Viscoelastic Properties of Polymeric Microneedles Determined by Micromanipulation Measurements and Mathematical Modelling
Abstract
1. Introduction
2. Materials and Methods
2.1. HA Microneedles Loaded with Lidocaine
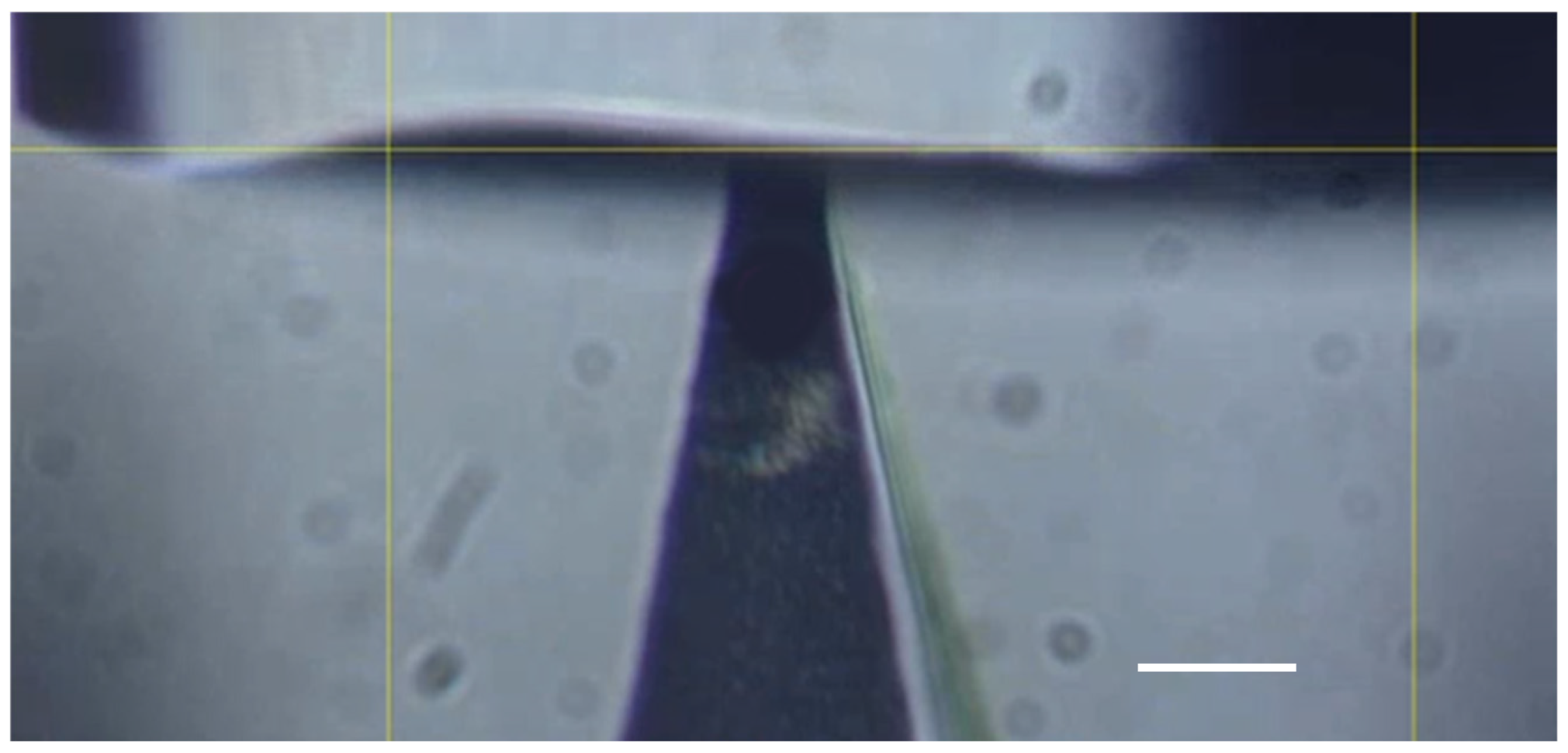
2.2. Micromanipulation of Single Microneedles
2.3. Modelling of the Viscoelastic Behaviour of Single Microneedles
2.3.1. Derivation of the Viscoelastic Model
2.3.2. Determination of Viscoelastic Parameters Based on Numerical Optimization
2.3.3. Prediction of the Loading Force Using the Viscoelastic Parameters
3. Results and Discussion
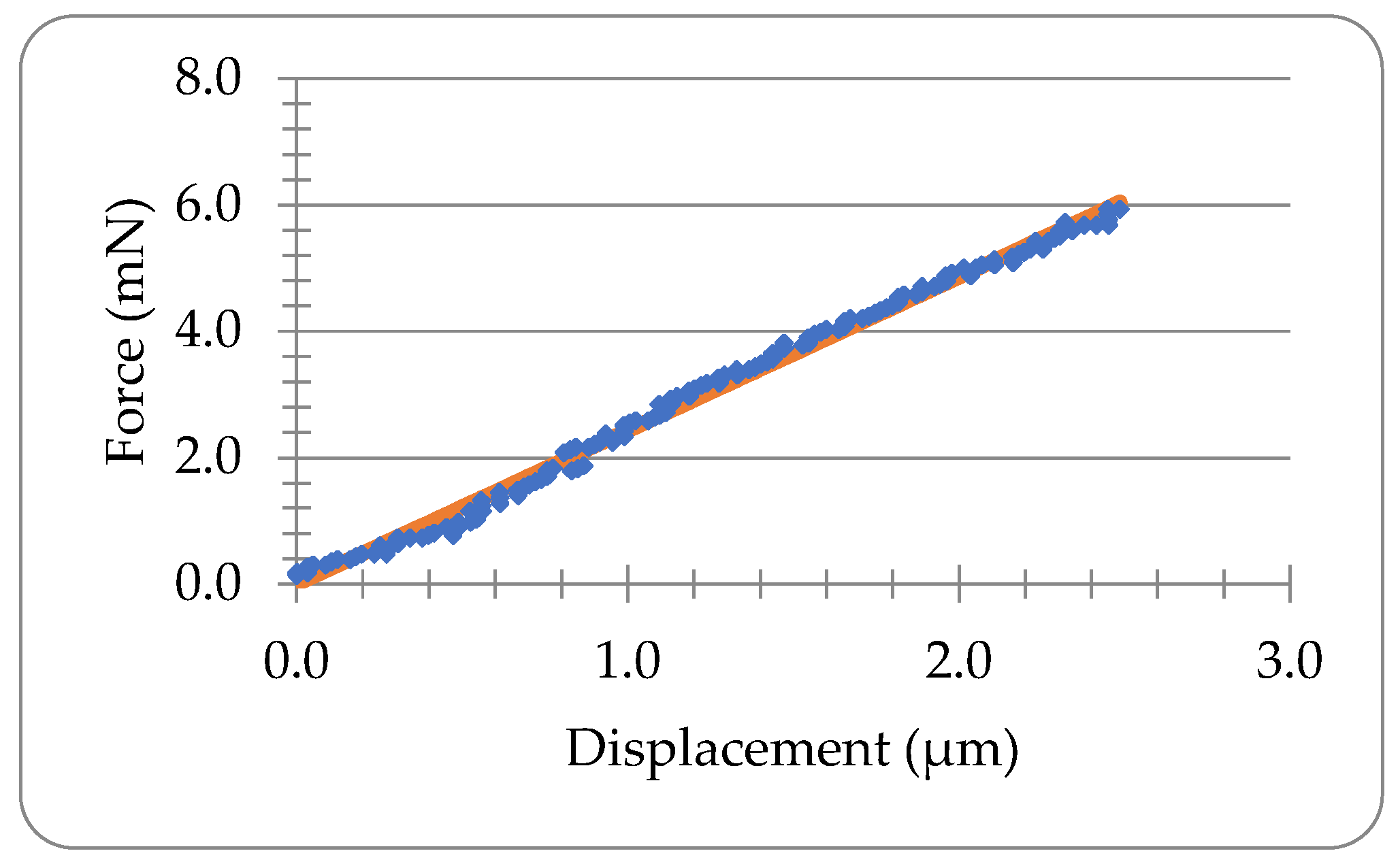
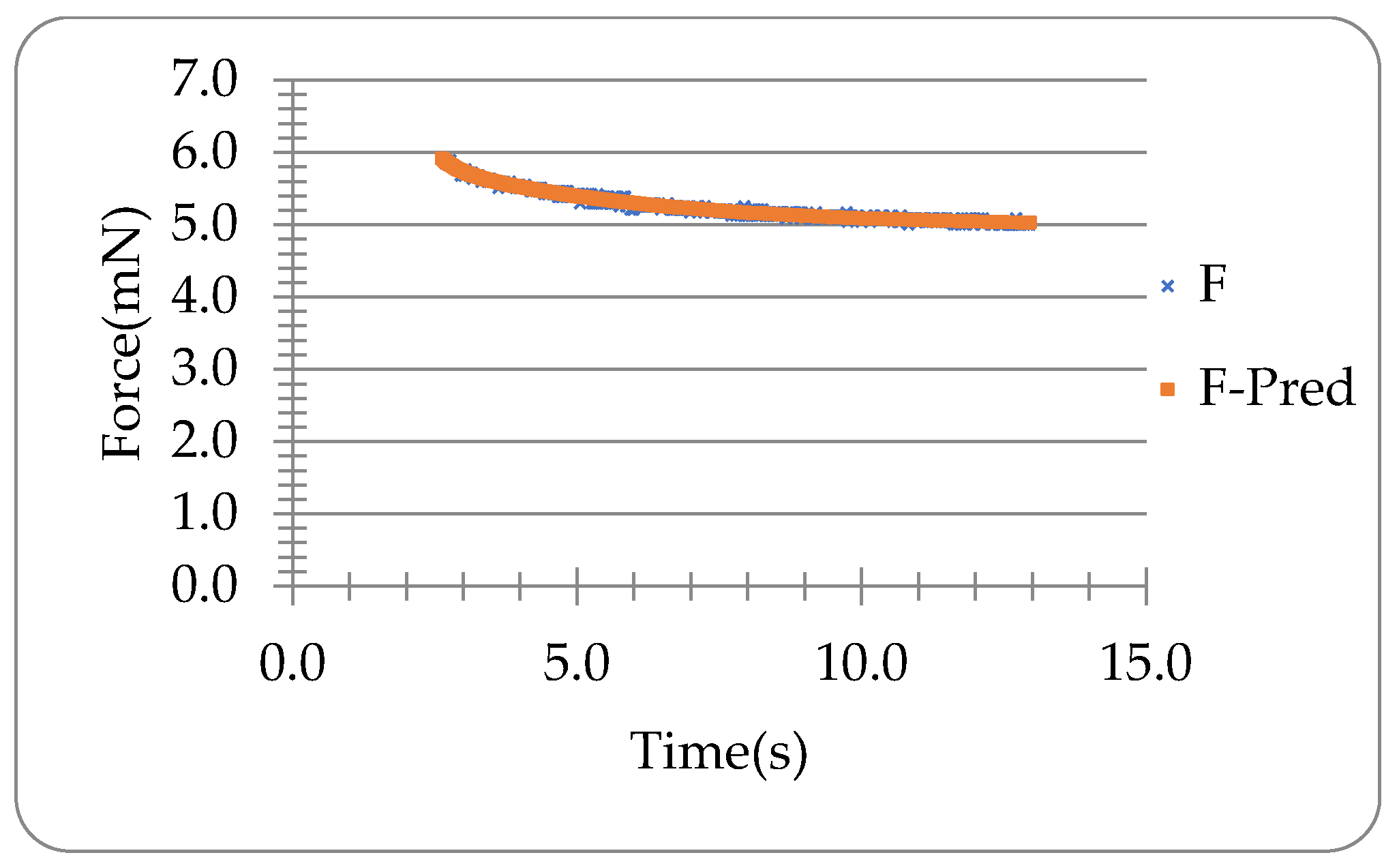
3.1. Force-Time Data of Lido-HA300kDa Microneedles under Compression and Holding
3.2. Elastic Analysis
3.3. Viscoelastic Analysis
4. Further Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| area of the microneedle bottom (m2) | |
| relaxed force (Equation (10)) (N) | |
| proportional constants (Equation (10)) (N) | |
| compliance of the force transducer (m/N) | |
| proportional constants (Equation (9)) (N) | |
| coefficient of determination | |
| Young’s modulus (Pa) | |
| instantaneous Young’s modulus (Pa) | |
| long-term Young’s modulus (Pa) | |
| predicted force from models (N) | |
| the ith force (N) | |
| time-dependent force (N) | |
| shear modulus (Pa) | |
| instantaneous shear modulus (Pa) | |
| long-term shear modulus (Pa) | |
| time-dependent shear modulus (Pa) | |
| height of the microneedle (m) | |
| height of the missing tip assuming the microneedle is perfectly sharp (m) | |
| degree number in the viscoelastic analysis | |
| number of data points during the holding | |
