Sintering, Microstructure, and Mechanical Properties of TiTaNbZrHf High-Entropy Alloys Prepared by Cold Isostatic Pressing and Pressure-Less Sintering of Hydrides
Abstract
1. Introduction
2. Experimental Procedures
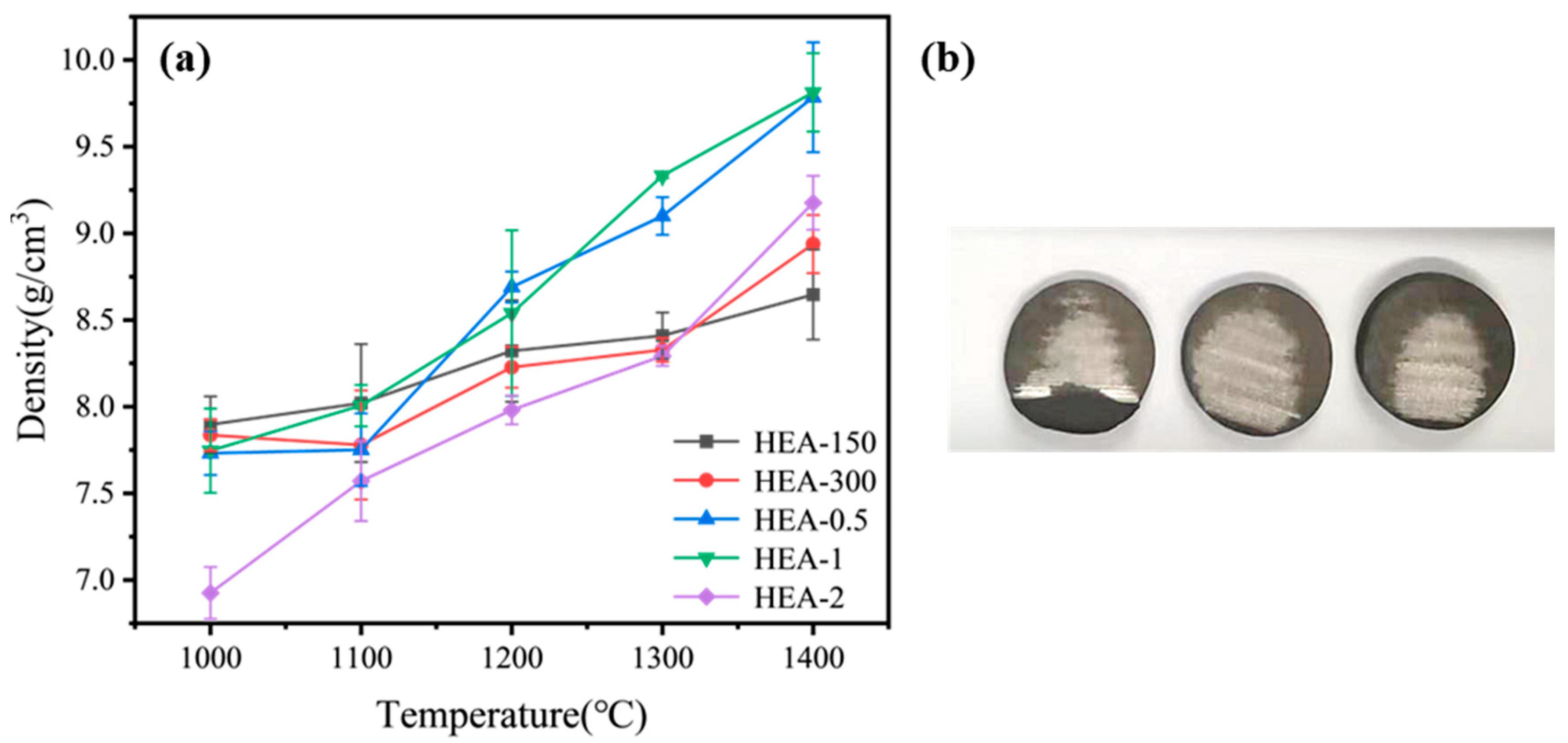
3. Results and Discussion
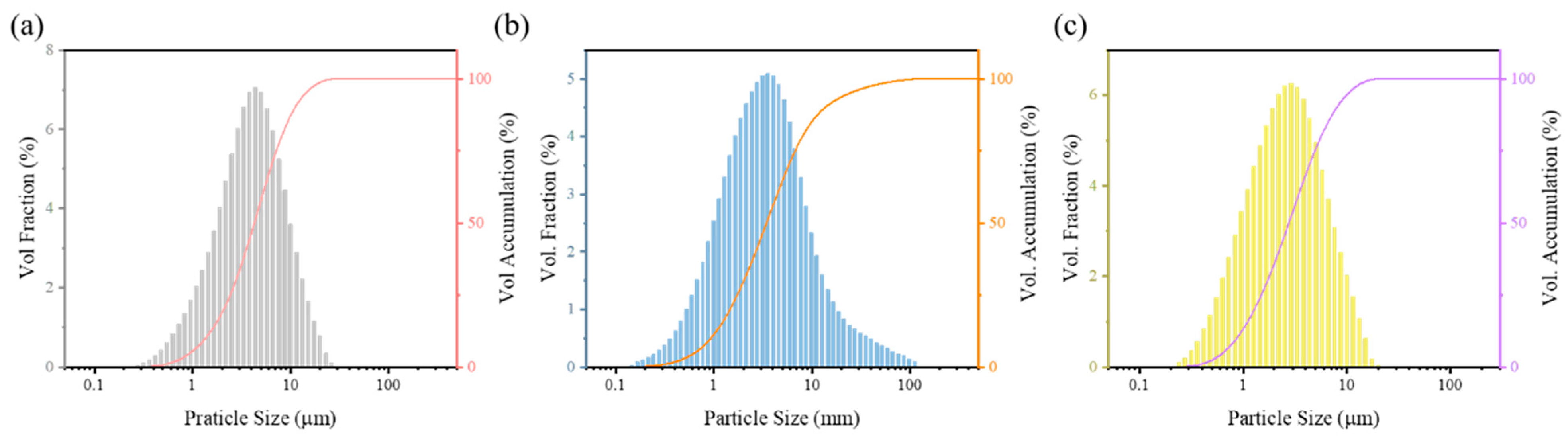
3.1. HEA Powders
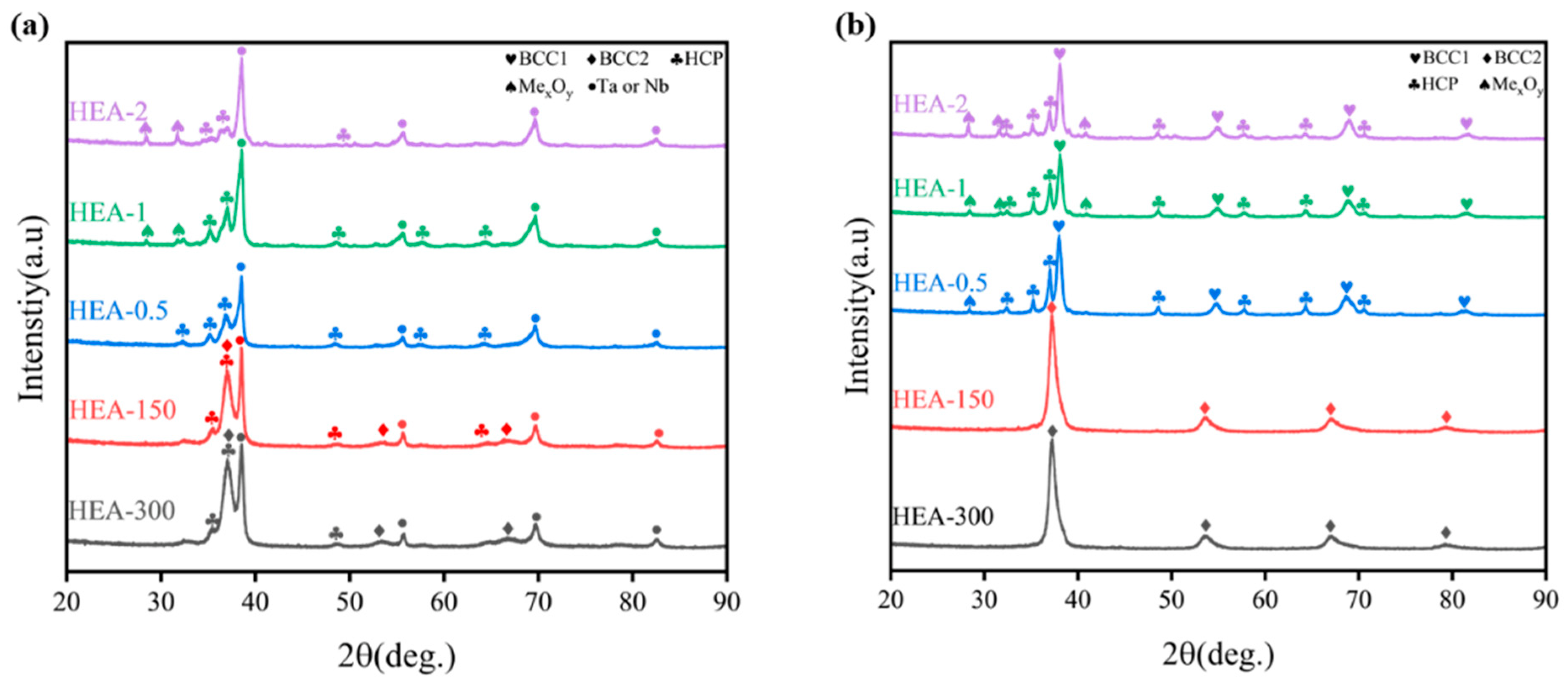
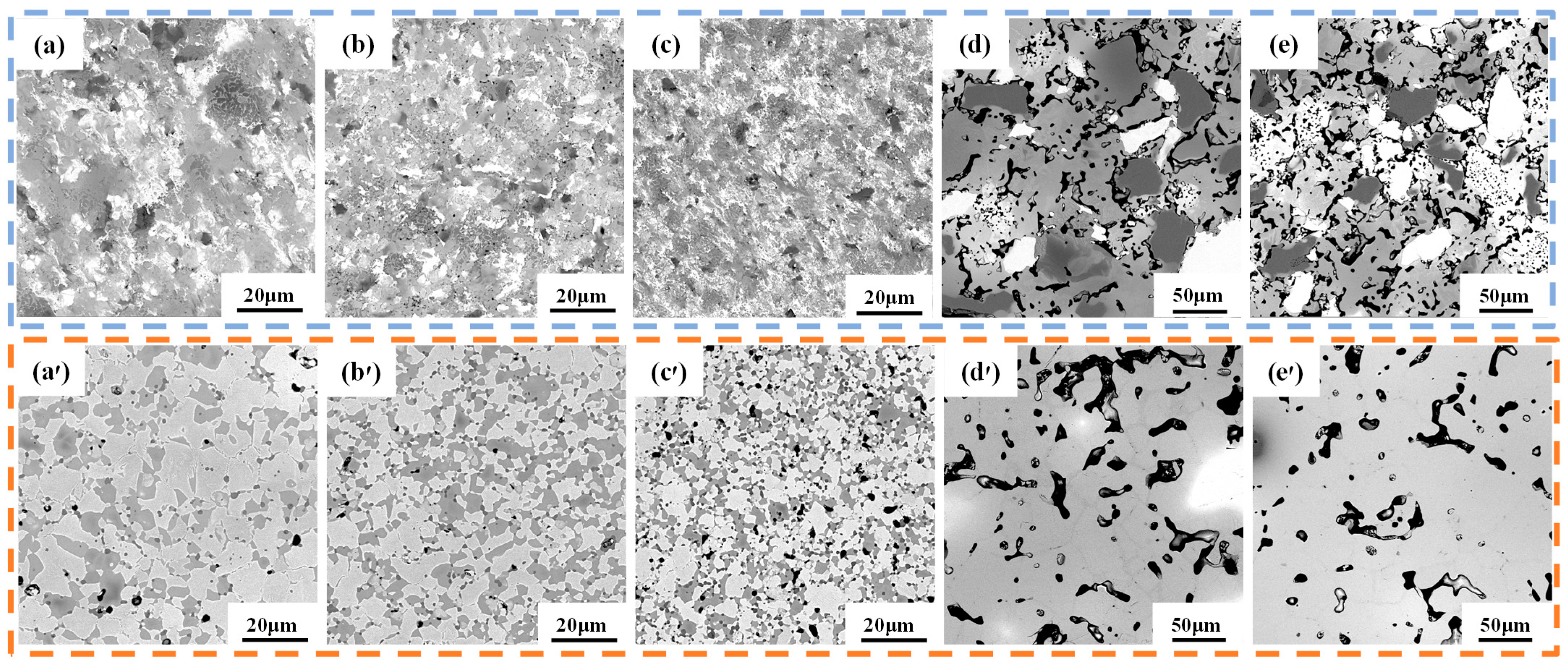
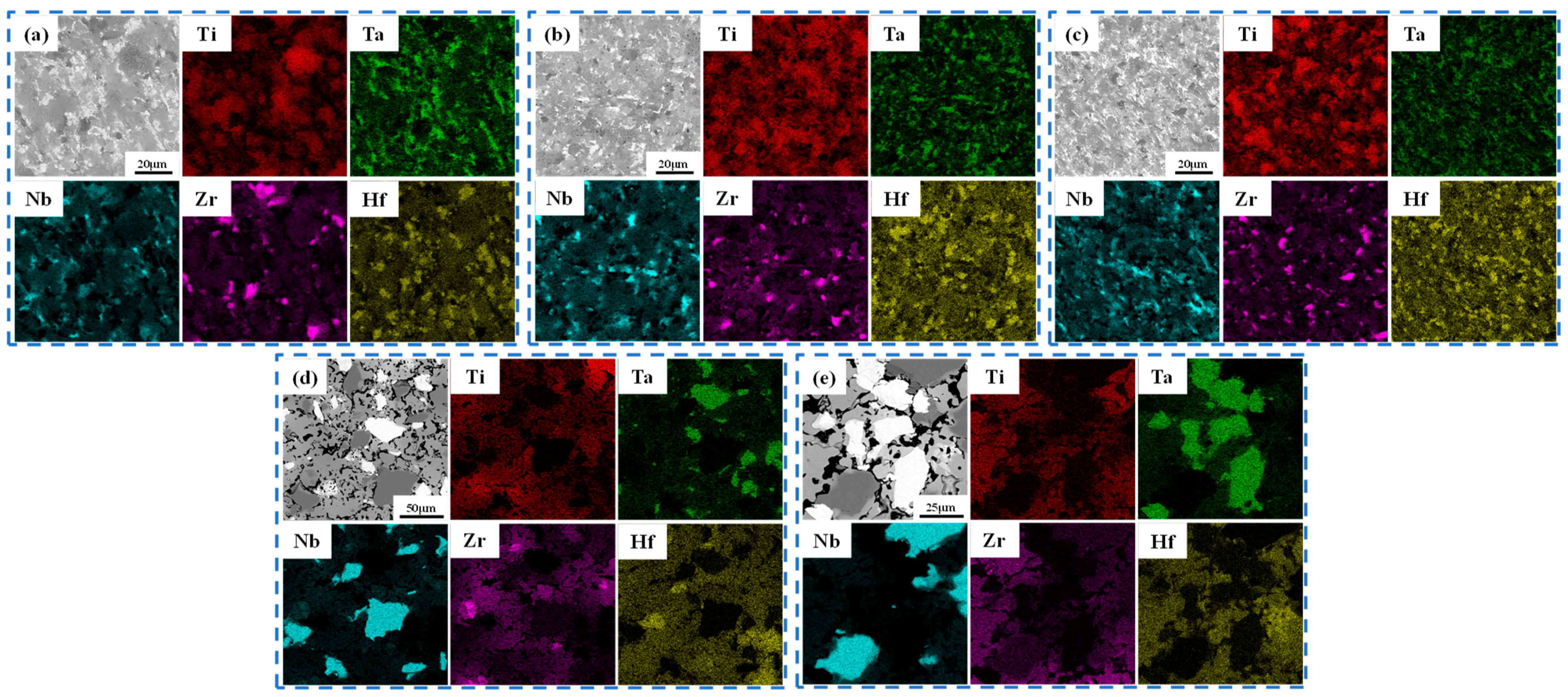
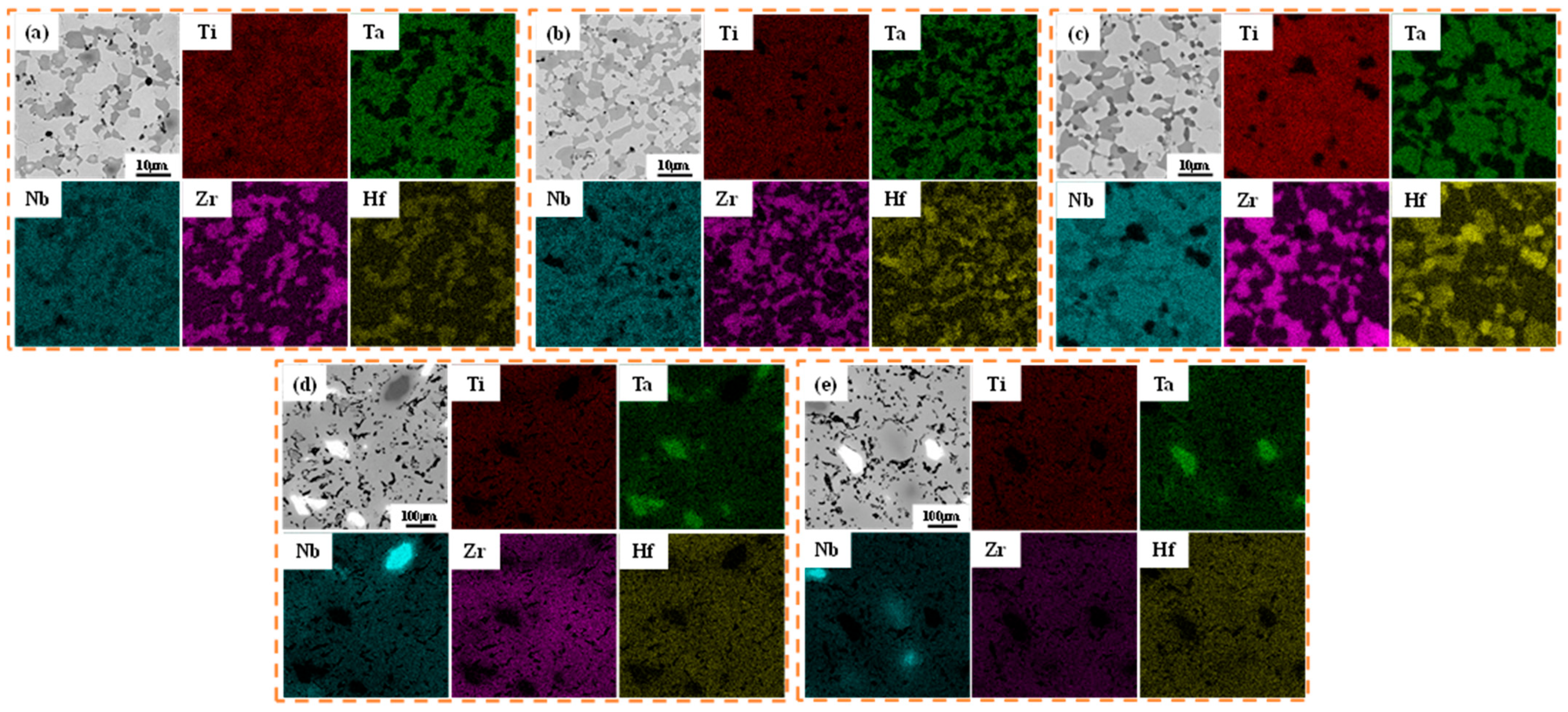
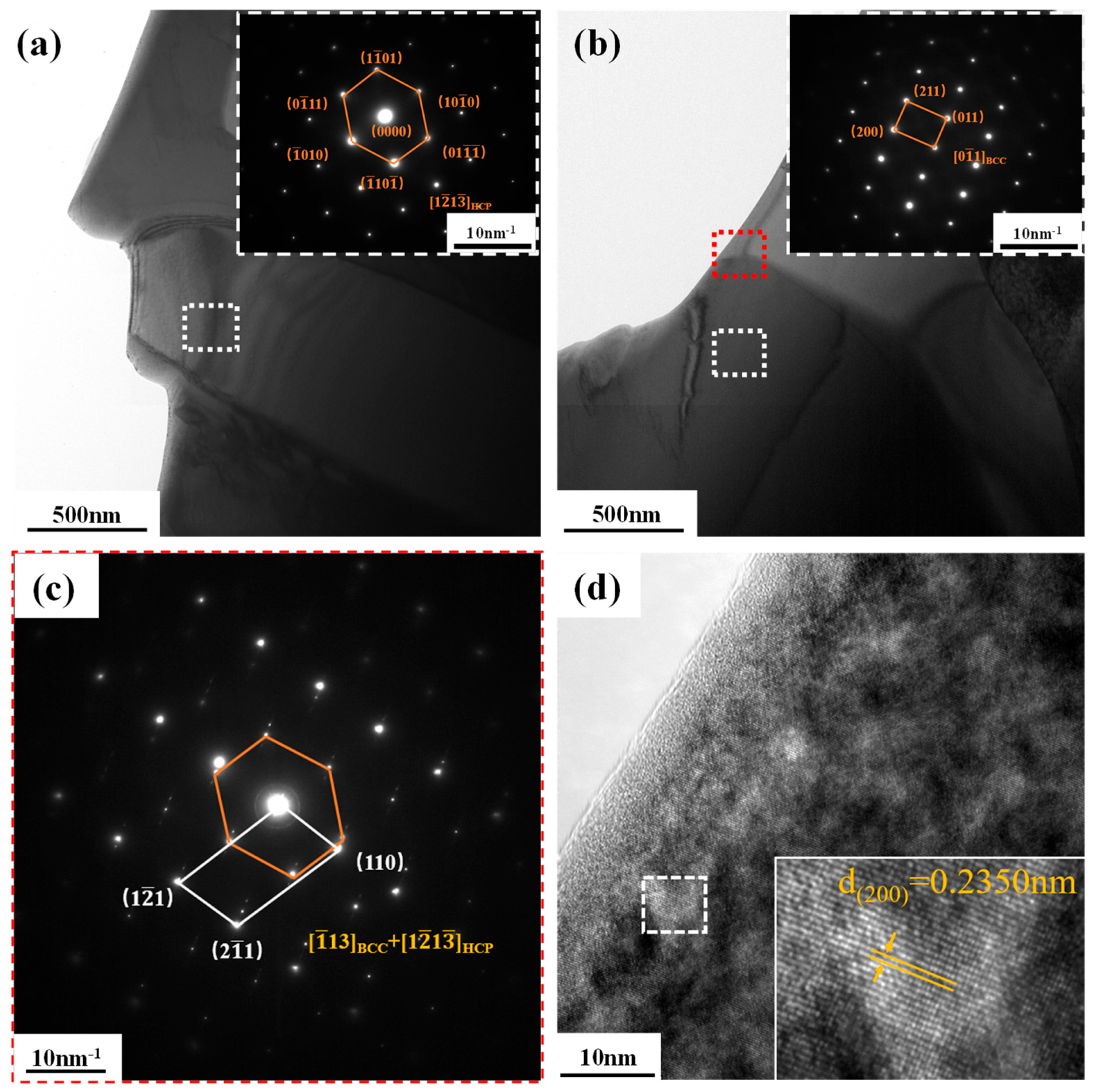
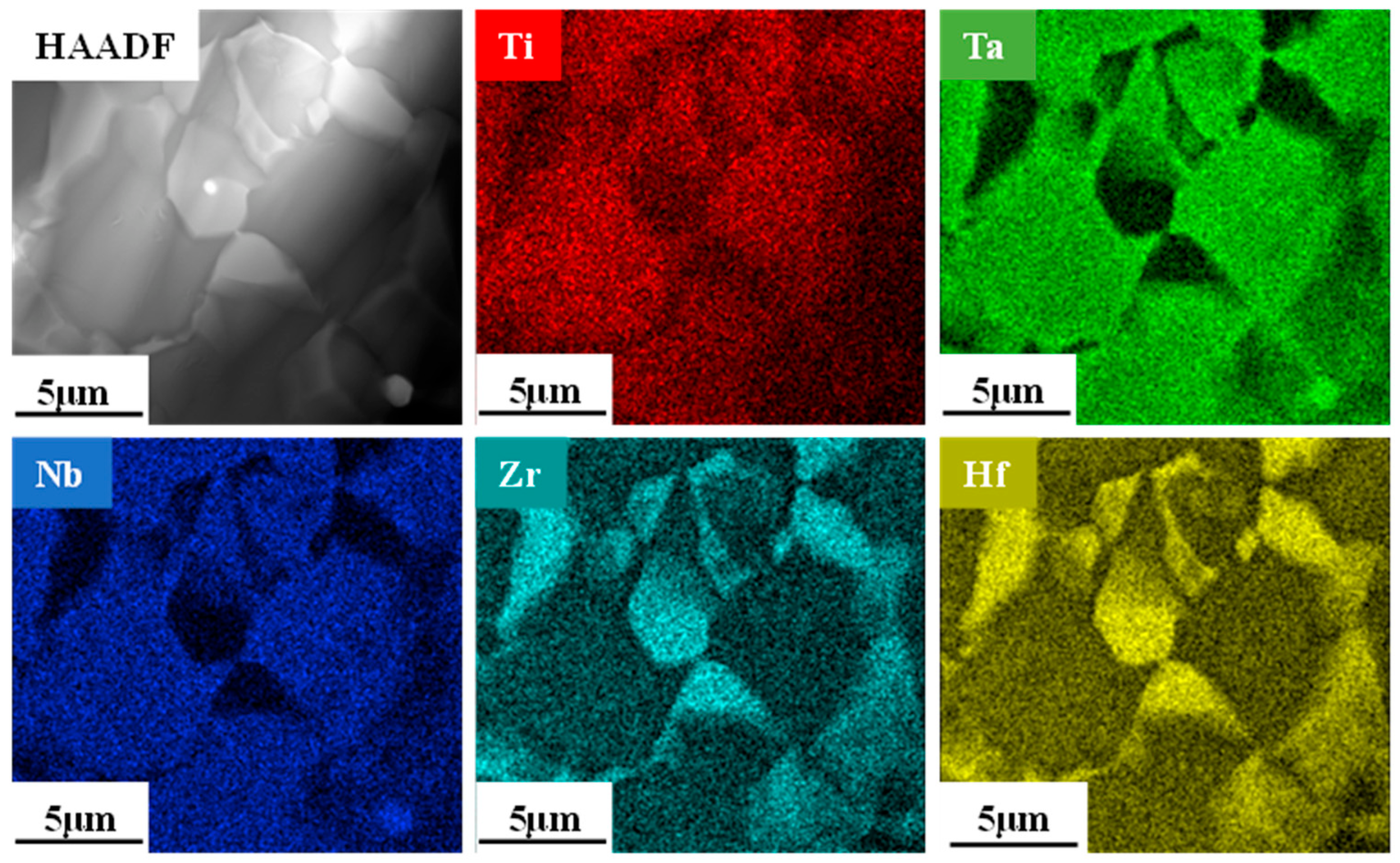
3.2. Phase and Microstructure of TiTaNbZrHf HEA
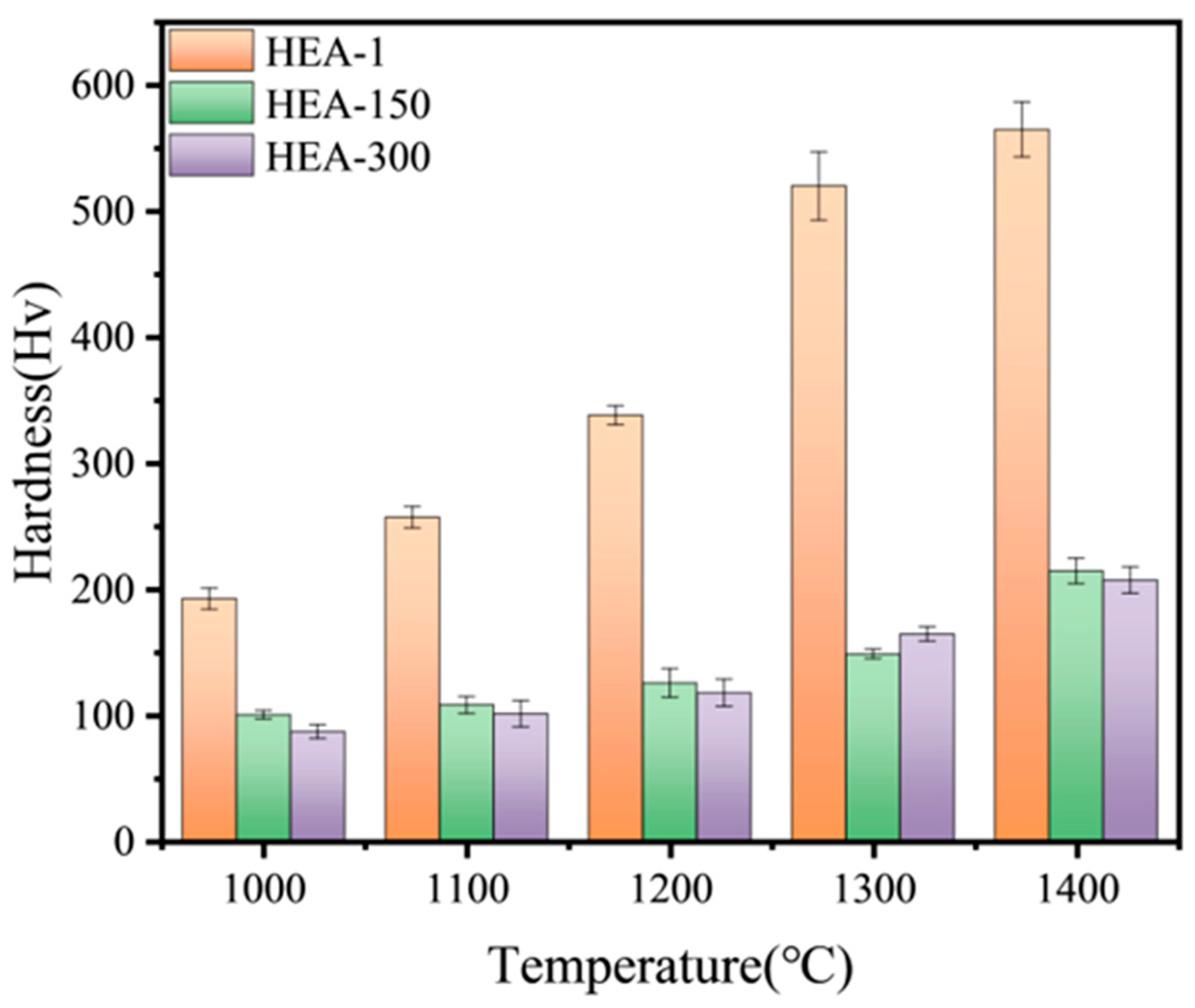
3.3. Mechanical Properties of the TiTaNbZrHf HEAs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Lin, S.-J.; Chin, T.-S.; Gan, J.-Y.; Chen, S.-K.; Shun, T.-T.; Tsau, C.-H.; Chou, S.-Y. Formation of simple crystal structures in Cu-Co-Ni-Cr-Al-Fe-Ti-V alloys with multiprincipal metallic elements. Met. Mater. Trans. A 2004, 35, 2533–2536. [Google Scholar] [CrossRef]
- Yeh, J.-W. Recent progress in high-entropy alloys. Ann. Chim. Sci. Matér. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Y.; Liu, B.; Zhang, W. Precipitation behavior during hot deformation of powder metallurgy Ti-Nb-Ta-Zr-Al high entropy alloys. Intermetallics 2018, 100, 95–103. [Google Scholar] [CrossRef]
