Abstract
In this work, isothermal sections of the Ln2O3–Cr2O3–B2O3 (Ln = Gd–Lu) ternary oxide systems at 900, 1000, and 1100 °C were constructed by determining the phase relations by using a powder X-ray diffraction technique. As a result, these systems were divided into subsidiary subsystems. Two types of double borates, LnCr3(BO3)4 (Ln = Gd–Er) and LnCr(BO3)2 (Ln = Ho–Lu), were observed in the investigated systems. Regions of phase stability for LnCr3(BO3)4 and LnCr(BO3)2 were determined. It was shown that the LnCr3(BO3)4 compounds crystallized in rhombohedral and monoclinic polytype modifications up to 1100 °C; above this temperature and up to the melting points, the monoclinic modification was predominantly formed. The LnCr3(BO3)4 (Ln = Gd–Er) and LnCr(BO3)2 (Ln = Ho–Lu) compounds were characterized by using a powder X-ray diffraction method and thermal analysis.
1. Introduction
The Ln2O3–Cr2O3–B2O3 (Ln = Gd–Lu) ternary oxide systems have not been studied so far in terms of their phase relations in an air atmosphere. However, compounds synthesized in binary systems corresponding to the sides of these ternary diagrams are of great interest in terms of both their crystal structures and their physicochemical properties.
The reactivity of the binary oxide systems of Ln2O3–B2O3, Cr2O3–B2O3, and Ln2O3–Cr2O3 has been analyzed in a number of publications, although some of these systems have not yet been sufficiently investigated. Most studies refer to binary diagrams of Ln2O3–B2O3 (Ln = Y, La–Nd, Sm–Lu), where the compounds of Ln(BO2)3, LnBO3, Ln3BO6, and LnB5O9 are described [1,2,3,4]. Lanthanide metaborates, Ln(BO2)3 (Ln = La–Nd, Sm–Dy), exhibit two structural modifications under normal pressure: α-Ln(BO2)3, where Ln = La–Nd, Sm–Tb (space group (sp. gr.) I2/a) [5,6,7,8,9,10,11], and β-Ln(BO2)3, where Ln = Tb–Dy with sp. gr. Pnma [12,13]. It was found that the metaborates of Ln(BO2)3 (Ln = La, Nd) melt congruently, while the metaborates of Ln(BO2)3 (Ln = Sm–Gd) melt incongruently at 1000–1150 °C [1,2]. In the latter case, melting occurs with the formation of LnBO3 (Ln = Eu, Gd) and B2O3 [1]. Rare-earth orthoborates, LnBO3 (Ln = Y, La–Nd, Sm–Lu), are isostructural to three CaCO3 modifications at normal pressure depending on the Ln-cation: aragonite (λ-LnBO3, with Ln = La–Nd), vaterite (π- and/or μ-LnBO3, with Ln = Y, Sm–Lu), and calcite (β-LuBO3) [1,14,15,16,17]. They melt congruently in the temperature range of 1600–1700 °C [1,2]. Rare-earth oxyborates with the general formula of Ln3BO6 (Ln = Y, La–Nd, Sm–Lu) are the most understudied compounds in this diagram. Initially, oxyborates were found in the La2O3–B2O3 system, and their formula was determined as Ln3BO6 based on phase identification [2]. However, the structural formulas of Ln3BO6 (Ln = La, Nd), Ln3BO6 (Ln = Y, Gd), and Yb3BO6 were further revised as Ln26(BO3)8O27 (Ln = La, Nd) [18,19], Ln17.33(BO3)4(B2O5)2O16 (Ln = Y, Gd) [20,21], and Yb26(BO3)4(B2O5)2(B4O11)O24 [22]. Thus, the oxyborate family is divided into three groups: Ln3BO6 (Ln = La–Nd) with sp. gr. P21/c, Ln3BO6 (Ln = Y, Sm–Tm) with sp. gr. Cm, and Ln3BO6 (Ln = Yb, Lu) with sp. gr. C2/m. Levin et al. [2] found that La3BO6 oxyborate melts incongruently at 1386 °C. Lanthanide pentaborates, LnB5O9 (Ln = La–Nd, Sm–Er), were obtained through the decomposition of Ln[B8O11(OH)5] (Ln = La–Nd), Ln[B9O13(OH)4] (Ln = Pr, Nd, Sm, Eu), and Ln[B6O9(OH)3] (Ln = Sm–Er) [3,4]. These compounds have two structural modifications: β-LnB5O9 (Ln = La, Ce) (sp. gr. P21/c) and α-LnB5O9 (Ln = Pr, Nd, Sm–Er) (sp. gr. I41/acd). Pentaborates decompose at 800–900 °C into metaborates Ln(BO2)3 (Ln = Sm–Tb) and orthoborates LnBO3 (Ln = Dy–Er). The representatives of the Ln2O3–B2O3 system are considered to be promising candidates for luminescent and nonlinear optical materials [23,24,25].
The binary system of Cr2O3–B2O3 has been investigated less. Two solid phases, CrBO3 [26] and Cr3BO6 [27], were reported to exist in it. Chromium orthoborate, CrBO3, has a calcite-type structure (sp. gr. ) and decomposes at 1220 °C to form Cr2O3 and B2O3. Bither et al. [28] reported that CrBO3 appeared to be a low-temperature antiferromagnetic material with the Néel temperature (TN) of 15 K. Chromium oxyborate, Cr3BO6, crystallizes in the sp. gr. Pnma. This compound demonstrates low thermal stability in air and decomposes into Cr2O3 and CrBO3 at 800 °C. The electrochemical properties of these materials were investigated, and it was shown that Cr3BO6 could be interesting as a negative electrode for lithium-ion batteries [27].
According to data from the literature, only LnCrO3 phases were found in the binary systems of Ln2O3–Cr2O3 (Ln = Y, La–Nd, Sm–Lu) [29]. They exhibited perovskite-type structure with the sp. gr. Pbnm [30,31] and demonstrated high congruent melting points at 2300–2400 °C [32]. These compounds are antiferromagnetically ordered with TN in the temperature range of 110–290 K [33,34]. Multiferroic properties were observed for some rare-earth orthochromites [35,36]. LnCrO3 materials are p-type semiconductors, which are valuable for sensor applications [37].
In the Ln2O3–Cr2O3–B2O3 (Ln = Y, La–Nd, Sm–Lu) ternary systems, two double borates were found: LnCr3(BO3)4 with a huntite-type structure (sp. gr. R32) [38,39] and LnCr(BO3)2 with a dolomite-type structure (sp. gr. ) [40,41]. Both borate families are still poorly studied. Rare-earth chromium borates of the huntite family were synthesized from multicomponent flux melts [42] and through solid-state reactions [43]. In addition to their rhombohedral structure, LnCr3(BO3)4 compounds also have a monoclinic modification with sp. gr. C2/c [44,45,46]. These materials have been found to exhibit antiferromagnetic ordering with TN in the temperature range of 6.5–10 K [43,47,48,49]. Several studies of LnCr(BO3)2 compounds have been performed, including the refinement of their crystal structure and measurements of antiferromagnetic ordering temperatures (6.1–8.1 K) [50,51].
