Long-Term Performance of Ultrafiltration Membranes: Corrosion Fouling Aspect
Abstract
1. Introduction
2. Materials and Methods
2.1. Ultrafiltration Membranes
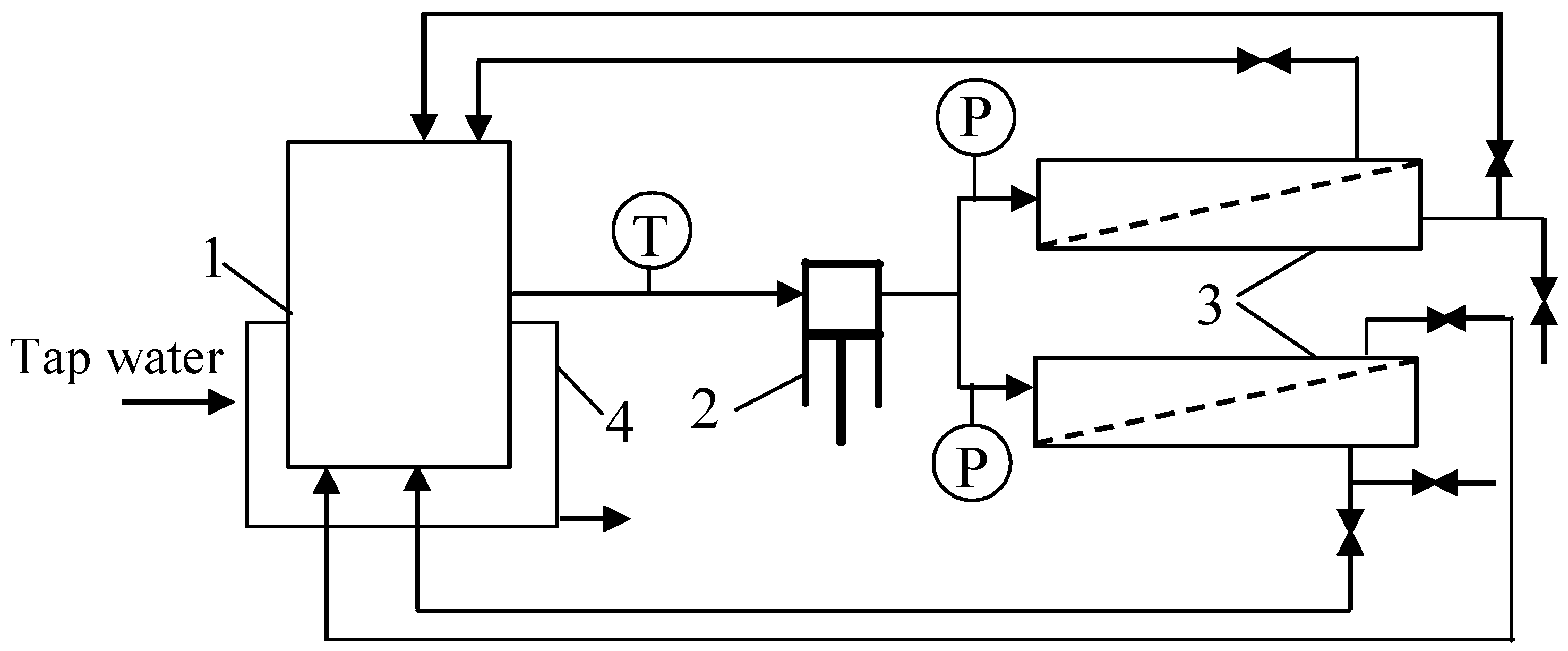
2.2. Ultrafiltration Units
2.2.1. Pilot-Scale Unit
2.2.2. Laboratory-Scale Unit
2.3. Feed Solutions
2.4. Analytical Methods
3. Results and Discussion
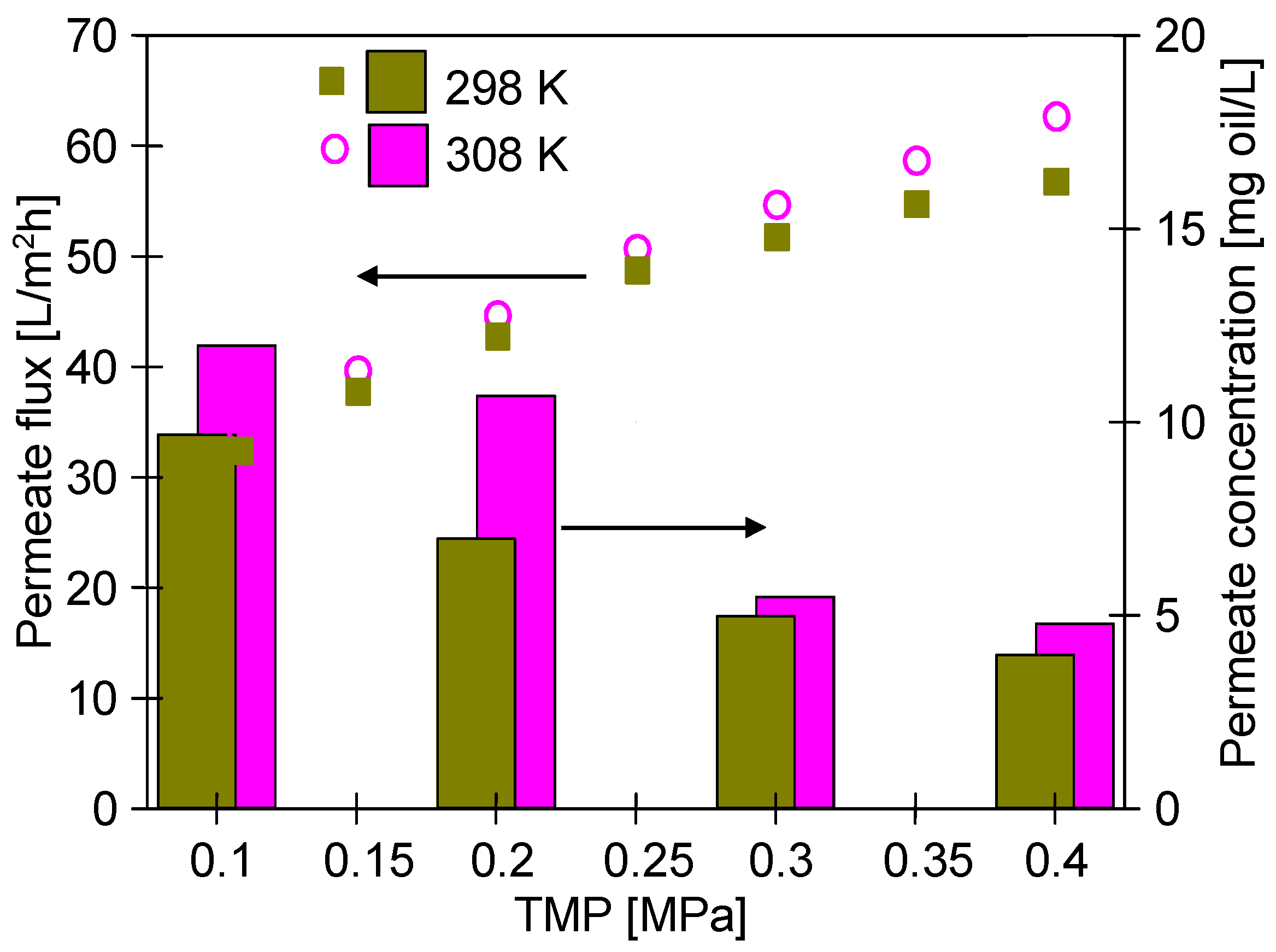
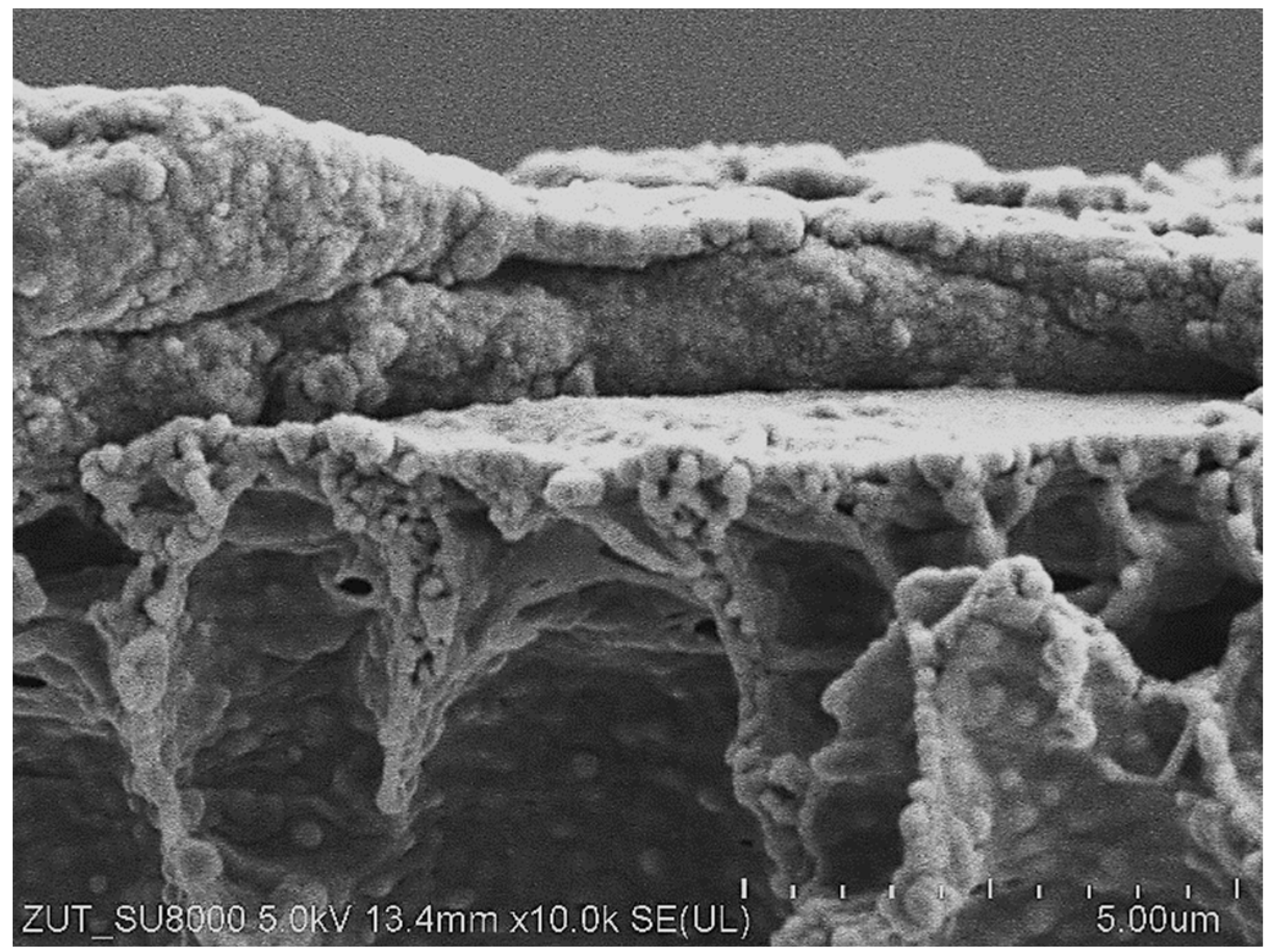
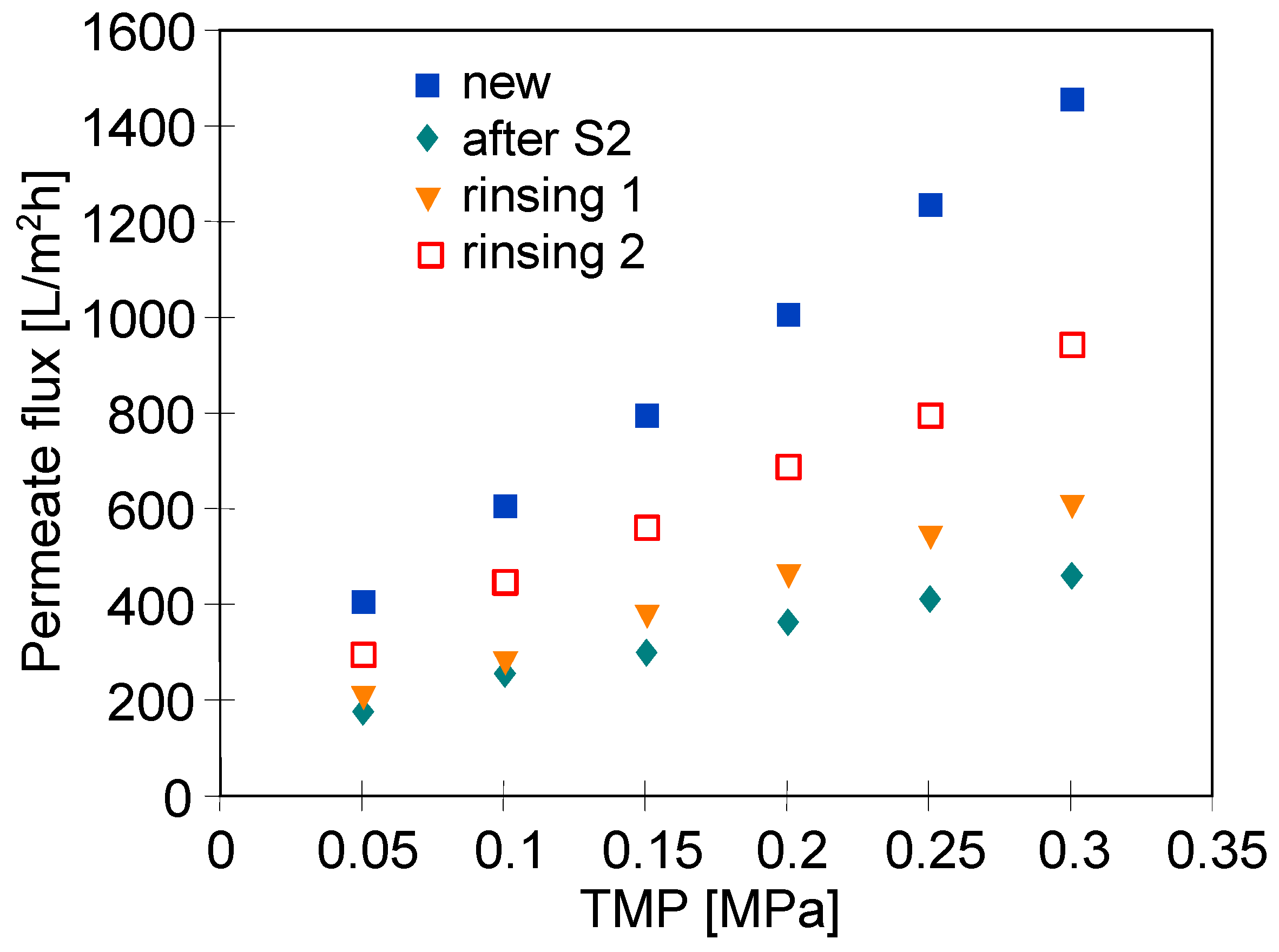
3.1. Pilot-Scale Unit
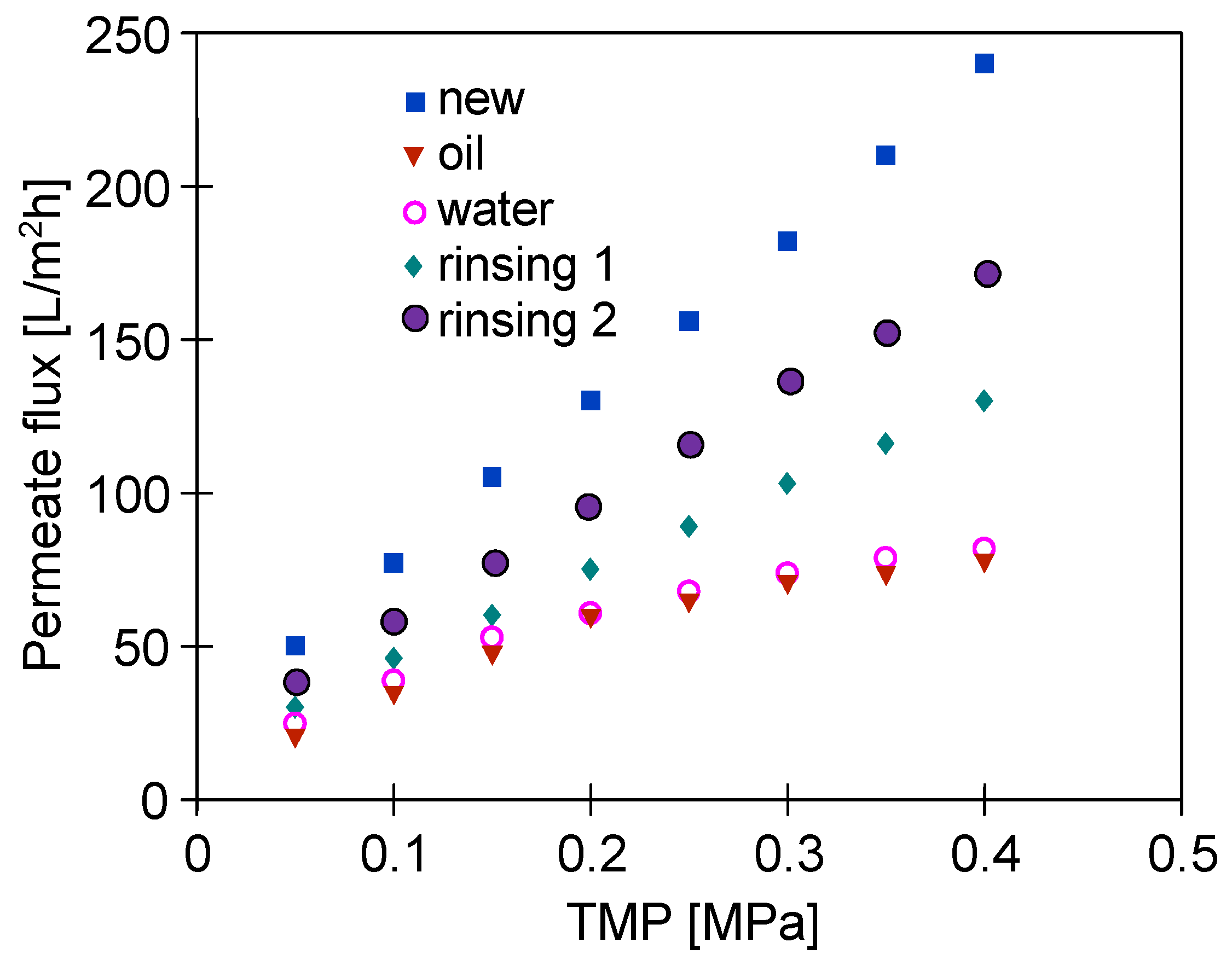
3.2. Laboratory-Scale Unit
4. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esmaeili, H.; Esmaeilzadeh, F.; Mowla, D. Effect of Surfactant on Stability and Size Distribution of Gas Condensate Droplets in Water. J. Chem. Eng. Data 2014, 59, 1461–1467. [Google Scholar] [CrossRef]
- Chen, W.; Peng, J.; Su, Y.; Zheng, L.; Wang, L.; Jiang, Z. Separation of Oil/Water Emulsion Using Pluronic F127 Modified Polyethersulfone Ultrafiltration Membranes. Sep. Purif. Technol. 2009, 66, 591–597. [Google Scholar] [CrossRef]
- Bratskaya, S.; Avramenko, V.; Schwarz, S.; Philippova, I. Enhanced Flocculation of Oil-in-Water Emulsions by Hydrophobically Modified Chitosan Derivatives. Colloids Surf. A Physicochem. Eng. Asp. 2006, 275, 168–176. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, W.; Li, X.; Wang, Z. Surface Engineering of Filter Membranes with Hydrogels for Oil-in-Water Emulsion Separation. Sep. Purif. Technol. 2023, 304, 122340. [Google Scholar] [CrossRef]
- Allende, D.; Pando, D.; Matos, M.; Carleos, C.E.; Pazos, C.; Benito, J.M. Optimization of a Membrane Hybrid Process for Oil-in-Water Emulsions Treatment Using Taguchi Experimental Design. Desalination Water Treat. 2016, 57, 4832–4841. [Google Scholar] [CrossRef]