- Xiang, T.; Cai, Z.; Du, P.; Li, K.; Zhang, Z.; Xie, G. Dual phase equal-atomic NbTaTiZr high-entropy alloy with ultra-fine grain and excellent mechanical properties fabricated by spark plasma sintering. J. Mater. Sci. Technol. 2021, 90, 150–158. [Google Scholar] [CrossRef]
- Jayaraj, J.; Thinaharan, C.; Ningshen, S.; Mallika, C.; Kamachi Mudali, U. Corrosion behavior and surface film characterization of TaNbHfZrTi high entropy alloy in aggressive nitric acid medium. Intermetallics 2017, 89, 123–132. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Senkov, O.N.; Scott, J.M.; Senkova, S.V.; Meisenkothen, F.; Miracle, D.B.; Woodward, C.F. Microstructure and elevated temperature properties of a refractory TaNbHfZrTi alloy. J. Mater. Sci. 2012, 47, 4062–4074. [Google Scholar] [CrossRef]
- Juan, C.-C.; Tseng, K.-K.; Hsu, W.-L.; Tsai, M.-H.; Tsai, C.-W.; Lin, C.-M.; Chen, S.-K.; Lin, S.-J.; Yeh, J.-W. Solution strengthening of ductile refractory HfMo x NbTaTiZr high-entropy alloys. Mater. Lett. 2016, 175, 284–287. [Google Scholar] [CrossRef]
- Zlotea, C.; Sow, M.A.; Ek, G.; Couzinié, J.P.; Perrière, L.; Guillot, I.; Bourgon, J.; Møller, K.T.; Jensen, T.R.; Akiba, E.; et al. Hydrogen sorption in TiZrNbHfTa high entropy alloy. J. Alloys Compd. 2019, 775, 667–674. [Google Scholar] [CrossRef]
