TiSiCN as Coatings Resistant to Corrosion and Neutron Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Deposition of the Coatings
2.2. Corrosion Testing and Characterization of the Coatings
2.3. Fast Neutrons Irradiation and the Neutron Activation Analysis (NAA)
3. Results and Discussions
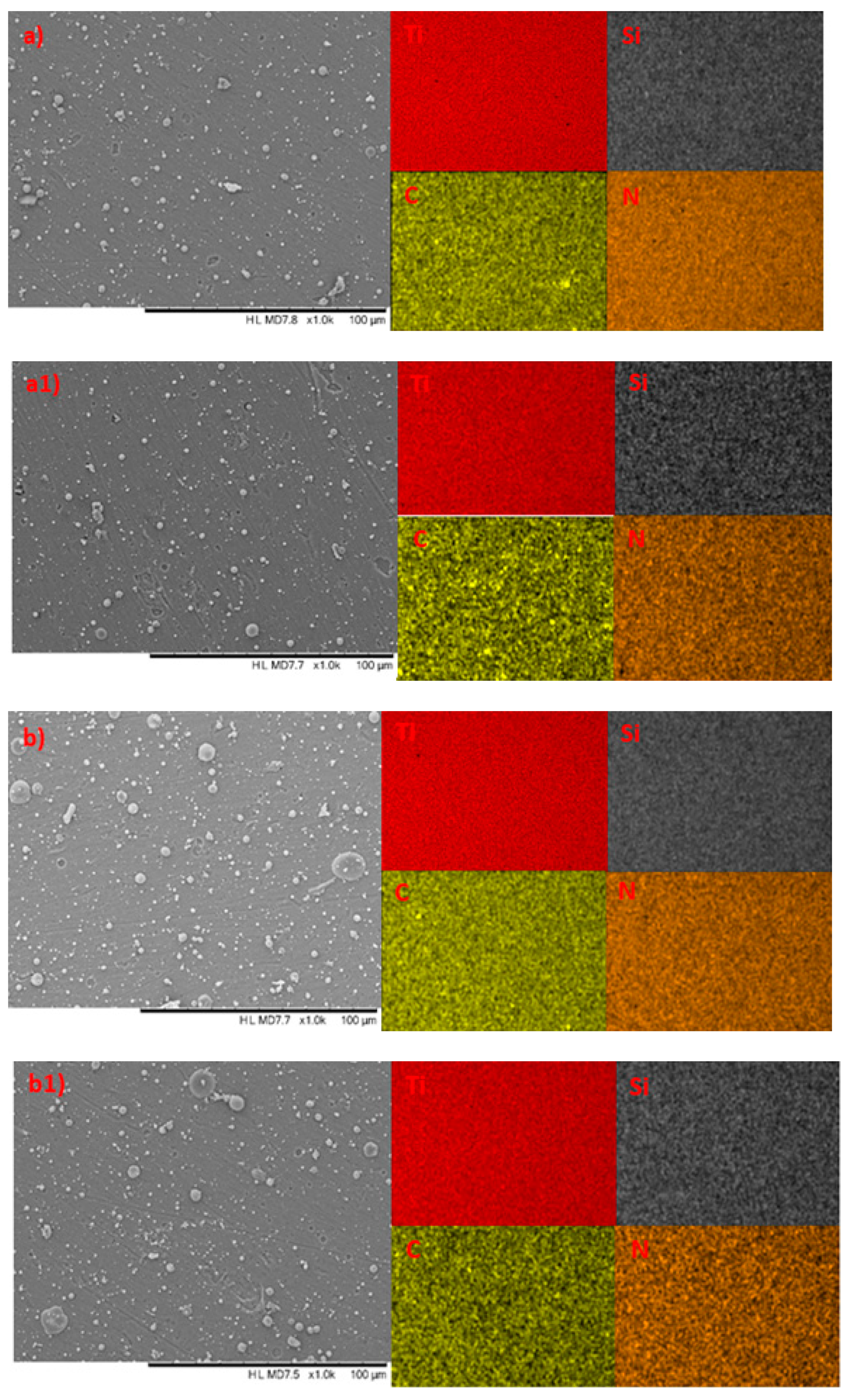
3.1. Elemental Composition Surface Morphology (EDS)
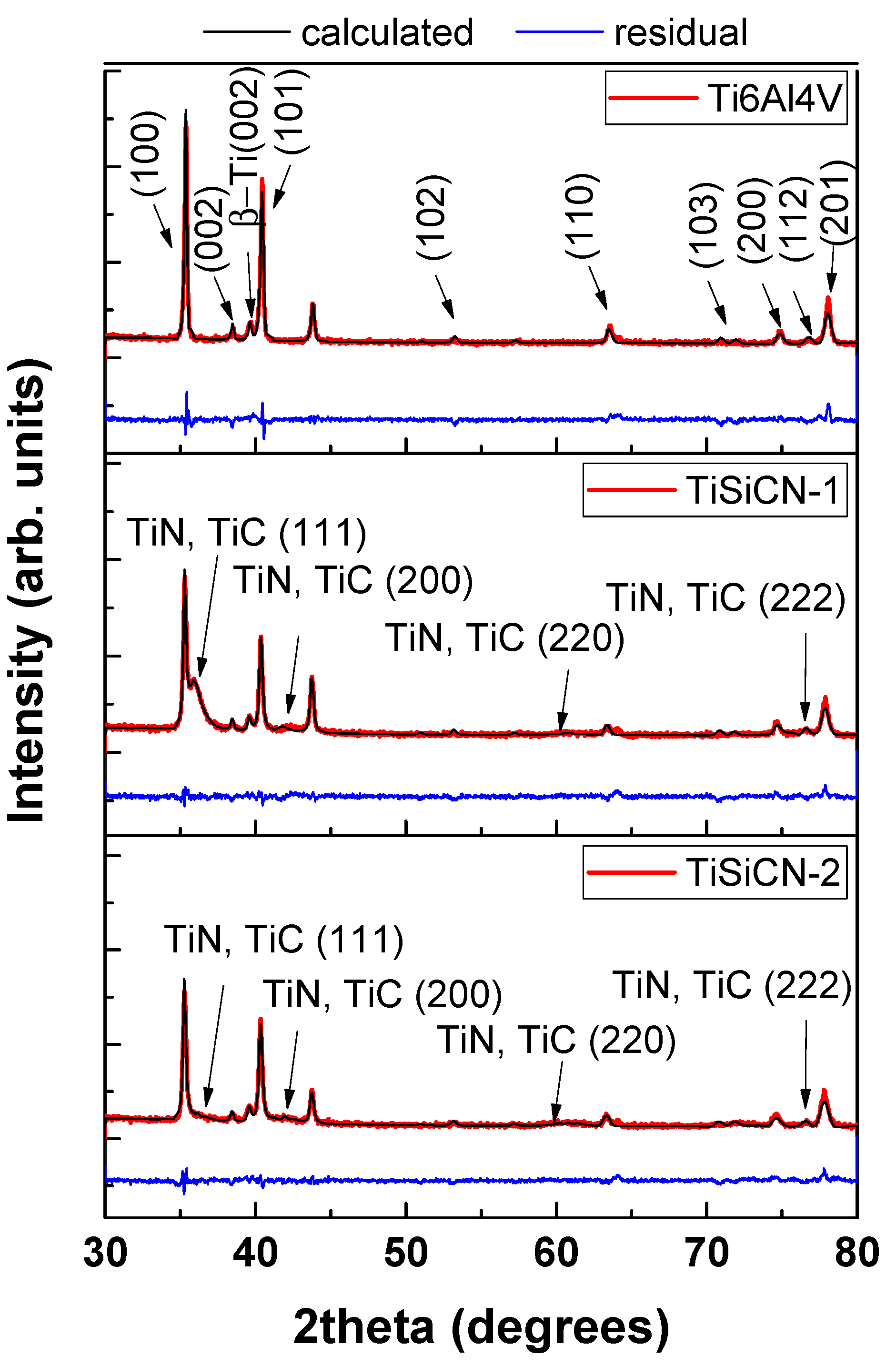
3.2. X-ray Diffraction Analysis of TiSiCN
3.3. Surface Morphology
3.4. Surface Roughness
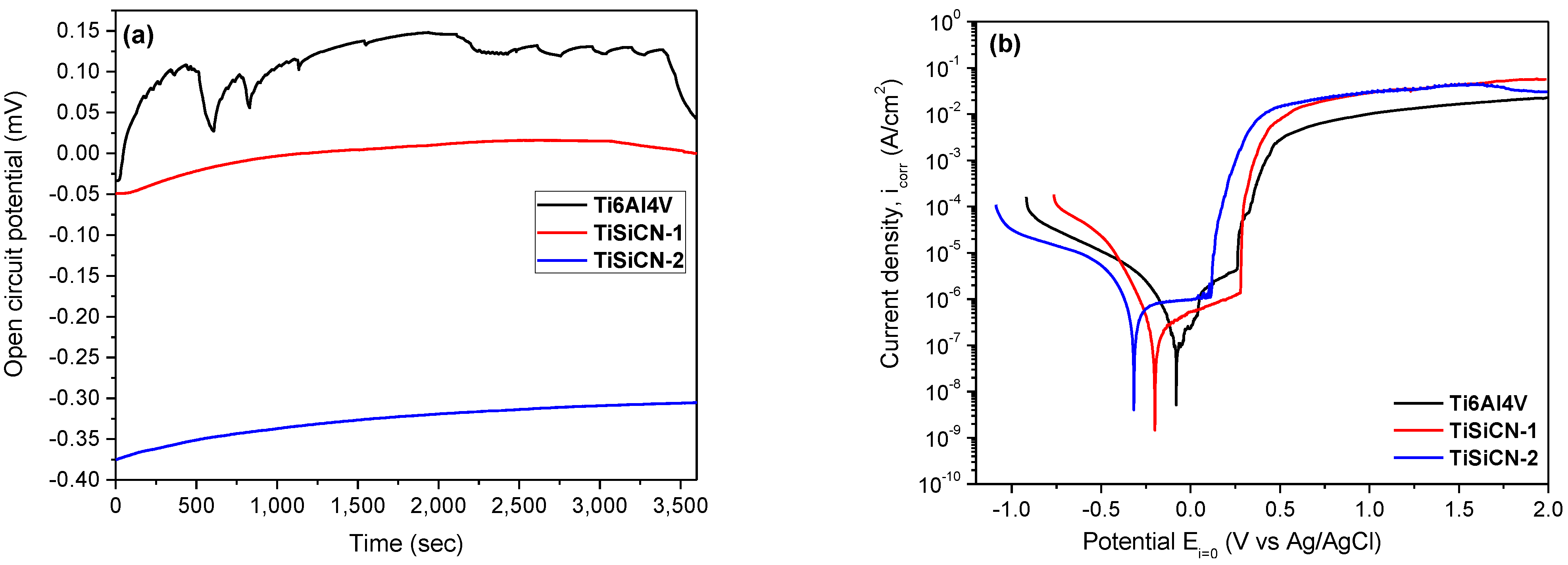
3.5. Corrosion Behaviour
- (1)
- Electropositivity signifies good resistance to corrosion: more electropositive corrosion potential value (Ecorr) means that the material is nobler in the used electrolyte. Figure 5b reveals that the most noble corrosion potential was measured with uncoated Ti6Al4V alloy. TiSiCN-2 coatings exhibit the more electronegative value, indicating poor corrosion resistance;
- (2)
- Surfaces with a low icorr value demonstrated a good corrosion resistance: all coatings presented lower icorr values compared to the uncoated Ti6Al4V alloy. TiSiCN-1 coating showed a lower icorr value compared to TiSiCN-2 coating, including the uncoated substrate;
- (3)
- Surfaces having higher anticorrosive properties demonstrate high polarization resistance (Rp) values. Considering the Rp values in Table 4, note that both coatings exhibit higher Rp value than the uncoated substrate;
- (4)
- Porosity of the coatings was also considered: surfaces with low porosity have good anticorrosive properties. TiSiCN-1 coating has low porosity, indicating that the increase in C/N ratio leads to an increase in porosity, meaning the loss of corrosion resistance;
- (5)
- The protective efficiency (Pe) was also considered: the highest Pe value was obtained for the TiSiCN-1 coating.
