Microstructures and Enhanced Mechanical Properties of (Zr, Ti)(C, N)-Based Nanocomposites Fabricated by Reactive Hot-Pressing at Low Temperature
Abstract
1. Introduction
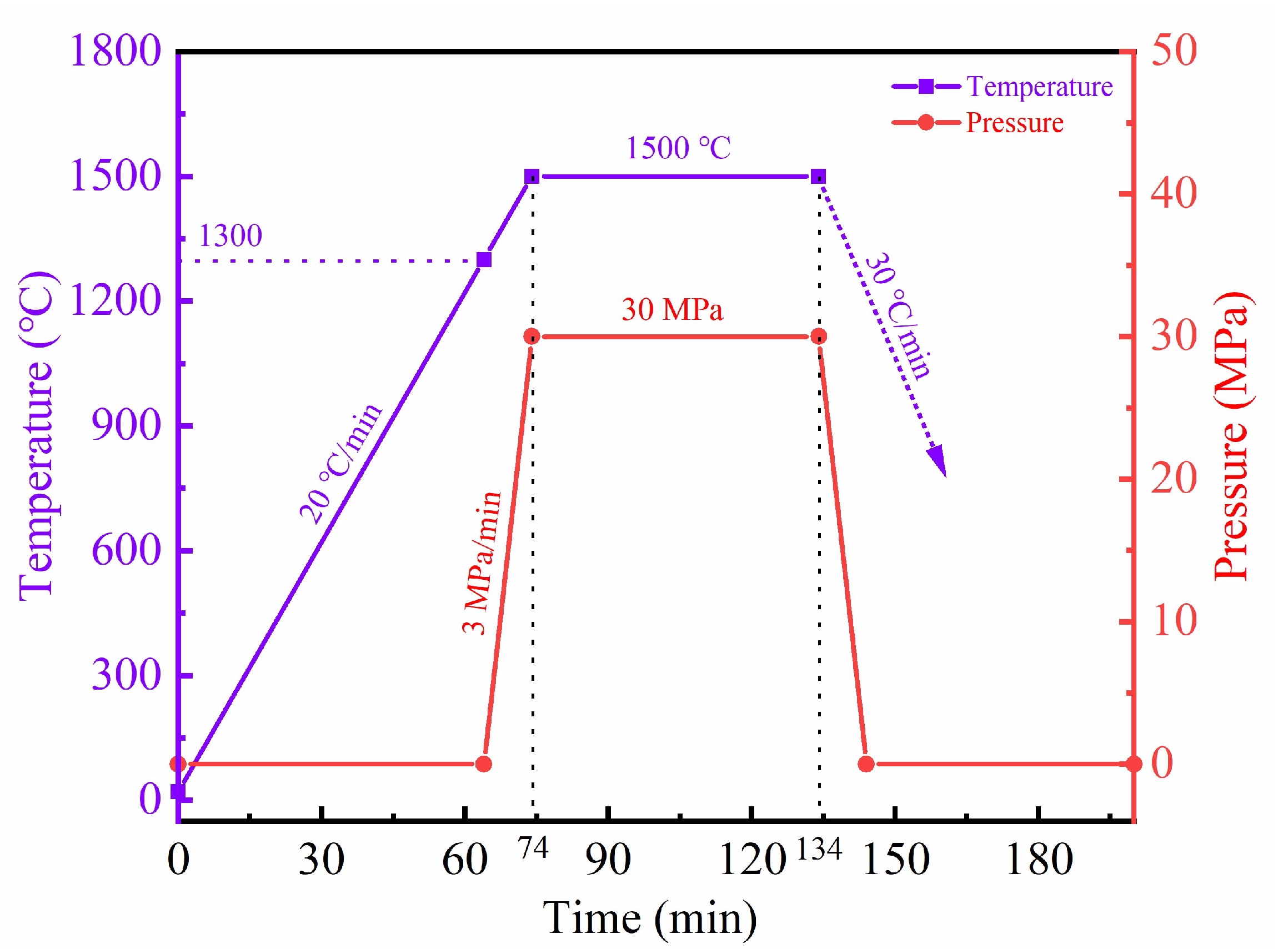
2. Materials and Methods
3. Results and Discussion
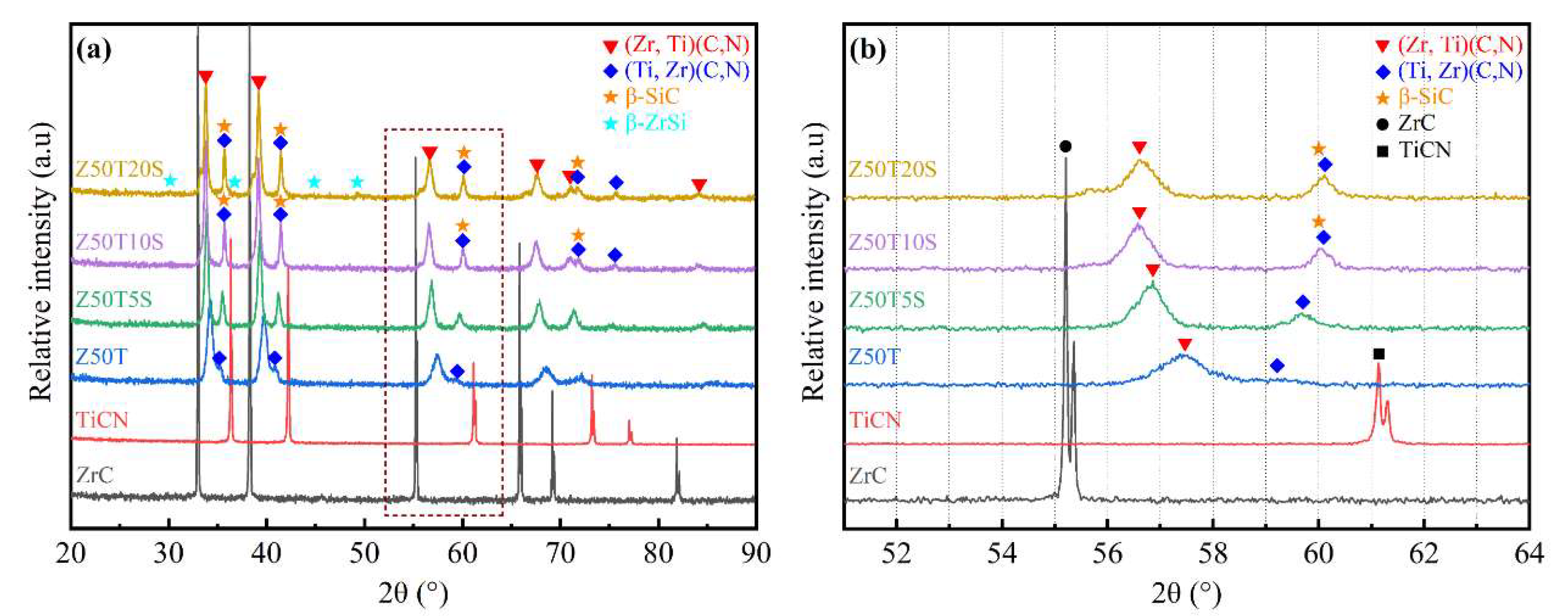
3.1. Phase Constitutions
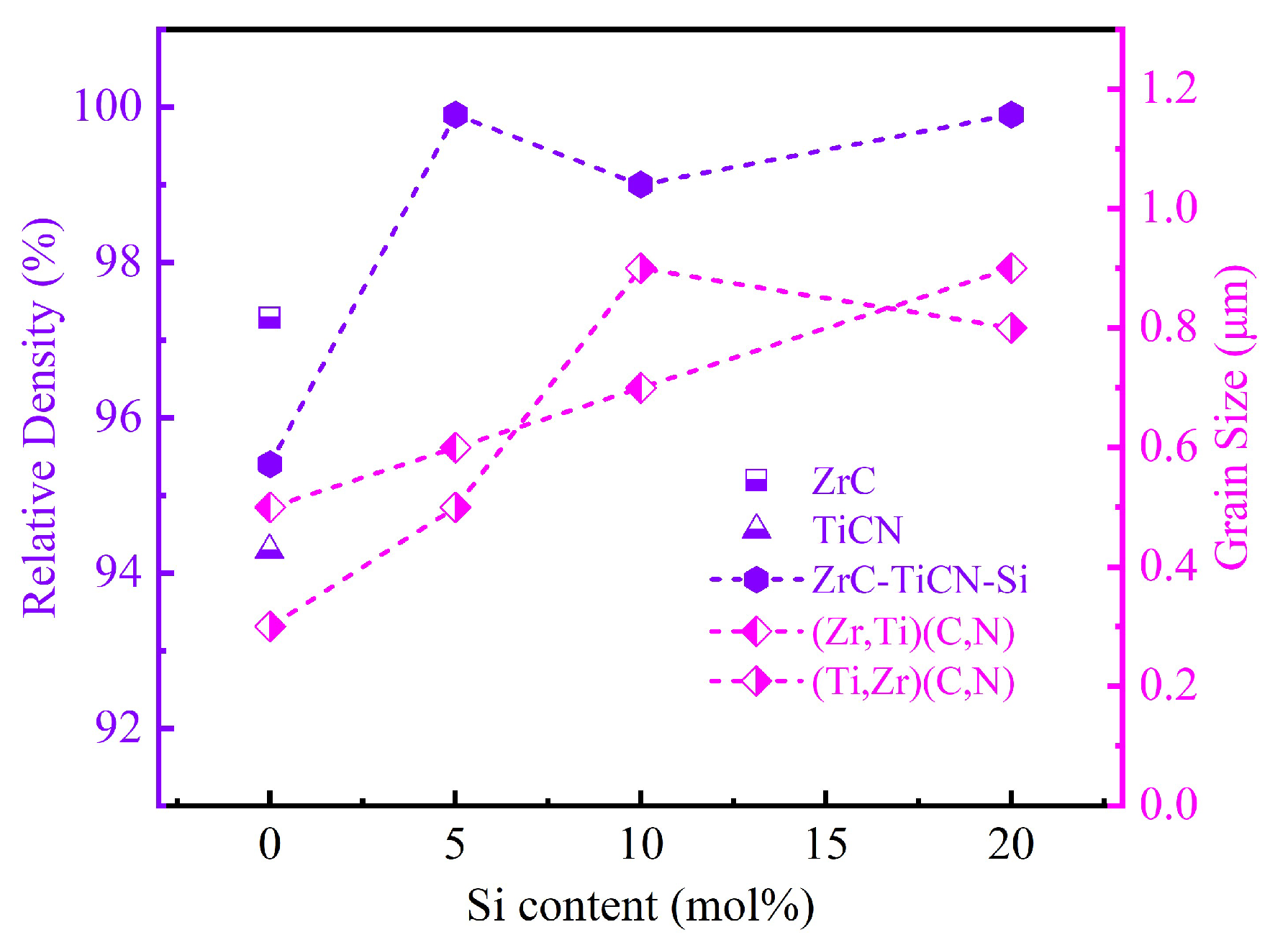
3.2. Relative Densities
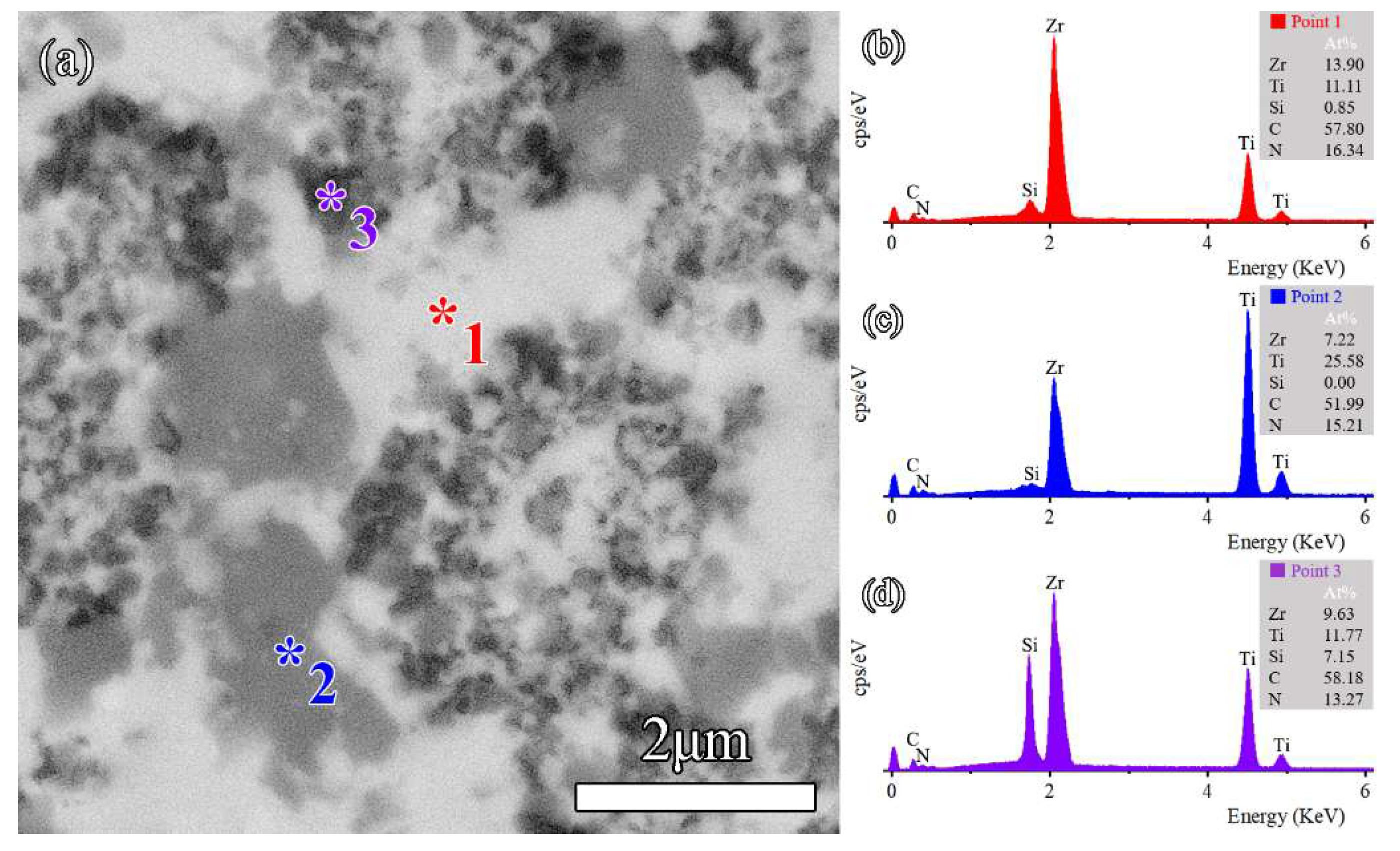
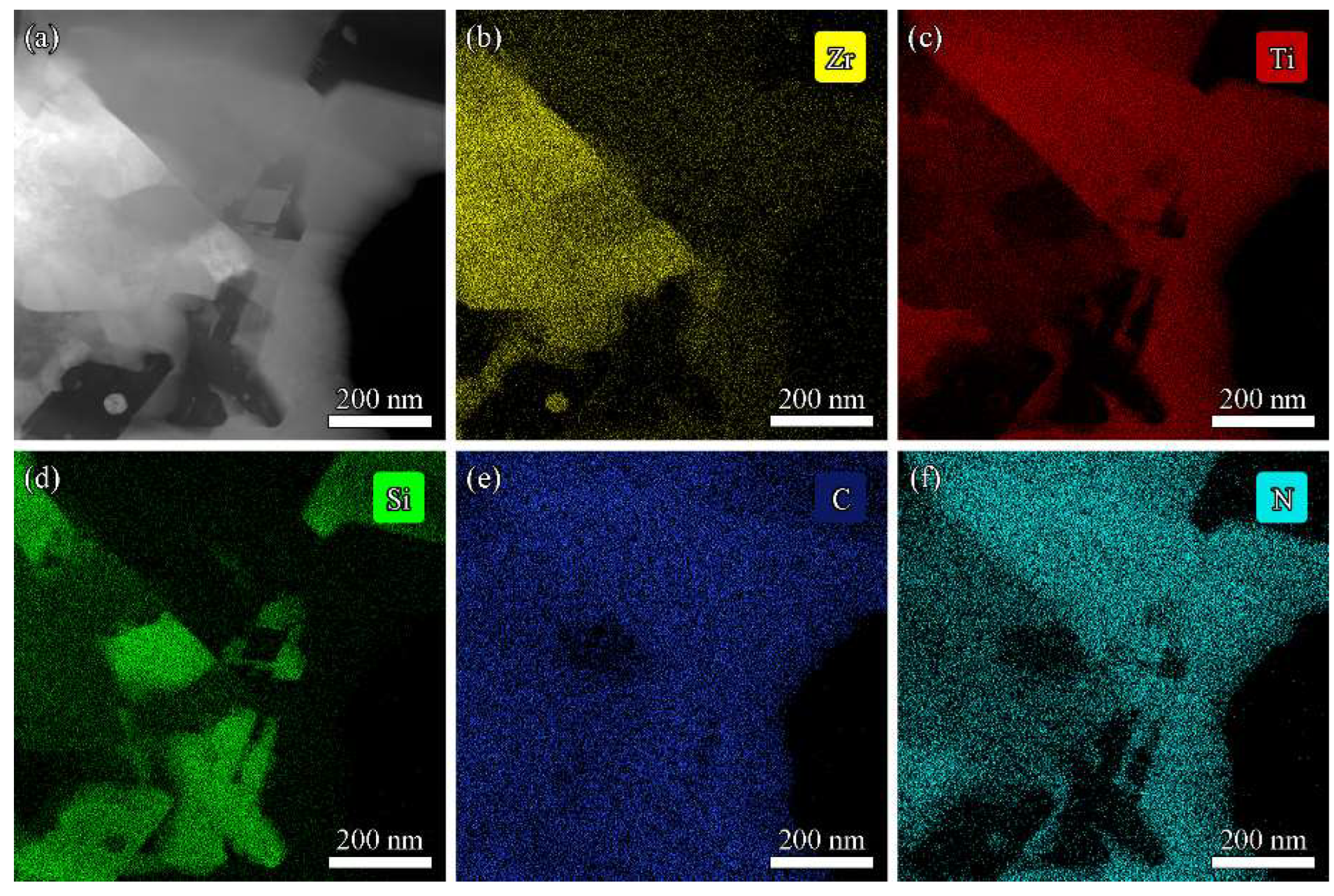
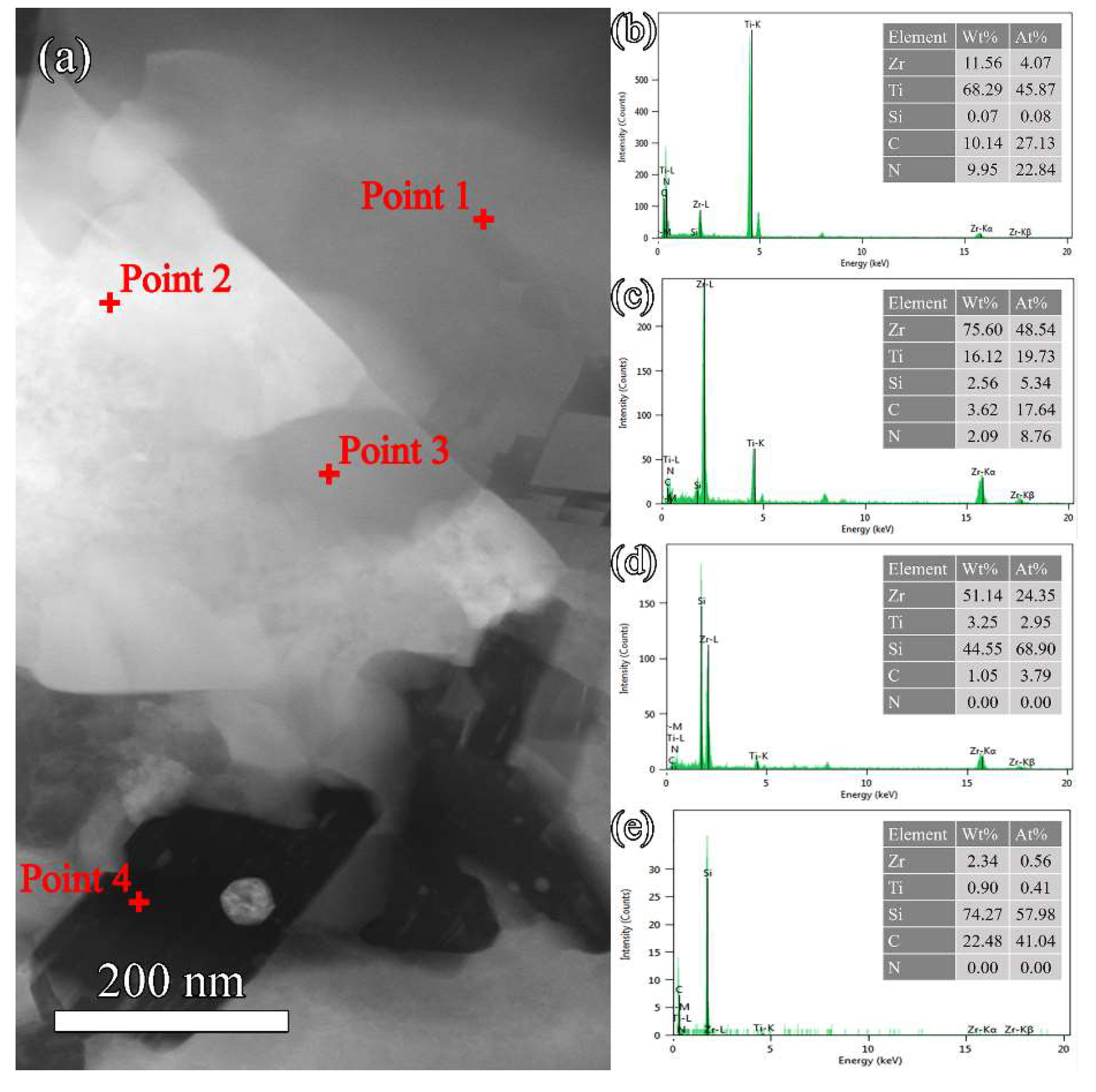
3.3. Microstructure
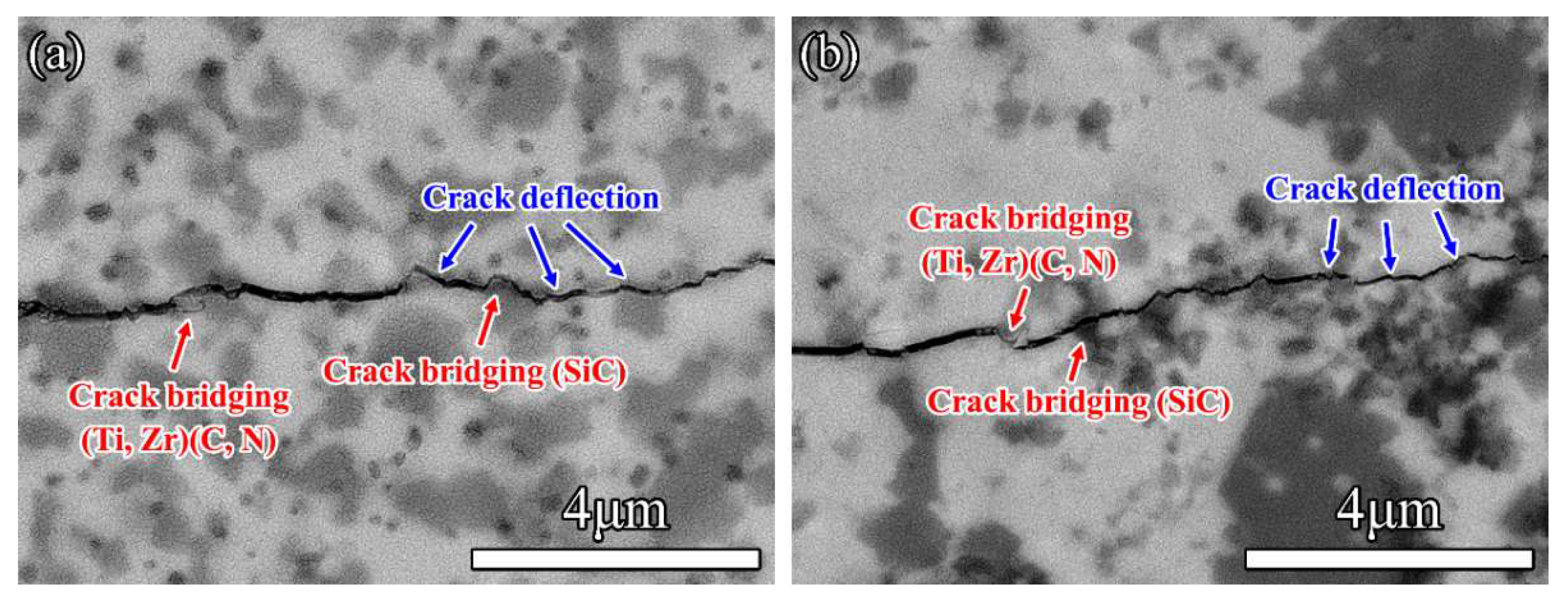
3.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silvestroni, L.; Sciti, D.; Kling, J.; Lauterbach, S.; Kleebe, H.-J. Sintering Mechanisms of Zirconium and Hafnium Carbides Doped with MoSi2. J. Am. Ceram. Soc. 2009, 92, 1574–1579. [Google Scholar] [CrossRef]
- Song, G.-M.; Wang, Y.-J.; Zhou, Y. The mechanical and thermophysical properties of ZrC/W composites at elevated temperature. Mater. Sci. Eng. A 2002, 334, 223–232. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Hilmas, G.E. Ultra-high temperature ceramics: Materials for extreme environments. Scr. Mater. 2017, 129, 94–99. [Google Scholar] [CrossRef]
- Katoh, Y.; Vasudevamurthy, G.; Nozawa, T.; Snead, L.L. Properties of zirconium carbide for nuclear fuel applications. J. Nucl. Mater 2013, 441, 718–742. [Google Scholar] [CrossRef]
- Lipke, D.W.; Zhang, Y.S.; Liu, Y.J.; Church, B.C.; Sandhage, K.H. Near net-shape/net-dimension ZrC/W-based composites with complex geometries via rapid prototyping and Displacive Compensation of Porosity. J. Eur. Ceram. Soc. 2010, 30, 2265–2277. [Google Scholar] [CrossRef]
- Wang, Y.G.; Liu, Q.M.; Liu, J.; Zhang, L.T.; Cheng, L.F. Deposition Mechanism for Chemical Vapor Deposition of Zirconium Carbide Coatings. J. Am. Ceram. Soc. 2008, 91, 1249–1252. [Google Scholar] [CrossRef]
- Wei, B.; Wang, D.; Wang, Y.; Zhang, H.; Peng, S.; Xu, C.; Song, G.; Zhou, Y. Corrosion kinetics and mechanisms of ZrC(1-x) ceramics in high temperature water vapor. RSC Adv. 2018, 8, 18163–18174. [Google Scholar] [CrossRef]
