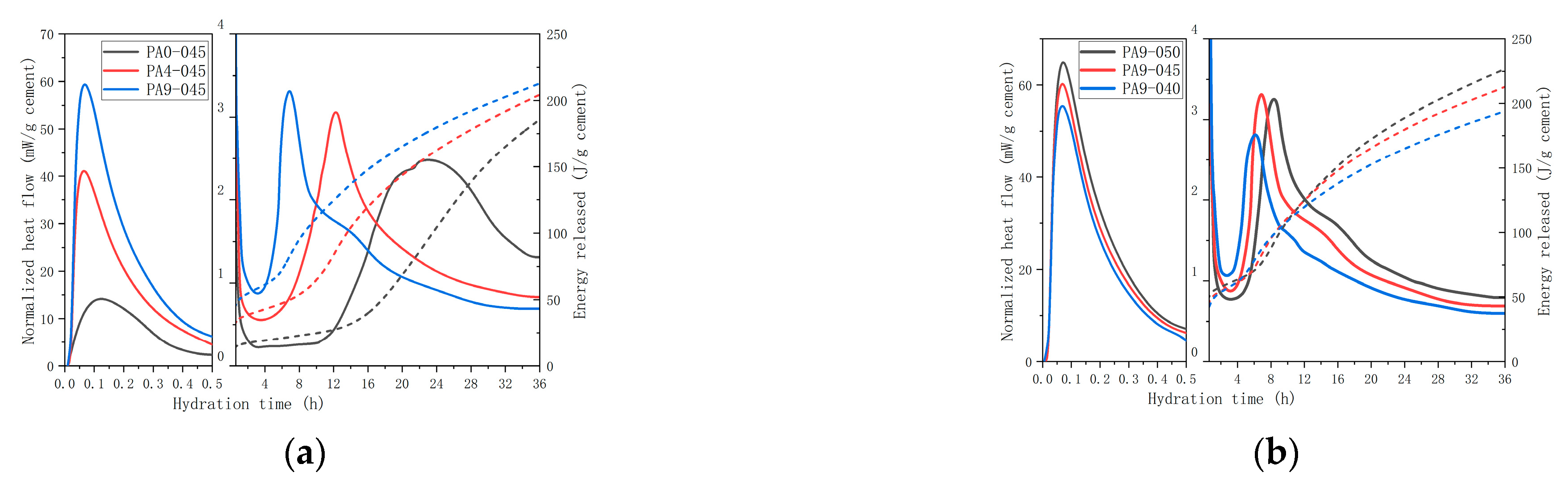
3.1. Isothermal Calorimetry
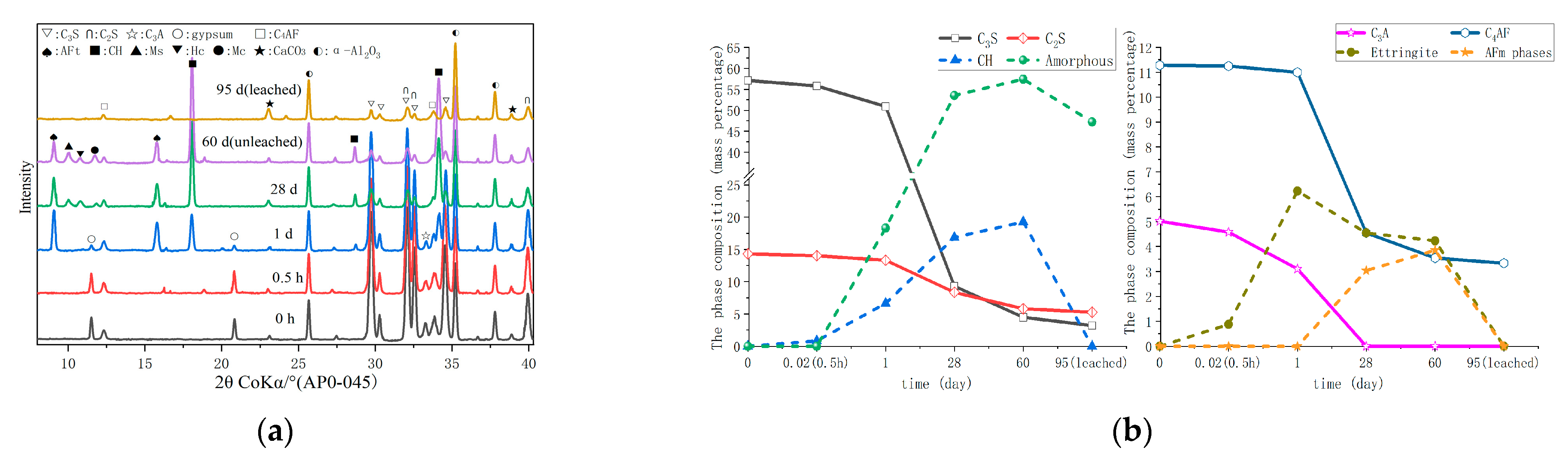
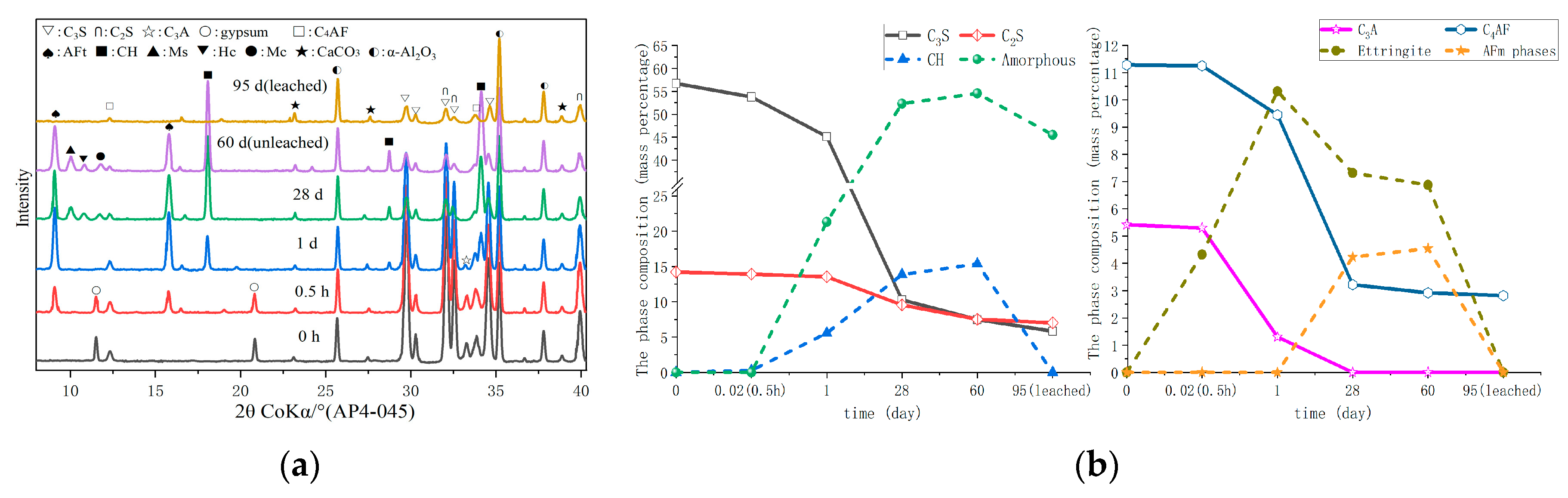
