Experimental Study on the Dissolution Characteristics and Microstructure of Carbonate Rocks under the Action of Thermal–Hydraulic–Chemical Coupling
Abstract
1. Introduction
2. Test Equipment and Experimental Methodology
2.1. Test Samples
2.2. Test Device
2.2.1. Dissolution Test Equipment
2.2.2. CT Scanning Equipment
2.2.3. NMR Equipment
2.3. Test Plan
2.3.1. Orthogonal Dissolution Test Scheme
2.3.2. CT Scanning Test Scheme
2.3.3. NMR Test Scheme
2.4. Test Steps
2.4.1. Initial CT Scans of Carbonate Rocks
2.4.2. Dissolution of Carbonate Rocks
2.4.3. Second CT Scanning of Carbonate Rocks
2.4.4. The NMR of Carbonate Rocks
3. Results and Discussion
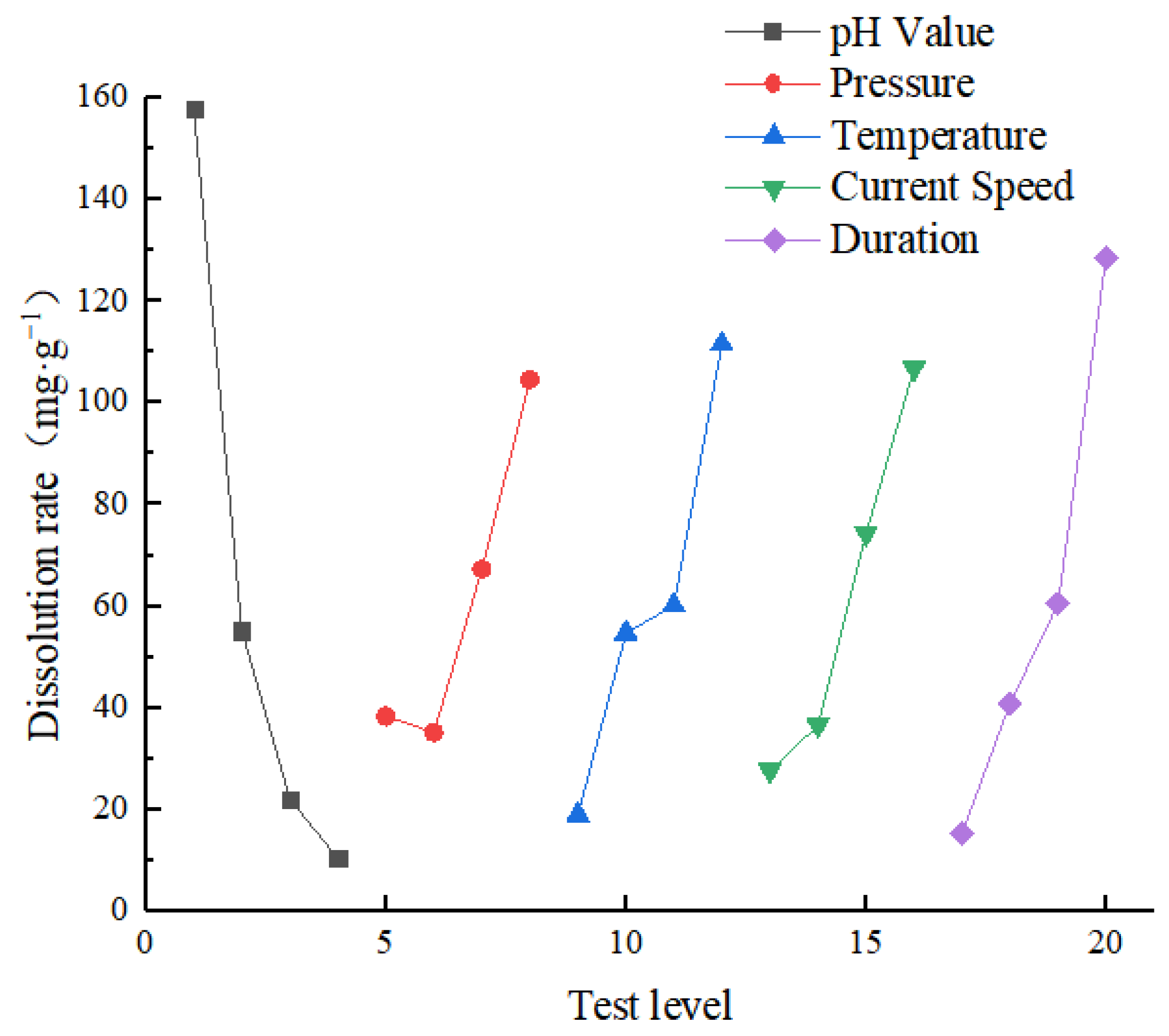
3.1. Analysis of Dissolution Results and Influencing Factors
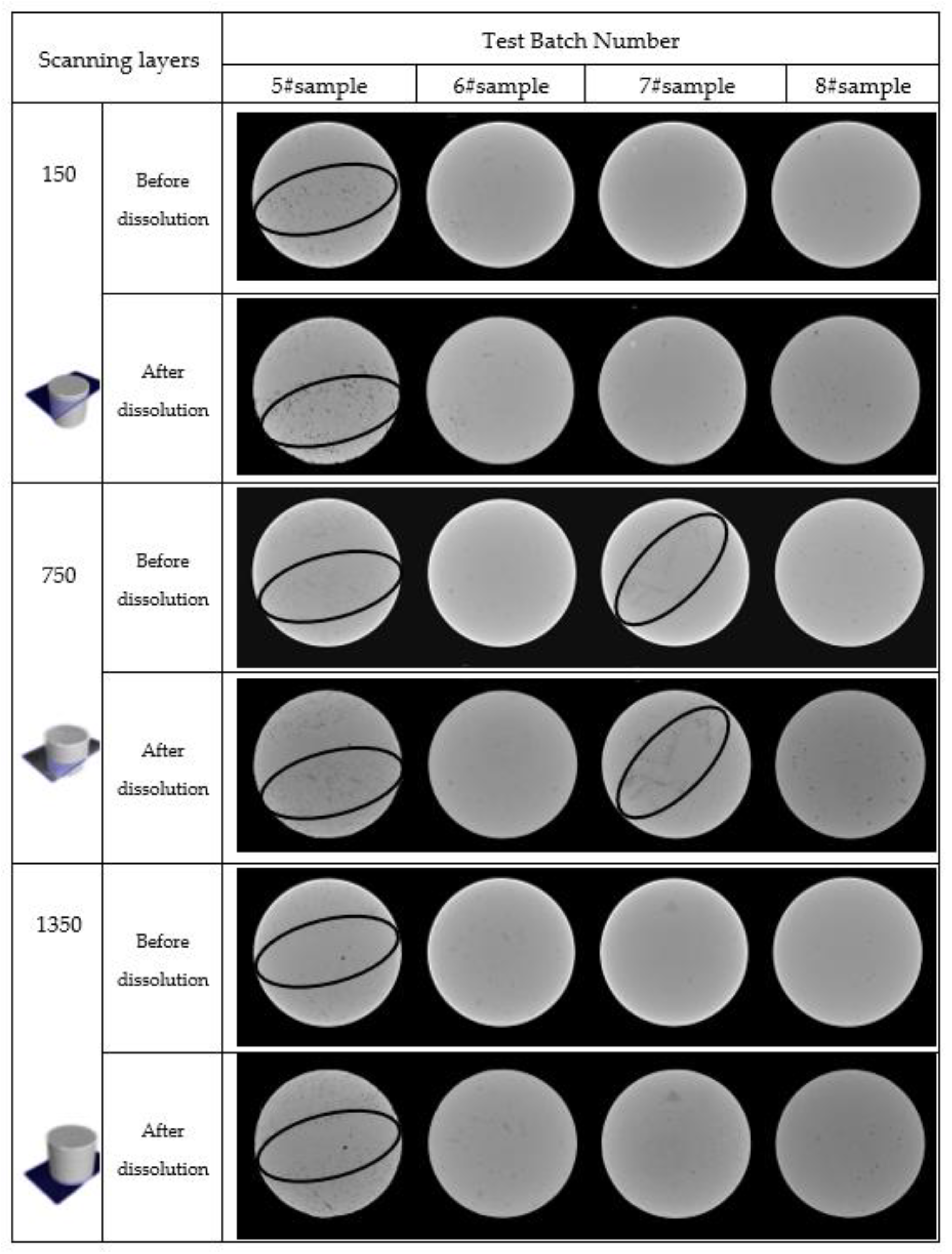
3.2. Analysis of CT Scan Test Results
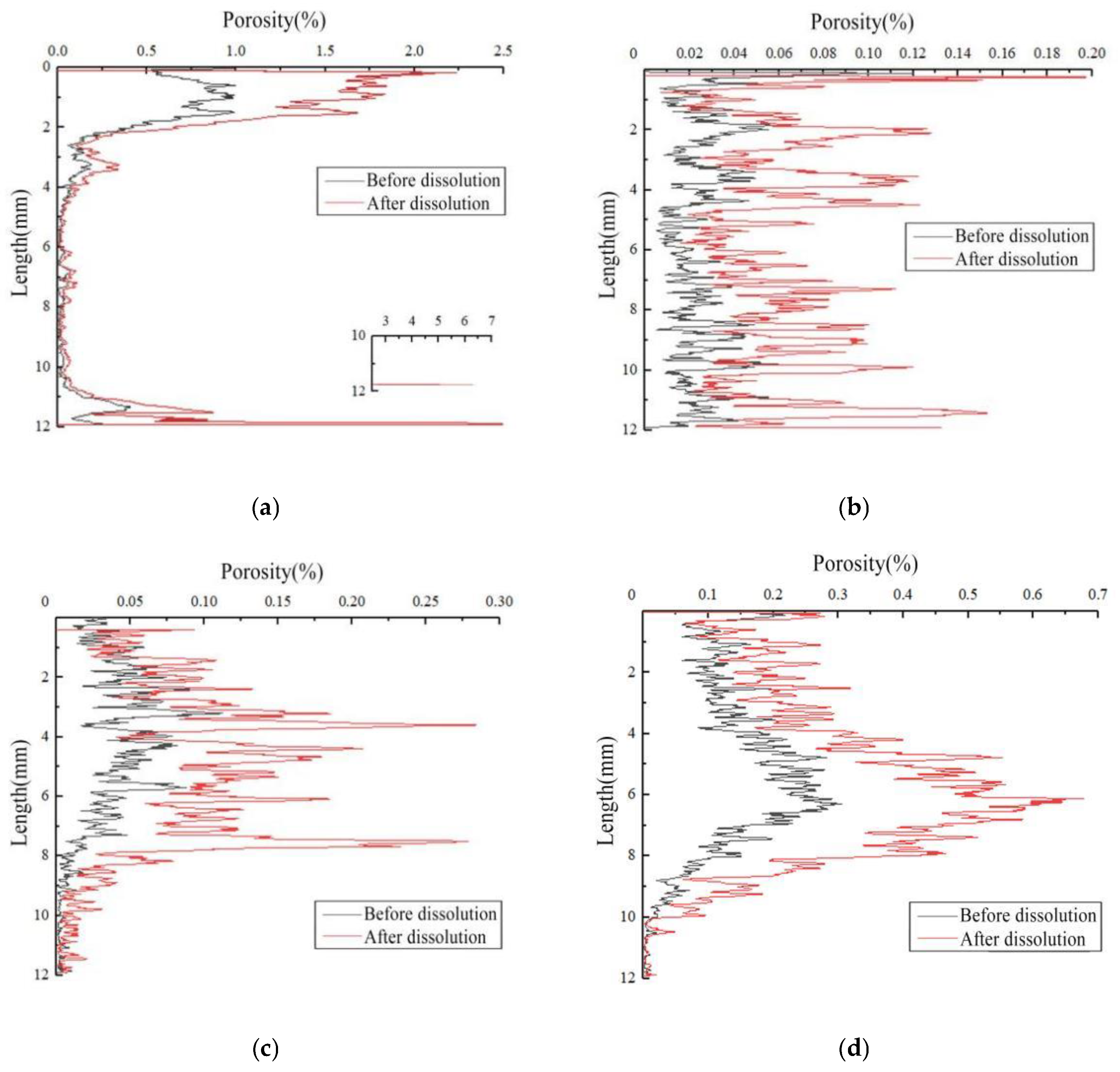
3.2.1. Spatial Distribution Characteristics of Pores
3.2.2. Quantitative Characterization of Pore Structure
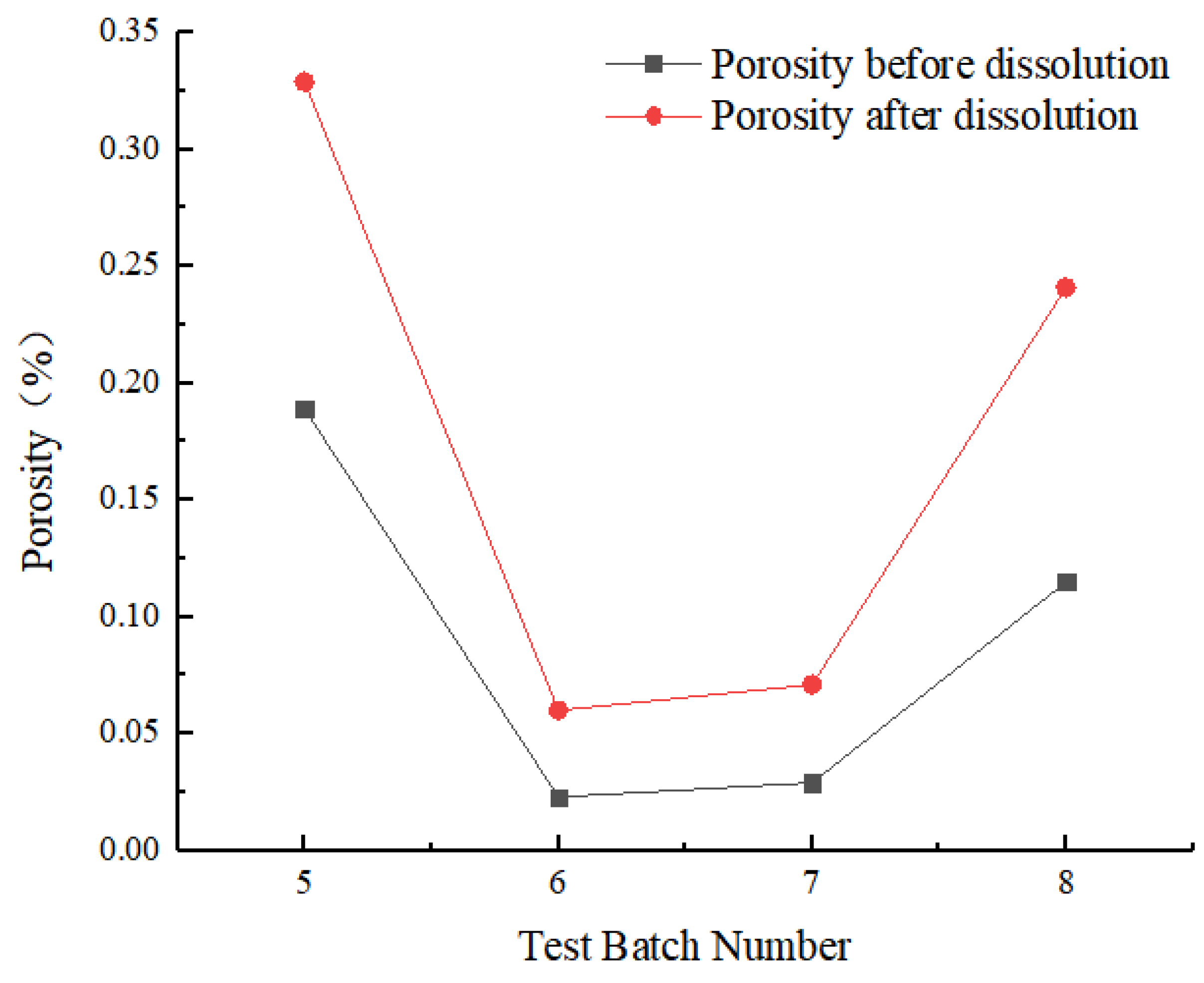
- Porosity change
- 2.
- Pore number and volume change
- 3.