- Shi, L.; Lei, Y.; Huang, J.; Shi, Y.; Yi, K.; Zhou, H. Ultrafiltration of Oil-in-Water Emulsions Using Ceramic Membrane: Roles Played by Stabilized Surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123948. [Google Scholar] [CrossRef]
- Tian, J.; Trinh, T.A.; Kalyan, M.N.; Ho, J.S.; Chew, J.W. In-Situ Monitoring of Oil Emulsion Fouling in Ultrafiltration via Electrical Impedance Spectroscopy (EIS): Influence of Surfactant. J. Membr. Sci. 2020, 616, 118527. [Google Scholar] [CrossRef]
- Kirschner, A.Y.; Cheng, Y.-H.; Paul, D.R.; Field, R.W.; Freeman, B.D. Fouling Mechanisms in Constant Flux Crossflow Ultrafiltration. J. Membr. Sci. 2019, 574, 65–75. [Google Scholar] [CrossRef]
- Tummons, E.N.; Chew, J.W.; Fane, A.G.; Tarabara, V.V. Ultrafiltration of Saline Oil-in-Water Emulsions Stabilized by an Anionic Surfactant: Effect of Surfactant Concentration and Divalent Counterions. J. Membr. Sci. 2017, 537, 384–395. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, T.; Gutierrez, L.; Ma, J.; Croué, J.-P. Influence of Surface Properties of Filtration-Layer Metal Oxide on Ceramic Membrane Fouling during Ultrafiltration of Oil/Water Emulsion. Environ. Sci. Technol. 2016, 50, 4668–4674. [Google Scholar] [CrossRef]
- Kasemset, S.; He, Z.; Miller, D.J.; Freeman, B.D.; Sharma, M.M. Effect of Polydopamine Deposition Conditions on Polysulfone Ultrafiltration Membrane Properties and Threshold Flux during Oil/Water Emulsion Filtration. Polymer 2016, 97, 247–257. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Application of Ultrafiltration Ceramic Membrane for Separation of Oily Wastewater Generated by Maritime Transportation. Sep. Purif. Technol. 2021, 261, 118259. [Google Scholar] [CrossRef]