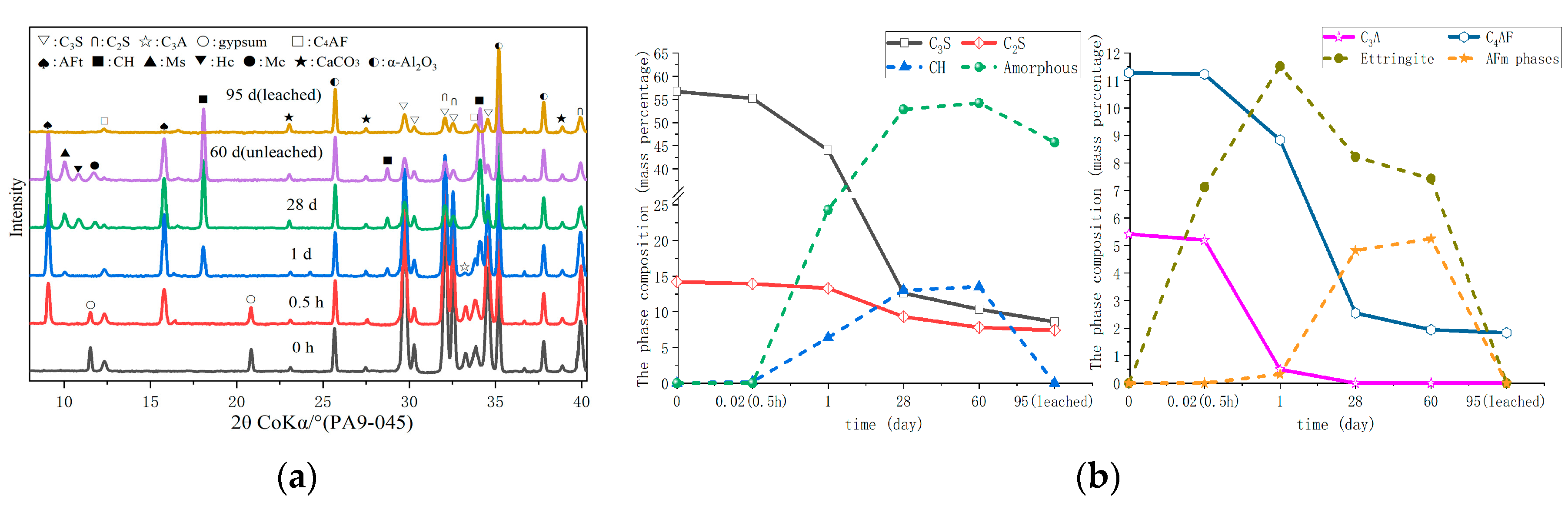
3.2. Hydrate Assemblage
3.3. Microstructure
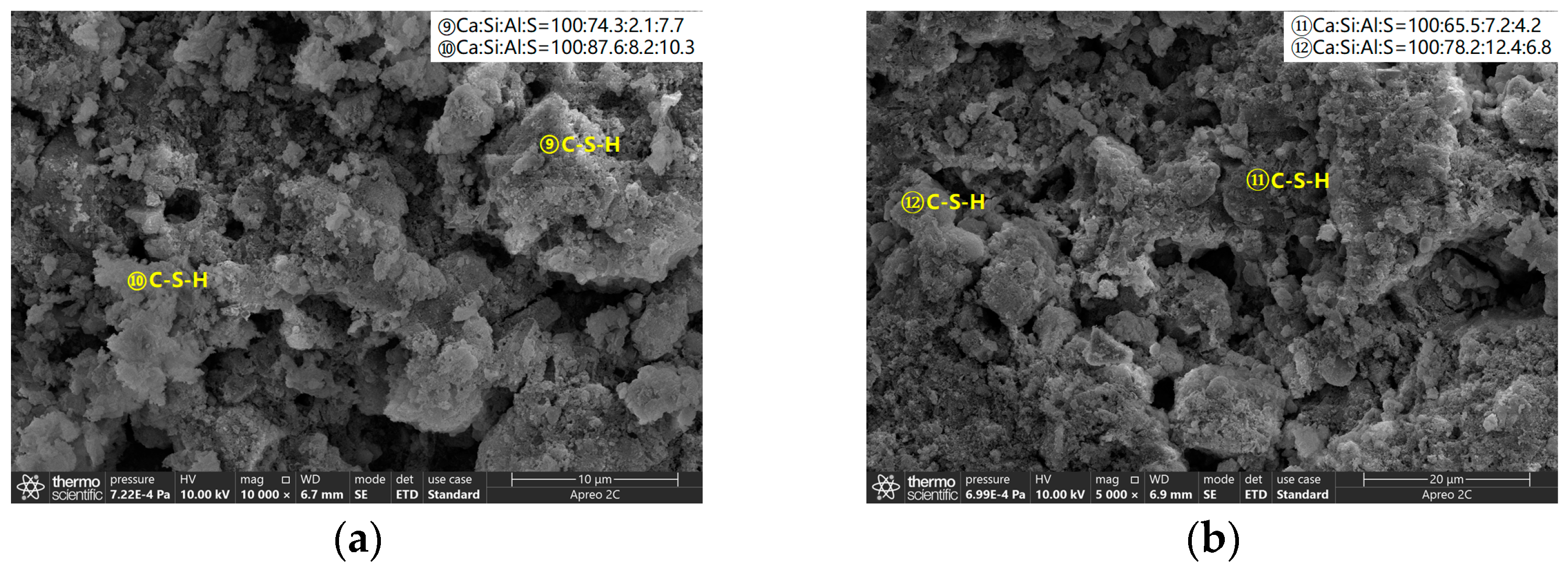