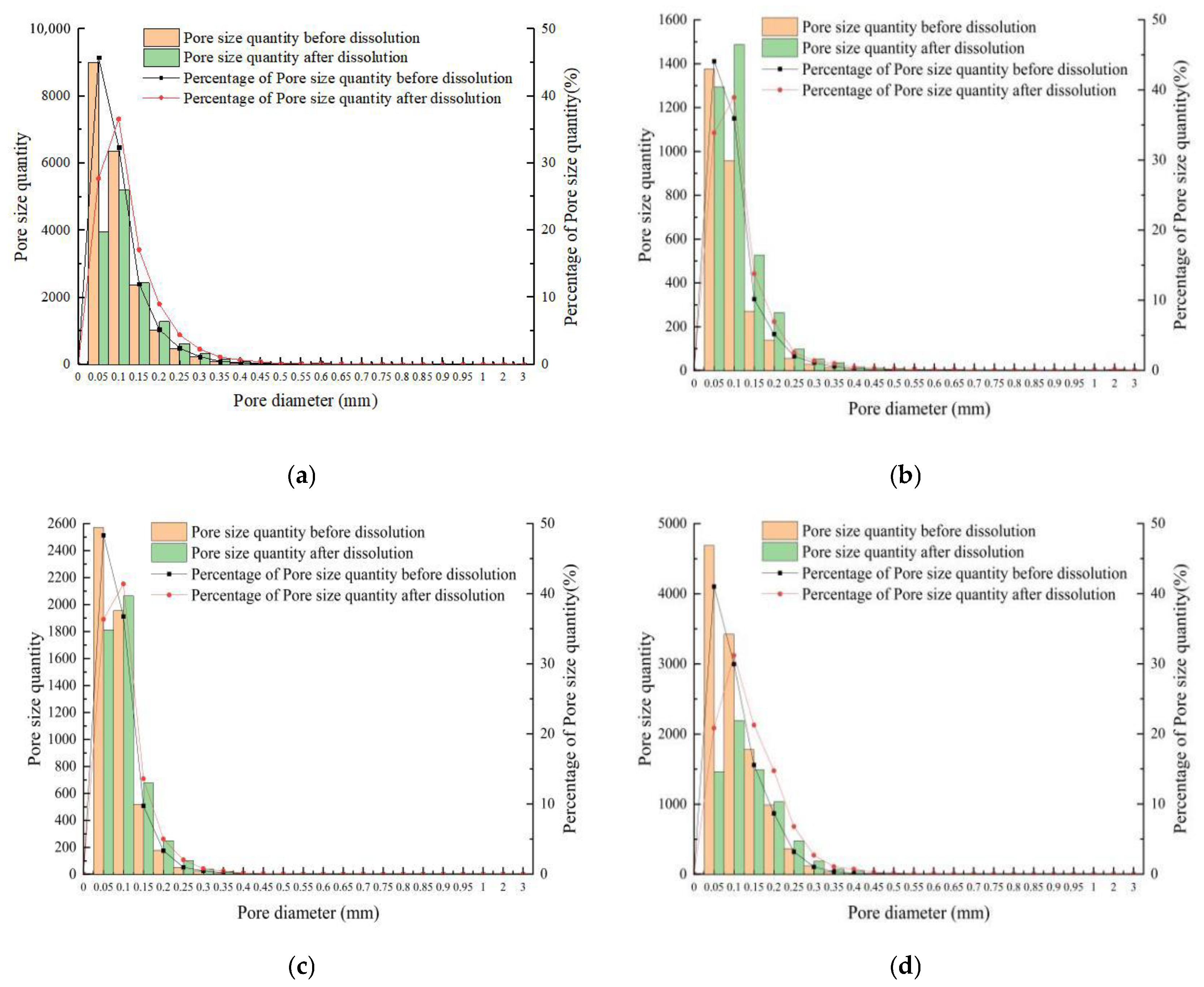
- Change in pore size distribution
3.3. Analysis of NMR Test Results
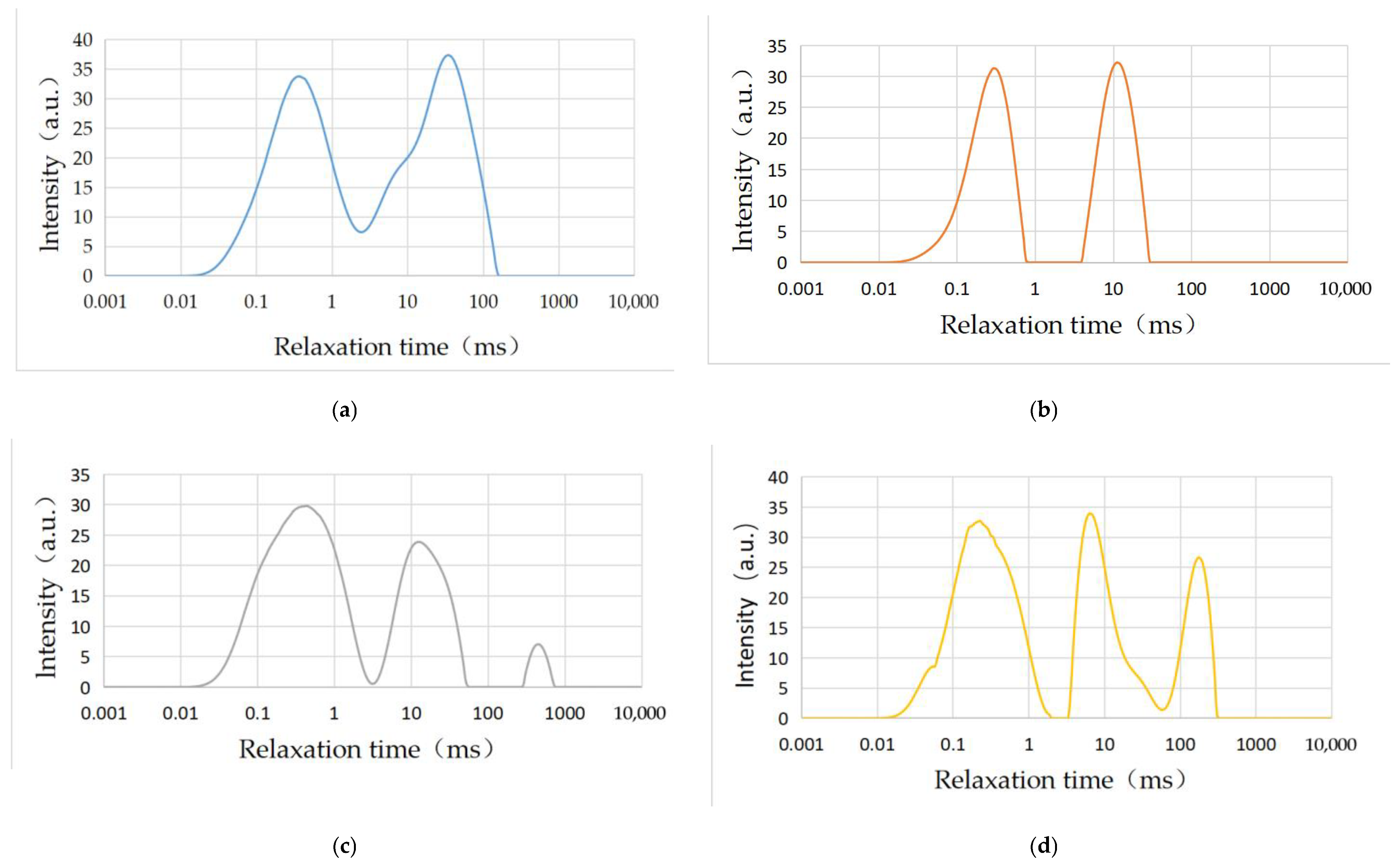
3.3.1. T2 Spectrum Analysis
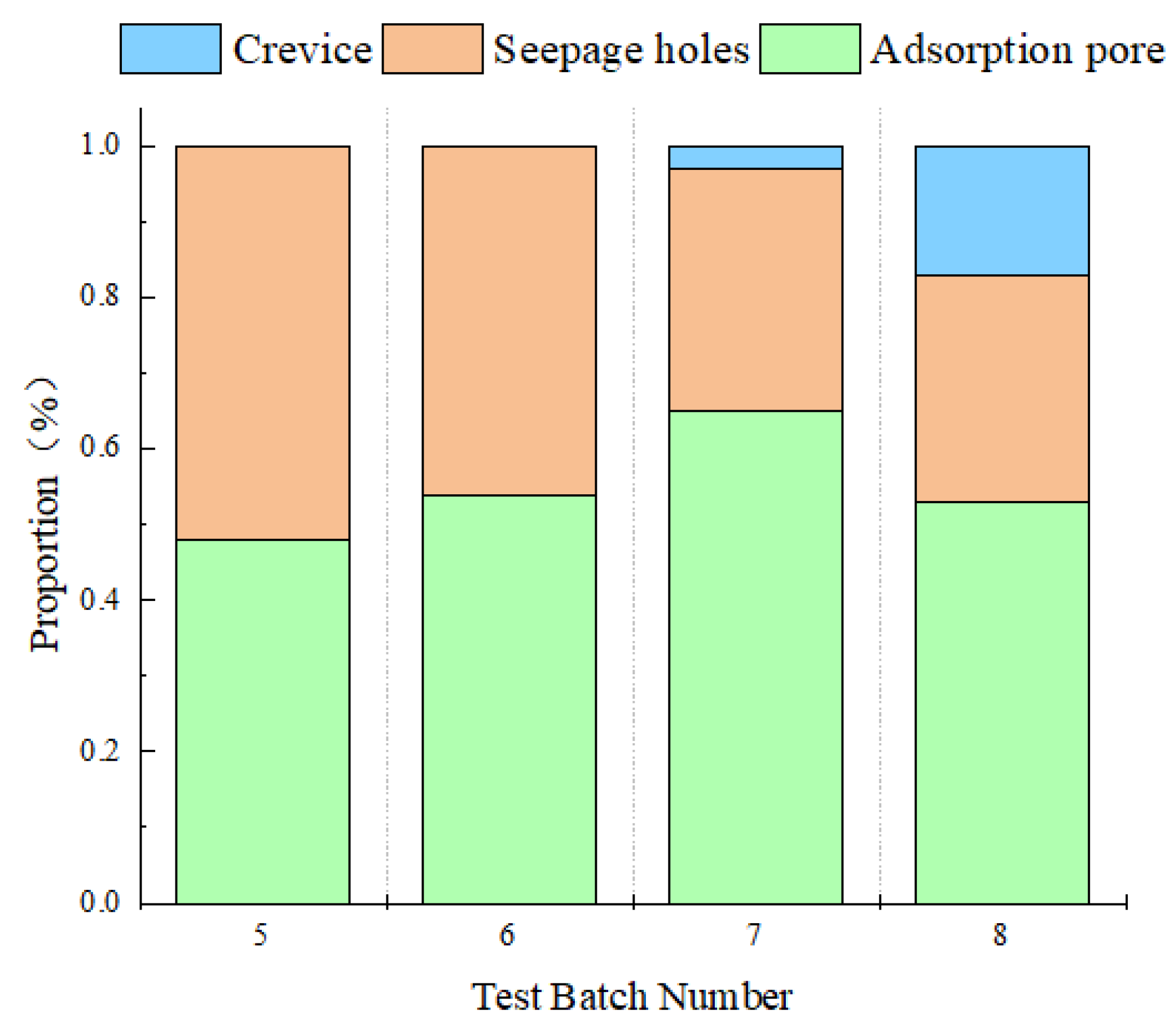
3.3.2. Pore Structure Analysis
4. Summary and Conclusions
- The highest dissolution rate can be achieved under working condition 4 (when the five influence factors of the test are set at pH 4.6, 8 MPa, 85 °C, 75 mL·min−1, and 21 days). The rate is 364.43 mg/g. The lowest dissolution rate can be attained under working condition 16 (when five influence factors of the test are set at pH 7.0, 8 MPa, 25 °C, 55 mL·min−1, and 7 days). The lowest rate is 3.69 mg/g. The dissolution rate is proportional to the flow velocity, temperature, and hydrodynamic pressure and inversely proportional to the pH. Furthermore, the sensitivity, significance, and contribution rate of each influencing factor to each parameter were determined using range analysis and variance analysis. The most influential factor in the dissolution effect is pH, followed by time, temperature, current speed, and hydrodynamic pressure. Moreover, pH has the greatest contribution rate to the erosion rate of rock samples, accounting for 42.36%, followed by time, temperature, current speed, and hydrodynamic pressure.
- Carbonate rocks have a complex pore space system with different pore compositions at the same facilities. The initial porosity of the four rock samples (5#–8#) was 0.189%, 0.023%, 0.029%, and 0.115%, and an increase of 0.140%, 0.038%, 0.042%, and 0.126%, respectively, was observed after the dissolution. The dissolution can improve overall porosity, and the initial porosity of carbonate rock significantly affected the increase in the dissolution-induced porosity. The change in porosity runs throughout the entire sample; the porosity of the upper part is greater than that of the lower part [67].
- The total number of pores decreases, while the volume of pores increases after rock dissolution. The number and percentage distribution of pores before and after dissolution increase and decrease as the single pore volume increases [68]. The percentage distribution of pore volume before dissolution has the characteristics of right deviation. Moreover, the percentage distribution of pore volume after dissolution has the characteristics of left deviation, and the peak distribution is flatter than before dissolution. When the single pore volume exceeds 0.0001 mm3, the pore volume percentage significantly increases. After dissolution, the average pore size of rock samples increased with the same distribution trend. The corresponding pore size decreased as the single pore size increased before and after dissolution. The PSD of carbonate rocks is logarithmic right deviation before dissolution and slightly left deviation after dissolution. The increased PSD skewness confirms the increase in overall pore size, and the decreased PSD kurtosis shows more aperture ratios scattered around the median before dissolution [69].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morse, J.W.; Arvidson, R.S.; Lüttge, A. Calcium Carbonate Formation and Dissolution. Chem. Rev. 2007, 107, 342–381. [Google Scholar] [CrossRef] [PubMed]
- Burdige, D.J.; Hu, X.; Zimmerman, R.C. The Widespread Occurrence of Coupled Carbonate Dissolution/Reprecipitation in Surface Sediments on the Bahamas Bank. Am. J. Sci. 2010, 310, 492–521. [Google Scholar] [CrossRef]
- Lunstrum, A.; Berelson, W. CaCO3 dissolution in carbonate-poor shelf sands increases with ocean acidification and porewater residence time. Geochim. Cosmochim. Acta 2022, 329, 168–184. [Google Scholar] [CrossRef]
- Cai, W.J.; Feely, R.A.; Testa, J.M.; Li, M.; Evans, W.; Alin, S.R.; Xu, Y.Y.; Pelletier, G.; Ahmed, A.; Greeley, D.J.; et al. Natural and Anthropogenic Drivers of Acidification in Large Estuaries. Annu. Rev. Mar. Sci. 2021, 13, 23–55. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.E.B.; La Bruna, V.; Rustichelli, A.; Bezerra, F.H.R.; Xavier, M.M.; Audra, P.; Barbosa, J.A.; Antonino, A.C.D. Structural and Sedimentary Discontinuities Control the Generation of Karst Dissolution Cavities in a Carbonate Sequence, Potiguar Basin, Brazil. Mar. Pet. Geol. 2021, 123, 104753. [Google Scholar] [CrossRef]