3.6. Neutron Activation Analysis for Determination of Fast Neutrons Flux Density
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, J.-F.; Huang, S.-Y.; Lin, J.-H.; Yang, F.-C.; Chang, C.-L. Mechanical Properties of TiN Deposited in Synchronous Bias Mode through High-Power Impulse Magnetron Sputtering. Surf. Coat. Technol. 2022, 434, 128201. [Google Scholar] [CrossRef]
- Braic, M.; Braic, V.; Balaceanu, M.; Zoita, C.N.; Kiss, A.; Vladescu, A.; Popescu, A.; Ripeanu, R. Structure and Properties of Zr/ZrCN Coatings Deposited by Cathodic Arc Method. Mater. Chem. Phys. 2011, 126, 818–825. [Google Scholar] [CrossRef]
- Bull, S.J.; Bhat, D.G.; Staia, M.H. Properties and Performance of Commercial TiCN Coatings. Part 1: Coating Architecture and Hardness Modelling. Surf. Coat. Technol. 2003, 163–164, 499–506. [Google Scholar] [CrossRef]
- Silva, E.; Rebelo de Figueiredo, M.; Franz, R.; Escobar Galindo, R.; Palacio, C.; Espinosa, A.; Calderon, V.S.; Mitterer, C.; Carvalho, S. Structure–Property Relations in ZrCN Coatings for Tribological Applications. Surf. Coat. Technol. 2010, 205, 2134–2141. [Google Scholar] [CrossRef]
- Mesquita, R.A.; Schuh, C.A. Tool Steel Coatings Based on Niobium Carbide and Carbonitride Compounds. Surf. Coat. Technol. 2012, 207, 472–479. [Google Scholar] [CrossRef]
- Warcholinski, B.; Gilewicz, A.; Kuklinski, Z.; Myslinski, P. Hard CrCN/CrN Multilayer Coatings for Tribological Applications. Surf. Coat. Technol. 2010, 204, 2289–2293. [Google Scholar] [CrossRef]
- Constantin, L.; Braic, M.; Dinu, M.; Balaceanu, M.; Braic, V.; Farcau, C.; Vladescu, A. Effects of Zr, Nb, or Si Addition on the Microstructural, Mechanical, and Corrosion Resistance of TiCN Hard Coatings. Mater. Corros. 2016, 67, 929–938. [Google Scholar] [CrossRef]
- He, J.; Nan, Z.; Mi, P.; Chen, K.; Qin, Y. In Situ Nanostructured (TiCr)CN Coating by Reactive Plasma Spraying. Ceram. Int. 2018, 44, 712–717. [Google Scholar] [CrossRef]
- Ma, S.L.; Ma, D.Y.; Guo, Y.; Xu, B.; Wu, G.Z.; Xu, K.W.; Chu, P.K. Synthesis and Characterization of Super Hard, Self-Lubricating Ti–Si–C–N Nanocomposite Coatings. Acta Mater. 2007, 55, 6350–6355. [Google Scholar] [CrossRef]
- Xie, X.; Li, J.; Dong, M.; Zhang, H.; Wang, L. Structure and Properties of TiSiCN Coatings with Different Bias Voltages by Arc Ion Plating. Surf. Topogr. 2018, 6, 014003. [Google Scholar] [CrossRef]
- Wang, H.; Ou, Y.; Zhang, X.; Liao, B.; Ou, X.; Luo, J.; Pang, P.; Chen, L.; Hua, Q.; Bao, M. Tribocorrosion Behaviors of TiSiCN Nanocomposite Coatings Deposited by High Power Impulse Magnetron Sputtering. Mater. Res. Express 2020, 7, 076407. [Google Scholar] [CrossRef]
- Bondar, O.V.; Postolnyi, B.O.; Kravchenko, Y.A.; Shypylenko, A.P.; Sobol, O.V.; Beresnev, V.M.; Kuzmenko, A.P.; Zukowski, P. Fabrication and Research of Superhard (Zr-Ti-Cr-Nb)N Coatings. Acta Phys. Pol. A 2015, 128, 867–871. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Xiao, J.; Pi, J.; He, G.; Chen, L.; Zeng, Y.; Jiang, J. Effects of Si Addition on Structure and Mechanical Properties of TiAlSiCN Coatings. Surf. Coat. Technol. 2019, 362, 21–26. [Google Scholar] [CrossRef]