- Poyai, T.; Khiewpuckdee, P.; Wongrueng, A.; Painmanakul, P.; Chawaloesphonsiya, N. Enhancement of Crossflow Ultrafiltration for the Treatment of Stabilized Oily Emulsions. Eng. J. 2019, 23, 15–27. [Google Scholar] [CrossRef]
- Pezeshk, S.; Ojagh, S.M.; Rezaei, M.; Shabanpour, B. Fractionation of Protein Hydrolysates of Fish Waste Using Membrane Ultrafiltration: Investigation of Antibacterial and Antioxidant Activities. Probiotics Antimicro. Prot. 2019, 11, 1015–1022. [Google Scholar] [CrossRef]
- Agi, A.; Junin, R.; Alqatta, A.Y.M.; Gbadamosi, A.; Yahya, A.; Abbas, A. Ultrasonic Assisted Ultrafiltration Process for Emulsification of Oil Field Produced Water Treatment. Ultrason. Sonochemistry 2019, 51, 214–222. [Google Scholar] [CrossRef]
- Halleb, A.; Yokoyama, F.; das Neves, M.A.; Nakajima, M. Effects of Surfactants and Oil-in-Water Emulsions on Reverse Osmosis Membrane Performance. Euro-Mediterr. J. Environ. Integr. 2021, 6, 44. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Clarification of 1,3-Propanediol Fermentation Broths by Using a Ceramic Fine UF Membrane. Membranes 2020, 10, 319. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Boyraz, E.; Maryska, J.; Kucerova, K. A Review on Membrane Technology and Chemical Surface Modification for the Oily Wastewater Treatment. Materials 2020, 13, 493. [Google Scholar] [CrossRef]