- Zhang, C. Carbonate Rock Dissolution Rates in Different Landuses and Their Carbon Sink Effect. Chin. Sci. Bull. 2011, 56, 3759–3765. [Google Scholar] [CrossRef]
- Meng, J.Z.; Chen, S.L.; Wang, J.X.; Chen, Z.; Zhang, J.Y. Development and application of carbonate dissolution test equipment under thermal–hydraulic–chemical coupling condition. Materials 2022, 15, 7383. [Google Scholar] [CrossRef]
- Sjöberg, E.L.; Rickard, D.T. Temperature dependence of calcite dissolution kinetics between 1 and 62 °C at pH 2.7 to 8.4 in aqueous solutions. Geochim. Cosmochim. Acta 1984, 48, 485–493. [Google Scholar] [CrossRef]
- Amrhein, C.; Jurinak, J.J.; Moore, W.M. Kinetics of Calcite Dissolution as Affected by Carbon Dioxide Partial Pressure. Soil Sci. Soc. Am. J. 1985, 49, 1393–1398. [Google Scholar] [CrossRef]
- Buhmann, D.; Dreybrodt, W. Calcite dissolution kinetics in the system H2O–CO2–CaCO3 with participation of foreign ions. Chem. Geol. 1987, 64, 89–102. [Google Scholar] [CrossRef]
- Ma, K.; Liu, G. Three-dimensional discontinuous deformation analysis of failure mechanisms and movement characteristics of slope rockfalls. Rock Mech. Rock Eng. 2022, 55, 275–296. [Google Scholar] [CrossRef]
- Plummer, L.N.; Parkhurst, D.L.; Wigley, T.M.L. The kinetics of calcite dissolution in CO2–water systems at 5 to 60 °C and 0.0 to 1.0 atm CO2. Am. J. Sci. 1978, 278, 179–216. [Google Scholar] [CrossRef]
- Plummer, L.N.; Parkhurst, D.L.; Wigley, T.M.L. Critical review of the kinetics of calcite dissolution and precipitation. In Chemical Modeling in Aqueous Systems; Amer Chemical Society: Washington, DC, USA, 1979; Volume 93, pp. 537–573. [Google Scholar]
- Liu, L.H.; Ma, Y.S.; Liu, B.; Wang, C.L. Hydrothermal dissolution of Ordovician carbonates rocks and its dissolution mechanism in Tarim Basin, China. Carbonates Evaporites 2017, 32, 525–537. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, Q.; Gu, Z.; Lu, Y.; Sheng, Z. The Dissolution Mechanism and Karst Development of Carbonate Rocks in Karst Rocky Desertification Area of Zhenfeng–Guanling–Huajiang County, Guizhou, China. Carbonates Evaporites 2019, 34, 45–51. [Google Scholar] [CrossRef]
- Zhang, K.N.; Zhu, K.F.; He, Y.; Zhang, Y.Y. Experimental study on karst development characteristics of calcrete and analysis of its dissolution mechanism. Carbonates Evaporites 2022, 37, 41. [Google Scholar] [CrossRef]
- Alkattan, M.; Oelkers, E.H.; Dandurand, J.L.; Schott, J. An experimental study of calcite and limestone dissolution rates as a function of pH from −1 to 3 and temperature from 25 to 80 °C. Chem. Geol. 1998, 151, 199–214. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Golubev, S.V.; Schott, J. Dissolution Kinetics of Calcite, Dolomite and Magnesite at 25 °C and 0 to 50 Atm PCO2. Chem. Geol. 2005, 217, 239–255. [Google Scholar] [CrossRef]
- Sjöberg, E.L.; Rickard, D. The Influence of Experimental Design on the Rate of Calcite Dissolution. Geochim. Cosmochim. Acta 1983, 47, 2281–2285. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, Y.; Zhang, F. Laboratory Simulation Experiment on Dissolution of Limestone under Hydrodynamic Pressure. Carbonates Evaporites 2013, 28, 3–11. [Google Scholar] [CrossRef]
- Mousavi Maddah, S.M.; Hafezi Moghaddas, N.; Ghafoori, M. Solubility Variations of Tirgan Limestone in Northeast of Iran with Change of PH and Temperature. Carbonates Evaporites 2020, 35, 9. [Google Scholar] [CrossRef]
- Fan, M.; Jiang, X.Q.; Liu, W.X.; Zhang, J.Y.; Chen, H.Y. Dissolution of carbonate rocks in CO2 solution under the different temperatures. Acta Sedimentol. Sin. 2007, 25, 825–830. [Google Scholar]
- Gao, J.W. Experimental Simulation of Dissolution of Carbonate Rocks in Acid Solution. Liaoning Chem. Ind. 2016, 45, 254–256. [Google Scholar]
- Shao, D.M. Influence of temperature on dissolution rate in Ordovician carbonate rock in different water flow rate. Coal Geol. Explor. 2012, 40, 62–65. [Google Scholar]
- Rosenbauer, R.J.; Koksalan, T.; Palandri, J.L. Experimental Investigation of CO2–Brine–Rock Interactions at Elevated Temperature and Pressure: Implications for CO2 Sequestration in Deep-Saline Aquifers. Fuel Process. Technol. 2005, 86, 1581–1597. [Google Scholar] [CrossRef]