| , | half side length of a quadrangular microneedle base and tip (m) |
| time (s) | |
| rising time (time taken to compress a microneedle) (s) | |
| acquisition time (sampling time) (s) | |
| compression speed (m/s) | |
| Greek Symbols | |
| displacement (m) | |
| maximum displacement before relaxation (m) | |
| relaxation times (s) | |
| ramp correction factors (Equation (13)) | |
| Poisson’s ratio |
References
- Yang, J.; Liu, X.; Fu, Y.; Song, Y. Recent advances of microneedles for biomedical applications: Drug delivery and beyond. Acta Pharm. Sin. B 2019, 9, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 581–587. [Google Scholar] [CrossRef]
- Chang, H.; Zheng, M.; Yu, X.; Than, A.; Seeni, R.Z.; Kang, R.; Tian, J.; Khanh, D.P.; Liu, L.; Chen, P.; et al. A Swellable Microneedle Patch to Rapidly Extract Skin Interstitial Fluid for Timely Metabolic Analysis. Adv. Mater. 2017, 29, 1702243. [Google Scholar] [CrossRef]
- McCrudden, M.T.C.; McAlister, E.; Courtenay, A.J.; González-Vázquez, P.; Raj Singh, T.R.; Donnelly, R.F. Microneedle applications in improving skin appearance. Exp. Dermatol. 2015, 24, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Faraji Rad, Z.; Prewett, P.; Davies, G. An overview of microneedle applications, materials, and fabrication methods. Beilstein J. Nanotechnol. 2021, 12, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, S.H.; Lee, B.Y.; Kim, S.J.; Sung, C.Y.; Jang, N.K.; Kim, J.D.; Jeong, D.H.; Ryu, H.Y.; Lee, S. A comparative study of dissolving hyaluronic acid microneedles with trehalose and poly(vinyl pyrrolidone) for efficient peptide drug delivery. Biomater. Sci. 2018, 6, 2566–2570. [Google Scholar] [CrossRef]