The great variety of properties specific to borates appearing both in binary Ln2O3–B2O3 (Ln = Gd–Lu), Cr2O3–B2O3, and Ln2O3–Cr2O3 (Ln = Gd–Lu) and in ternary Ln2O3–Cr2O3–B2O3 (Ln = Gd–Lu) diagrams makes such systems interesting and challenging for further investigation. Therefore, the purpose of this work is to study the phase relations in Ln2O3–Cr2O3–B2O3 (Ln = Gd–Lu) ternary systems, the phase formation of promising magnetic materials in them, and the determination of the structural and thermal properties of the latter.
2. Materials and Methods
The ternary diagrams were studied through the solid-state reaction (SSR) in a high-temperature muffle furnace in air. The initial reagents were oxides (Ln2O3 (Ln = Gd, Dy–Lu) (99.9%), Tb4O7 (99.9%), Cr2O3 (99.9%)) and orthoboric acid (H3BO3 (99.9%)). The oxides were preliminarily calcined before mixing to remove water. After that, they were mixed with orthoboric acid in the required proportions in an agate mortar. A 5 mol.% excess of H3BO3 was added to compensate for the loss of B2O3 due to volatilization during heating. This mixture was pressed into tablets (12 mm in diameter) and held in alumina crucibles at 400 °C to decompose the orthoboric acid. Then, the mixture was ground again in acetone, heated at a rate of 4 °C/min, and held at 24 h in the temperature range of 800–1300 °C. After each heating stage, the samples were left in the furnace until they cooled to room temperature; then, the phase composition was determined with a powder X-ray diffraction (PXRD) method. The heating was repeated until the PXRD patterns remained absolutely stable. New tablets were used for each sintering temperature.
PXRD was carried out by using an ARL X’tra diffractometer (Thermo Fisher Scientific, Basel, Switzerland) equipped with a MYTHEN2 R 1D detector (Dectris, Baden-Daettwil, Switzerland). The diffraction patterns of the SSR products were recorded in continuous mode at room temperature by using CuKα1,2 radiation (λ = 1.540562 Å) in the range of 10° ≤ 2θ ≤ 70° with a scan speed of 8° per minute, U = 40 kV, and I = 40 mA. Long PXRD scans were collected in the range of 10° ≤ 2θ ≤ 90° for LnCr3(BO3)4 and LnCr(BO3)2 to carry out a quantitative analysis.
Compounds were identified by matching the experimental PXRD patterns to those of the ICDD PDF-2 powder diffraction database release 2020 [52].
The refinement of the lattice parameters of the rare-earth chromium borates and dysprosium metaborate was carried out through the Le Bail method by using the JANA2006 software package [53]. All parameters were refined with the least-square method. The pseudo-Voigt function was used as the peak profile function. The initial structural parameters were the structural data for DyCr3(BO3)4 (monoclinic, sp. gr. C2/c, a = 7.394 Å, b = 9.450 Å, c = 11.357 Å, α = γ = 90°, β = 103.9°; rhombohedral, sp. gr. R32, a = 9.461 Å, c = 7.488 Å, α = β = 90°; γ = 120°) [49] and α-Tb(BO2)3 (monoclinic, sp. gr. C2/c, a = 6.21781(5) Å, b = 8.02564(6) Å, c = 7.80659(4) Å, α = γ = 90°, β = 93.6935(7)°) [11].
Differential scanning calorimetry (DSC) was performed by means of STA 449 F5 Jupiter equipment (Netzsch, Selb, Germany) in the temperature range of 50–1500 °C with a heating rate of 20 °C/min in Ar gas flow. PtRh crucibles were used in the DSC experiments.
3. Results and Discussion
3.1. Phase Formation in the Ln2O3–Cr2O3–B2O3 (Ln = Gd–Lu) Systems
3.1.1. Ln2O3–Cr2O3–B2O3 (Ln = Gd, Tb) Ternary Systems
The phase relations in the Ln2O3–Cr2O3–B2O3 (Ln = Gd, Tb) ternary systems at 900, 1000, and 1100 °C (Figure 1, Table 1) were determined based on an analysis of 24 samples (eight samples per isothermal section). Each diagram had seven different subsidiary subsystems. Double borates LnCr3(BO3)4 (GdCr3(BO3)4 [PDF-2 #01-085-4144]) were established in both systems. Metaborates (Gd(BO2)3 [PDF-2 #01-086-3642] and Tb(BO2)3 [PDF-2 #01-072-3639]) crystallized in the α-modification. There was no α-Gd(BO2)3 compound in the cross-section at 1100 °C (Figure 1b), which was due to its melting at 1085 °C [54]. The metaborate α-Tb(BO2)3 was not observed at temperatures above 900 °C due to its lower melting point than that of α-Gd(BO2)3. The results obtained correlated well with the decrease in the melting point in the Ln(BO2)3 series from La to Gd [1,2]. Orthoborates (GdBO3 [PDF-2 #01-089-6546] and TbBO3 [PDF-2 #01-080-3937]) crystallized in a vaterite modification. The data from the PDF-2 #01-074-3085 card were used to determine the Gd2O3 phase. Oxyborates Ln3BO6 (Ln = Gd, Tb) were identified based on the PXRD data of the isostructural compound Y3BO6 [PDF-2 #00-034-0291].
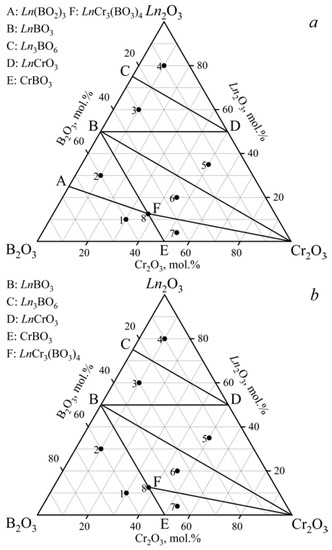
Figure 1.
Phase relations in the systems: (a) Gd2O3–Cr2O3–B2O3 at 900 and 1000 °C, Tb2O3–Cr2O3–B2O3 at 900 °C; (b) Gd2O3–Cr2O3–B2O3 at 1100 °C, Tb2O3–Cr2O3–B2O3 at 1000 and 1100 °C.

Table 1.
Phase compositions of specimens in the Ln2O3–Cr2O3–B2O3 (Ln = Gd, Tb) systems as indicated by PXRD.
The stability of the LnCr3(BO3)4 compounds in the range of 800–1250 °C was investigated (Figure 2). Double borates of LnCr3(BO3)4 coexisted with LnBO3, α-Ln(BO2)3, and Cr2O3 at 900 °C, and in the temperature range of 1000–1200 °C, they existed with LnBO3 and Cr2O3. The compounds of LnBO3 and Cr2O3 [PDF-2 #00-038-1479] were observed at temperatures of 1250 °C, while LnBO3, α-Ln(BO2)3, and Cr2O3 were formed at 800 °C.