- Yang, Y.K.; Liu, B.; Qin, S.; Luo, P.; Zhang, D.M.; Zhou, M.H.; Shi, K.B.; Tian, Y.J. Re-recognition of deep carbonate dissolution based on the observation of in-situ simulation experiment. Acta Sci. Nat. Univ. Pekin. 2014, 50, 316–322. [Google Scholar]
- Shou, J.F.; She, M.; Shen, A.J. Experimental study of carbonate dissolution modification effect under deep conditions. Bull. Mineral. Petrol. Geochem. 2016, 35, 860–867. [Google Scholar]
- Zhao, Y.J.; Wang, F.G.; Li, C.S.; Wu, F.S.; Cao, Y.Q. Carbonate Karst Erosion Experiment Research of Guizhou Huangguoshu Tunnel. Coal Technol. 2016, 35, 310–312. [Google Scholar]
- Zhou, J.W.; Yang, X.G.; Fu, W.X.; Xu, J.; Li, H.T.; Zhou, H.W.; Liu, J.F. Experimental test and fracture damage mechanical characteristics of brittle rock under uniaxial cyclic loading and unloading conditions. Chin. J. Rock Mech. Eng. 2010, 29, 1172–1183. [Google Scholar]
- De Boever, E.; Varloteaux, C.; Nader, F.H.; Foubert, A.; Békri, S.; Youssef, S.; Rosenberg, E. Quantification and Prediction of the 3D Pore Network Evolution in Carbonate Reservoir Rocks. Oil Gas Sci. Technol. Rev. IFP Energ. Nouv. 2012, 67, 161–178. [Google Scholar] [CrossRef]
- Hidajat, I.; Mohanty, K.K.; Flaum, M.; Hirasaki, G. Study of Vuggy Carbonates Using X-ray CT Scanner and NMR. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 29 September 2002. [Google Scholar]
- Zhang, F.; Sui, X.; Duan, C.; Deng, H.; Gao, S.; Li, Y. NMR Experimental Study on Carbonate Reservoirs with High Porosity and Low Permeability. Well Logging Technol. 2018, 42, 497–502+529. [Google Scholar]
- Jora, M.Z.; Nunes de Souza, R.; Lucas-Oliveira, E.; Speglich, C.; Bonagamba, T.J.; Sabadini, E. Static Acid Dissolution of Carbonate Outcrops Investigated by 1H NMR and X-ray Tomography. J. Pet. Sci. Eng. 2021, 207, 109124. [Google Scholar] [CrossRef]
- Zhang, T.; Ni, X.; Huang, L.; Zhou, J.; Yu, Z.; Zhou, H.; Yi, Y.; Kang, T.; Zhang, C. Experimental Research Based on NMR and SIP for Pore Evolution during Dissolution of Dolomite Reservoirs. J. China Univ. Pet. (Ed. Nat. Sci.) 2022, 46, 70–79. [Google Scholar]
- Xiao, L. Application of NMR Log Data to Formation Pore Structure Evaluation. Xinjiang Pet. Geol. 2008, 29, 260–263. [Google Scholar]
- Fheed, A. The Impact of Fossils on Diagenetically Controlled Reservoir Quality: The Zechstein Brońsko Reef (Upper Permian, W Poland). ASGP 2019, 89, 47–81. [Google Scholar] [CrossRef]
- Fheed, A.; Kłodowski, K.; Krzyżak, A. Fracture Orientation and Fluid Flow Direction Recognition in Carbonates Using Diffusion-Weighted Nuclear Magnetic Resonance Imaging: An Example from Permian. J. Appl. Geophys. 2020, 174, 103964. [Google Scholar] [CrossRef]
- Fheed, A.; Świerczewska, A.; Strzelecki, P.J.; Radzik-Hotloś, N.; Gebus-Czupyt, B. Influence of Substrate Morphology on Reef Diagenesis: Examples from the Zechstein Limestone Formation (SE Wolsztyn Ridge, W Poland). Mar. Pet. Geol. 2022, 136, 105442. [Google Scholar] [CrossRef]
- Matenoglou, G.; Kelessidis, V.C.; Garcia, A.P.; Heidari, Z.; Fardis, M.; Karayanni, M.; Maris, T.G. Evaluation of the Wettability and the Petrophysical Properties in Carbonate Reservoirs by Advanced NMR and MRI Techniques. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 7–10 November 2016. [Google Scholar]
- Krzyżak, A.T.; Mazur, W.; Fheed, A.; Węglarz, W.P. Prospects and Challenges for the Spatial Quantification of the Diffusion of Fluids Containing 1H in the Pore System of Rock Cores. JGR Solid Earth 2022, 127, e2021JB023299. [Google Scholar] [CrossRef]
- Kingsley, P.B. Introduction to Diffusion Tensor Imaging Mathematics: Part II. Anisotropy, Diffusion-Weighting Factors, and Gradient Encoding Schemes. Concepts Magn. Reson. 2006, 28A, 123–154. [Google Scholar] [CrossRef]
- Fheed, A.; Krzyżak, A.; Świerczewska, A. Exploring a Carbonate Reef Reservoir—Nuclear Magnetic Resonance and Computed Microtomography Confronted with Narrow Channel and Fracture Porosity. J. Appl. Geophys. 2018, 151, 343–358. [Google Scholar] [CrossRef]