- Sutrisna, P.D.; Kurnia, K.A.; Siagian, U.W.R.; Ismadji, S.; Wenten, I.G. Membrane Fouling and Fouling Mitigation in Oil–Water Separation: A Review. J. Environ. Chem. Eng. 2022, 10, 107532. [Google Scholar] [CrossRef]
- Ahmad, T.; Guria, C.; Mandal, A. A Review of Oily Wastewater Treatment Using Ultrafiltration Membrane: A Parametric Study to Enhance the Membrane Performance. J. Water Process Eng. 2020, 36, 101289. [Google Scholar] [CrossRef]
- Luján-Facundo, M.J.; Mendoza-Roca, J.A.; Cuartas-Uribe, B.; Álvarez-Blanco, S. Cleaning Efficiency Enhancement by Ultrasounds for Membranes Used in Dairy Industries. Ultrason. Sonochemistry 2016, 33, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Faibish, R.S.; Cohen, Y. Fouling and Rejection Behavior of Ceramic and Polymer-Modified Ceramic Membranes for Ultrafiltration of Oil-in-Water Emulsions and Microemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2001, 191, 27–40. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Swaminathan, J.; Guillen-Burrieza, E.; Arafat, H.A.; Lienhard, V.J.H. Scaling and Fouling in Membrane Distillation for Desalination Applications: A Review. Desalination 2015, 356, 294–313. [Google Scholar] [CrossRef]
- Xu, X.; Liu, S.; Liu, Y.; Smith, K.; Cui, Y. Corrosion of Stainless Steel Valves in a Reverse Osmosis System: Analysis of Corrosion Products and Metal Loss. Eng. Fail. Anal. 2019, 105, 40–51. [Google Scholar] [CrossRef]
- Ling, R.; Yu, L.; Pham, T.P.T.; Shao, J.; Chen, J.P.; Reinhard, M. Catalytic Effect of Iron on the Tolerance of Thin-Film Composite Polyamide Reverse Osmosis Membranes to Hydrogen Peroxide. J. Membr. Sci. 2018, 548, 91–98. [Google Scholar] [CrossRef]