- Golizadeh, M.; Kuptsov, K.A.; Shvyndina, N.V.; Shtansky, D.V. Multilayer SiBCN/TiAlSiCN and AlOx/TiAlSiCN Coatings with High Thermal Stability and Oxidation Resistance. Surf. Coat. Technol. 2017, 319, 277–285. [Google Scholar] [CrossRef]
- Shtansky, D.V.; Kuptsov, K.A.; Kiryukhantsev-Korneev, P.V.; Sheveiko, A.N.; Fernandez, A.; Petrzhik, M.I. Comparative Investigation of Al- and Cr-Doped TiSiCN Coatings. Surf. Coat. Technol. 2011, 205, 4640–4648. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, F.; Leng, Y.; Wei, R.; Huang, N. Microstructure and Tribological Properties of Ti(Cr)SiCN Coating Deposited by Plasma Enhanced Magnetron Sputtering. Vacuum 2013, 89, 168–173. [Google Scholar] [CrossRef]
- Xin, X.; Tengfei, Z.; Yu, W.; Yan, Y.; Yongxiang, L.; Dong, X.; Nan, H. Microstructure and Mechanical Properties of Ti(Cr)SiC(O)N Coatings Deposited by Plasma Enhanced Magnetron Sputtering. Rare Met. Mater. Eng. 2017, 46, 1762–1767. [Google Scholar] [CrossRef]
- Chaparro, W.A.; Martin, C.R.; López, E.V. Synergy between Erosion-Corrosion of Steel AISI 4140 Covered by a Multilayer TiCN /TiNbCN, at an Impact Angle of 90°. Dyna 2013, 80, 101–108. [Google Scholar]
- Caicedo, J.C.; Guerrero, A.; Aperador, W. Determination of Multilayer Effect Evidence on Metal Carbon-Nitride System. J. Alloys Compd. 2019, 785, 178–190. [Google Scholar] [CrossRef]
- Bondarev, A.V.; Kiryukhantsev-Korneev, P.V.; Levashov, E.A.; Shtansky, D.V. Tribological Behavior and Self-Healing Functionality of TiNbCN-Ag Coatings in Wide Temperature Range. Appl. Surf. Sci. 2017, 396, 110–120. [Google Scholar] [CrossRef]
- Li, W.; Liu, P.; Xue, Z.; Ma, F.; Zhang, K.; Chen, X.; Feng, R.; Liaw, P.K. Microstructures, Mechanical Behavior and Strengthening Mechanism of TiSiCN Nanocomposite Films. Sci. Rep. 2017, 7, 2140. [Google Scholar] [CrossRef] [PubMed]
- El-Rahman, A.M.A.; Wei, R. A Comparative Study of Conventional Magnetron Sputter Deposited and Plasma Enhanced Magnetron Sputter Deposited Ti–Si–C–N Nanocomposite Coatings. Surf. Coat. Technol. 2014, 241, 74–79. [Google Scholar] [CrossRef]
- Abraham, S.; Choi, E.Y.; Kang, N.; Kim, K.H. Microstructure and Mechanical Properties of Ti-Si-C-N Films Synthesized by Plasma-Enhanced Chemical Vapor Deposition. Surf. Coat. Technol. 2007, 202, 915–919. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Dang, C.; Wang, Y.; Zhu, Y. Influence of Carbon Contents on the Structure and Tribocorrosion Properties of TiSiCN Coatings on Ti6Al4V. Tribol. Int. 2017, 109, 285–296. [Google Scholar] [CrossRef]
- Wei, R. Plasma Enhanced Magnetron Sputter Deposition of Ti–Si–C–N Based Nanocomposite Coatings. Surf. Coat. Technol. 2008, 203, 538–544. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, S.; Xu, K. Effects of Carbon Content and Annealing Temperature on the Microstructure and Hardness of Super Hard Ti–Si–C–N Nanocomposite Coatings Prepared by Pulsed d.c. PCVD. Surf. Coat. Technol. 2007, 201, 5240–5243. [Google Scholar] [CrossRef]