- Hounsfield, G.N. Computerized transverse axial scanning (tomography): Part I. Description of system. Br. J. Radiol. 1973, 46, 1016–1022. [Google Scholar] [CrossRef]
- Raynaud, S.; Fabre, D.; Mazerolle, F.; Geraud, Y.; Latière, H.J. Analysis of the Internal Structure of Rocks and Characterization of Mechanical Deformation by a Non-Destructive Method: X-ray Tomodensitometry. Tectonophysics 1989, 159, 149–159. [Google Scholar] [CrossRef]
- Ding, W.H.; Wu, Y.Q.; Pu, Y.B.; Cui, Z.X.; Cao, G.Z. History and present situation of X-ray computerized tomography (CT) of rocks. Seismol. Geol. 2003, 25, 467–476. [Google Scholar]
- Berg, S.; Ott, H.; Klapp, S.A.; Schwing, A.; Neiteler, R.; Brussee, N.; Makurat, A.; Leu, L.; Enzmann, F.; Schwarz, J.O.; et al. Real-Time 3D Imaging of Haines Jumps in Porous Media Flow. Proc. Natl. Acad. Sci. USA 2013, 110, 3755–3759. [Google Scholar] [CrossRef] [PubMed]
- Wildenschild, D.; Sheppard, A.P. X-ray Imaging and Analysis Techniques for Quantifying Pore-Scale Structure and Processes in Subsurface Porous Medium Systems. Adv. Water Resour. 2013, 51, 217–246. [Google Scholar] [CrossRef]
- Blunt, M.J.; Bijeljic, B.; Dong, H.; Gharbi, O.; Iglauer, S.; Mostaghimi, P.; Paluszny, A.; Pentland, C. Pore-scale imaging and modelling. Adv. Water Resour. 2013, 51, 197–216. [Google Scholar] [CrossRef]
- Luquot, L.; Gouze, P. Experimental determination of porosity and permeability changes induced by injection of CO2 into carbonate rocks. Chem. Geol. 2009, 265, 148–159. [Google Scholar] [CrossRef]
- Garing, C.; Luquot, L.; Pezard, P.A.; Gouze, P. Electrical and flow properties of highly heterogeneous carbonate rocks. AAPG Bull. 2014, 98, 49–66. [Google Scholar] [CrossRef]
- Bird, M.B.; Butler, S.L.; Hawkes, C.D.; Kotzer, T. Numerical modeling of fluid and electrical currents through geometries based on synchrotron X-ray tomographic images of reservoir rocks using Avizo and COMSOL. Comput. Geosci. 2014, 73, 6–16. [Google Scholar] [CrossRef]
- Bultreys, T.; Van Hoorebeke, L.; Cnudde, V. Multi-Scale, Micro-Computed Tomography-Based Pore Network Models to Simulate Drainage in Heterogeneous Rocks. Adv. Water Resour. 2015, 78, 36–49. [Google Scholar] [CrossRef]
- Cnudde, V.; Boone, M.N. High-Resolution X-ray Computed Tomography in Geosciences: A Review of the Current Technology and Applications. Earth-Sci. Rev. 2013, 123, 1–17. [Google Scholar] [CrossRef]
- Rabbani, A.; Ayatollahi, S.; Kharrat, R.; Dashti, N. Estimation of 3-D pore network coordination number of rocks from watershed segmentation of a single 2-D image. Adv. Water Resour. 2016, 94, 264–277. [Google Scholar] [CrossRef]
- Dong, H.; Touati, M.; Blunt, M. Pore Network Modeling: Analysis of Pore Size Distribution of Arabian Core Samples. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 11–14 March 2007. [Google Scholar]
- Freire-Gormaly, M.; Ellis, J.S.; MacLean, H.L.; Bazylak, A. Pore Structure Characterization of Indiana Limestone and Pink Dolomite from Pore Network Reconstructions. Oil Gas Sci. Technol. Rev. IFP Energ. Nouv. 2016, 71, 33. [Google Scholar] [CrossRef]
- Noiriel, C.; Gouze, P.; Madé, B. 3D analysis of geometry and flow changes in a limestone fracture during dissolution. J. Hydrol. 2013, 486, 211–223. [Google Scholar] [CrossRef]
- Rötting, T.S.; Luquot, L.; Carrera, J.; Casalinuovo, D.J. Changes in porosity, permeability, water retention curve and reactive surface area during carbonate rock dissolution. Chem. Geol. 2015, 403, 86–98. [Google Scholar] [CrossRef]
- Krebs, M.; Lungwitz, B.; Souza, A.; Pépin, A.; Montoya, S.; Schlicht, P.; Boyd, A.; Vidoto, E.; Polli, R.; Bonagamba, T. The First Visualization of Acid Treatments on Carbonates with 3D Nuclear-Magnetic-Resonance Imaging. SPE J. 2015, 20, 678–688. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D.; Cai, Y.; Li, J. Advanced characterization of pores and fractures in coals by nuclear magnetic resonance and X-ray computed tomography. Sci. China Earth Sci. 2010, 53, 854–862. [Google Scholar] [CrossRef]
- Fredd, C.N.; Fogler, H.S. Optimum conditions for wormhole formation in carbonate porous media: Influence of transport and reaction. SPE J. 1999, 4, 196–205. [Google Scholar] [CrossRef]