- Karime, M.; Bouguecha, S.; Hamrouni, B. RO Membrane Autopsy of Zarzis Brackish Water Desalination Plant. Desalination 2008, 220, 258–266. [Google Scholar] [CrossRef]
- Dow, N.; Gray, S.; Li, J.; Zhang, J.; Ostarcevic, E.; Liubinas, A.; Atherton, P.; Roeszler, G.; Gibbs, A.; Duke, M. Pilot Trial of Membrane Distillation Driven by Low Grade Waste Heat: Membrane Fouling and Energy Assessment. Desalination 2016, 391, 30–42. [Google Scholar] [CrossRef]
- Gryta, M. Effect of Iron Oxides Scaling on the MD Process Performance. Desalination 2007, 216, 88–102. [Google Scholar] [CrossRef]
- Gryta, M. Resistance of Polypropylene Membrane to Oil Fouling during Membrane Distillation. Membranes 2021, 11, 552. [Google Scholar] [CrossRef]
- Karakulski, K.; Gryta, M. The Application of Ultrafiltration for Treatment of Ships Generated Oily Wastewater. Chem. Pap. 2017, 71, 1165–1173. [Google Scholar] [CrossRef]
- Zhu, L.; Dong, X.; Xu, M.; Yang, F.; Guiver, M.D.; Dong, Y. Fabrication of Mullite Ceramic-Supported Carbon Nanotube Composite Membranes with Enhanced Performance in Direct Separation of High-Temperature Emulsified Oil Droplets. J. Membr. Sci. 2019, 582, 140–150. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Wang, H.; Wang, M. The Application of Nanofiltration Membrane for Recovering Lithium from Salt Lake Brine. Desalination 2019, 468, 114081. [Google Scholar] [CrossRef]
- He, D.; Xiao, Y.; Li, X.; Jin, Y. Treatment of Oil/Water Emulsion by Polyethylene Glycol Ultrafiltration Membrane. J. Cent. South Univ. Technol. 2005, 12, 542–545. [Google Scholar] [CrossRef]
- Hesampour, M.; Krzyzaniak, A.; Nyström, M. The Influence of Different Factors on the Stability and Ultrafiltration of Emulsified Oil in Water. J. Membr. Sci. 2008, 325, 199–208. [Google Scholar] [CrossRef]
