Effect of Ti on Characterization and Properties of CoCrFeNiTix High Entropy Alloy Prepared Via Electro-Deoxidization of the Metal Oxides and Vacuum Hot Pressing Sintering Process
Abstract
:1. Introduction
2. Materials Methods
2.1. Selection and Proportioning of Raw Materials
2.2. Electro-Deoxidation Process of Products
2.3. Vacuum Hot Pressing Sintering
2.4. Characterization and Test
3. Results and Discussion
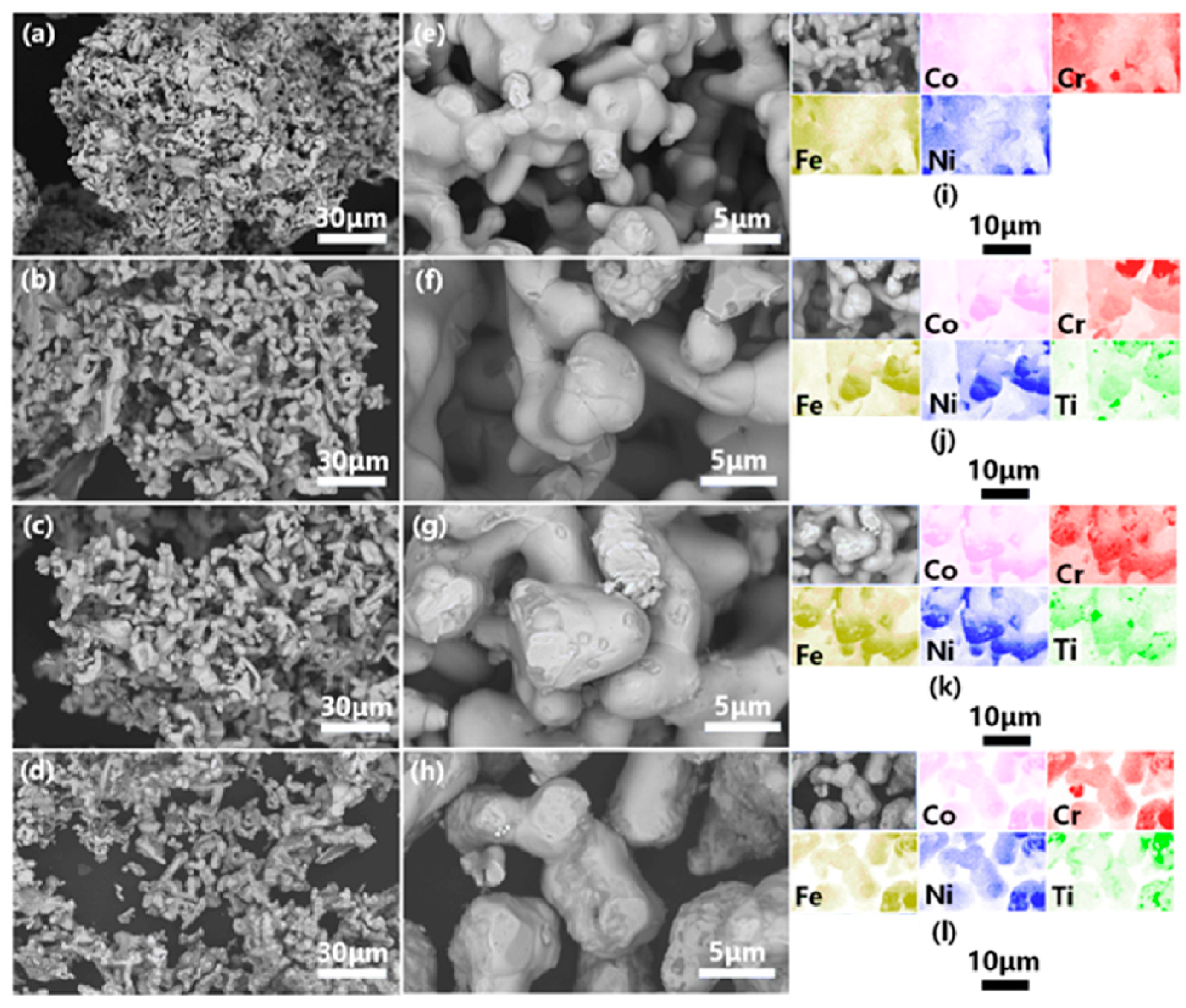
3.1. Electro-Deoxidization of the Mixed Oxides Powders
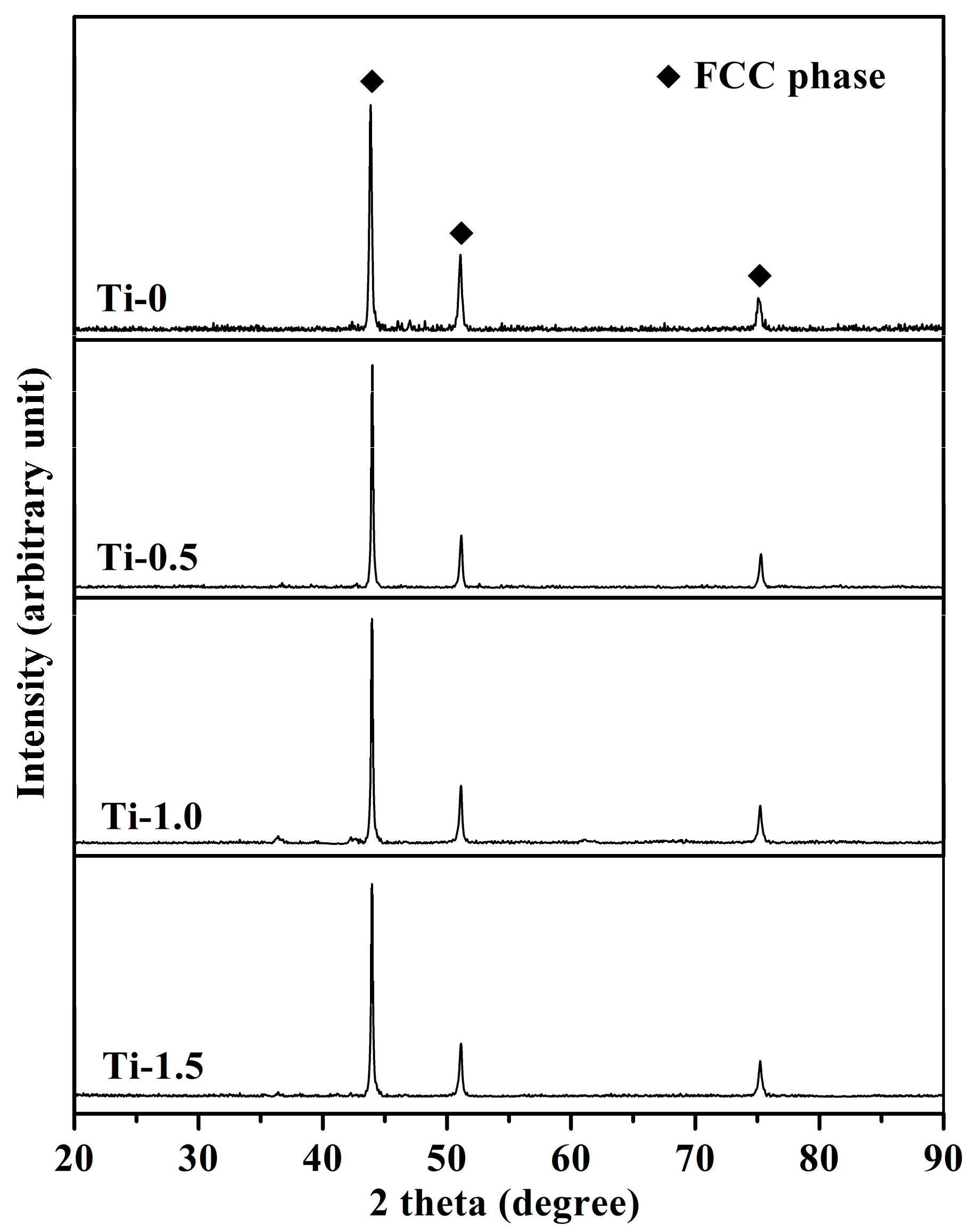
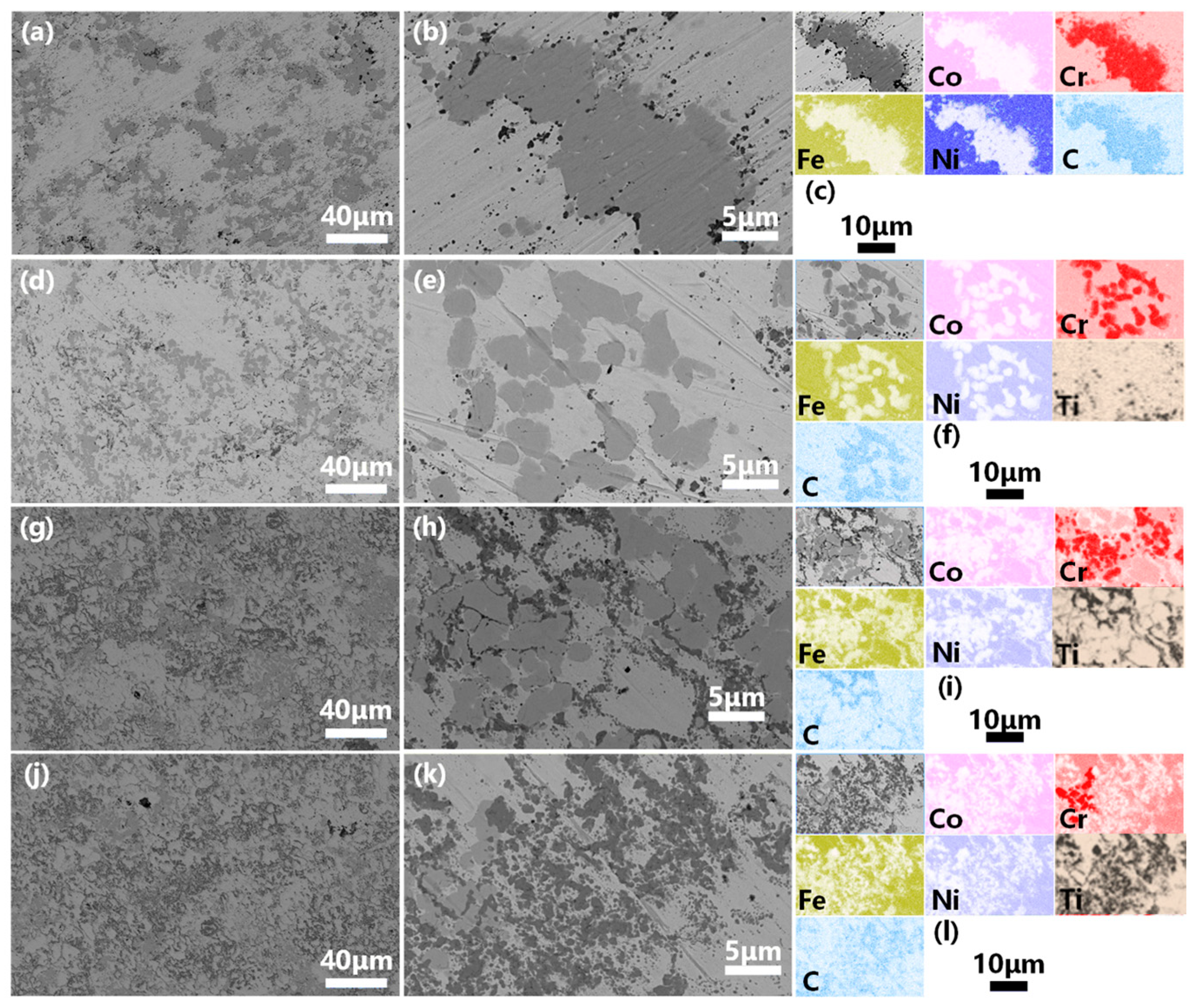
3.2. Structural and Morphological Characterization of the VHPS Product
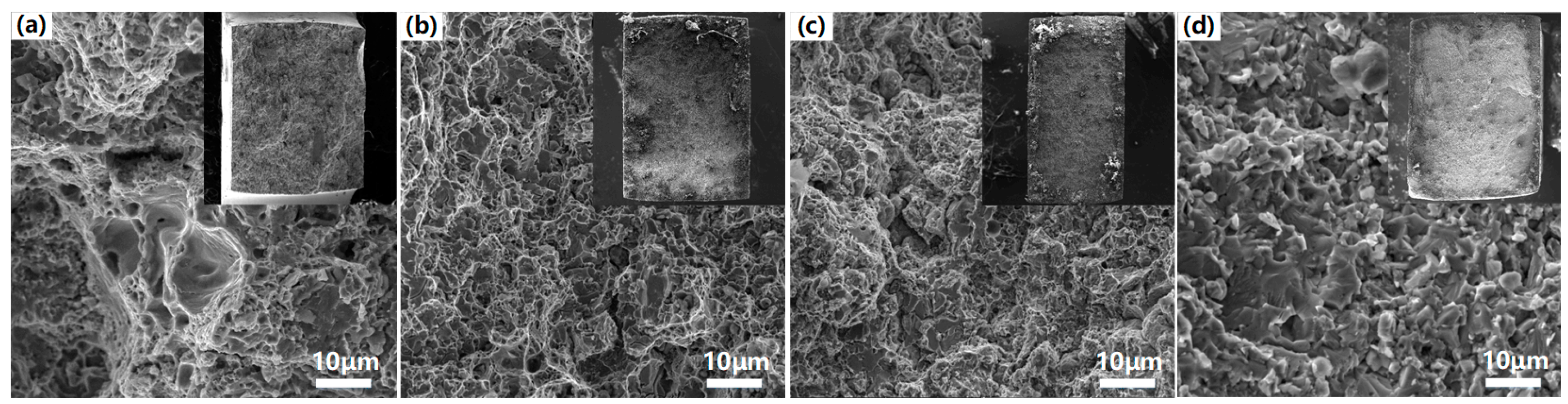
3.3. Mechanical Properties and Corrosion Behavior of the VHPS Products
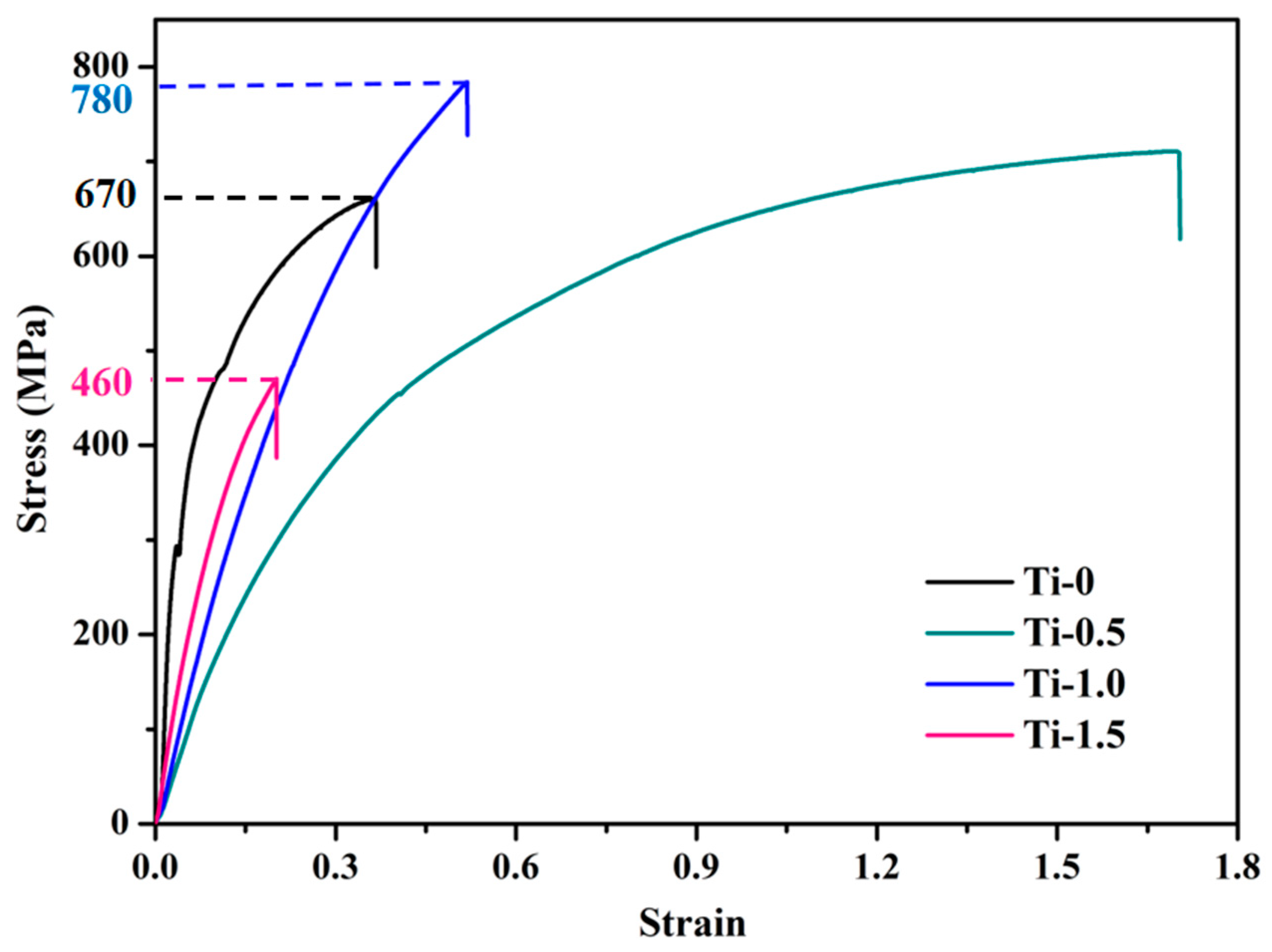
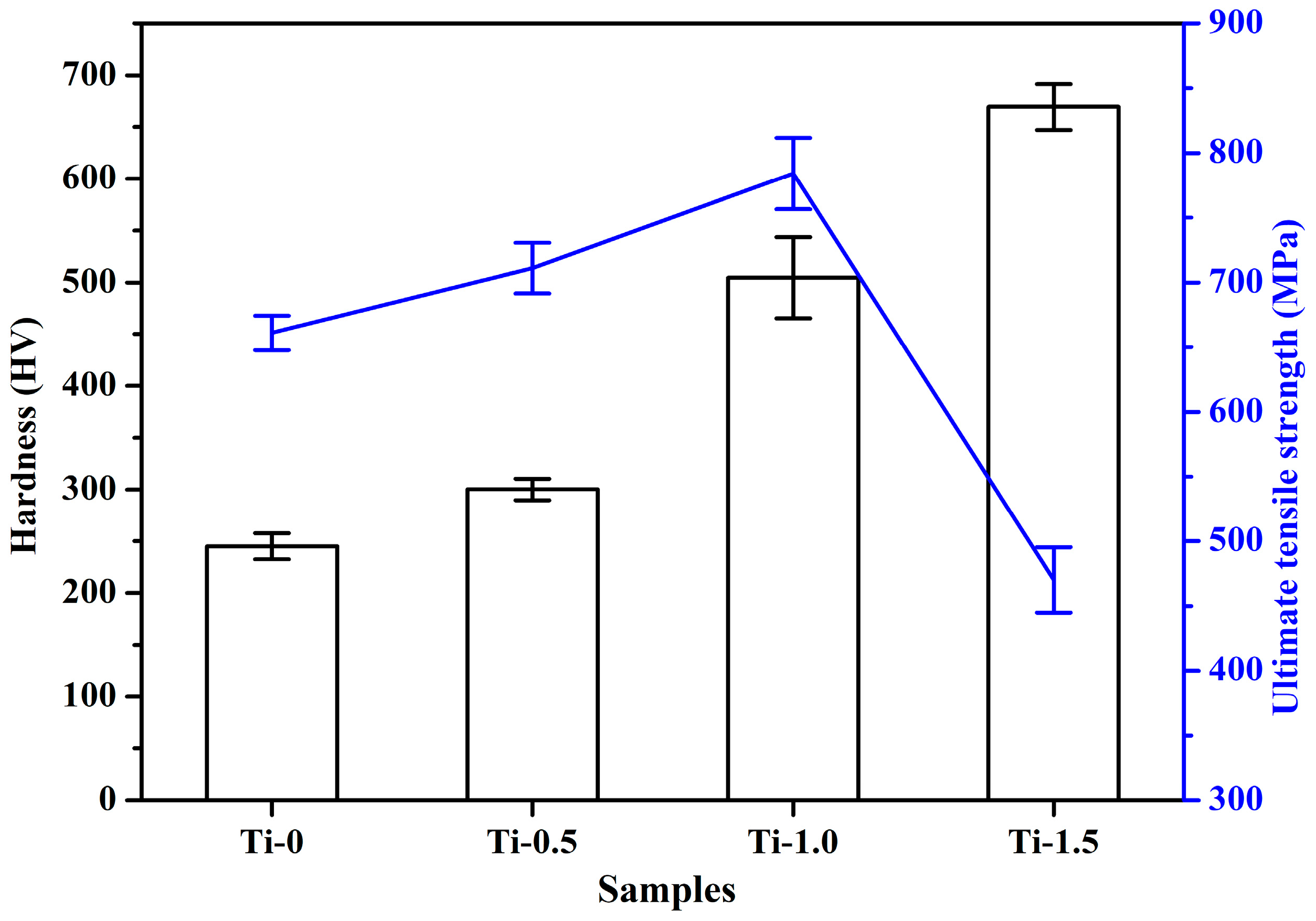
3.3.1. Tensile and Hardness Results