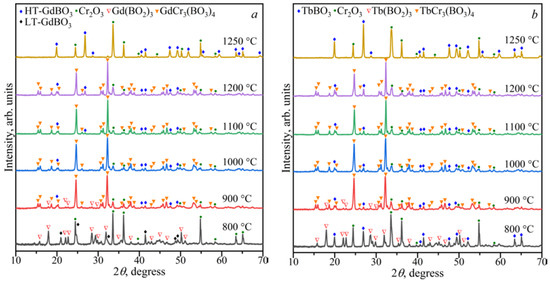
Figure 2.
PXRD patterns of the compositions of Gd2O3:3Cr2O3:4B2O3 (a) and Tb2O3:3Cr2O3:4B2O3 (b) in the temperature range of 800–1250 °C.
Thus, in the investigated ternary systems, the reactions proceeded according to the following equations:
Ln2O3 + B2O3 → 2LnBO3 (800–1250 °C)
Ln2O3 + 3B2O3 → 2α-Ln(BO2)3 (800–1000 °C for Ln = Gd,
800–900 °C for Ln = Tb)
800–900 °C for Ln = Tb)
Ln2O3 + 3Cr2O3 + 4B2O3 → 2LnCr3(BO3)4 (900–1200 °C)
Figure 2a shows that the borate GdBO3 crystallized in the low-temperature (LT, PDF-2 #01-089-6545) modification at 800 °C, while the high-temperature (HT) vaterite modification of GdBO3 formed above 900 °C. This was consistent with the data of Ren et al. [17].
3.1.2. The Dy2O3–Cr2O3–B2O3 Ternary System
The phase relations in the Dy2O3–Cr2O3–B2O3 ternary system at 900, 1000, and 1100 °C (Figure 3, Table 2) were determined based on 21 experimental compositions (seven specimens for each isothermal section). The system exhibited six subsidiary subsystems. The dysprosium chromium borate, DyCr3(BO3)4, was observed. DyBO3 [PDF-2 #01-074-1933] demonstrated the vaterite modification. The powder data from the PDF-2 #01-083-9837 card were used to determine the Dy2O3 phase. Dy(BO2)3 was determined from the similarity with the PXRD patterns of known metaborates of Ln(BO2)3 (Ln = Gd, Tb). The oxyborate Dy3BO6 was isostructural with Y3BO6. All compounds established in the considered diagram were stable up to 1100 °C.
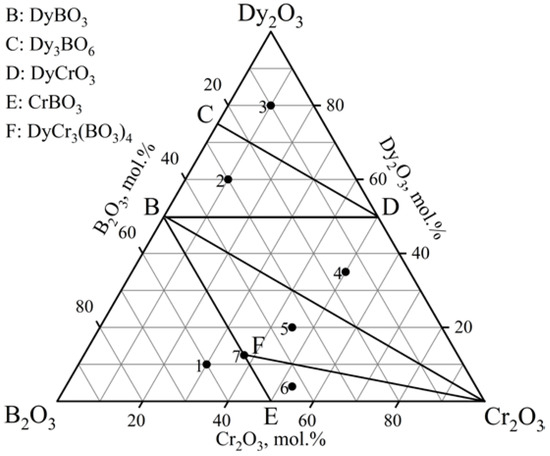
Figure 3.
Phase relations in the Dy2O3–Cr2O3–B2O3 system at 900, 1000, and 1100 °C.

Table 2.
Phase compositions of specimens in the Dy2O3–Cr2O3–B2O3 system as indicated by PXRD.
Vicat et al. [40] reported the synthesis of the double borate DyCr(BO3)2 with a dolomite-type structure. However, the existence of this phase is doubtful. For example, Doi et al. [50] synthesized only LnCr(BO3)2 (Ln = Y, Ho—Lu) borates. Our results confirm these data.
The study of the stability of DyCr3(BO3)4 was carried out in the range of 800–1300 °C. It was found that above 1250 °C, DyBO3 and Cr2O3 were formed (Figure 4). DyCr3(BO3)4 coexisted with DyBO3 and Cr2O3 in the range of 900–1250 °C. The compounds DyBO3, α-Dy(BO2)3, and Cr2O3 were observed below 900 °C.
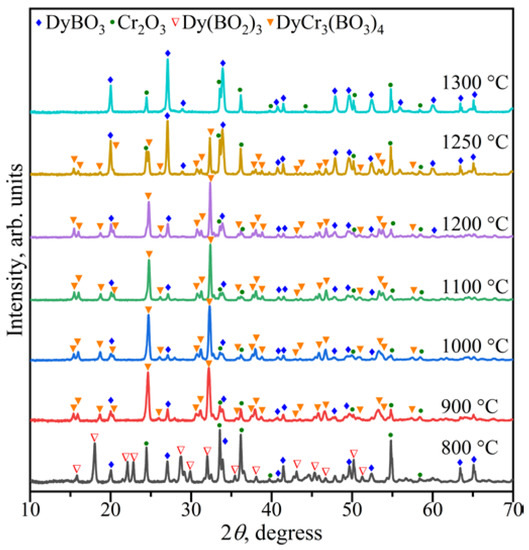
Figure 4.
PXRD patterns of the Dy2O3: 3Cr2O3:4B2O3 composition in the temperature range of 800–1300 °C.
Consequently, the following reactions proceeded:
Dy2O3 + B2O3 → 2DyBO3 (800–1300 °C)
Dy2O3 + 3B2O3 → 2α-Dy(BO2)3 (800 °C)
Dy2O3 + 3Cr2O3 + 4B2O3 → 2DyCr3(BO3)4 (900–1250 °C)
3.1.3. The Ln2O3–Cr2O3–B2O3 (Ln = Ho, Er) Ternary Systems
The phase relations in the Ln2O3–Cr2O3–B2O3 (Ln = Ho, Er) ternary systems at 900, 1000, and 1100 °C (Figure 5, Table 3) on the basis of 27 samples (nine specimens per isothermal section) were investigated. There were seven subsidiary subsystems in each of the systems considered. LnCr3(BO3)4 and LnCr(BO3)2 (HoCr(BO3)2 [PDF-2 #01-082-9333] and ErCr(BO3)2 [PDF-2 #01-082-9334]) borates were found. The orthoborates (HoBO3 [PDF-2 #01-074-1934] and ErBO3 [PDF-2 #01-013-0486] were isostructural to the vaterite modification. The Ho2O3 and Er2O3 phases were determined by using the powder data from the PDF-2 #01-074-3085 and PDF-2 #00-013-0387 cards, respectively. The PXRD data of the isostructural compound Y3BO6 were used to determine the Ln3BO6 phases. All compounds of these systems were stable up to 1100 °C.
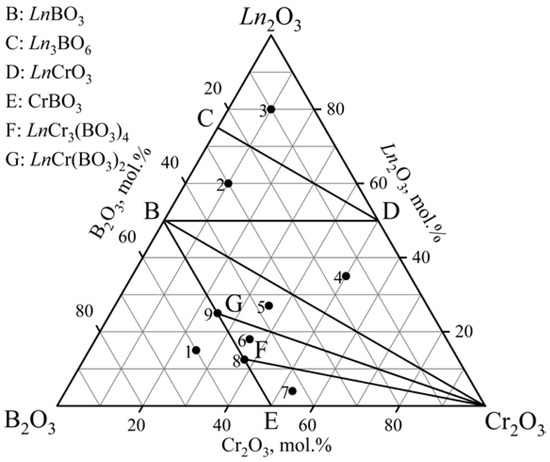
Figure 5.