- Motallebzadeh, A.; Peighambardoust, N.S.; Sheikh, S.; Murakami, H.; Guo, S.; Canadinc, D. Microstructural, mechanical and electrochemical characterization of TiZrTaHfNb and Ti1.5ZrTa0.5Hf0.5Nb0.5 refractory high-entropy alloys for biomedical applications. Intermetallics 2019, 113, 106572. [Google Scholar] [CrossRef]
- Senkov, O.N.; Semiatin, S.L. Microstructure and properties of a refractory high-entropy alloy after cold working. J. Alloys Compd. 2015, 649, 1110–1123. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.-P. Development and exploration of refractory high entropy alloys—A review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef]
- Dirras, G.; Gubicza, J.; Heczel, A.; Lilensten, L.; Couzinié, J.P.; Perrière, L.; Guillot, I.; Hocini, A. Microstructural investigation of plastically deformed Ti20Zr20Hf20Nb20Ta20 high entropy alloy by X-ray diffraction and transmission electron microscopy. Mater. Charact. 2015, 108, 1–7. [Google Scholar] [CrossRef]
- Wang, S.; Wu, M.; Shu, D.; Zhu, G.; Wang, D.; Sun, B. Mechanical instability and tensile properties of TiZrHfNbTa high entropy alloy at cryogenic temperatures. Acta Mater. 2020, 201, 517–527. [Google Scholar] [CrossRef]
- Couzinié, J.P.; Dirras, G.; Perrière, L.; Chauveau, T.; Leroy, E.; Champion, Y.; Guillot, I. Microstructure of a near-equimolar refractory high-entropy alloy. Mater. Lett. 2014, 126, 285–287. [Google Scholar] [CrossRef]