3.3.2. Potentiodynamic Polarization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.D.; Zhang, L.J.; Fan, J.T.; Yu, P.F.; Li, G. Novel Co-free CrFeNiNb0.1Tix high-entropy alloys with ultra high hardness and strength. J. Mater. Sci. Eng. A 2019, 764, 138212. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Curado, T.M.; Zeng, Z.; Lopes, J.G.; Rossinyol, E.; Park, J.M.; Schell, N.; Braz Fernandes, F.M.; Kim, H.S. Gas tungsten arc welding of as-rolled CrMnFeCoNi high entropy alloy. J. Mater. Des. 2020, 189, 108505. [Google Scholar] [CrossRef]
- Hou, Y.X.; Liu, T.; He, D.D.; Li, Z.J.; Chen, L.; Su, H.H.; Fu, P.X.; Dai, P.Q.; Huang, W.D. Sustaining strength-ductility synergy of SLM Fe50Mn30Co10Cr10 metastable high-entropy alloy by Si addition. J. Intermet. 2020, 774, 138940. [Google Scholar] [CrossRef]
- Holmström, E.; Lizárraga, R.; Linder, D.; Salmasi, A.; Wang, W.; Kaplan, B.; Mao, H.H.; Larsson, H.; Larsson, L. High entropy alloys: Substituting for cobalt in cutting edge technology. J. Appl. Mater. Today 2018, 12, 322–329. [Google Scholar] [CrossRef]
- Cantor, B. Multicomponent high-entropy Cantor alloys. Prog. Mater. Sci. 2021, 120, 100754. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. J. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Liu, X.W.; Liu, L.; Liu, G.; Wu, X.X.; Lu, D.H.; Yao, J.Q.; Jiang, W.M.; Fan, Z.T.; Zhang, W.B. The role of carbon in grain refinement of cast CrFeCoNi high-entropy alloys. Metall. Mater. Trans. A 2018, 49, 2151–2160. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.H.; Xie, Y.H.; Chan, S.L.I.; Liang, J.M.; Wang, J. Multiple deformation mechanisms induced by pre-twinning in CoCrFeNi high entropy alloy. Scr. Mater. 2022, 207, 114266. [Google Scholar] [CrossRef]