- Fredd, C.N.; Fogler, H.S. Alternative stimulation fluids and their impact on carbonate acidizing. SPE J. 1998, 3, 34–41. [Google Scholar] [CrossRef]
- Fredd, C.N.; Fogler, H.S. Influence of Transport and Reaction on Wormhole Formation in Porous Media. AIChE J. 1998, 44, 1933–1949. [Google Scholar] [CrossRef]
- Golfier, F.; Bazin, B.; Zarcone, C.; Lernorm, R.; Lasseux, D.; Quintard, M. Acidizing carbonate reservoirs: Numerical modelling of wormhole propagation and comparison to experiments. In Proceedings of the SPE 68922, SPE European Formation Damage Conference, The Hague, The Netherlands, 21–22 May 2001. [Google Scholar]
- Tansey, J.; Balhoff, M.T. Pore Network Modeling of Reactive Transport and Dissolution in Porous Media. Transp. Porous Media 2016, 113, 303–327. [Google Scholar] [CrossRef]
- Guo, G.; Xing, L.; Li, H. Quantitative Characterization of Different Coal Rank Reservoirs Permeability Based on NMR and X-CT Technology. Geol. Surv. China 2020, 7, 103–108. (In Chinese) [Google Scholar]
- Oliveira, M.F.S.; Lima, I.; Borghi, L.; Lopes, R.T. X-ray Microtomography Application in Pore Space Reservoir Rock. Appl. Radiat. Isot. 2012, 70, 1376–1378. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Su, X.F.; Wang, J.J.; Song, Y.; Wang, M.F.; Lang, Y.F. Multi-scale fine characterization of coal pore-fracture structure based on X-ray micro-CT scanning: A case study of Mabidong Block, southern Qinshui Basin. Pet. Geol. Recovery Effic. 2019, 26, 66–72. [Google Scholar]
- Andriamihaja, S.; Padmanabhan, E.; Ben-Awuah, J.; Sokkalingam, R. Static Dissolution-Induced 3D Pore Network Modification and Its Impact on Critical Pore Attributes of Carbonate Rocks. Pet. Explor. Dev. 2019, 46, 374–383. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, S. Application of NMR and X-CT Technology in the Pore Structure Study of Shale Gas Reservoirs. Adv. Earth Sci. 2014, 29, 624–631. [Google Scholar]
- Li, N.; Wang, K.; Zhang, G.; Wu, H.; Zhao, L.; Wu, L.; Feng, Q.; Feng, Z. Prediction of gas production in carbonates based on CT analysis and nuclear magnetic resonance (NMR) logging. Pet. Explor. Dev. 2015, 42, 167–174. [Google Scholar] [CrossRef]
- Tian, H.; Wang, G.; Feng, Q.; Li, C.; Tian, M. Review and Prospective of Complex Pore Structure of Carbonate Reservoir. Sci. Technol. Eng. 2020, 20, 11825–11833. [Google Scholar]
- Jiang, S. Study on the Characteristics of the Micro-Pore Structure and the Comprehensive Reservoir Evaluation Method for the Low Permeability Carbonate Reservoir in One Typical Oil Field. Master’s Thesis, University of Chinese Academy of Sciences, Langfang, China, 2015. [Google Scholar]
- Huang, J.; Xu, K.; Guo, S.; Guo, H. Comprehensive Study on Pore Structures of Shale Reservoirs Based on SEM, NMR and X-CT. Geoscience 2015, 29, 198–205. [Google Scholar]
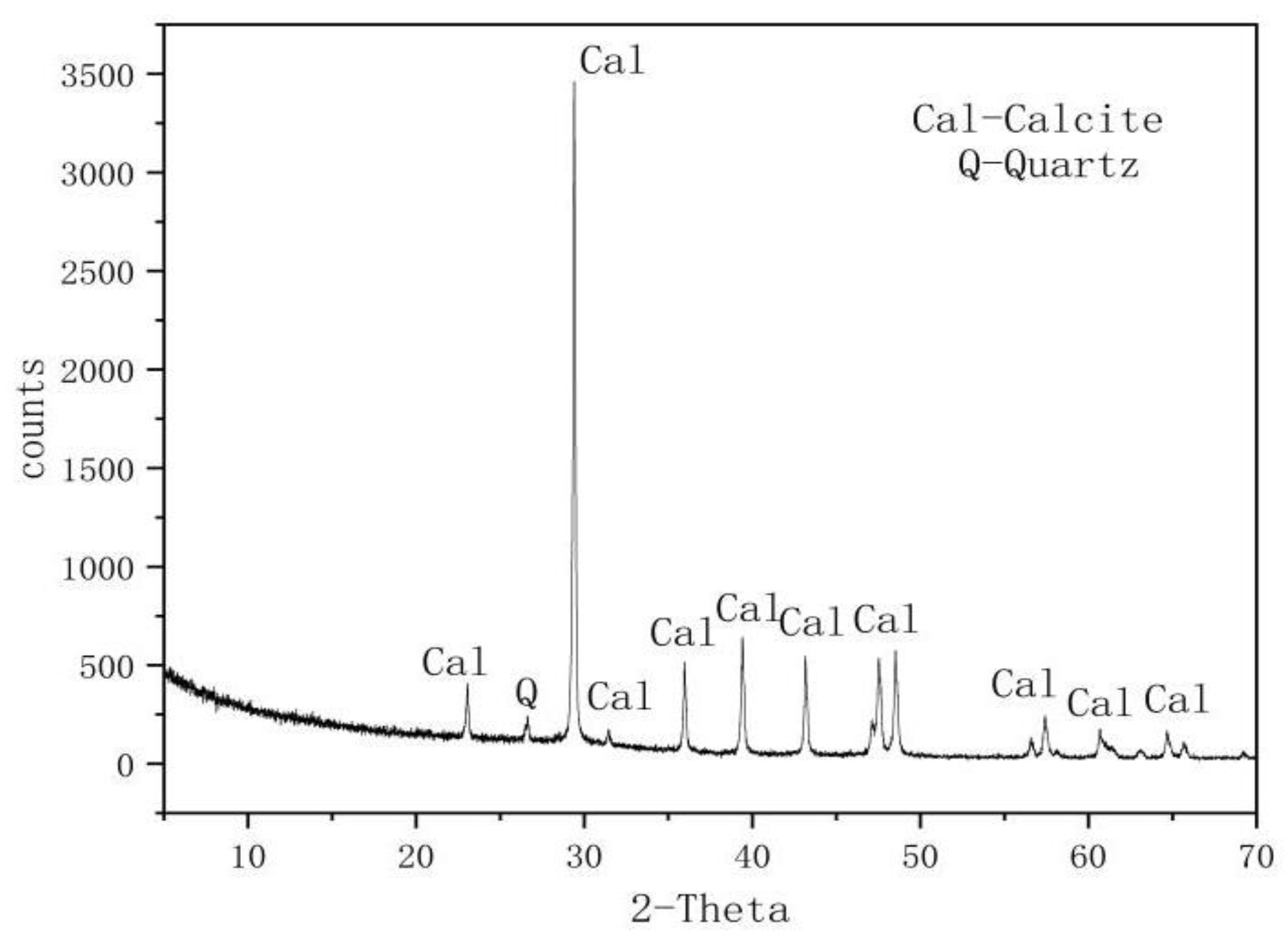


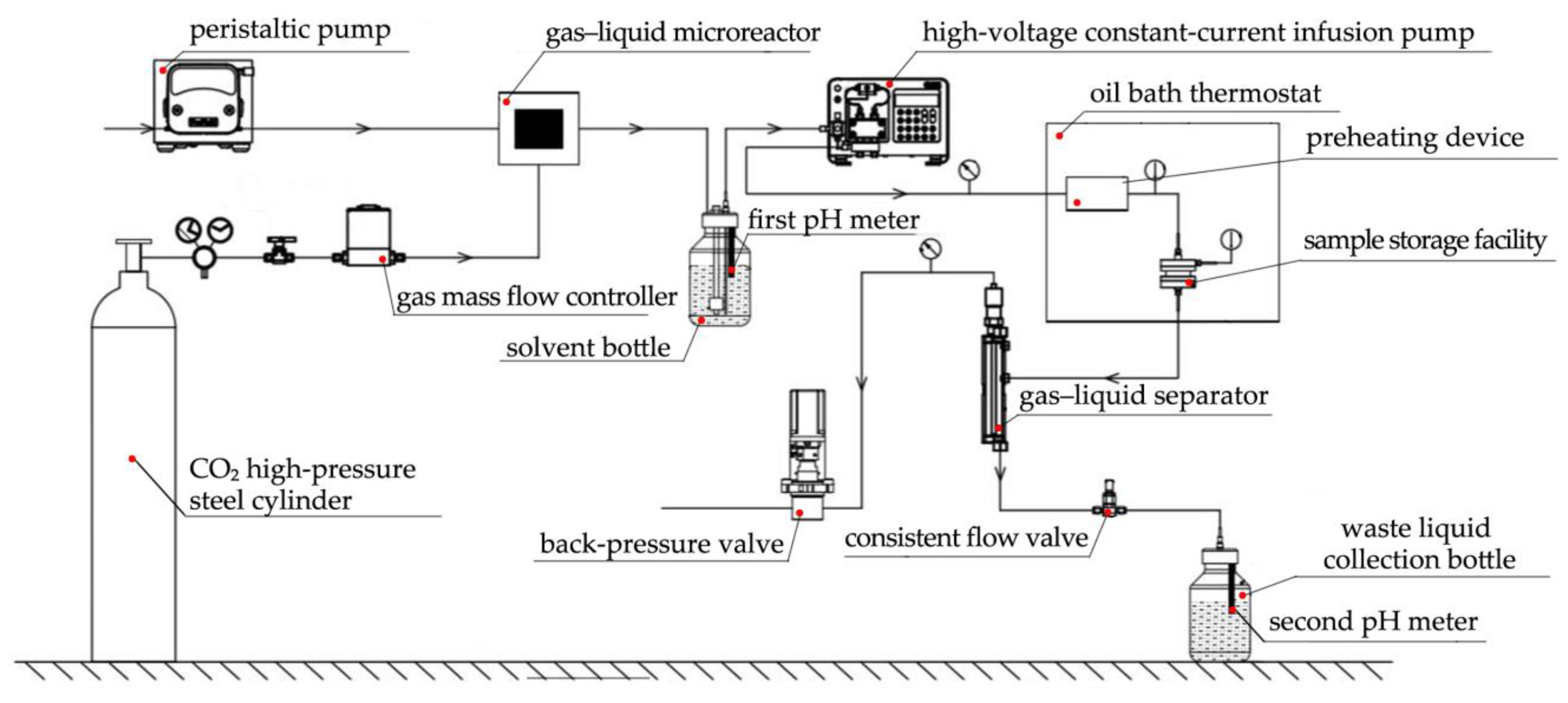

| Mineral Composition | Quartz | Calcite | Else |
|---|---|---|---|
| Mineral content (%) | 1.5 | 96.5 | 2.0 |
| Chemical Components | SrO | Cl | Loss on Ignition | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mineral content (%) | 0.532 | 55.41 | 0.206 | 43.54 | 0.050 | 0.053 | 0.052 | 0.040 | 0.117 |
| Level | Dissolution Conditions | ||||
|---|---|---|---|---|---|
| pH Value | Pressure (MPa) | Temperature (°C) | Current Speed (mL·min−1) | Duration (d) | |
| 1 | 4.6 | 2 | 25 | 15 | 3 |
| 2 | 5.4 | 4 | 45 | 35 | 7 |
| 3 | 6.2 | 6 | 65 | 55 | 14 |
| 4 | 7.0 | 8 | 85 | 75 | 21 |
| Experimental Scheme | Dissolution Conditions Parameters | ||||
|---|---|---|---|---|---|
| pH Value | Pressure (MPa) | Temperature (°C) | Current Speed (mL·min−1) | Duration (d) | |
| 1 | 4.6 | 2 | 25 | 15 | 3 |
| 2 | 4.6 | 4 | 45 | 35 | 7 |
| 3 | 4.6 | 6 | 65 | 55 | 14 |
| 4 | 4.6 | 8 | 85 | 75 | 21 |
| 5 | 5.4 | 2 | 45 | 55 | 21 |
| 6 | 5.4 | 4 | 25 | 75 | 14 |
| 7 | 5.4 | 6 | 85 | 15 | 7 |
| 8 | 5.4 | 8 | 65 | 35 | 3 |
| 9 | 6.2 | 2 | 65 | 75 | 7 |
| 10 | 6.2 | 4 | 85 | 55 | 3 |
| 11 | 6.2 | 6 | 25 | 35 | 21 |
| 12 | 6.2 | 8 | 45 | 15 | 14 |
| 13 | 7.0 | 2 | 85 | 35 | 14 |
| 14 | 7.0 | 4 | 65 | 15 | 21 |
| 15 | 7.0 | 6 | 45 | 75 | 3 |
| 16 | 7.0 | 8 | 25 | 55 | 7 |