- Du, G.; Zhang, Z.; He, P.; Zhang, Z.; Sun, X. Determination of the mechanical properties of polymeric microneedles by micromanipulation. J. Mech. Behav. Biomed. Mater. 2021, 117, 104384. [Google Scholar] [CrossRef]
- Fonseca, D.F.; Vilela, C.; Pinto, R.J.; Bastos, V.; Oliveira, H.; Catarino, J.; Faísca, P.; Rosado, C.; Silvestre, A.J.; Freire, C.S. Bacterial nanocellulose-hyaluronic acid microneedle patches for skin applications: In vitro and in vivo evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111350. [Google Scholar] [CrossRef]
- Du, G.; He, P.; Zhao, J.; He, C.; Jiang, M.; Zhang, Z.; Zhang, Z.; Sun, X. Polymeric microneedle-mediated transdermal delivery of melittin for rheumatoid arthritis treatment. J. Control. Release 2021, 336, 537–548. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, H.; Wang, L.; Shen, D.; Ni, Z.; Ren, S.; Lu, Y.; Chen, X.; Yang, J.; Hong, Y. Research progress on cosmetic microneedle systems: Preparation, property and application. Eur. Polym. J. 2022, 163, 110942. [Google Scholar] [CrossRef]
- Aldawood, F.K.; Andar, A.; Desai, S. A comprehensive review of microneedles: Types, materials, processes, characterizations and applications. Polymers 2021, 13, 2815. [Google Scholar] [CrossRef] [PubMed]
- Loizidou, E.Z.; Williams, N.A.; Barrow, D.A.; Eaton, M.J.; McCrory, J.; Evans, S.L.; Allender, C.J. Structural characterisation and transdermal delivery studies on sugar microneedles: Experimental and finite element modelling analyses. Eur. J. Pharm. Biopharm. 2015, 89, 224–231. [Google Scholar] [CrossRef]
- Davis, S.P.; Landis, B.J.; Adams, Z.H.; Allen, M.G.; Prausnitz, M.R. Insertion of microneedles into skin: Measurement and prediction of insertion force and needle fracture force. J. Biomech. 2004, 37, 1155–1163. [Google Scholar] [CrossRef]