- Kiss, Z.L.; Kertész, S.; Beszédes, S.; Hodúr, C.; László, Z. Investigation of Parameters Affecting the Ultrafiltration of Oil-in-Water Emulsion Wastewater. Desalination Water Treat. 2013, 51, 4914–4920. [Google Scholar] [CrossRef]
- Matos, M.; Lobo, A.; Fernández, E.; Benito, J.M.; Pazos, C.; Coca, J. Recycling of Oily Ultrafiltration Permeates to Reformulate O/W Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2008, 331, 8–15. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Ultrafiltration of Stable Oil-in-Water Emulsion by Polysulfone Membrane. J. Membr. Sci. 2008, 325, 427–437. [Google Scholar] [CrossRef]
- Yi, X.S.; Yu, S.L.; Shi, W.X.; Sun, N.; Jin, L.M.; Wang, S.; Zhang, B.; Ma, C.; Sun, L.P. The Influence of Important Factors on Ultrafiltration of Oil/Water Emulsion Using PVDF Membrane Modified by Nano-Sized TiO2/Al2O3. Desalination 2011, 281, 179–184. [Google Scholar] [CrossRef]
- Salud Camilleri-Rumbau, M.; Popovic, O.; Briceño, K.; Errico, M.; Søtoft, L.F.; Christensen, K.V.; Norddahl, B. Ultrafiltration of Separated Digestate by Tubular Membranes: Influence of Feed Pretreatment on Hydraulic Performance and Heavy Metals Removal. J. Environ. Manag. 2019, 250, 109404. [Google Scholar] [CrossRef]
- Abd-Razak, N.H.; Chew, Y.M.J.; Bird, M.R. Orange Juice Ultrafiltration: Characterisation of Deposit Layers and Membrane Surfaces after Fouling and Cleaning. Int. J. Food Eng. 2021, 17, 837–850. [Google Scholar] [CrossRef]