- Maiti, S.; Steurer, W. Structural-disorder and its effect on mechanical properties in single-phase TaNbHfZr high-entropy alloy. Acta Mater. 2016, 106, 87–97. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Q.; Yang, J.; Li, X.; Sui, X.; Gu, Y.; Liu, Y. Synthesis and thermal stability of a nanocrystalline MoNbTaTiV refractory high-entropy alloy via mechanical alloying. Int. J. Refract. Met. Hard Mater. 2019, 84, 104988. [Google Scholar] [CrossRef]
- Lukac, F.; Dudr, M.; Musalek, R.; Klecka, J.; Cinert, J.; Cizek, J.; Chraska, T.; Cizek, J.; Melikhova, O.; Kuriplach, J.; et al. Spark plasma sintering of gas atomized high-entropy alloy HfNbTaTiZr. J. Mater. Res. 2018, 33, 3247–3257. [Google Scholar] [CrossRef]
- Kang, B.; Lee, J.; Ryu, H.J.; Hong, S.H. Ultra-high strength WNbMoTaV high-entropy alloys with fine grain structure fabricated by powder metallurgical process. Mater. Sci. Eng. A 2018, 712, 616–624. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Y.; Li, Y.; Liu, B.; Wang, J.; Du, M.; Liu, R. Precipitation strengthening in a hot-worked TiNbTa0.5ZrAl0.5 refractory high entropy alloy. Mater. Lett. 2019, 246, 186–189. [Google Scholar] [CrossRef]