- Park, J.-H.; Allen, M.; Prausnitz, M. Biodegradable polymer microneedles: Fabrication, mechanics and transdermal drug delivery. J. Control. Release 2005, 104, 51–66. [Google Scholar] [CrossRef]
- Park, J.-H.; Allen, M.; Prausnitz, M. Polymer Microneedles for Controlled-Release Drug Delivery. Pharm. Res. 2006, 23, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Gittard, S.D.; Chen, B.; Xu, H.; Ovsianikov, A.; Chichkov, B.; Monteiro-Riviere, N.; Narayan, R.J. The effects of geometry on skin penetration and failure of polymer microneedles. J. Adhes. Sci. Technol. 2013, 27, 227–243. [Google Scholar] [CrossRef]
- Wang, Q.L.; Ren, J.W.; Chen, B.Z.; Jin, X.; Zhang, C.Y.; Guo, X.D. Effect of humidity on mechanical properties of dissolving microneedles for transdermal drug delivery. J. Ind. Eng. Chem. 2018, 59, 251–258. [Google Scholar] [CrossRef]
- Dardano, P.; Caliò, A.; Di Palma, V.; Bevilacqua, M.F.; Di Matteo, A.; De Stefano, L. A Photolithographic Approach to Polymeric Microneedles Array Fabrication. Materials 2015, 8, 8661–8673. [Google Scholar] [CrossRef]
- Boyer, J.; Kramer, P. Measuring the Water Status of Plants and Soils; Academic Press Inc.: San Diego, CA, USA, 1995. [Google Scholar]
- Heinrich, V.; Rawicz, W. Automated, high-resolution micropipet aspiration reveals new insight into the physical properties of fluid membranes. Langmuir ACS J. Surf. Colloids 2005, 21, 1962–1971. [Google Scholar] [CrossRef]
- Neuman, K.C.; Nagy, A. Single-molecule force spectroscopy: Optical tweezers, magnetic tweezers and atomic force microscopy. Nat. Methods 2008, 5, 491–505. [Google Scholar] [CrossRef]