Phase relations in the Ln2O3–Cr2O3–B2O3 (Ln = Ho, Er) system at 900, 1000, and 1100 °C.

Table 3.
Phase compositions of specimens in the Ln2O3–Cr2O3–B2O3 (Ln = Ho, Er) systems as indicated by PXRD.
Our results confirm the existence of the ErCr3(BO3)4 compound, which was reported only by Kurazhkovskaya et al. [45].
The study of the stability of the borates LnCr3(BO3)4 and LnCr(BO3)2 in the range of 800–1250 °C showed that LnBO3 and Cr2O3 were formed above 1200 °C (Figure 6 and Figure 7). The HoCr3(BO4)3, HoBO3, and Cr2O3 phases were stable at 1200 °C for the Ho2O3:3Cr2O3:4B2O3 and Ho2O3:Cr2O3:2B2O3 compositions. The ErCr(BO3)2, ErBO3, and Cr2O3 phases were stable at 1200 °C for the Er2O3:3Cr2O3:4B2O3 and Er2O3:Cr2O3:2B2O3 compositions. The LnCr3(BO3)4, LnCr(BO3)2, LnBO3, and Cr2O3 phases coexisted from 900 to 1100 °C for the Ln2O3:3Cr2O3:4B2O3 (Ln = Ho, Er) and Ln2O3:Cr2O3:2B2O3 (Ln = Ho, Er) compositions. The LnBO3 and Cr2O3 compounds were observed below 900 °C.
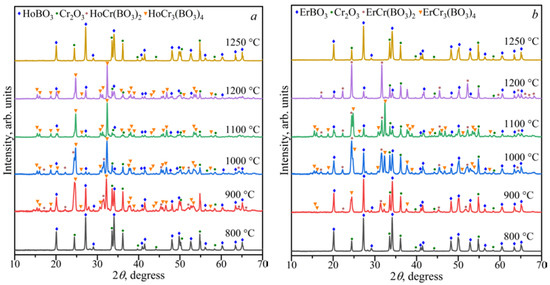
Figure 6.
PXRD patterns of the Ho2O3:3Cr2O3:4B2O3 (a) and Er2O3:3Cr2O3:4B2O3 (b) compositions in the temperature range of 800–1250 °C.
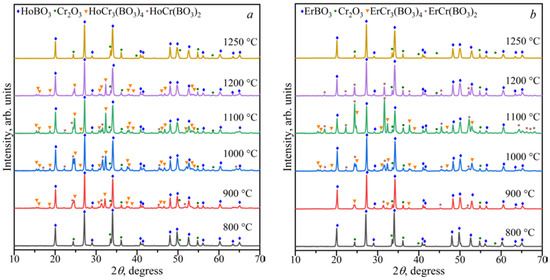
Figure 7.
PXRD patterns of the Ho2O3:Cr2O3:2B2O3 (a) and Er2O3:Cr2O3:2B2O3 (b) compositions in the temperature range of 800–1250 °C.
Thus, the following reactions occurred:
Ln2O3 + B2O3 → 2LnBO3 (800–1250 °C)
Ln2O3 + Cr2O3 + 2B2O3 → 2LnCr(BO3)2 (900–1200 °C for Ln = Er and 900–1100 °C for Ln = Ho)
Ln2O3 + 3Cr2O3 + 4B2O3 → 2LnCr3(BO3)4 (900–1200 °C for Ln = Ho and 900–1100 °C for Ln = Er)
3.1.4. The Ln2O3–Cr2O3–B2O3 (Ln = Tm–Lu) Ternary Systems
The phase relations in the Ln2O3–Cr2O3–B2O3 (Ln = Tm–Lu) ternary systems were defined at 1000 °C (Table 4, Figure 8) based on the phase compositions for 21 samples (seven samples per system). There were six subsidiary subsystems. The double borates (TmCr(BO3)2 [PDF-2 #01-082-9335], YbCr(BO3)2 [PDF-2 #01-082-9336], and LuCr(BO3)2 [PDF-2 #01-082-9337]) were found. The orthoborates TmBO3 [PDF-2 #00-013-0482] and YbBO3 [PDF-2 #01-076-7335] were isostructural to the vaterite modification, and LuBO3 crystallized in the vaterite [PDF-2 #01-085-7631] and calcite [PDF-2 #01-085-7534] modifications. The Tm2O3, Yb2O3, and Lu2O3 phases were determined by using the powder data from the PDF-2 #00-041-1090, PDF-2 #00-041-1106, PDF-2 #00-012-0728 cards, respectively. The oxyborate Tm3BO6 was determined from the PXRD patterns of the isostructural compound Y3BO6, and Ln3BO6 (Ln = Yb, Lu) was established through similarity with the PXRD patterns from the literature [22].

Table 4.
Phase compositions of specimens in the Ln2O3–Cr2O3–B2O3 (Ln = Tm–Lu) systems as indicated by PXRD.
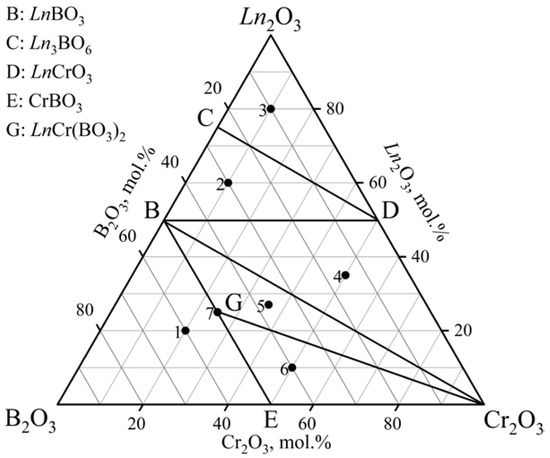
Figure 8.
Phase relations in the Ln2O3–Cr2O3–B2O3 (Ln = Tm–Lu) system at 1000 °C.
Leonyuk et al. [39] reported the formation of YbCr3(BO3)4. Nevertheless, the existence of this phase is in doubt. In studies on the synthesis and characterization of LnCr3(BO3)4 compounds by using vibrational spectroscopy, the authors of [44,45,46] reported huntite-type borates with Ln = La, Pr, Nd, Sm–Er, which corresponded with our data.
The study of the stability LnCr(BO3)2 borates in the range of 800–1200 °C showed that above 1150 °C, the LnBO3 and Cr2O3 phases formed (Figure 9). At temperatures from 900 to 1150 °C, LnCr(BO3)2 borates coexisted with LnBO3, CrBO3, and Cr2O3. The LnBO3 and Cr2O3 compounds were observed below 900 °C.