- Malek, J.; Zyka, J.; Lukac, F.; Vilemova, M.; Vlasak, T.; Cizek, J.; Melikhova, O.; Machackova, A.; Kim, H.S. The Effect of Processing Route on Properties of HfNbTaTiZr High Entropy Alloy. Materials 2019, 12, 4022. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Nagano, K.; Saxena, K.K.; Fujiwara, H.; Ameyama, K. Application of Hydride Process in Achieving Equimolar TiNbZrHfTa BCC Refractory High Entropy Alloy. Crystals 2020, 10, 1020. [Google Scholar] [CrossRef]
- Wang, S.-P.; Xu, J. (TiZrNbTa)-Mo high-entropy alloys: Dependence of microstructure and mechanical properties on Mo concentration and modeling of solid solution strengthening. Intermetallics 2018, 95, 59–72. [Google Scholar] [CrossRef]
- Neslušan, M.; Minárik, P.; Čilliková, M.; Kolařík, K.; Rubešová, K. Barkhausen noise emission in tool steel X210Cr12 after semi-solid processing. Mater. Charact. 2019, 157, 109891. [Google Scholar] [CrossRef]
- Sharma, B.; Vajpai, S.; Ameyama, K. An Efficient Powder Metallurgy Processing Route to Prepare High-Performance β-Ti–Nb Alloys Using Pure Titanium and Titanium Hydride Powders. Metals 2018, 8, 516. [Google Scholar] [CrossRef]
- Shkodich, N.; Sedegov, A.; Kuskov, K.; Busurin, S.; Scheck, Y.; Vadchenko, S.; Moskovskikh, D. Refractory High-Entropy HfTaTiNbZr-Based Alloys by Combined Use of Ball Milling and Spark Plasma Sintering: Effect of Milling Intensity. Metals 2020, 10, 1268. [Google Scholar] [CrossRef]