3.3.1. SEM
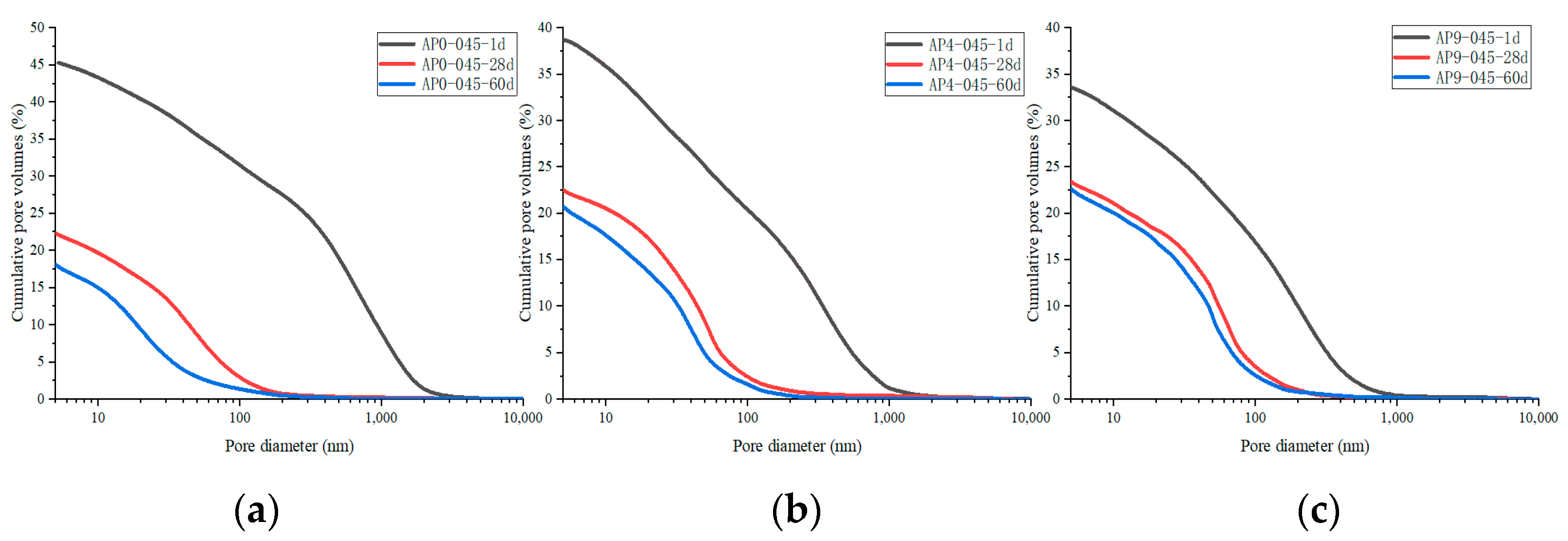
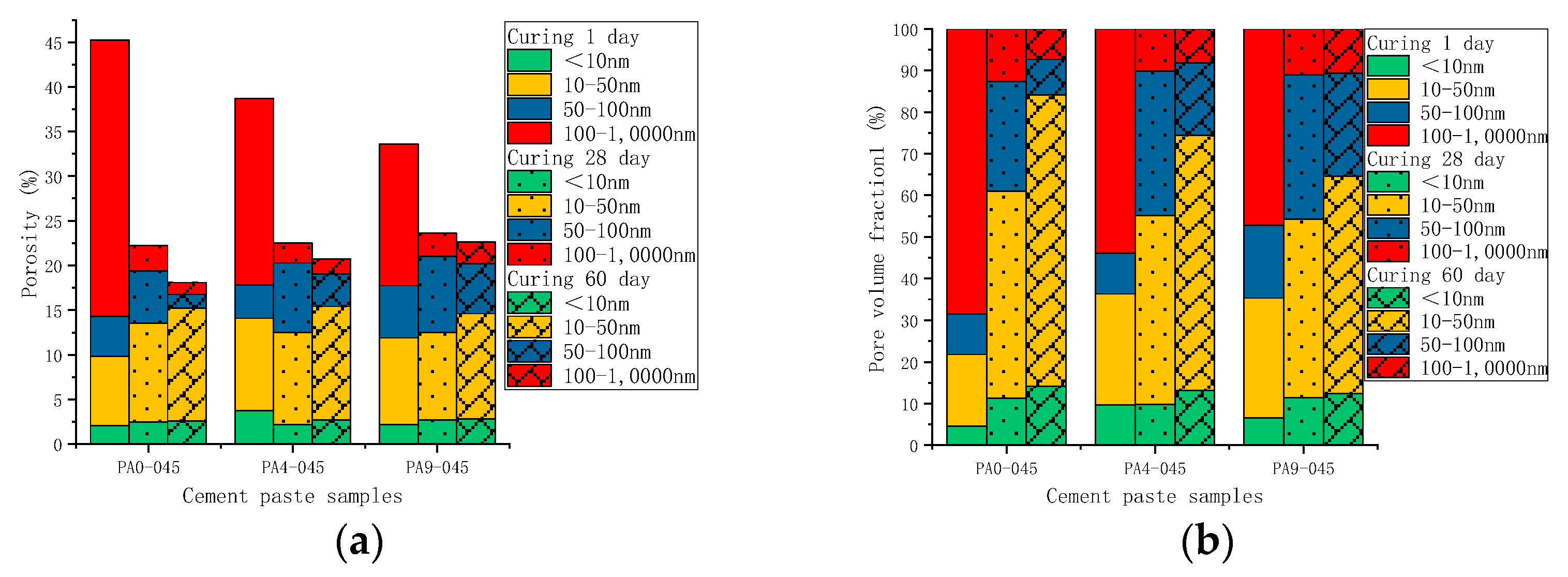
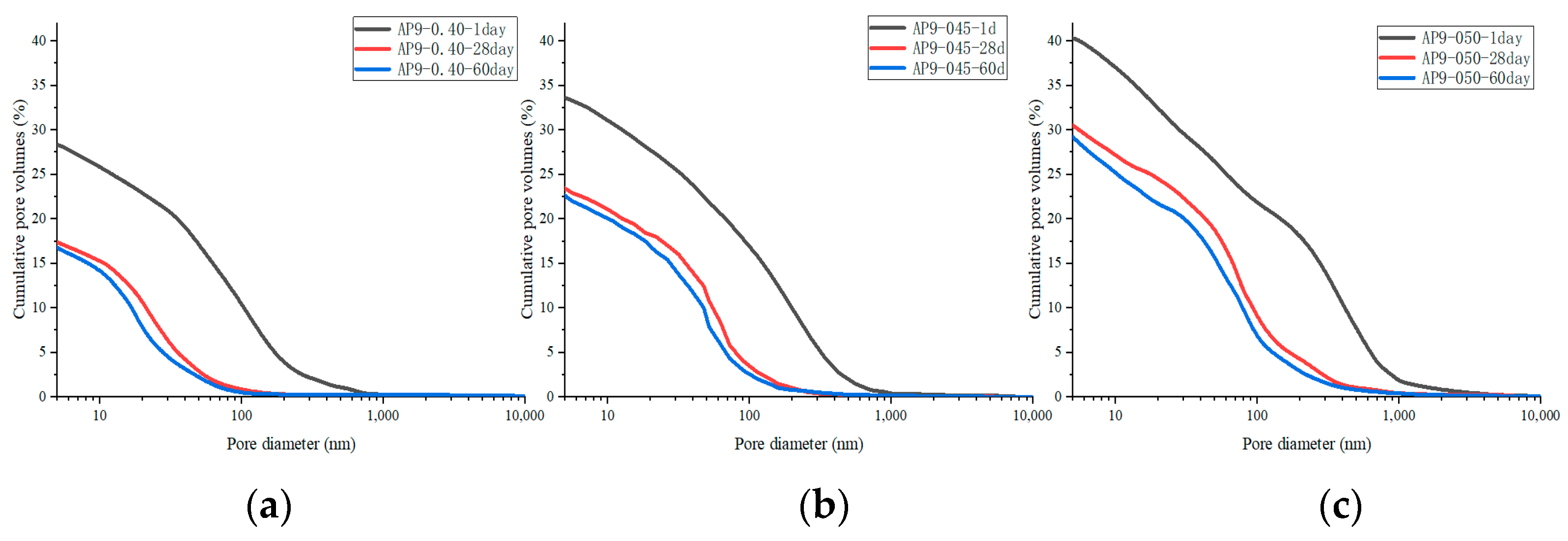