- Jeon, J.-H.; Choi, S.R.; Chung, W.S.; Kim, K.H. Synthesis and Characterization of Quaternary Ti–Si–C–N Coatings Prepared by a Hybrid Deposition Technique. Surf. Coat. Technol. 2004, 188–189, 415–419. [Google Scholar] [CrossRef]
- Lin, H.-M.; Duh, J.-G.; Wei, R.; Rincon, C.; Lee, J.-W. The Effect of Microstructure and Composition on Mechanical Properties in Thick-Layered Nanocomposite Ti–Si–C–N Coatings. Surf. Coat. Technol. 2010, 205, 1460–1464. [Google Scholar] [CrossRef]
- Onoprienko, A.A.; Ivashchenko, V.I.; Dub, S.N.; Khyzhun, O.Y.; Timofeeva, I.I. Microstructure and Mechanical Properties of Hard Ti–Si–C–N Films Deposited by Dc Magnetron Sputtering of Multicomponent Ti/C/Si Target. Surf. Coat. Technol. 2011, 205, 5068–5072. [Google Scholar] [CrossRef]
- Vepřek, S.; Reiprich, S.; Shizhi, L. Superhard Nanocrystalline Composite Materials: The TiN/Si 3 N 4 System. Appl. Phys. Lett. 1995, 66, 2640–2642. [Google Scholar] [CrossRef]
- Endler, I.; Höhn, M.; Schmidt, J.; Scholz, S.; Herrmann, M.; Knaut, M. Ternary and Quarternary TiSiN and TiSiCN Nanocomposite Coatings Obtained by Chemical Vapor Deposition. Surf. Coat. Technol. 2013, 215, 133–140. [Google Scholar] [CrossRef]
- Marchin, N.; Ashrafizadeh, F. Surface Structural Characterization of TiSiN and TiSiCN Coatings Produced by Cathodic Arc Physical Vapor Deposition. Iran. J. Surf. Sci. Eng. 2019, 39, 47–58. [Google Scholar]
- Eriksson, A. Cathodic Arc Synthesis of Ti-Si-C-N Thin Films–Plasma Analysis and Microstructure Formation. Ph.D. Thesis, Linköping University Sweden, Linköping, Sweden, 2012. [Google Scholar]
- Pruncu, C.I.; Braic, M.; Dearn, K.D.; Farcau, C.; Watson, R.; Constantin, L.R.; Balaceanu, M.; Braic, V.; Vladescu, A. Corrosion and Tribological Performance of Quasi-Stoichiometric Titanium Containing Carbo-Nitride Coatings. Arab. J. Chem. 2017, 10, 1015–1028. [Google Scholar] [CrossRef]
- Meyer, M.K. Composite Fuel (Cermet, Cercer). In Comprehensive Nuclear Materials; Elsevier: Amsterdam, The Netherlands, 2012; pp. 257–273. ISBN 9780081028667. [Google Scholar]
- Konys, J.; Krauss, W.; Holstein, N. Aluminum-Based Barrier Development for Nuclear Fusion Applications. Corrosion 2011, 67, 026002-1–026002-6. [Google Scholar] [CrossRef]
- Elsener, B.; Rota, A.; Böhni, H. Impedance Study on the Corrosion of PVD and CVD Titanium Nitride Coatings. Mater. Sci. Forum 1991, 44–45, 29–38. [Google Scholar] [CrossRef]
- Pederson, R. Microstructure and Phase Transformation of Ti-6Al-4V. Ph.D. Thesis, Luleå University of Technology, Luleå, Sweden, 2002. [Google Scholar]
- Hatem, A.; Lin, J.; Wei, R.; Torres, R.D.; Laurindo, C.; de Souza, G.B.; Soares, P. Tribocorrosion Behavior of Low Friction TiSiCN Nanocomposite Coatings Deposited on Titanium Alloy for Biomedical Applications. Surf. Coat. Technol. 2018, 347, 1–12. [Google Scholar] [CrossRef]
- Qin, C.P.; Zheng, Y.G.; Wei, R. Cavitation Erosion Behavior of Nanocomposite Ti–Si–C–N and Ti/Ti–Si–C–N Coatings Deposited on 2Cr13 Stainless Steel Using a Plasma Enhanced Magnetron Sputtering Process. Surf. Coat. Technol. 2010, 204, 3530–3538. [Google Scholar] [CrossRef]