- Senkov, O.N.; Scott, J.M.; Senkova, S.V.; Miracle, D.B.; Woodward, C.F. Microstructure and room temperature properties of a high-entropy TaNbHfZrTi alloy. J. Alloys Compd. 2011, 509, 6043–6048. [Google Scholar] [CrossRef]
- Senkov, O.N.; Pilchak, A.L.; Semiatin, S.L. Effect of Cold Deformation and Annealing on the Microstructure and Tensile Properties of a HfNbTaTiZr Refractory High Entropy Alloy. Met. Mater. Trans. A 2018, 49, 2876–2892. [Google Scholar] [CrossRef]
- Yao, J.Q.; Liu, X.W.; Gao, N.; Jiang, Q.H.; Li, N.; Liu, G.; Zhang, W.B.; Fan, Z.T. Phase stability of a ductile single-phase BCC Hf0.5Nb0.5Ta0.5Ti1.5Zr refractory high-entropy alloy. Intermetallics 2018, 98, 79–88. [Google Scholar] [CrossRef]
- Larianovsky, N.; Katz-Demyanetz, A.; Eshed, E.; Regev, M. Microstructure, Tensile and Creep Properties of Ta20Nb20Hf20Zr20Ti20 High Entropy Alloy. Materials 2017, 10, 883. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Liu, X.; Wu, Y.; Wang, H.; Jiang, S.; Wang, S.; Hui, X.; Wu, Y.; Gault, B.; Kontis, P.; et al. Enhanced strength and ductility in a high-entropy alloy via ordered oxygen complexes. Nature 2018, 563, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Q.; Lin, D.; Chen, X.; Wang, T.; Wang, W.Y.; Wang, Y.; Hui, X. Phase Stability and Deformation Behavior of TiZrHfNbO High-Entropy Alloys. Front. Mater. 2020, 7, 589052. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Qian, M.; Shi, Z.; Song, T.; Huang, L.; Zou, J. A novel quaternary equiatomic Ti-Zr-Nb-Ta medium entropy alloy (MEA). Intermetallics 2018, 101, 39–43. [Google Scholar] [CrossRef]
- Su, I.A.; Tseng, K.-K.; Yeh, J.-W.; El-Sayed, B.; Liu, C.-H.; Wang, S.-H. Strengthening mechanisms and microstructural evolution of ductile refractory medium-entropy alloy Hf20Nb10Ti35Zr35. Scr. Mater. 2022, 206, 114225. [Google Scholar] [CrossRef]
- Yasuda, H.Y.; Yamada, Y.; Cho, K.; Nagase, T. Deformation behavior of HfNbTaTiZr high entropy alloy singe crystals and polycrystals. Mater. Sci. Eng. A 2021, 809, 140983. [Google Scholar] [CrossRef]
- Eleti, R.R.; Stepanov, N.; Yurchenko, N.; Klimenko, D.; Zherebtsov, S. Plastic deformation of solid-solution strengthened Hf-Nb-Ta-Ti-Zr body-centered cubic medium/high-entropy alloys. Scr. Mater. 2021, 200, 113927. [Google Scholar] [CrossRef]


| Materials | Preparation Methods | Phase Structure | Yield Strength (MPa) | Plastic Strain (%) |
|---|---|---|---|---|
| TiZrNbHfTa [28] | As-cast | BCC | 929 | >50 |
| TaNbHfZr [17] | As-cast | BCC | 1315 | 21.6 |
| TiZrNbTa [34] | As-cast | BCC | 1100 ± 90 | 48 ± 6 |
| TiTaNbZrHf | PM | BCC + HCP | 1400 | 20 |
| Hf20Nb10Zr35Ti35 [35] | As-cast | BCC | 524 | 17.5 |
| TiTaNbZrHf [36] | PM | BCC | 940 | >50 |