- Vasudevamurthy, G.; Knight, T.W.; Roberts, E.; Adams, T.M. Laboratory production of zirconium carbide compacts for use in inert matrix fuels. J. Nucl. Mater 2008, 374, 241–247. [Google Scholar] [CrossRef]
- Ushakov, S.V.; Navrotsky, A.; Hong, Q.J.; van de Walle, A. Carbides and Nitrides of Zirconium and Hafnium. Materials 2019, 12, 2728. [Google Scholar] [CrossRef]
- Zhang, M.; Wei, B.; Wang, D.; Fang, W.; Chen, L.; Wang, Y. Novel (Zr, Ti)(C, N)–SiC ceramics via reactive hot-pressing at low temperature. Ceram. Int. 2022, 48, 29641–29651. [Google Scholar] [CrossRef]
- Li, Y.; Katsui, H.; Goto, T. Phase decomposition of (Ti, Zr)(C, N) solid solutions prepared by spark plasma sintering. J. Eur. Ceram. Soc. 2019, 39, 4588–4594. [Google Scholar] [CrossRef]
- Li, Y.; Katsui, H.; Goto, T. Preparation of ZrCN−TiCN solid solutions by spark plasma sintering. Ceram. Int. 2017, 43, 16965–16971. [Google Scholar] [CrossRef]
- Liang, L.Q.; Wei, B.X.; Wang, D.; Fang, W.B.; Chen, L.; Wang, Y.J. Densification, microstructures, and mechanical properties of (Zr, Ti)(C, N) ceramics fabricated by spark plasma sintering. J. Eur. Ceram. Soc. 2022, 42, 6445–6456. [Google Scholar] [CrossRef]
- Larijani, M.M.; Zanjanbar, M.B.; Majdabadi, A. The effect of carbon fraction in Zr(C, N) films on the nano-structural properties and hardness. J. Alloy. Compd. 2010, 492, 735–738. [Google Scholar] [CrossRef]
- Huo, S.; Wang, Y.; Yao, M.; Chen, L.; Kong, Q.; Ouyang, J.; Fu, Y.; Zhou, Y. Reactive sintering behavior and enhanced densification of (Ti,Zr)B2–(Zr,Ti)C composites. J. Eur. Ceram. Soc. 2020, 40, 4373–4380. [Google Scholar] [CrossRef]
- Niu, B.; Zhang, F.; Ji, W.; Zhang, J.Y.; Fu, Z.Y.; Wang, W.M. Effect of solid solution formation on densification of spark plasma sintered ZrC ceramics with TiC as sintering aid. Adv. Appl. Ceram. 2016, 115, 55–59. [Google Scholar] [CrossRef]