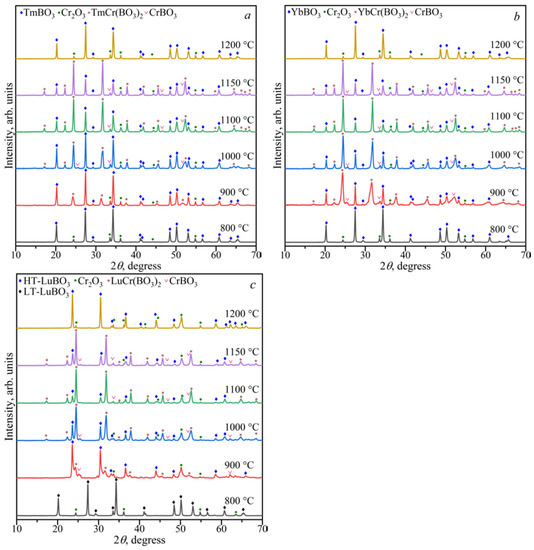
Figure 9.
PXRD patterns of solids phases of the Tm2O3:Cr2O3:2B2O3 (a), Yb2O3:Cr2O3:2B2O3 (b), and Lu2O3:Cr2O3:2B2O3 (c) compositions in the range of 800–1200 °C.
Therefore, the following reactions occurred:
Ln2O3 + B2O3 → 2LnBO3 (800–1200 °C)
Cr2O3 + B2O3 → 2CrBO3 (900–1150 °C)
Ln2O3 + Cr2O3 + 2B2O3 → 2LnCr(BO3)2 (900–1150 °C)
In the Lu2O3–Cr2O3–B2O3 system, at 800 °C, the borate LuBO3 was obtained only in a vaterite-type structure (LT modification of LuBO3); in the temperature range of 900–1000 °C, there were vaterite- and calcite-type structures, and at higher temperatures, they crystallized only in a calcite-type structure (HT modification of LuBO3). The obtained materials were consistent with the data obtained by the authors of [15]. It should be noted that the borate LuBO3 with only the calcite structure in the isothermal section at 1000 °C was observed for regions with a high content of boron oxide (regions 1 and 5 in Figure 8). The coexistence of calcite and vaterite modifications of the borate LuBO3 was observed in other regions.
3.2. Powder X-ray Diffraction
The Le Bail fit was used to confirm the structural similarity of the borates within the families of LnCr3(BO3)4 and LnCr(BO3)2. All huntite-type LnCr3(BO3)4 borates turned out to be polytypes and contained a significant number of rhombohedral (sp. gr. R32, α-LnCr3(BO3)4) and monoclinic (sp. gr. C2/c, β-LnCr3(BO3)4) modifications. The refined lattice parameters are presented in Table 5 and Table 6 for α-, β-LnCr3(BO3)4 and LnCr(BO3)2, respectively. As an example, the convergences of the Le Bail fittings for α-, β-GdCr3(BO3)4 and LuCr(BO3)2 are shown in Figure 10. After the refinement, there was a good agreement between the calculated and experimental diffraction patterns with low-reliability factors. Peaks of impurity phases of LnBO3, CrBO3, and Cr2O3 existed in the diffraction patterns. The decrease in the values of a, b, and c for both groups of compounds was due to a decrease in the ionic radii from Gd3+ to Er3+ [55]. The small deviation of the lattice parameters of LnCr(BO3)2 (Table 6) from those presented by Doi at el. [50] can be attributed to the disorder of Ln3+ and Cr3+ ions in the crystal structure.

Table 5.
Lattice parameters of α-, β-LnCr3(BO3)4 (Ln = Gd–Er) borates (sp. gr. R32 and sp. gr. C2/c).

Table 6.
Lattice parameters of LnCr(BO3)2 (Ln = Ho–Lu) borates (sp. gr. ).
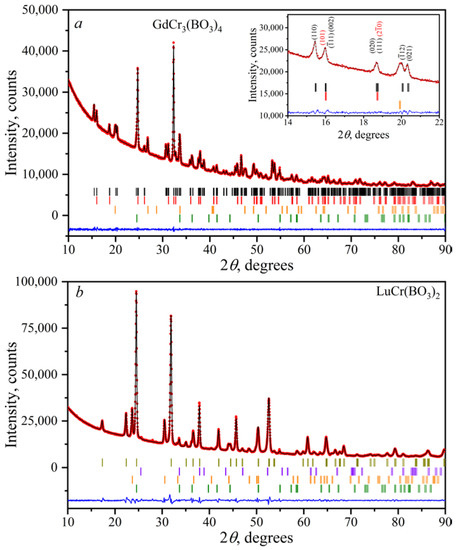
Figure 10.
Final convergence of the Le Bail refinements for α-, β-GdCr3(BO3)4 (a) and LuCr(BO3)2 (b). The calculated and observed diffraction profiles are shown with a black solid line and red circles, respectively. The bottom blue trace is a plot of the difference between the calculated and observed intensities. Black, red, orange, green, dark yellow, and violet vertical marks denote Bragg reflections due to the main and impurity β-GdCr3(BO3)4, α-GdCr3(BO3)4, LnBO3 (Ln = Gd, Lu), Cr2O3, LuCr(BO3)2, and CrBO3 phases, respectively.
It is noted that with an increase in the synthesis temperature, the amount of the monoclinic β-LnCr3(BO3)4 modification increased, with a simultaneous decrease in the rhombohedral α-LnCr3(BO3)4 one. This is shown in Figure 11 by using DyCr3(BO3)4 as an example. Thus, this confirms the high-temperature nature of the monoclinic modification.
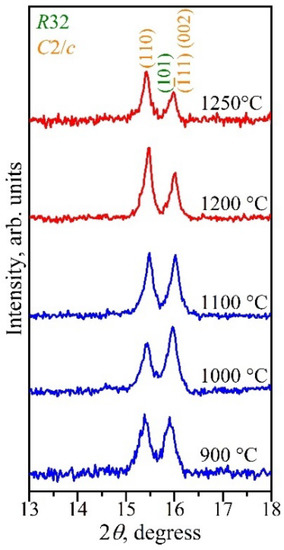
Figure 11.
Bragg reflections , , , and of α-DyCr3(BO3)4 and β-DyCr3(BO3)4 borates synthesized at different temperatures.
Mukherjee et al. [11] reported that they obtained a mixture of α-Dy(BO2)3 and DyBO3. However, they did not give structural parameters for the metaborate α-Dy(BO2)3. The metaborate α-Dy(BO2)3 was also refined with the Le Bail method. The lattice parameters were a = 6.1982 Å, b = 8.0213 Å, c = 7.8021 Å, β = 93.249°, and V = 386.27 Å3. Rwp = 0.77 and Rp = 1.03 became the final values of the R-factors. The additional phases were DyBO3 and Cr2O3. The obtained lattice parameters correlated with the trend towards a decrease in the lattice parameters of the α-Ln(BO2)3 (Ln = La–Nd, Sm–Tb) metaborates with the decrease in the ionic radius of the rare-earth ions [11].