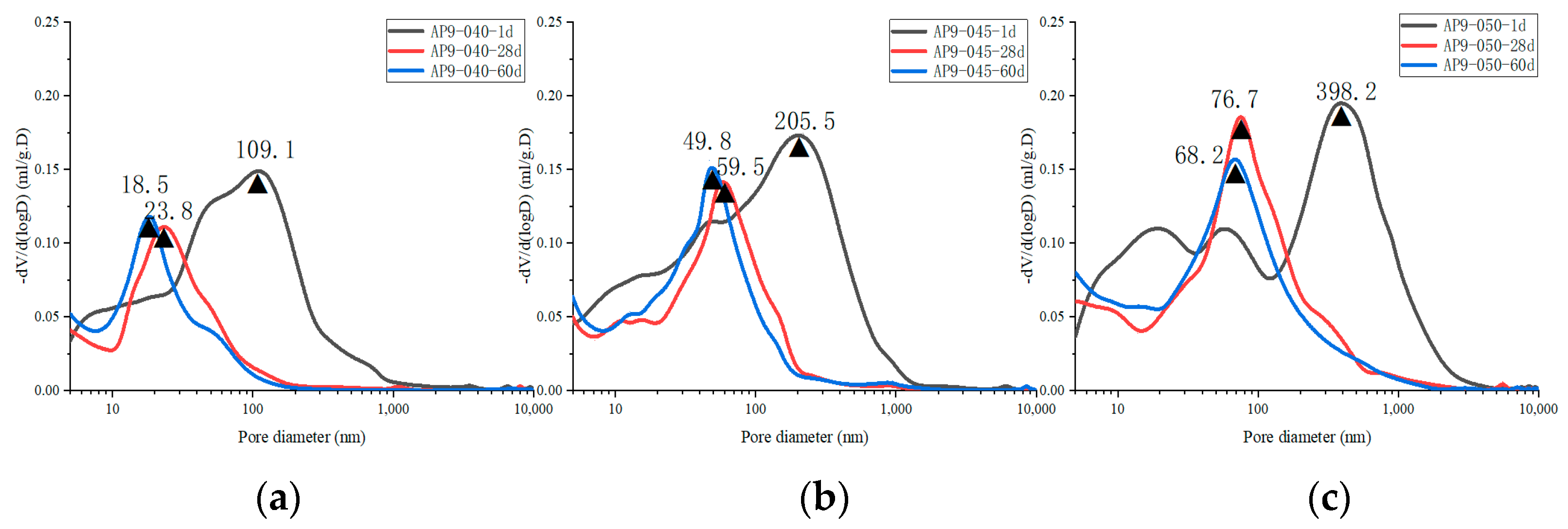
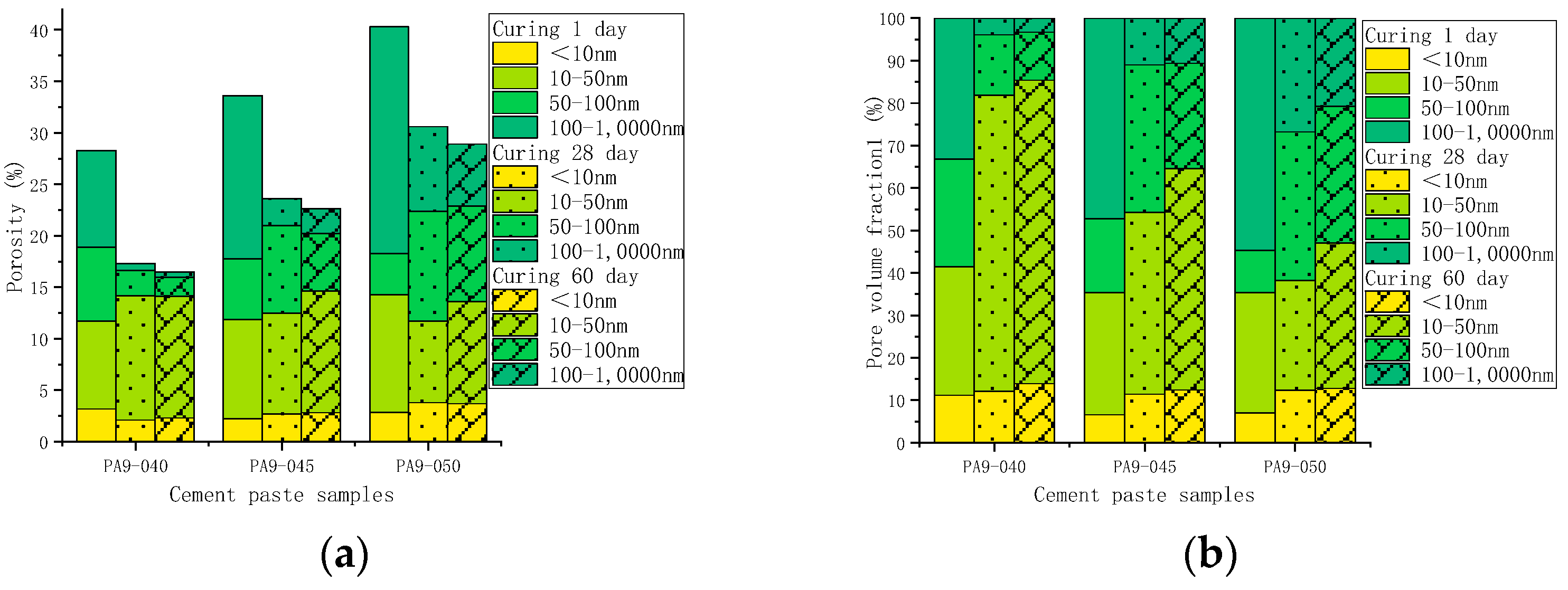
3.3.2. Pore Structure
- The pore structure in the hydration stage
- 2.