- Qiu, L.; Du, Y.; Wu, L.; Wang, S.; Zhu, J.; Cheng, W.; Tan, Z.; Yin, L.; Liu, Z.; Layyous, A. Microstructure, Mechanical Properties and Cutting Performances of TiSiCN Super-Hard Nanocomposite Coatings Deposited Using CVD Method under the Guidance of Thermodynamic Calculations. Surf. Coat. Technol. 2019, 378, 124956. [Google Scholar] [CrossRef]
- Lin, J.; Wei, R.; Bitsis, D.C.; Lee, P.M. Development and Evaluation of Low Friction TiSiCN Nanocomposite Coatings for Piston Ring Applications. Surf. Coat. Technol. 2016, 298, 121–131. [Google Scholar] [CrossRef]
- Abd El-Rahman, A.M.; Wei, R.; Raaif, M.; El-Hossary, F.M.; Hammad Fawey, M.; Abo El-kassem, M. Effect of N2/TMS Gas Ratio on Mechanical and Erosion Performances of Ti-Si-C-N Nanocomposite Coatings. J. Mater. Eng. Perform. 2020, 29, 3324–3333. [Google Scholar] [CrossRef]
- Marchin, N.; Ashrafizadeh, F. Effect of Carbon Addition on Tribological Performance of TiSiN Coatings Produced by Cathodic Arc Physical Vapour Deposition. Surf. Coat. Technol. 2021, 407, 126781. [Google Scholar] [CrossRef]
- Du, H.; Liu, P.; Li, W.; Zhang, K.; Ma, F.; Chen, X.; Yang, J.; Feng, R.; Liaw, P.K. Effects of C/Si Ratios on Structures and Behaviors of NbSiCN Nanocomposite Films Synthesized by Reactive Magnetron Sputtering. Mater. Charact. 2020, 167, 110466. [Google Scholar] [CrossRef]
- Wan, Q.; Wu, Z.Y.; Liu, Y.; Yang, B.; Liu, H.D.; Ren, F.; Wang, P.; Xiao, Y.Y.; Zhang, J.; Zhang, G.D. Lead-Bismuth Eutectic (LBE) Corrosion Mechanism of Nano-Amorphous Composite TiSiN Coatings Synthesized by Cathodic Arc Ion Plating. Corros. Sci. 2021, 183, 109264. [Google Scholar] [CrossRef]
- Wan, Q.; Chen, Y.M.; Liu, H.D.; Yang, B. Investigation on Oxidation Behaviors of Ti-Si-N Coating at High Temperature. Anti-Corros. Methods Mater. 2018, 65, 125–130. [Google Scholar] [CrossRef]
- Finšgar, M.; Petovar, B.; Xhanari, K.; Maver, U. The Corrosion Inhibition of Certain Azoles on Steel in Chloride Media: Electrochemistry and Surface Analysis. Corros. Sci. 2016, 111, 370–381. [Google Scholar] [CrossRef]
- Evgeny, B.; Hughes, T.; Eskin, D. Effect of Surface Roughness on Corrosion Behaviour of Low Carbon Steel in Inhibited 4 M Hydrochloric Acid under Laminar and Turbulent Flow Conditions. Corros. Sci. 2016, 103, 196–205. [Google Scholar] [CrossRef]
- Pan, J.; Thierry, D.; Leygraf, C. Electrochemical Impedance Spectroscopy Study of the Passive Oxide Film on Titanium for Implant Application. Electrochim. Acta 1996, 41, 1143–1153. [Google Scholar] [CrossRef]
- Milošev, I.; Metikoš-Huković, M.; Strehblow, H.-H. Passive Film on Orthopaedic TiAlV Alloy Formed in Physiological Solution Investigated by X-Ray Photoelectron Spectroscopy. Biomaterials 2000, 21, 2103–2113. [Google Scholar] [CrossRef]
- Qin, P.; Chen, L.Y.; Liu, Y.J.; Jia, Z.; Liang, S.X.; Zhao, C.H.; Sun, H.; Zhang, L.C. Corrosion and Passivation Behavior of Laser Powder Bed Fusion Produced Ti-6Al-4V in Static/Dynamic NaCl Solutions with Different Concentrations. Corros. Sci. 2021, 191, 109728. [Google Scholar] [CrossRef]
- Bignon, Q.; Martin, F.; Auzoux, Q.; Miserque, F.; Tabarant, M.; Latu-Romain, L.; Wouters, Y. Oxide Formation on Titanium Alloys in Primary Water of Nuclear Pressurised Water Reactor. Corros. Sci. 2019, 150, 32–41. [Google Scholar] [CrossRef]