- Borja, L. CoCrFeNi increases strength at cryogenic temperatures. MRS Bull. 2019, 44, 150–151. [Google Scholar] [CrossRef]
- Li, W.; Liu, H.Y.; Yin, P.H.; Yan, W.; Wang, W.; Shan, Y.Y.; Yang, K. Special tetrahedral twins in a cryogenically deformed CoCrFeNi high-entropy alloy. J. Mater. Sci. Technol. 2022, 100, 129–136. [Google Scholar] [CrossRef]
- Huo, W.Y.; Fang, F.; Zhou, H.; Xie, Z.H.; Shang, J.K.; Jiang, J.Q. Remarkable strength of CoCrFeNi high-entropy alloy wires at cryogenic and elevated temperatures. Scr. Mater. 2017, 141, 125–128. [Google Scholar] [CrossRef]
- Zhong, X.Z.; Zhang, Q.M.; Xie, J.; Wu, M.Z.; Jiang, F.Q.; Yan, Y.M.; Wang, Z.W. Mechanical properties and microstructure of the Al0.3CoCrFeNiTi0.3 high entropy alloy under dynamic compression. J Mater. Sci. Eng. A 2021, 465, 11–19. [Google Scholar] [CrossRef]
- Karimzadeh, M.; Malekan, M.; Mirzadeh, H.; Li, L.; Saini, N. Effects of titanium addition on the microstructure and mechanical properties of quaternary CoCrFeNi high entropy alloy. J. Mater. Sci. Eng. A 2022, 856, 143971. [Google Scholar] [CrossRef]
- Cui, P.; Ma, Y.M.; Zhang, L.J.; Zhang, M.D.; Fan, J.T.; Dong, W.Q.; Yu, P.F.; Li, G.; Liu, R.P. Effect of Ti on microstructures and mechanical properties of high entropy alloys based on CoFeMnNi system. J. Mater. Sci. Eng. A 2018, 737, 198–204. [Google Scholar] [CrossRef]
- Liu, S.Y.; Grohol, C.M.; Shin, Y.C. High throughput synthesis of CoCrFeNiTi high entropy alloys via directed energy deposition. J. Alloys Compd. 2022, 916, 165469. [Google Scholar] [CrossRef]
- Yang, T.H.; Cai, B.; Shi, Y.J.; Wang, M.X.; Zhang, G.P. Preparation of nanostructured CoCrFeMnNi high entropy alloy by hot pressing sintering gas atomized powders. J. Micron. 2021, 147, 103082. [Google Scholar] [CrossRef]