3.3. Thermal Analysis
A thermal analysis was carried out to determine the melting points of rare-earth chromium borates. Figure 12 shows fragments of the DSC curves for the Ln2O3:3Cr2O3:4B2O3 (Ln = Gd–Er) and Ln2O3:Cr2O3:2B2O3 (Ln = Ho–Lu) compositions in the range of the melting points of the LnCr3(BO3)4 and LnCr(BO3)2 borates. The DSC curves had one or two endopeaks. One peak was observed for the Ln2O3:3Cr2O3:4B2O3 (Ln = Gd–Dy) and Ln2O3:Cr2O3:2B2O3 (Ln = Tm–Lu) compositions, and two were observed for the Ln2O3:3Cr2O3:4B2O3 (Ln = Ho, Er) and Ln2O3:Cr2O3:2B2O3 (Ln = Ho, Er) compositions. In the first case, one peak was explained by the melting of LnCr3(BO3)4 or LnCr(BO3)2 (the melting points are presented in Table 7). In the second case, the picture was more complicated. The coexistence of two borates, LnCr3(BO3)4 and LnCr(BO3)2, was found for the Ln2O3:3Cr2O3:4B2O3 (Ln = Ho, Er) and Ln2O3:Cr2O3:2B2O3 (Ln = Ho, Er) compositions (Section 3.1). Thus, the two peaks on the DSC curve were explained by the alternate melting of these two compounds. Table 7 shows that the melting point of the LnCr3(BO3)4 (Ln = Gd–Dy) borates gradually decreased as the atomic number of the rare-earth element in these compounds increased. At the same time, the melting point of the LnCr(BO3)2 (Ln = Tm–Lu) borates increased. Based on this, we attributed the low-temperature peak in the DSC curves to LnCr3(BO3)4 (Ln = Ho, Er), and we attributed the high-temperature peak to LnCr(BO3)2 (Ln = Ho, Er). In this case, we were guided by the peak value of the melting temperature, since, in our opinion, it reflected it better (there was a very wide peak for the ErCr3(BO3)4 borate, which was possibly due to the polycrystalline nature of the samples).
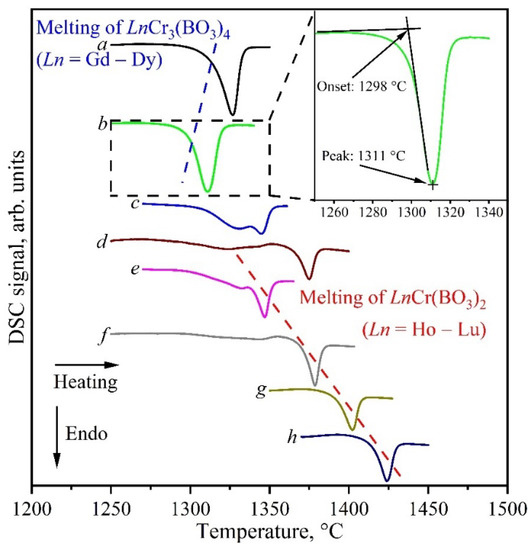
Figure 12.
Fragments of the DSC curves of the Ln2O3:3Cr2O3:4B2O3 (Ln = Gd (a), Dy (b), Ho (c), Er (d)) and Ln2O3:Cr2O3:2B2O3 (Ln = Ho (e), Er (f), Tm (g), Yb (h)) compositions showing the melting points of the LnCr3(BO3)4 and LnCr(BO3)2 borates (the inset shows the notation used at the melting peak).

Table 7.
Melting points of the LnCr3(BO3)4 (Ln = Gd–Er) and LnCr(BO3)2 (Ln = Ho–Lu) borates.
According to DSC and PXRD data (Section 3.1), the thermally induced processes can be illustrated with the following reactions:
2LnCr3(BO3)4 → 2LnBO3 + 3Cr2O3 + 3B2O3 (Ln = Gd–Dy) (1310–1330 °C)
4LnCr3(BO3)4 → 2LnCr(BO3)2 + 2LnBO3 + 5Cr2O3 + 5B2O3 (Ln = Ho–Er) (1320–1330 °C)
2LnCr(BO3)2 → 2LnBO3 + Cr2O3 + B2O3 (Ln = Ho–Lu) (1330–1450 °C)
4. Conclusions
The Ln2O3–Cr2O3–B2O3 (Ln = Gd–Lu) ternary oxide systems were studied with PXRD. In these systems, it was found that the LnCr3(BO3)4 borates were stable up to Ln = Er, after which LnCr(BO3)2 borates crystallized. It was established that LnCr3(BO3)4 borates crystallized in rhombohedral (α-LnCr3(BO3)4) and monoclinic (β-LnCr3(BO3)4) polytype modifications. The latter prevailed at temperatures above 1100 °C. The melting points of LnCr3(BO3)4 (Ln = Gd–Er) were slightly higher than those for LnAl3(BO3)4 (Ln = Gd–Er). They increased with a decrease in the ionic radius of rare-earth ions for LnCr(BO3)2 (Ln = Ho–Lu) borates, while for LnCr3(BO3)4 (Ln = Gd–Er) borates, they decreased to Ln = Dy and then increased.
Author Contributions
Conceptualization, N.K. and V.M.; methodology, N.K., V.M. and E.V.; software, N.K.; validation, N.K., V.M., E.M., E.V., K.B. and E.K.; formal analysis, N.K., V.M., E.M., E.V. and K.B.; investigation, N.K., V.M. and E.M.; resources, V.M. and E.V.; data curation, N.K. and V.M.; writing—original draft preparation, N.K., V.M. and E.V.; writing—review and editing, N.K., V.M., E.M., E.V. and K.B.; visualization, N.K.; supervision, V.M.; project administration, V.M. and K.B.; funding acquisition, N.K. and K.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the research project FFUU-2022-0003 of the Institute of Spectroscopy of the Russian Academy of sciences. N.K. and K.B. are members of the Leading Scientific School of the Russian Federation (grant of the President of the Russian Federation NSh-776.2022.1.2).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data can be obtained from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Levin, E.M. Liquid Immiscibility in Oxide Systems. In Phase Diagrams: Materials Science and Technology; Alper, A.M., Ed.; Academic Press: New York, NY, USA, 1970; Volume 3, pp. 143–236. ISBN 978-0-12-053203-2. [Google Scholar]
- Levin, E.M.; Robbins, C.R.; Waring, J.L. Immiscibility and the System Lanthanum Oxide-Boric Oxide. J. Am. Ceram. Soc. 1961, 44, 87–91. [Google Scholar] [CrossRef]
- Li, L.; Lu, P.; Wang, Y.; Jin, X.; Li, G.; Wang, Y.; You, L.; Lin, J. Synthesis of Rare Earth Polyborates Using Molten Boric Acid as a Flux. Chem. Mater. 2002, 14, 4963–4968. [Google Scholar] [CrossRef]
- Li, L.; Jin, X.; Li, G.; Wang, Y.; Liao, F.; Yao, G.; Lin, J. Novel Rare Earth Polyborates. 2. Syntheses and Structures. Chem. Mater. 2003, 15, 2253–2260. [Google Scholar] [CrossRef]
- Ysker, J.S.; Hoffmann, W. Die Kristallstruktur des La[B3O6]: Ein neuer Ketten-Borat-Typ. Naturwissenschaften 1970, 57, 129. [Google Scholar] [CrossRef]