- Milošev, I.; Kosec, T.; Strehblow, H.-H. XPS and EIS Study of the Passive Film Formed on Orthopaedic Ti–6Al–7Nb Alloy in Hank’s Physiological Solution. Electrochim. Acta 2008, 53, 3547–3558. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of Effective Capacitance and Film Thickness from Constant-Phase-Element Parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Córdoba-Torres, P. Relationship between Constant-Phase Element (CPE) Parameters and Physical Properties of Films with a Distributed Resistivity. Electrochim. Acta 2017, 225, 592–604. [Google Scholar] [CrossRef]




| Elemental Composition (at.%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Coatings | Ti | Si | C | N | O | V | Al | (C + N)/Metal + Si | C/N |
| TiSiCN-1 | 42.30 | 5.17 | 15.91 | 34.36 | 2.12 | 0 | 0.14 | 0.61 | 0.46 |
| TiSiCN-2 | 44.12 | 4.57 | 29.16 | 19.19 | 2.90 | 0 | 0.10 | 0.71 | 1.52 |
| Substrate and Samples | Before Corrosion | After Corrosion | ||
|---|---|---|---|---|
| Ra (nm) | Sk | Ra (nm) | Sk | |
| Ti6Al4V | 36.69 ± 9.7 | −0.11 ± 0.1 | 24.94 ± 2.7 | −0.15 ± 0.1 |
| TiSiCN-1 | 473.92 ± 19.0 | 1.99 ± 0.3 | 462.85 ± 35.3 | 1.71 ± 0.3 |
| TiSiCN-2 | 545.55 ± 33.5 | 1.50 ± 0.3 | 544.59 ± 52.4 | 2.16 ± 0.6 |
| Sample | Rs (Ω cm2) | Qcoat (μF s(α−1) cm−2) | αcoat | Rpore (kΩ cm2) | Qdl (μF s(α−1) cm−2) | αdl | Rct (kΩ cm2) | χ2 |
|---|---|---|---|---|---|---|---|---|
| Ti6Al4V | 9 | 76.17 | 0.53 | 4 | 5004 | 1.0 | - | 4.0 × 10−4 |
| TiSiCN-1 | 8 | 89.65 | 0.77 | 3626 | 12.21 | 1.0 | - | 2.0 × 10−4 |
| TiSiCN-2 | 5 | 120.71 | 0.63 | 17 | 10.65 | 0.98 | - | 4.0 × 10−4 |
| Substrate and Samples | Ecorr (mV) | icorr (nA) | Rp (Ω × 10−3) | Pe (%) | P |
|---|---|---|---|---|---|
| Ti6Al4V | −78 | 222.123 | 0.195 | - | - |
| TiSiCN-1 | −199 | 25.281 | 8.795 | 88.6 | 0.016 |
| TiSiCN-2 | −316 | 94.347 | 1.816 | 57.5 | 0.058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirzayev, M.N.; Parau, A.C.; Slavov, L.; Dinu, M.; Neov, D.; Slavkova, Z.; Popov, E.P.; Belova, M.; Hasanov, K.; Aliyev, F.A.; et al. TiSiCN as Coatings Resistant to Corrosion and Neutron Activation. Materials 2023, 16, 1835. https://doi.org/10.3390/ma16051835
Mirzayev MN, Parau AC, Slavov L, Dinu M, Neov D, Slavkova Z, Popov EP, Belova M, Hasanov K, Aliyev FA, et al. TiSiCN as Coatings Resistant to Corrosion and Neutron Activation. Materials. 2023; 16(5):1835. https://doi.org/10.3390/ma16051835
Chicago/Turabian StyleMirzayev, Matlab N., Anca C. Parau, Lyubomir Slavov, Mihaela Dinu, Dimitar Neov, Zdravka Slavkova, Evgeni P. Popov, Maria Belova, Kanan Hasanov, Fuad A. Aliyev, and et al. 2023. "TiSiCN as Coatings Resistant to Corrosion and Neutron Activation" Materials 16, no. 5: 1835. https://doi.org/10.3390/ma16051835
APA StyleMirzayev, M. N., Parau, A. C., Slavov, L., Dinu, M., Neov, D., Slavkova, Z., Popov, E. P., Belova, M., Hasanov, K., Aliyev, F. A., & Vladescu, A. (2023). TiSiCN as Coatings Resistant to Corrosion and Neutron Activation. Materials, 16(5), 1835. https://doi.org/10.3390/ma16051835





.jpg)