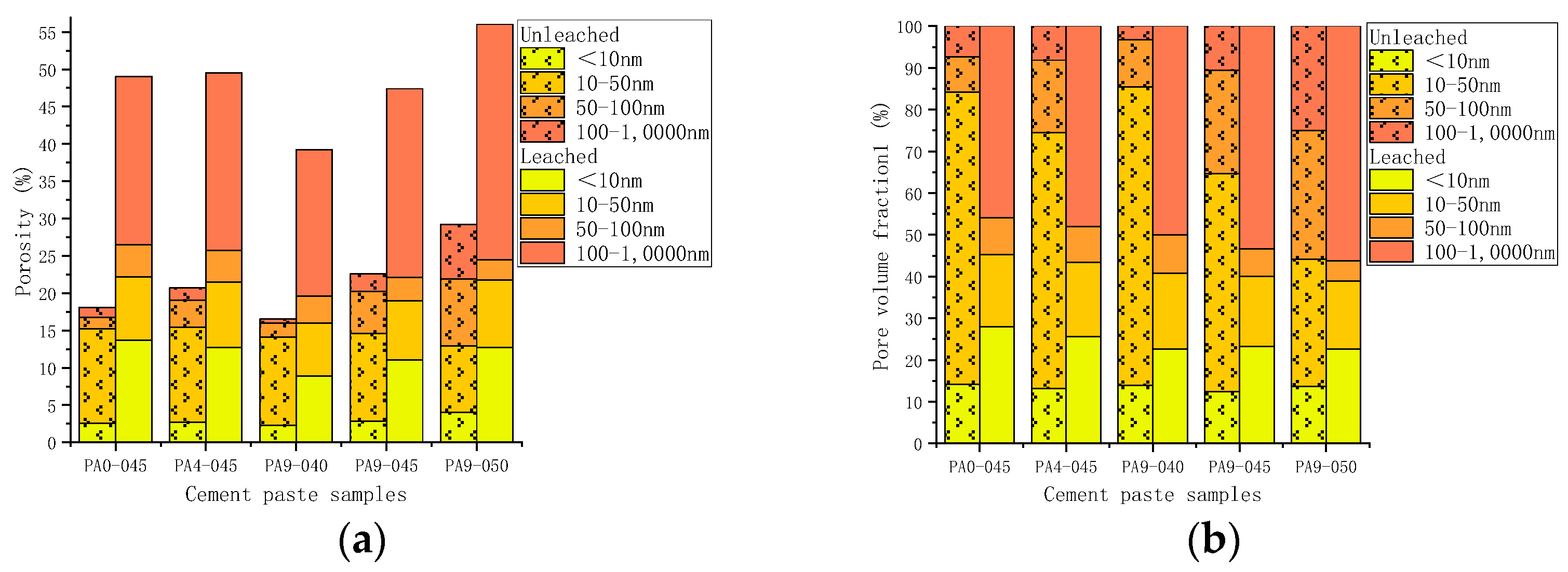
- The pore structure before and after leaching
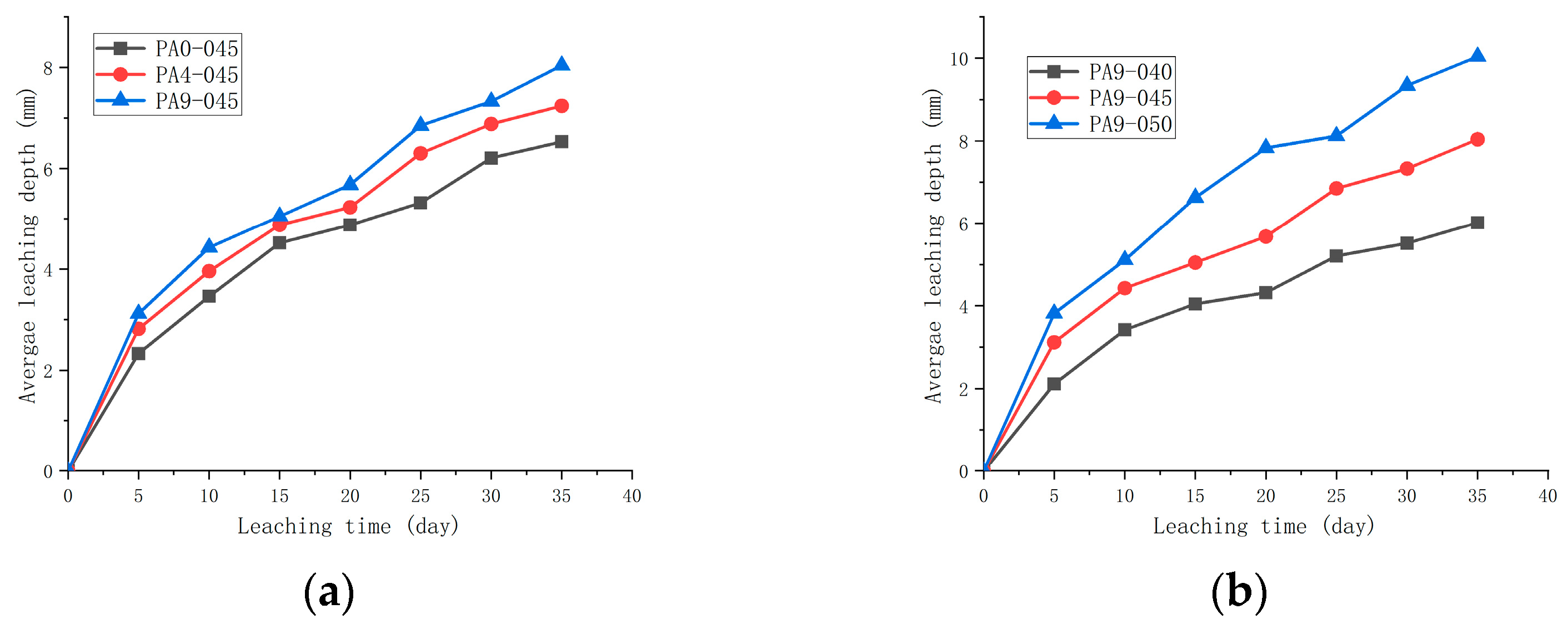
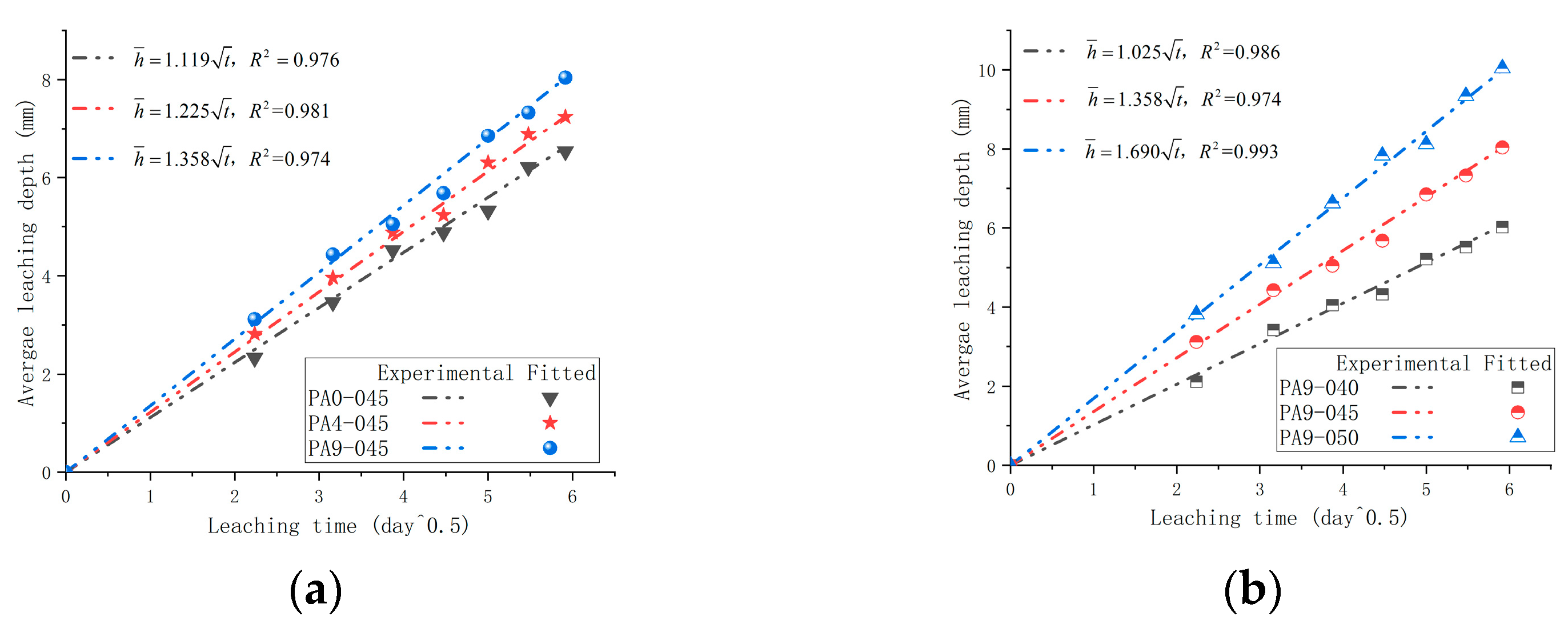
3.4. The Leaching Length
4. Discussion
5. Conclusions
- (1)
- The AS accelerator changes the pore structure of cement by altering its hydration kinetics. A cement paste containing a higher dose of the AS accelerator has a lower porosity and a smaller critical pore size in the early stage, and a higher porosity and a larger critical pore size in the later stage;
- (2)
- The hydration kinetics of AS cement pastes are not significantly affected by w/c. As the w/c decreases, the porosity decreases, and the critical pore size of the cement with the AS accelerator decreases;
- (3)
- Leaching changes the microstructure of cement. Compared with unleached ones, the porosity of leached samples increased by 93.1–170.1%. The higher the w/c, the higher the porosity of the leached samples. Despite samples containing different doses of the AS accelerator, the porosity of the leached samples with the same w/c is slightly similar;
- (4)
- It is clear that the AS accelerator affects the leaching resistance of shotcrete. As compared with unaccelerated cement samples, cement leaching resistance decreases by 9.5~21.3% when the AS accelerator dosage is 4~9%;
- (5)
- The reduction in the w/c contributes to leaching resistance. The reduction in w/c from 0.45 to 0.40 results in a 24.5% improvement in leaching resistance based on cement with a w/c of 0.45 and an AS accelerator content of 9%.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Wang, M.; Yu, L.; Guo, X.; Wang, Z.; Jiang, Y. Bond-slip behavior between corroded I-shaped steel and concrete in a subsea tunnel. Eng. Fail. Anal. 2021, 120, 105061. [Google Scholar] [CrossRef]
- Carde, C.; Franois, R.; Torrenti, J.M. Leaching of Both Calcium Hydroxide and C-S-H from Cement Paste: Modeling the Mechanical Behavior. Cem. Concr. Res. 1996, 26, 1257–1268. [Google Scholar] [CrossRef]
- Rozière, E.; Loukili, A. Performance-based assessment of concrete resistance to leaching. Cem. Concr. Compos. 2011, 33, 451–456. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Colina, H.; Torrenti, J.M.; Boulay, C.; Nedjar, B. Chemo-mechanical coupling behaviour of leached concrete: Part I: Experimental results. Nucl. Eng. Des. 2007, 237, 2083–2089. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Nedjar, B.; Torrenti, J.M. Chemo-mechanical coupling behaviour of leached concrete: Part II, Modelling. Nucl. Eng. Des. 2007, 237, 2083–2089. [Google Scholar] [CrossRef]
- Phung, Q.T.; Maes, N.; Jacques, D.; De Schutter, G.; Ye, G. Investigation of the changes in microstructure and transport properties of leached cement pastes accounting for mix composition. Cem. Concr. Res. 2016, 79, 217–234. [Google Scholar] [CrossRef]