- Abd-Razak, N.H.; Pihlajamäki, A.; Virtanen, T.; John Chew, Y.M.; Bird, M.R. The Influence of Membrane Charge and Porosity upon Fouling and Cleaning during the Ultrafiltration of Orange Juice. Food Bioprod. Process. 2021, 126, 184–194. [Google Scholar] [CrossRef]
- Madeira, T.; Marçal, C.; Cardoso, S.M.; Gando-Ferreira, L.M.; Costa, R. Ultrafiltration of Fucus Vesiculosus Extracts Under Different Operating Conditions. Waste Biomass Valorization 2022, 13, 4447–4458. [Google Scholar] [CrossRef]
- Weis, A.; Bird, M.R.; Nyström, M.; Wright, C. The Influence of Morphology, Hydrophobicity and Charge upon the Long-Term Performance of Ultrafiltration Membranes Fouled with Spent Sulphite Liquor. Desalination 2005, 175, 73–85. [Google Scholar] [CrossRef]
- Vasyliev, G.S. The Influence of Flow Rate on Corrosion of Mild Steel in Hot Tap Water. Corros. Sci. 2015, 98, 33–39. [Google Scholar] [CrossRef]
- Kumar, R.; Kachwaha, M.; Verma, S.; Patidar, D. Quick Detection of Iron in Contaminated Water before Feeding to RO Membranes. SN Appl. Sci. 2019, 1, 427. [Google Scholar] [CrossRef]
- Berradi, M.; Elrhayam, Y.; Harfi, A.E. Cleaning Cycle Optimization of Ultrafiltration Membranes by the Method of Experiment Design. Moroc. J. Chem. 2015, 3, 222–228. [Google Scholar]
- Antón, E.; Álvarez, J.R.; Palacio, L.; Prádanos, P.; Hernández, A.; Pihlajamäki, A.; Luque, S. Ageing of Polyethersulfone Ultrafiltration Membranes under Long-Term Exposures to Alkaline and Acidic Cleaning Solutions. Chem. Eng. Sci. 2015, 134, 178–195. [Google Scholar] [CrossRef]
- Arkhangelsky, E.; Kuzmenko, D.; Gitis, N.V.; Vinogradov, M.; Kuiry, S.; Gitis, V. Hypochlorite Cleaning Causes Degradation of Polymer Membranes. Tribol. Lett. 2007, 28, 109–116. [Google Scholar] [CrossRef]