- Goubin, F.; Montardi, Y.; Deniard, P.; Rocquefelte, X.; Brec, R.; Jobic, S. Optical Properties of CeBO3 and CeB3O6 Compounds: First-Principles Calculations and Experimental Results. J. Solid State Chem. 2004, 177, 89–100. [Google Scholar] [CrossRef]
- Sieke, C.; Nikelski, T.; Schleid, T. Pr(BO2)3 Und PrCl(BO2)2: Zwei Meta-Borate Des Praseodyms Im Vergleich. Z. Anorg. Und Allg. Chem. 2002, 628, 819. [Google Scholar] [CrossRef]
- Müller-Bunz, H.; Nikelski, T.; Schleid, T. Einkristalle Des Neodym(III)-Meta-Borats Nd(BO2)3 Und -Ortho-Borats Nd[BO3]/Single Crystals of the Neodymium(III) Meta-Borate Nd(BO2)3 and Ortho-Borate Nd[BO3]. Z. Nat. B 2003, 58, 375–380. [Google Scholar] [CrossRef]
- Goriounova, A.; Held, P.; Becker, P.; Bohatý, L. Monoclinic Modification of Polymorphic TbB3O6. Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, i83–i85. [Google Scholar] [CrossRef]
- Bambauer, H.U.; Weidelt, J.; Ysker, J.-S. Röntgenographische Und Optische Untersuchungen an Boraten Seltener Erden SE(BO2)3. Z. Krist. Cryst. Mater. 1969, 130, 207–213. [Google Scholar] [CrossRef]
- Mukherjee, P.; Suard, E.; Dutton, S.E. Magnetic Properties of Monoclinic Lanthanide Metaborates, Ln(BO2)3, Ln = Pr, Nd, Gd, Tb. J. Phys. Condens. Matter 2017, 29, 405807. [Google Scholar] [CrossRef]
- Nikelski, T.; Schleid, T. Synthese Und Kristallstruktur von Terbium(III)-Meta-Oxoborat Tb(BO2)3 (≡ TbB3O6). Z. Anorg. Und Allg. Chem. 2003, 629, 1017–1022. [Google Scholar] [CrossRef]
- Nikelski, T.; Schäfer, M.C.; Huppertz, H.; Schleid, T. Crystal Structure of Dysprosium Meta-Oxoborate, β-Dy(BO2)3, via Normal-Pressure Synthesis. Z. Krist. New Cryst. Struct. 2008, 223, 177–178. [Google Scholar] [CrossRef]
- Levin, E.M.; Roth, R.S.; Martin, J.B. Polymorphism of ABO3 Type Rare Earth Borates. Am. Mineral. 1961, 46, 1030–1055. [Google Scholar]
- Wu, Y.; Ding, D.; Pan, S.; Yang, F.; Ren, G. Research on Phase Transition Behavior of Lutetium Orthoborate LuBO3. Phase Transit. 2011, 84, 315–324. [Google Scholar] [CrossRef]
- Fedorov, P.P. Morphotropism of Rare-Earth Orthoborates RBO3. J. Struct. Chem. 2019, 60, 679–691. [Google Scholar] [CrossRef]
- Ren, M.; Lin, J.H.; Dong, Y.; Yang, L.Q.; Su, M.Z.; You, L.P. Structure and Phase Transition of GdBO3. Chem. Mater. 1999, 11, 1576–1580. [Google Scholar] [CrossRef]
- Lin, J.H.; Su, M.Z.; Wurst, K.; Schweda, E. The Structure of La26(BO3)8O27: A Structure with a Distorted Fluorite Type Arrangement of Atoms. J. Solid State Chem. 1996, 126, 287–291. [Google Scholar] [CrossRef]
- Noirault, S.; Célérier, S.; Joubert, O.; Caldes, M.T.; Piffard, Y. Effects of Water Uptake on the Inherently Oxygen-Deficient Compounds Ln26O27□(BO3)8 (Ln = La, Nd). Inorg. Chem. 2007, 46, 9961–9967. [Google Scholar] [CrossRef]
- Lin, J.H.; You, L.P.; Lu, G.X.; Yang, L.Q.; Su, M.Z. Structural and Luminescent Properties of Eu3+ Doped Gd17.33(BO3)4(B2O5)2O16. J. Mater. Chem. 1998, 8, 1051–1054. [Google Scholar] [CrossRef]
- Lin, J.H.; Zhou, S.; Yang, L.Q.; Yao, G.Q.; Su, M.Z.; You, L.P. Structure and Luminescent Properties of Y17.33(BO3)4(B2O5)2O16. J. Solid State Chem. 1997, 134, 158–163. [Google Scholar] [CrossRef]
- Yang, M.; Li, K.; Su, J.; Huang, Q.; Bao, W.; You, L.; Li, Z.; Wang, Y.; Jiang, Y.; Liao, F.; et al. Study on the Crystal Structure of the Rare Earth Oxyborate Yb26B12O57 from Powder X-ray and Neutron Diffraction. J. Alloys Compd. 2011, 509, 4707–4713. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Z.; Zhang, X.; Marathe, A.; Cordes, D.B.; Weeks, B.; Chaudhuri, J. Tunable Photoluminescence and Energy Transfer of YBO3:Tb3+, Eu3+ for White Light Emitting Diodes. J. Mater. Chem. C 2013, 1, 7202. [Google Scholar] [CrossRef]
- Yang, R.; Sun, X.; Jiang, P.; Gao, W.; Cong, R.; Yang, T. Sol-Gel Syntheses of Pentaborate β-LaB5O9 and the Photoluminescence by Doping with Eu3+, Tb3+, Ce3+, Sm3+, and Dy3+. J. Solid State Chem. 2018, 258, 212–219. [Google Scholar] [CrossRef]
- Zhu, Q.; Fan, Z.; Wang, S.; Xiahou, J.; Li, J. Uniform Colloidal Spheres for RE3BO6 (RE = Eu-Yb, Y) and Excitation-dependent Luminescence of Y3BO6:Eu3+ Red Phosphor. J. Am. Ceram. Soc. 2019, 102, 7448–7461. [Google Scholar] [CrossRef]
- Tombs, N.C.; Croft, W.J.; Mattraw, H.C. Preparation and Properties of Chromium Borate. Inorg. Chem. 1963, 2, 872–873. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Nazar, L.F. Synthesis, Structure, and Solid-State Electrochemical Properties of Cr3BO6: A New Chromium(III) Borate with the Norbergite Structure. J. Mater. Chem. 2001, 11, 3228–3233. [Google Scholar] [CrossRef]
- Bither, T.A.; Frederick, C.G.; Gier, T.E.; Weiher, J.F.; Young, H.S. Ferromagnetic VBO3 and Antiferromagnetic CrBO3. Solid State Commun. 1970, 8, 109–112. [Google Scholar] [CrossRef]
- Schneider, S.J.; Roth, R.S.; Waring, J.L. Solid State Reactions Involving Oxides of Trivalent Cations. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1961, 65A, 345–374. [Google Scholar] [CrossRef] [PubMed]
- Quezel-Ambrunaz, S.; Mareschal, M. Paramètres cristallins des chromites de terres rares. Bull. Soc. Fr. Mineral. Cristallogr. 1963, 86, 204–205. [Google Scholar] [CrossRef]