| Test Batch Number | Test Specimen Number | Dissolution Rate (mg·g−1) | Test Batch Number | Test Specimen Number | Dissolution Rate (mg·g−1) |
|---|---|---|---|---|---|
| 1 | 1–1 | 12.17 | 9 | 9–1 | 24.51 |
| 1–2 | 14.67 | 9–2 | 22.06 | ||
| 1–3 | 12.08 | 9–3 | 22.00 | ||
| 1–4 | 12.22 | 9–4 | 22.28 | ||
| Average value | 12.78 | Average value | 22.71 | ||
| 2 | 2–1 | 65.69 | 10 | 10–1 | 12.25 |
| 2–2 | 95.12 | 10–2 | 12.32 | ||
| 2–3 | 65.38 | 10–3 | 14.74 | ||
| 2–4 | 93.14 | 10–4 | 12.29 | ||
| Average value | 79.78 | Average value | 12.90 | ||
| 3 | 3–1 | 155.34 | 11 | 11–1 | 19.75 |
| 3–2 | 200.00 | 11–2 | 24.45 | ||
| 3–3 | 141.49 | 11–3 | 22.00 | ||
| 2–4 | 202.93 | 11–4 | 22.06 | ||
| Average value | 174.76 | Average value | 22.07 | ||
| 4 | 4–1 | 344.66 | 12 | 12–1 | 22.17 |
| 4–2 | 373.17 | 12–2 | 24.51 | ||
| 4–3 | 373.13 | 12–3 | 24.57 | ||
| 4–4 | 366.75 | 12–4 | 24.57 | ||
| Average value | 364.43 | Average value | 23.96 | ||
| 5 | 5–1 | 108.37 | 13 | 13–1 | 9.85 |
| 5–2 | 105.65 | 13–2 | 14.67 | ||
| 5–3 | 105.13 | 13–3 | 12.17 | ||
| 5–4 | 103.96 | 13–4 | 12.17 | ||
| Average value | 105.78 | Average value | 12.22 | ||
| 6 | 6–1 | 32.02 | 14 | 14–1 | 12.25 |
| 6–2 | 31.71 | 14–2 | 19.85 | ||
| 6–3 | 26.57 | 14–3 | 17.16 | ||
| 6–4 | 34.40 | 14–4 | 17.16 | ||
| Average value | 31.15 | Average value | 16.59 | ||
| 7 | 7–1 | 59.11 | 15 | 15–1 | 9.51 |
| 7–2 | 59.11 | 15–2 | 9.50 | ||
| 7–3 | 54.19 | 15–3 | 7.82 | ||
| 7–4 | 56.23 | 15–4 | 9.40 | ||
| Average value | 57.16 | Average value | 9.16 | ||
| 8 | 8–1 | 29.27 | 16 | 16–1 | 4.90 |
| 8–2 | 27.30 | 16–2 | 2.46 | ||
| 8–3 | 22.11 | 16–3 | 4.90 | ||
| 8–4 | 26.89 | 16–4 | 2.47 | ||
| Average value | 26.40 | Average value | 3.69 |
| Level | pH Value | Pressure (MPa) | Temperature (°C) | Current Speed (mL·min−1) | Duration (d) |
|---|---|---|---|---|---|
| 1 | 157.938 | 38.373 | 17.422 | 27.623 | 15.310 |
| 2 | 55.123 | 35.105 | 54.670 | 35.117 | 40.835 |
| 3 | 20.410 | 65.787 | 60.115 | 74.282 | 60.523 |
| 4 | 10.415 | 104.620 | 111.678 | 106.862 | 127.218 |
| Range | 147.523 | 69.515 | 94.256 | 79.239 | 111.908 |
| Factor | Deviation Quadratic Sum | Degree of Freedom | F Comparison | F Critical Value | Contribution Rate (%) |
|---|---|---|---|---|---|
| pH Value | 54,551.244 | 3 | 2.118 | 3.290 | 42.36 |
| Pressure (MPa) | 12,432.704 | 3 | 0.483 | 3.290 | 9.65 |
| Temperature (°C) | 18,032.225 | 3 | 0.700 | 3.290 | 14.00 |
| Current Speed (mL·min−1) | 16,255.007 | 3 | 0.631 | 3.290 | 12.62 |
| Duration (d) | 27,516.741 | 3 | 1.068 | 3.290 | 21.37 |
| Deviation | 128,787.92 | 15 | - | - | - |
| Rock Sample | Total Spectral Area | Wave Peaks | Peak Point Time | Peak Area | Peak Proportion (%) |
|---|---|---|---|---|---|
| 5# | 2106.97 | 1 | 0.369 | 1012.26 | 0.48 |
| 2 | 34.489 | 1094.71 | 0.52 | ||
| 6# | 1075.75 | 1 | 0.289 | 583.43 | 0.54 |
| 2 | 11.098 | 492.32 | 0.56 | ||
| 7# | 1601.11 | 1 | 0.289 | 1034.44 | 0.65 |
| 2 | 12.034 | 512.41 | 0.32 | ||
| 3 | 460.592 | 53.66 | 0.03 | ||
| 8# | 1792.93 | 1 | 0.227 | 946.82 | 0.53 |
| 2 | 6.295 | 546.39 | 0.30 | ||
| 3 | 174.263 | 299.72 | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, J.; Chen, S.; Wang, J.; Chen, Z.; Zhang, J. Experimental Study on the Dissolution Characteristics and Microstructure of Carbonate Rocks under the Action of Thermal–Hydraulic–Chemical Coupling. Materials 2023, 16, 1828. https://doi.org/10.3390/ma16051828
Meng J, Chen S, Wang J, Chen Z, Zhang J. Experimental Study on the Dissolution Characteristics and Microstructure of Carbonate Rocks under the Action of Thermal–Hydraulic–Chemical Coupling. Materials. 2023; 16(5):1828. https://doi.org/10.3390/ma16051828
Chicago/Turabian StyleMeng, Jinzhu, Sili Chen, Junxiang Wang, Zhi Chen, and Jingyu Zhang. 2023. "Experimental Study on the Dissolution Characteristics and Microstructure of Carbonate Rocks under the Action of Thermal–Hydraulic–Chemical Coupling" Materials 16, no. 5: 1828. https://doi.org/10.3390/ma16051828
APA StyleMeng, J., Chen, S., Wang, J., Chen, Z., & Zhang, J. (2023). Experimental Study on the Dissolution Characteristics and Microstructure of Carbonate Rocks under the Action of Thermal–Hydraulic–Chemical Coupling. Materials, 16(5), 1828. https://doi.org/10.3390/ma16051828