- Jebli, M.; Jamin, F.; Pelissou, C.; Malachanne, E.; Youssou, F.I.M. Leaching effect on mechanical properties of cement-aggregate interface. Cem. Concr. Compos. 2018, 87, 10–19. [Google Scholar] [CrossRef]
- Bo, M.A.; Cz, A.; Wz, B.; Xu, Z.; Pla, D.J.T.-W.S. Influence of cracks at the invert on the mechanical behavior of the tunnel structures. Thin-Walled Struct. 2021, 161, 107405. [Google Scholar] [CrossRef]
- Rosenqvist, M.; Bertron, A.; Fridh, K.; Hassanzadeh, M. Concrete alteration due to 55years of exposure to river water: Chemical and mineralogical characterisation. Cem. Concr. Res. 2017, 92, 110–120. [Google Scholar] [CrossRef]
- Lagerblad, J.T.B. Leaching of 90-Year Old Concrete Mortar in Contact with Stagnant Water; Swedish Nuclear Fuel and WasteManagement Co: Stockholm, Sweden, 1998. [Google Scholar]
- Wang, M.; Zhang, Y.; Yu, L.; Dong, Y.; Zhou, G.J.M. Experimental Study on Bond-Slip Behavior between Corroded I-Shaped Steel and Concrete in Subsea Tunnel. Materials 2019, 12, 2863. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Yu, L.; Guo, X.; Wang, Z.; Li, C. Experimental and numerical research on the influence of steel arch frame corrosion on security of supporting system in subsea tunnel. Tunn. Undergr. Space Technol. 2022, 120, 104253. [Google Scholar] [CrossRef]
- Adenot, F. Durabilité du béton: Caractérisation des modélisation des processus physique et chimiques de dégradation du ciment. Ph.D. Thesis, Université d’Orléans, Orléans, France, 1992. [Google Scholar]
- Berner, U.R. Modelling the Incongruent Dissolution of Hydrated Cement Minerals. Radiochem. Acta 1988, 44-45, 387–394. [Google Scholar] [CrossRef]
- Phung, Q.T.; Maes, N.; Jacques, D.; Perko, J.; De Schutter, G.; Ye, G. Modelling the evolution of microstructure and transport properties of cement pastes under conditions of accelerated leaching. Constr. Build. Mater. 2016, 115, 179–192. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, C.; Zeng, Q.; Yang, C.; Luo, C.; Yang, K. Deterioration of mortars exposed to sulfate attack under electrical field. Constr. Build. Mater. 2016, 117, 121–128. [Google Scholar] [CrossRef]
- Hiroshi Saitoa, A.D. Leaching tests on different mortars using accelerated electrochemical method. Cem. Concr. Res. 2000, 30, 1815–1825. [Google Scholar] [CrossRef]
- Faucon, P.; Gerard, B.; Jacquinot, J.F.; Marchand, J. Water attack of a cement paste: Towards an improved accelerated test? Adv. Cem. Res. 1998, 10, 67–73. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, L.; Yan, Z.; Qi, P.; Yi, X. Predicting the calcium leaching behavior of cement pastes in aggressive environments. Constr. Build. Mater. 2012, 29, 88–96. [Google Scholar]
- Wan, K.; Yan, L.; Wei, S. Experimental and modelling research of the accelerated calcium leaching of cement paste in ammonium nitrate solution. Constr. Build. Mater. 2013, 40, 832–846. [Google Scholar] [CrossRef]