- Li, Y.; Katsui, H.; Goto, T. Spark plasma sintering of TiC–ZrC composites. Ceram. Int. 2015, 41, 7103–7108. [Google Scholar] [CrossRef]
- Kang, S. Stability of N in Ti(CN) Solid Solutions for Cermet Applications. Powder Metall. 2013, 40, 139–142. [Google Scholar] [CrossRef]
- Chamberlain, A.L.; Fahrenholtz, W.G.; Hilmas, G.E. Low-temperature densification of zirconium diboride ceramics by reactive hot pressing. J. Am. Ceram. Soc. 2006, 89, 3638–3645. [Google Scholar] [CrossRef]
- Wei, B.; Chen, L.; Wang, Y.; Zhang, H.; Peng, S.; Ouyang, J.; Wang, D.; Zhou, Y. Densification, mechanical and thermal properties of ZrC1−x ceramics fabricated by two-step reactive hot pressing of ZrC and ZrH2 powders. J. Eur. Ceram. Soc. 2018, 38, 411–419. [Google Scholar] [CrossRef]
- Amiri, S.H.; Kakroudi, M.G.; Vafa, N.P.; Asl, M.S. Synthesis and Sintering of Ti3SiC2–SiC Composites through Reactive Hot-Pressing of TiC and Si Precursors. Silicon 2021, 14, 4227–4235. [Google Scholar] [CrossRef]
- Wang, X.G.; Zhang, G.J.; Xue, J.X.; Tang, Y.; Huang, X.; Xu, C.M.; Wang, P.L. Reactive Hot Pressing of ZrC-SiC Ceramics at Low Temperature. J. Am. Ceram. Soc. 2013, 96, 32–36. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Liu, H.; Zhou, Y.B.; Sha, W.H.; Huang, Y.H.; Huang, Z.R. Enhancement mechanical properties of in-situ preparated B4C-based composites with small amount of (Ti3SiC2+Si). Ceram. Int. 2022, 48, 12006–12013. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, J.; Huang, Y.; Chen, J.; Liu, X.; Huang, Z. Harmonized toughening and strengthening in pressurelessly reactive-sintered Ta0.8Hf0.2C-SiC composite. J. Eur. Ceram. Soc. 2018, 38, 5610–5614. [Google Scholar] [CrossRef]
- Kong, F.L.; Dong, F.H.; Duan, M.J.; Habibi, M.; Safarpour, H.; Tounsi, A. On the vibrations of the Electrorheological sandwich disk with composite face sheets considering pre and post-yield regions. Thin-Walled Struct. 2022, 179, 109631. [Google Scholar] [CrossRef]