- van der Maaden, K.; Sekerdag, E.; Jiskoot, W.; Bouwstra, J. Impact-Insertion Applicator Improves Reliability of Skin Penetration by Solid Microneedle Arrays. AAPS J. 2014, 16, 681–684. [Google Scholar] [CrossRef]
- Zhang, Z.; Ferenczi, M.; Lush, A.C.; Thomas, C.R. A novel micromanipulation technique for measuring the bursting strength of single mammalian cells. Appl. Microbiol. Biotechnol. 1991, 36, 208–210. [Google Scholar] [CrossRef]
- Zhang, Z.; Saunders, R.; Thomas, C. Mechanical strength of single microcapsules determined by a novel micromanipulation technique. J. Microencapsul. 1999, 16, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Stenson, J.; Thomas, C. Chapter 2 Micromanipulation in Mechanical Characterisation of Single Particles. In Advances in Chemical Engineering; Li, J., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 29–85. [Google Scholar]
- Zhang, Z.; He, Y.; Zhang, Z. Micromanipulation and Automatic Data Analysis to Determine the Mechanical Strength of Microparticles. Micromachines 2022, 13, 751. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, Z.; Stokes, J.R.; Zhou, Q.-Z.; Ma, G.-H.; Adams, M.J. Mechanical characterization of agarose micro-particles with a narrow size distribution. Powder Technol. 2009, 192, 122–130. [Google Scholar] [CrossRef]
- Bonfanti, A.; Kaplan, J.L.; Charras, G.; Kabla, A. Fractional viscoelastic models for power-law materials. Soft Matter 2020, 16, 6002–6020. [Google Scholar] [CrossRef]
- Mattice, J.M.; Lau, A.; Oyen, M.; Kent, R.W. Spherical indentation load-relaxation of soft biological tissues. J. Mater. Res. 2006, 21, 2003–2010. [Google Scholar] [CrossRef]