- Wan, K.; Li, L.; Sun, W. Solid–liquid equilibrium curve of calcium in 6 mol/L ammonium nitrate solution. Constr. Build. Mater. 2013, 53, 44–50. [Google Scholar]
- Zuo, X.-B.; Tang, Y.-J.; Yin, G.-J.; Jiang, K.; He, S.-L. Influence of Fly Ash and Its Partial Replacement by Slag on the Leaching Behavior of Blended Cement Pastes. J. Mater. Civ. Eng. 2017, 29, 04017187. [Google Scholar] [CrossRef]
- Galan, I.; Baldermann, A.; Kusterle, W.; Dietzel, M.; Mittermayr, F. Durability of shotcrete for underground support-Review and update. Constr. Build. Mater. 2019, 202, 465–493. [Google Scholar]
- Salvador, R.P.; Cavalaro, S.H.P.; Segura, I.; Figueiredo, A.D.; Pérez, J. Early age hydration of cement pastes with alkaline and alkali-free accelerators for sprayed concrete. Constr. Build. Mater. 2016, 111, 386–398. [Google Scholar] [CrossRef]
- Wang, J.; Xie, Y.; Zhong, X.; Li, L. Test and simulation of cement hydration degree for shotcrete with alkaline and alkali-free accelerators. Cem. Concr. Compos. 2020, 112, 103684. [Google Scholar] [CrossRef]
- Baquerizo, L.G.; Matschei, T.; Scrivener, K.L.; Saeidpour, M.; Wadsö, L. Hydration states of AFm cement phases. Cem. Concr. Res. 2015, 73, 143–157. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The AFm phase in Portland cement. Cem. Concr. Res. 2007, 37, 118–130. [Google Scholar] [CrossRef]
- Minard, H.; Garrault, S.; Regnaud, L.; Nonat, A. Mechanisms and parameters controlling the tricalcium aluminate reactivity in the presence of gypsum. Cem. Concr. Res. 2007, 37, 1418–1426. [Google Scholar] [CrossRef]
- Xu, Q.; Stark, J. Early hydration of ordinary Portland cement with an alkaline shotcrete accelerator. Adv. Cem. Res. 2005, 17, 1–8. [Google Scholar] [CrossRef]
- Galobardes, I. Characterization and Control of Wet Mix Sprayed Concrete with Accelerator. Ph.D. Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2013. [Google Scholar]
- Salvador, R.P.; Cavalaro, S.; Cincotto, M.A.; Figueiredo, A. Parameters controlling early age hydration of cement pastes containing accelerators for sprayed concrete. Cem. Concr. Res. 2016, 89, 230–248. [Google Scholar]
- Galobardes, I.; Cavalaro, S.H.; Aguado, A.; Garcia, T. Estimation of the modulus of elasticity for sprayed concrete. Constr. Build. Mater. 2014, 53, 48–58. [Google Scholar] [CrossRef]
- Salvador, R.P.; Cavalaro, S.H.P.; Monte, R. Relation between chemical processes and mechanical properties of sprayed cementitious matrices containing accelerators. Cem. Concr. Compos. 2017, 79, 117–132. [Google Scholar] [CrossRef]
- Garboczi, E.J.; Bentz, D.P. Modelling of the microstructure and transport properties of concrete. Constr. Build. Mater. 1996, 10, 293–300. [Google Scholar] [CrossRef]
- GB/T 35159-2017; Accelerator for Shotcrete. Chinese Standard Press: Beijing, China, 2017.