| TiTaNbZrHf [37] | As-cast | BCC | 1000 | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Liu, P.; Dong, Z.; Liu, H.; Wang, J.; Guo, X.; Xia, Y.; Wang, Q. Sintering, Microstructure, and Mechanical Properties of TiTaNbZrHf High-Entropy Alloys Prepared by Cold Isostatic Pressing and Pressure-Less Sintering of Hydrides. Materials 2023, 16, 1759. https://doi.org/10.3390/ma16051759
Chen Y, Liu P, Dong Z, Liu H, Wang J, Guo X, Xia Y, Wang Q. Sintering, Microstructure, and Mechanical Properties of TiTaNbZrHf High-Entropy Alloys Prepared by Cold Isostatic Pressing and Pressure-Less Sintering of Hydrides. Materials. 2023; 16(5):1759. https://doi.org/10.3390/ma16051759
Chicago/Turabian StyleChen, Yubing, Peidong Liu, Zhaowang Dong, Hanning Liu, Junjie Wang, Xueyi Guo, Yang Xia, and Qinmeng Wang. 2023. "Sintering, Microstructure, and Mechanical Properties of TiTaNbZrHf High-Entropy Alloys Prepared by Cold Isostatic Pressing and Pressure-Less Sintering of Hydrides" Materials 16, no. 5: 1759. https://doi.org/10.3390/ma16051759
APA StyleChen, Y., Liu, P., Dong, Z., Liu, H., Wang, J., Guo, X., Xia, Y., & Wang, Q. (2023). Sintering, Microstructure, and Mechanical Properties of TiTaNbZrHf High-Entropy Alloys Prepared by Cold Isostatic Pressing and Pressure-Less Sintering of Hydrides. Materials, 16(5), 1759. https://doi.org/10.3390/ma16051759