- Heidari, F.; Taheri, K.; Sheybani, M.; Janghorban, M.; Tounsi, A. On the mechanics of nanocomposites reinforced by wavy/defected/aggregated nanotubes. Steel Compos. Struct. 2021, 38, 533–545. [Google Scholar]
- Nassira, D.; Abdelmoumen Anis, B.; Abdelhakim, K.; Mahmoud, M.S.; Fouad, B.; Abdeldjebbar, T.; Abdelouahed, T.; Kouider Halim, B.; Mahmoud, S.R. Large cylindrical deflection analysis of FG carbon nanotube-reinforced plates in thermal environment using a simple integral HSDT. Steel Compos. Struct. Int. J. 2022, 42, 779–789. [Google Scholar]
- Evans, A.G.; Charles, E.A. Fracture Toughness Determinations by Indentation. J. Am. Ceram. Soc. 1976, 59, 371–372. [Google Scholar] [CrossRef]
- Pogrebnjak, A.; Ivashchenko, V.; Maksakova, O.; Buranich, V.; Konarski, P.; Bondariev, V.; Zukowski, P.; Skrynskyy, P.; Sinelnichenko, A.; Shelest, I.; et al. Comparative measurements and analysis of the mechanical and electrical properties of Ti-Zr-C nanocomposite: Role of stoichiometry. Measurement 2021, 176, 109223. [Google Scholar] [CrossRef]
- Okamoto, H. The Si-Zr (Silicon-Zirconium) system. J. Phase Equilib 1990, 11, 513–519. [Google Scholar] [CrossRef]
- Madelung, O. Phase Equilibria, Crystallographic and Thermodynamic Data of Binary Alloys Pu-Re-Zn-Zr. Landolt-B Örnstein—Group IV Phys. Chem. 1998, 5J, 1–3. [Google Scholar]
- Acicbe, R.B.; Goller, G. Densification behavior and mechanical properties of spark plasma-sintered ZrC–TiC and ZrC–TiC–CNT composites. J. Mater. Sci. 2012, 48, 2388–2393. [Google Scholar] [CrossRef]
- Feng, L.; Fahrenholtz, W.G.; Hilmas, G.E.; Watts, J.; Zhou, Y. Densification, microstructure, and mechanical properties of ZrC–SiC ceramics. J. Am. Ceram. Soc. 2019, 102, 5786–5795. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.J.; Li, Y.P.; Zhang, X.H.; Meng, Q.C. Microstructural evolution, mechanical and thermal properties of TiC-ZrC-Cr3C2composites. Int. J. Refract. Met. H 2019, 80, 188–194. [Google Scholar] [CrossRef]