- Geller, S. Crystallographic Studies of Perovskite-like Compounds. IV. Rare Earth Scandates, Vanadites, Galliates, Orthochromites. Acta Crystallogr. 1957, 10, 243–248. [Google Scholar] [CrossRef]
- Berjoan, R. Contribution to the Study of the Interactions between Oxygen and Lanthanum Oxide, Chromic Oxide, or Lanthanum Chromite. Rev. Int. Des Hautes Temp. Des Refract. 1976, 13, 119–135. [Google Scholar]
- Du, Y.; Cheng, Z.X.; Wang, X.-L.; Dou, S.X. Structure, Magnetic, and Thermal Properties of Nd1-xLaxCrO3 (0≤x≤1.0). J. Appl. Phys. 2010, 108, 093914. [Google Scholar] [CrossRef]
- Kumar, S.; Coondoo, I.; Rao, A.; Lu, B.-H.; Kuo, Y.-K.; Kholkin, A.L.; Panwar, N. Impact of Low Level Praseodymium Substitution on the Magnetic Properties of YCrO3 Orthochromites. Phys. B Condens. Matter 2017, 510, 104–108. [Google Scholar] [CrossRef]
- Serrao, C.R.; Kundu, A.K.; Krupanidhi, S.B.; Waghmare, U.V.; Rao, C.N.R. Biferroic YCrO3. Phys. Rev. B 2005, 72, 220101. [Google Scholar] [CrossRef]
- Sahu, J.R.; Serrao, C.R.; Ray, N.; Waghmare, U.V.; Rao, C.N.R. Rare Earth Chromites: A New Family of Multiferroics. J. Mater. Chem. 2007, 17, 42–44. [Google Scholar] [CrossRef]
- Siemons, M.; Simon, U. High Throughput Screening of the Propylene and Ethanol Sensing Properties of Rare-Earth Orthoferrites and Orthochromites. Sens. Actuators B Chem. 2007, 126, 181–186. [Google Scholar] [CrossRef]
- Ballman, A.A. A New Series of Synthetic Borates Isostructural with the Carbonate Mineral Huntite. Am. Mineral. 1962, 47, 1380–1383. [Google Scholar]
- Leonyuk, N.I.; Leonyuk, L.I. Growth and Characterization of RM3(BO3)4 Crystals. Prog. Cryst. Growth Charact. Mater. 1995, 31, 179–278. [Google Scholar] [CrossRef]
- Vicat, J.; Aléonard, S. Etude de borates MeMe′(BO3)2 de structure dolomite. Mater. Res. Bull. 1968, 3, 611–620. [Google Scholar] [CrossRef]
- Vicat, J.; Aléonard, S. Borates de terres rares TCr(BO3)2 de structure dolomite. Bull. Soc. Fr. Mineral. Crystallogr. 1968, 91, 293–295. [Google Scholar] [CrossRef]
- Kuzmin, N.N.; Maltsev, V.V.; Volkova, E.A.; Leonyuk, N.I.; Boldyrev, K.N.; Bludov, A.N. Growth and Spectroscopic and Magnetic Properties of TbCr3(BO3)4 Crystals. Inorg. Mater. 2020, 56, 828–835. [Google Scholar] [CrossRef]
- Gondek, Ł.; Szytuła, A.; Przewoźnik, J.; Żukrowski, J.; Prokhorov, A.; Chernush, L.; Zubov, E.; Dyakonov, V.; Duraj, R.; Tyvanchuk, Y. On the Peculiar Properties of Triangular-Chain EuCr3(BO3)4 Antiferromagnet. J. Solid State Chem. 2014, 210, 30–35. [Google Scholar] [CrossRef]
- Kurazhkovskaya, V.S.; Borovikova, E.Y.; Leonyuk, N.I.; Koporulina, E.V.; Belokoneva, E.L. Infrared Spectroscopy and the Structure of Polytypic Modifications of RM3(BO3)4 Borates (R—Nd, Gd, Y; M—Al, Ga, Cr, Fe). J. Struct. Chem. 2008, 49, 1035–1041. [Google Scholar] [CrossRef]
- Kurazhkovskaya, V.S.; Dobretsova, E.A.; Borovikova, E.Y.; Mal’tsev, V.V.; Leonyuk, N.I. Infrared Spectroscopy and the Structure of Rare-Earth Chromium Borates RCr3(BO3)4 (R = La—Er). J. Struct. Chem. 2011, 52, 699–707. [Google Scholar] [CrossRef]
- Borovikova, E.Y.; Dobretsova, E.A.; Boldyrev, K.N.; Kurazhkovskaya, V.S.; Maltsev, V.V.; Leonyuk, N.I. Vibrational Spectra and Factor Group Analysis of Rare-Earth Chromium Borates, RCr3(BO3)4, with R = La–Ho. Vib. Spectrosc. 2013, 68, 82–90. [Google Scholar] [CrossRef]
- Boldyrev, K.N.; Kuz’min, N.N.; Mukhin, A.A.; Ivanov, V.Y.; Dobretsova, E.A.; Popova, E.A.; Gavrilkin, S.Y.; Leonyuk, N.I.; Maltsev, V.V.; Malkin, B.Z.; et al. Thermal and Magnetic Properties and Optical Spectroscopy of SmCr3(BO3)4. Phys. Rev. Mater. 2021, 5, 104413. [Google Scholar] [CrossRef]
- Bludov, A.; Savina, Y.; Kobets, M.; Khrustalyov, V.; Savitsky, V.; Gnatchenko, S.; Zajarniuk, T.; Lynnyk, A.; Gutowska, M.U.; Szewczyk, A.; et al. Features of Magnetic and Magnetoelectric Properties, H-T Phase Diagram of GdCr3(BO3)4. J. Magn. Magn. Mater. 2020, 512, 167010. [Google Scholar] [CrossRef]
- Bludov, A.N.; Savina, Y.O.; Pashchenko, V.A.; Gnatchenko, S.L.; Zajarniuk, T.; Lynnyk, A.; Gutowska, M.U.; Szewczyk, A.; Kolodiy, I.V.; Mal’tsev, V.V.; et al. Magnetic Properties of DyCr3(BO3)4. Low Temp. Phys. 2020, 46, 697–703. [Google Scholar] [CrossRef]
- Doi, Y.; Satou, T.; Hinatsu, Y. Crystal Structures and Magnetic Properties of Lanthanide Containing Borates LnM(BO3)2 (Ln=Y, Ho–Lu; M=Sc, Cr). J. Solid State Chem. 2013, 206, 151–157. [Google Scholar] [CrossRef]
- Sinclair, R.; Zhou, H.D.; Lee, M.; Choi, E.S.; Li, G.; Hong, T.; Calder, S. Magnetic Ground States and Magnetodielectric Effect in RCr(BO3)2 (R = Y and Ho). Phys. Rev. B 2017, 95, 174410. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A Quality Materials Characterization Database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General Features. Z. Für Krist. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Cohen-Adad, M.T.; Aloui-Lebbou, O.; Goutaudier, C.; Panczer, G.; Dujardin, C.; Pedrini, C.; Florian, P.; Massiot, D.; Gerard, F.; Kappenstein, C. Gadolinium and Yttrium Borates: Thermal Behavior and Structural Considerations. J. Solid State Chem. 2000, 154, 204–213. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).