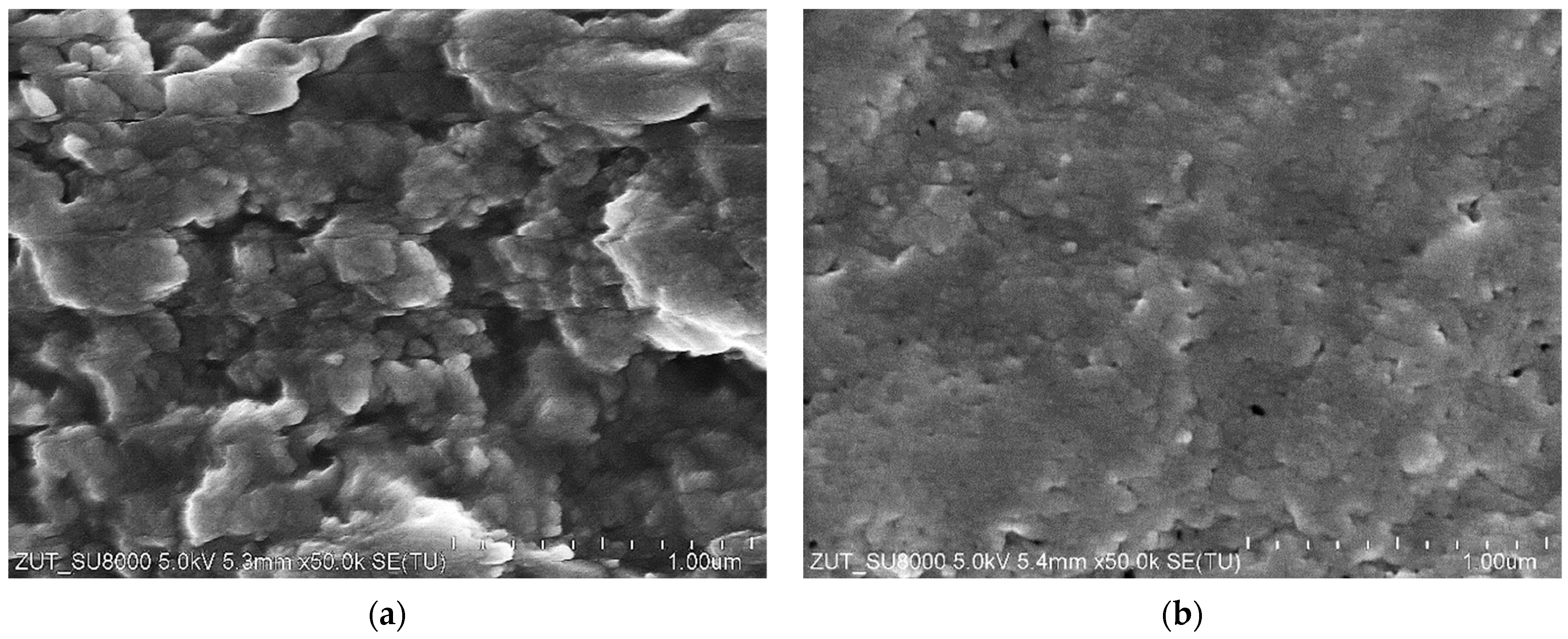

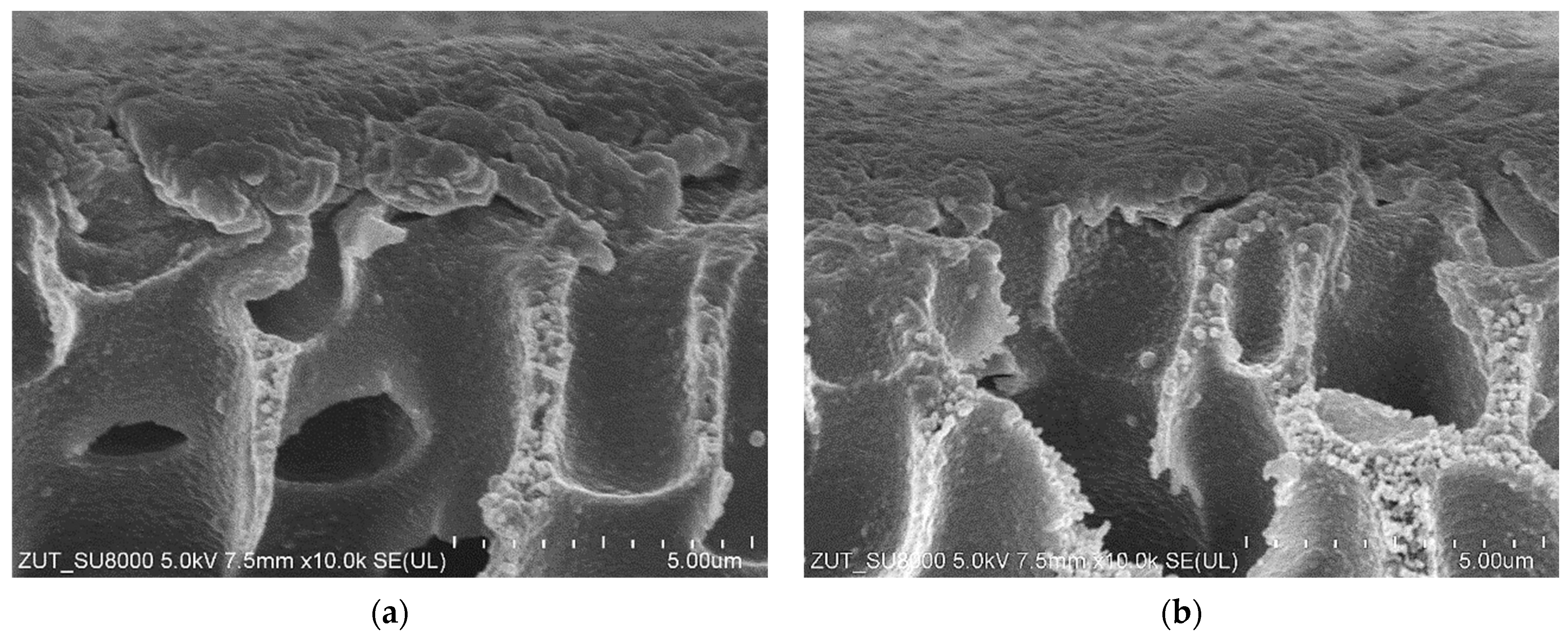

| Membrane | Manufacturer | Polymer | MWCO [kDa] | pH Range |
|---|---|---|---|---|
| FP100 | PCI | PVDF | 100 | 1.5–12.0 |
| UE50 | TriSep | PES | 100 | 2.0–12.0 |
| Ion | Na+ | K+ | Ca2+ | Mg2+ | NO3− | Cl− | SO42− |
|---|---|---|---|---|---|---|---|
| Tap water | 23.9 | 5.80 | 63.90 | 16.60 | 1.80 | 46.90 | 86.90 |
| NF permeate | 2.31 | 0.35 | 0.54 | 0.09 | 0.17 | 2.04 | 0.24 |
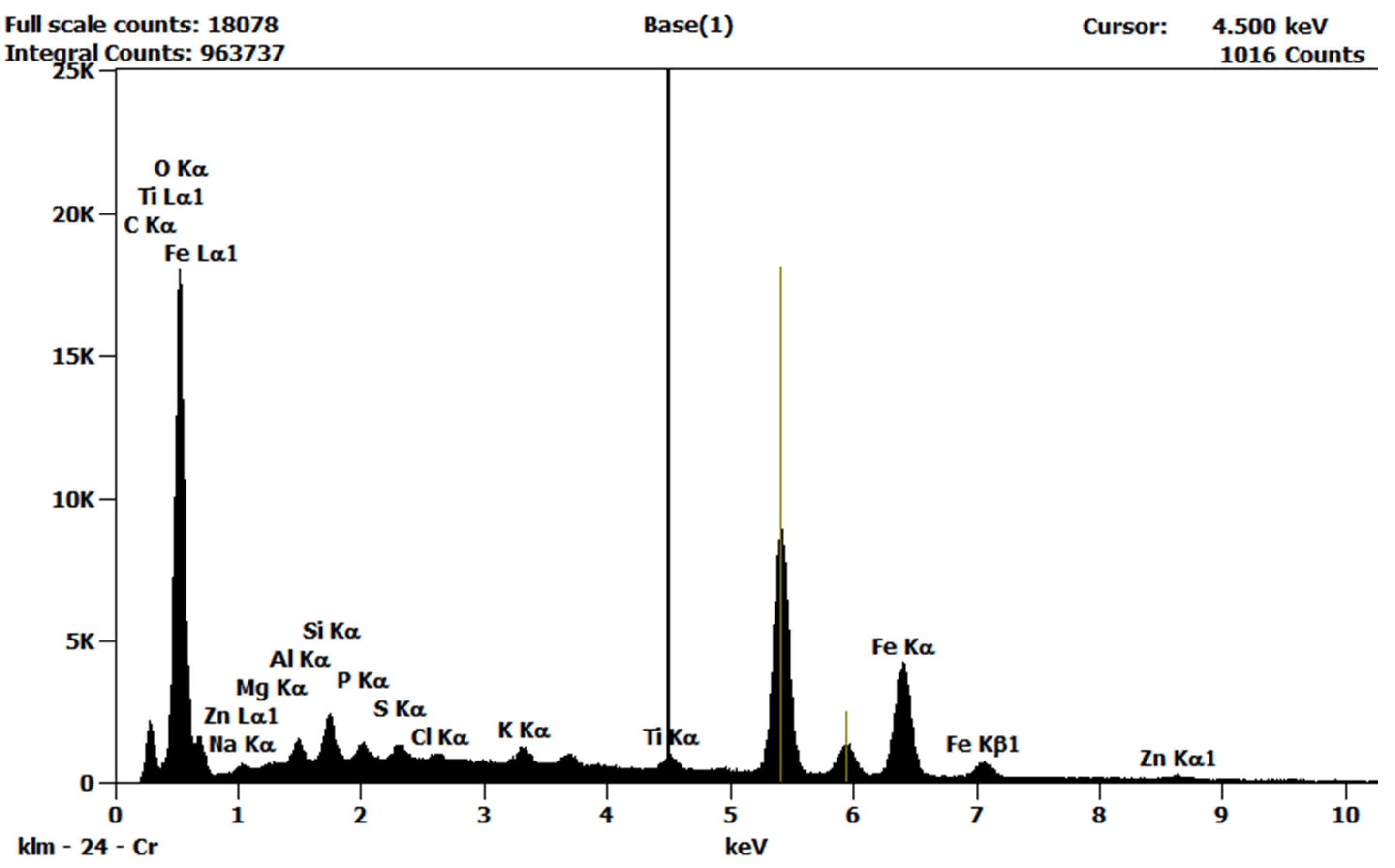
| Element | C | O | Fe | Na | Mg | Si | P | Zn | Cl | S |
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration [wt%] | 8.40 | 58.10 | 22.60 | 0.98 | 0.29 | 2.40 | 0.80 | 1.70 | 1.30 | 0.68 |
| Element | C | O | Fe | Na | Mg | Si | P | Zn | Cl | S |
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration [wt%] | 35.30 | 36.40 | 0.17 | - | - | 0.14 | - | 0.20 | - | 23.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczak, W.; Gryta, M. Long-Term Performance of Ultrafiltration Membranes: Corrosion Fouling Aspect. Materials 2023, 16, 1673. https://doi.org/10.3390/ma16041673
Tomczak W, Gryta M. Long-Term Performance of Ultrafiltration Membranes: Corrosion Fouling Aspect. Materials. 2023; 16(4):1673. https://doi.org/10.3390/ma16041673
Chicago/Turabian StyleTomczak, Wirginia, and Marek Gryta. 2023. "Long-Term Performance of Ultrafiltration Membranes: Corrosion Fouling Aspect" Materials 16, no. 4: 1673. https://doi.org/10.3390/ma16041673
APA StyleTomczak, W., & Gryta, M. (2023). Long-Term Performance of Ultrafiltration Membranes: Corrosion Fouling Aspect. Materials, 16(4), 1673. https://doi.org/10.3390/ma16041673