- Zhong, L.B.; Liu, L.M.; Worsch, C.; Gonzalez, J.; Springer, A.; Ye, F. Transient liquid phase sintering of tantalum carbide ceramics by using silicon as the sintering aid and its effects on microstructure and mechanical properties. Mater Chem. Phys. 2015, 149, 505–511. [Google Scholar] [CrossRef]
- Kong, Q.Y.; Huo, S.J.; Chen, L.; Wang, Y.J.; Ouyang, J.H.; Zhou, Y. Novel (Zr, Ti)B2-(Zr, Ti)C-SiC ceramics via reactive hot pressing. J. Eur. Ceram. Soc. 2022, 42, 4045–4052. [Google Scholar] [CrossRef]
- Liu, Q.; Han, W.; Han, J. Influence of SiCnp content on the microstructure and mechanical properties of ZrB2–SiC nanocomposite. Scr. Mater. 2010, 63, 581–584. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, Y.; Chen, L.; Zhou, Y. Microstructure, mechanical properties and thermal conductivity of (Ti0.5Nb0.5)C–SiC composites. Ceram. Int. 2022, 48, 6745–6749. [Google Scholar] [CrossRef]
- Ma, B.X.; Zhang, X.H.; Han, J.C.; Han, W.B. Microstructure and Mechanical Properties of ZrC-SiC-C-g Ceramic Prepared by Hot Pressing. Rare Met. Mat. Eng. 2009, 38, 890–893. [Google Scholar]
- Feng, L.; Fahrenholtz, W.G.; Hilmas, G.E. Effect of ZrB2 content on the densification, microstructure, and mechanical properties of ZrC-SiC ceramics. J. Eur. Ceram. Soc. 2020, 40, 220–225. [Google Scholar] [CrossRef]
- Zhao, L.; Jia, D.; Duan, X.; Yang, Z.; Zhou, Y. Low temperature sintering of ZrC–SiC composite. J. Alloy. Compd. 2011, 509, 9816–9820. [Google Scholar] [CrossRef]
- Moshtaghioun, B.M.; Morgado Chávez, J.M.; Cumbrera, F.L.; Gómez García, D. Titanium carbonitride fabricated by spark plasma sintering: Is it a ceramic model of carbon-induced Friedel-Fleisher strengthening effect? J. Eur. Ceram. Soc. 2021, 41, 6275–6280. [Google Scholar] [CrossRef]
- Braic, V.; Braic, M.; Balaceanu, M.; Vladescu, A.; Zoita, C.N.; Titorencu, I.; Jinga, V. (Zr,Ti)CN coatings as potential candidates for biomedical applications. Surf. Coat. Tech. 2011, 206, 604–609. [Google Scholar] [CrossRef]