- Cabanne, I. modelisation a long terme de l’evolution des trafics voyageurs a longue distance en france. Ph.D. Thesis, Ecole Normale Superieure de Cachan, Paris, France, 1999. [Google Scholar]
- Zhang, J.; Scherer, G.W. Comparison of methods for arresting hydration of cement. Cem. Concr. Res. 2011, 41, 1024–1036. [Google Scholar]
- Cline, J.P.; Dreele, R.B.V.; Winburn, R.; Stephens, P.W. Addressing the amorphous content issue in quantitative phase analysis: The certification of NIST standard reference material 676a. Powder Diffr. 2009, 24, 172. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials || Glossary; Crc Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Ouzia, A.; Scrivener, K. The needle model: A new model for the main hydration peak of alite. Cem. Concr. Res. 2019, 115, 339–360. [Google Scholar] [CrossRef]
- Juilland, P. Early Hydration of Cementitious Systems. Ph.D. Thesis, École Polytechnique Fédérale De Lausanne, Lausanne, Suisse, 2009. [Google Scholar]
- Hatakeyama, A.; Nawa, T. Study On Interactions Between Alite-C3A-Gypsum Hydrations. Cem. Sci. Concr. Technol. 2014, 68, 82–88. [Google Scholar]
- Quennoz, A.; Scrivener, K.L. Interactions between alite and C(3)A-gypsum hydrations in model cements. Cem. Concr. Res. 2013, 44, 46–54. [Google Scholar]
- Scrivener, K.L.; Nonat, A. Hydration of cementitious materials, present and future. Cem. Concr. Res. 2011, 41, 651–665. [Google Scholar]
- Kocaba, V.; Gallucci, E.; Scrivener, K.L. Methods for determination of degree of reaction of slag in blended cement pastes. Cem. Concr. Res. 2012, 42, 511–525. [Google Scholar]
- Lothenbach, B.; Saout, G.L.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 339–356. [Google Scholar]
- Liu, X.; Ma, B.; Tan, H.; Gu, B.; Zhang, T. Effect of aluminum sulfate on the hydration of Portland cement, tricalcium silicate and tricalcium aluminate. Constr. Build. Mater. 2020, 232, 117179. [Google Scholar]
- Bergold, S.T.; Goetz-Neunhoeffer, F.; Neubauer, J. Interaction of silicate and aluminate reaction in a synthetic cement system: Implications for the process of alite hydration. Cem. Concr. Res. 2017, 93, 32–44. [Google Scholar] [CrossRef]
- Park, H.-G.; Sung, S.-K.; Park, C.-G.; Won, J.-P. Influence of a C12A7 mineral-based accelerator on the strength and durability of shotcrete. Cem. Concr. Res. 2008, 38, 379–385. [Google Scholar] [CrossRef]
- Faucon, P.; Adenot, F.; Jorda, M.; Cabrillac, R. Behaviour of crystallised phases of Portland cement upon water attack. Mater. Struct. 1997, 30, 480–485. [Google Scholar]
- Faucon, P.; Adenot, F.; Jacquinot, J.F.; Petit, J.C.; Jorda, M. Long-term behaviour of cement pastes used for nuclear waste disposal: Review of physico-chemical mechanisms of water degradation. Cem. Concr. Res. 1998, 28, 847–857. [Google Scholar] [CrossRef]
- Puertas, F.; Goñi, S.; Hernández, M.S.; Varga, C.; Guerrero, A. Comparative study of accelerated decalcification process among C3S, grey and white cement pastes. Cem. Concr. Compos. 2012, 34, 384–391. [Google Scholar] [CrossRef]
- Constantinides, G.; Ulm, F.J. The effect of two types of C-S-H on the elasticity of cement-based materials: Results from nanoindentation and micromechanical modeling. Cem. Concr. Res. 2004, 34, 67–80. [Google Scholar]
- Han, X.; Wang, B.; Feng, J. Relationship between fractal feature and compressive strength of concrete based on MIP. Constr. Build. Mater. 2022, 322, 126504. [Google Scholar]
- Choi, Y.C.; Kim, J.; Choi, S. Mercury intrusion porosimetry characterization of micropore structures of high-strength cement pastes incorporating high volume ground granulated blast-furnace slag. Constr. Build. Mater. 2017, 137, 96–103. [Google Scholar]
- Hidalgo, A.; Petit, S.; Domingo, C.; Alonso, C.; Andrade, C. Microstructural characterization of leaching effects in cement pastes due to neutralisation of their alkaline nature. Cem. Concr. Res. 2007, 37, 63–70. [Google Scholar] [CrossRef]
- Pichler, C.; Saxer, A.; Lackner, R. Differential-scheme based dissolution/diffusion model for calcium leaching in cement-based materials accounting for mix design and binder composition. Cem. Concr. Res. 2012, 42, 686–699. [Google Scholar] [CrossRef]
- Berra, M.; Carassiti, F.; Mangialardi, T.; Paolini, A.E.; Sebastiani, M. Leaching behaviour of cement pastes containing nanosilica. Adv. Cem. Res. 2013, 25, 352–361. [Google Scholar] [CrossRef]
- Garg, A.; Aggarwal, P.; Aggarwal, Y.; Belarbi, M.O.; Chalak, H.D.; Tounsi, A.; Gulia, R. Machine learning models for predicting the compressive strength of concrete containing nano silica. Comput. Concr. 2022, 30, 33–42. [Google Scholar]
- Bourada, F.; Bousahla, A.A.; Tounsi, A.; Bedia, E.A.A.; Mahmoud, S.R.; Benrahou, K.H.; Tounsi, A. Stability and dynamic analyses of SW-CNT reinforced concrete beam resting on elastic-foundation. Comput. Concr. 2020, 25, 485–495. [Google Scholar] [CrossRef]
- Li, Z.Q.; Li, Z.; Huang, W.W.; Zhang, H.R.; Zhang, H. Fatigue damage analysis of ballastless slab track in heavy-haul railway tunnels. Undergr. Space 2022, 7, 440–452. [Google Scholar] [CrossRef]







| Composition | CaO | SiO2 | Al2O3 | Fe2O3 | Na2O | MgO | K2O | Ignition Loss |
|---|---|---|---|---|---|---|---|---|
| Content (wt%) | 63.21 | 20.35 | 4.52 | 3.75 | 0.06 | 1.78 | 0.74 | 1.67 |
| Clinker Mineral | C3S | C2S | C3A | C4AF |
|---|---|---|---|---|
| Content (wt%) | 57.11 | 14.32 | 5.02 | 11.28 |
| Composition | Al2O3 | SO42− | Na2O | pH (20 ℃) | Solid Content | Density (g/cm3) |
|---|---|---|---|---|---|---|
| Content (wt%) | 13.6 | 25.6 | - | 2.8 | 52.5 | 1.39 |
| Name | AP0-045 | AP4-045 | AP9-045 | AP9-040 | AP9-050 | |
|---|---|---|---|---|---|---|
| Cement | (g) | 100 | 100 | 100 | 100 | 100 |
| Deionized water | (g) | 45 | 45 | 45 | 40 | 50 |
| AS accelerator | (% bcw) | 0 | 4 | 9 | 9 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, X.; Wu, Q.; Yang, J.; Wang, W.; Liu, D.; Zhang, Y. Experiment Study on the Effect of Aluminum Sulfate-Based Alkali-Free Accelerator and the w/c on Cement Hydration and Leaching. Materials 2023, 16, 2165. https://doi.org/10.3390/ma16062165
Ling X, Wu Q, Yang J, Wang W, Liu D, Zhang Y. Experiment Study on the Effect of Aluminum Sulfate-Based Alkali-Free Accelerator and the w/c on Cement Hydration and Leaching. Materials. 2023; 16(6):2165. https://doi.org/10.3390/ma16062165
Chicago/Turabian StyleLing, Xuepeng, Quande Wu, Jie Yang, Wenzheng Wang, Dagang Liu, and Yiteng Zhang. 2023. "Experiment Study on the Effect of Aluminum Sulfate-Based Alkali-Free Accelerator and the w/c on Cement Hydration and Leaching" Materials 16, no. 6: 2165. https://doi.org/10.3390/ma16062165
APA StyleLing, X., Wu, Q., Yang, J., Wang, W., Liu, D., & Zhang, Y. (2023). Experiment Study on the Effect of Aluminum Sulfate-Based Alkali-Free Accelerator and the w/c on Cement Hydration and Leaching. Materials, 16(6), 2165. https://doi.org/10.3390/ma16062165