- Bergström, J. 6-Linear Viscoelasticity. In Mechanics of Solid Polymers; Bergström, J., Ed.; William Andrew Publishing: San Diego, CA, USA, 2015; pp. 309–351. [Google Scholar]
- Kalra, A.; Lowe, A.; Al-Jumaily, A. Mechanical behaviour of skin: A review. J. Mater. Sci. Eng. 2016, 5, 1–7. [Google Scholar]



| Abbreviation | Matrix |
|---|---|
| Lido-HA300kDa | Lidocaine + Hyaluronic acid (MW: 300,000 Da) Lidocaine to HA weight ratio 4:5 |
| Width (μm) | (mN) | (mN) | (mN) | (s) | (s) |
|---|---|---|---|---|---|
| 16.1 | 4.98 | 1.55 | 534.35 | 3.82 | 0.32 |
| (MPa) | (MPa) | (MPa) |
|---|---|---|
| 228.8 | 49.6 | 63.8 |
| (MPa) | (MPa) | (MPa) | (MPa) |
|---|---|---|---|
| 171.1 | 114.4 | 513.3 | 343.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Du, G.; Sun, X.; Zhang, Z. Viscoelastic Properties of Polymeric Microneedles Determined by Micromanipulation Measurements and Mathematical Modelling. Materials 2023, 16, 1769. https://doi.org/10.3390/ma16051769
Zhang Z, Du G, Sun X, Zhang Z. Viscoelastic Properties of Polymeric Microneedles Determined by Micromanipulation Measurements and Mathematical Modelling. Materials. 2023; 16(5):1769. https://doi.org/10.3390/ma16051769
Chicago/Turabian StyleZhang, Zhihua, Guangsheng Du, Xun Sun, and Zhibing Zhang. 2023. "Viscoelastic Properties of Polymeric Microneedles Determined by Micromanipulation Measurements and Mathematical Modelling" Materials 16, no. 5: 1769. https://doi.org/10.3390/ma16051769
APA StyleZhang, Z., Du, G., Sun, X., & Zhang, Z. (2023). Viscoelastic Properties of Polymeric Microneedles Determined by Micromanipulation Measurements and Mathematical Modelling. Materials, 16(5), 1769. https://doi.org/10.3390/ma16051769