- Martienssen, W.; Warlimont, H. Springer Handbook of Condensed Matter and Materials Data; Springer Berlin.Heidelberg: Berlin, Germany, 2005; pp. 431–476. [Google Scholar]
- Wang, K.; Xu, G.P.; Jiang, H.Y.; Wang, Q.D.; Ding, W.J. Effects of TiC0.5N0.5 nanoparticles on the microstructure, mechanical and thermal properties of TiC0.5N0.5/Al-Cu nanocomposites. J. Mater. Res. Technol. 2020, 9, 2044–2053. [Google Scholar] [CrossRef]
- Xu, C.H. Effects of particle size and matrix grain size and volume fraction of particles on the toughening of ceramic composite by thermal residual stress. Ceram. Int. 2005, 31, 537–542. [Google Scholar] [CrossRef]
- Chai, J.L.; Zhu, Y.B.; Gao, X.; Shen, T.L.; Niu, L.J.; Li, S.F.; Jin, P.; Cui, M.H.; Wang, Z.G. Effects of residual stress and intragranular particles on mechanical properties of hot-pressed Al2O3/SiC ceramic composites. Ceram. Int. 2022, 48, 23258–23265. [Google Scholar] [CrossRef]



| Code | Compositions of Composites | Sintering Parameters | |||
|---|---|---|---|---|---|
| ZrC:TiCN | ZrC (mol%) | TiCN (mol%) | Si (mol%) | ||
| ZrC | 100:0 | 100 | - | - | 2100 °C/30 MPa/1 h |
| TiCN | 0:100 | - | 100 | - | 2100 °C/30 MPa/1 h |
| Z50T | 50:50 | 50 | 50 | - | 2000 °C/30 MPa/1 h |
| Z50T5S | 50:50 | 47.5 | 47.5 | 5 | 1700 °C/30 MPa/1 h |
| Z50T10S | 50:50 | 45 | 45 | 10 | 1500 °C/30 MPa/1 h |
| Z50T20S | 50:50 | 40 | 40 | 20 | 1500 °C/30 MPa/1 h |
| Composition | 2θ (°) of (220) | 2θ (°) of ZrSi (002) | Lattice Parameters (nm) | ||
|---|---|---|---|---|---|
| ZrC/ (Zr,Ti)(C,N) | TiCN/ (Ti,Zr)(C,N) | ZrC/ (Zr,Ti)(C,N) | TiCN/ (Ti,Zr)(C,N) | ||
| ZrC (PDF # 00-035-0784) | 55.3 | - | - | 0.4693 | - |
| TiC0.51N0.49 (PDF # 04-006-0748) | - | 61.2 | - | - | 0.4278 |
| β-ZrSi (PDF # 04-004-7165) | - | - | 48.5 | - | - |
| ZrC | 55.3 | - | - | 0.4696 | - |
| TiCN | - | 61.2 | - | - | 0.4275 |
| Z50T | 57.5 | 59.1 | - | 0.4525 | 0.4418 |
| Z50T5S | 57.0 | 59.9 | - | 0.4561 | 0.4356 |
| Z50T10S | 56.7 | 60.2 | - | 0.4587 | 0.4350 |
| Z50T20S | 56.7 | 60.2 | 49.5 | 0.4589 | 0.4346 |
| Sample | Relative Density (%) | Grain Size (μm) | Phase Content by Image Analysis (vol%) | |||||
|---|---|---|---|---|---|---|---|---|
| ZrC/ (Zr, Ti)(C, N) | TiCN/ (Ti, Zr)(C, N) | SiC | ZrC/ (Zr, Ti)(C, N) | TiCN/ (Ti, Zr)(C, N) | SiC | ZrSi | ||
| ZrC | 97.3 | 1.3 ± 0.2 | - | - | 100 | - | - | - |
| TiCN | 94.3 | - | 24.2 ± 5.7 | - | - | 100 | - | - |
| Z50T | 95.4 | 0.5 ± 0.1 | 0.3 ± 0.1 | - | 88.1 | 11.9 | - | - |
| Z50T5S | 99.9 | 0.6 ± 0.2 | 0.5 ± 0.1 | 0.3 ± 0.1 | 64.6 | 31.2 | 4.2 | - |
| Z50T10S | 99.0 | 0.7 ± 0.2 | 0.9 ± 0.3 | 0.4 ± 0.1 | 57.1 | - | 9.8 | trace |
| Z50T20S | 99.9 | 0.9 ± 0.2 | 0.8 ± 0.1 | 0.3 ± 0.1 | 54.7 | - | 12.5 | - |
| Sample | Processing Parameters | Relative Density (%) | Grain Size (μm) | Flexural Strength (MPa) | Vickers Hardness (GPa) | Fracture Toughness (MPa·m1/2) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| ZrC/ (Zr,Ti)(C,N) | TiCN/ (Ti,Zr)(C,N) | SiC | |||||||
| ZrC | HP/2200 °C/1 h/30 MPa | 98.2 | ~40 | - | - | 210 ± 19 | 16.4 ± 1.3 | 3.1 ± 0.7 | [34] |
| ZrC-10 vol% SiC | HP/1900 °C/1 h/32 MPa | 98.2 | 1.4 ± 0.6 | - | 0.5 ± 0.2 | ~405 | 19.7 ± 0.6 | 2.9 ± 0.1 | [33] |
| ZrC-20 vol% SiC | HP/1900 °C/1 h/30 MPa | 95 | ~10 | - | ~4 | ~450 | ~19.6 | ~3.9 | [39] |
| ZrC-20 vol% SiC | HP/1900 °C/1 h/32 MPa | 98.3 | 1.0 ± 0.5 | - | 0.5 ± 0.2 | ~427 | ~21.2 | 2.9 ± 0.1 | [33] |
| ZrC-30 vol% SiC | HP/1900 °C/1 h/32 MPa | 99.3 | 1.5 ± 0.6 | - | 0.8 ± 0.5 | - | 23.0 ± 0.5 | 2.6 ± 0.2 | [40] |
| ZrC-30 vol% SiC | SPS/1800 °C/5 min/45 MPa | 96.1 | <1.0 | - | ~0.5 | 523 ± 20 | 18.8 ± 1.2 | 4.0 ± 0.3 | [41] |
| TiCN | SPS/1800 °C/5 min/75 MPa | 98.6 | - | 3.4 ± 1.5 | - | - | 21.8 ± 1.3 | 2.5 ± 0.3 | [42] |
| ZrC-90 mol% TiCN | SPS/2000 °C/5 min/50 MPa | 98.0 | - | - | - | - | 20.3 | 2.7 | [12] |
| ZrC-80 mol% TiCN | 99.0 | - | - | - | - | 22.2 | 2.0 | ||
| ZrC-5 mol% TiCN | SPS/2000 °C/10 min/40 MPa | 97.4 | 1.1 ± 0.2 | - | - | 378 ± 18 | 16.4 ± 2.0 | 2.3 ± 0.3 | [13] |
| ZrC-20 mol% TiCN | 97.8 | 1.3 ± 0.4 | - | - | 368 ± 43 | 20.0 ± 0.8 | 2.5 ± 0.2 | ||
| ZrC-50 mol% TiCN | 96.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | - | 305 ± 41 | 22.5 ± 1.0 | 3.4 ± 0.3 | ||
| ZrC | HP/2100 °C/30 MPa/1 h | 97.3 | 1.3 ± 0.2 | - | - | 312 ± 35 | 16.7 ± 1.0 | 2.8 ± 0.3 | This work |
| TiCN | HP/2100 °C/30 MPa/1 h | 94.3 | - | 24.2 ± 5.7 | - | 280 ± 31 | 21.0 ± 1.8 | 2.6 ± 0.5 | |
| Z50T | HP/2000 °C/30 MPa/1 h | 95.4 | 0.5 ± 0.1 | 0.3 ± 0.1 | - | 297 ± 49 | 26.9 ± 0.7 | 3.3 ± 0.1 | |
| Z50T5S | HP/1700 °C/30 MPa/1 h | 99.9 | 0.6 ± 0.2 | 0.5 ± 0.1 | 0.3 ± 0.1 | 508 ± 33 | 24.5 ± 0.7 | 3.8 ± 0.1 | |
| Z50T10S | HP/1500 °C/30 MPa/1 h | 99.0 | 0.7 ± 0.2 | 0.9 ± 0.3 | 0.4 ± 0.1 | 435 ± 28 | 22.4 ± 0.5 | 4.0 ± 0.3 | |
| Z50T20S | HP/1500 °C/30 MPa/1 h | 99.9 | 0.9 ± 0.2 | 0.8 ± 0.1 | 0.3 ± 0.1 | 330 ± 17 | 21.7 ± 0.7 | 4.4 ± 0.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Wei, B.; Liang, L.; Fang, W.; Chen, L.; Wang, Y. Microstructures and Enhanced Mechanical Properties of (Zr, Ti)(C, N)-Based Nanocomposites Fabricated by Reactive Hot-Pressing at Low Temperature. Materials 2023, 16, 2145. https://doi.org/10.3390/ma16062145
Zhang M, Wei B, Liang L, Fang W, Chen L, Wang Y. Microstructures and Enhanced Mechanical Properties of (Zr, Ti)(C, N)-Based Nanocomposites Fabricated by Reactive Hot-Pressing at Low Temperature. Materials. 2023; 16(6):2145. https://doi.org/10.3390/ma16062145
Chicago/Turabian StyleZhang, Mengmeng, Boxin Wei, Lanqing Liang, Wenbin Fang, Lei Chen, and Yujin Wang. 2023. "Microstructures and Enhanced Mechanical Properties of (Zr, Ti)(C, N)-Based Nanocomposites Fabricated by Reactive Hot-Pressing at Low Temperature" Materials 16, no. 6: 2145. https://doi.org/10.3390/ma16062145
APA StyleZhang, M., Wei, B., Liang, L., Fang, W., Chen, L., & Wang, Y. (2023). Microstructures and Enhanced Mechanical Properties of (Zr, Ti)(C, N)-Based Nanocomposites Fabricated by Reactive Hot-Pressing at Low Temperature. Materials, 16(6), 2145. https://doi.org/10.3390/ma16062145