- Mishra, R.K.; Shahi, R. A systematic approach for enhancing magnetic properties of CoCrFeNiTi-based high entropy alloys via stoichiometric variation and annealing. J. Alloys Compd. 2020, 821, 153534. [Google Scholar] [CrossRef]
- Li, S.; Lei, S.; Wu, Y.B.; Hu, S.S.; Liu, Y.F.; Xu, H.L. Effect of Ti content on Magnetic and Electrochemical Corrosion Properties of FeCoCrNi High Entropy Alloys. ECS. J. Solid. State. Sci. Technol. 2021, 10, 033003. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Han, B.; Li, M.Y.; Zhang, Q.; Hu, C.Y.; Niu, S.Y.; Li, Z.H.; Wang, Y. Investigation on solid particles erosion resistance of laser cladded CoCrFeNiTi high entropy alloy coating. J. Intermet. 2021, 131, 107111. [Google Scholar] [CrossRef]
- Zhao, Y.K.; Lau, K.B.; The, W.H.; Lee, J.J.; Wei, F.X.; Lin, M.; Wang, P.; Tan, C.C.; Ramamurty, U. Compositionally graded CoCrFeNiTix high-entropy alloys manufactured by laser powder bed fusion: A combinatorial assessment. J. Alloys Compd. 2021, 883, 160825. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Chen, Z.W.; Yang, K.; Le, W.; Naseem, S. Effects of aging treatment on the evolution of precipitated phase in CoCrFeNiTi0.6 high entropy alloys. J. Alloys Compd. 2021, 887, 161407. [Google Scholar] [CrossRef]
- Shun, T.T.; Hsieh, C.Y.; Hung, W.J.; Lee, C.F. Age Heat Treatment of the CoCrFeNiTi0.3 High-Entropy Alloy. J. Mater. Trans. 2018, 59, 730–733. [Google Scholar] [CrossRef]
- Lin, C.M.; Tsai, H.L. Effect of annealing treatment on microstructure and properties of high-entropy FeCoNiCrCu0.5 alloy. Mater. Chem. Phys. 2011, 128, 50–56. [Google Scholar] [CrossRef]
- Man, J.L.; Wu, B.L.; Duan, G.S.; Zhang, L.; Wang, G.; Li, Z.; Zou, N.F.; Liu, Y.D. The synergistic addition of Al, Ti, Mo and W to strengthen the equimolar CoCrFeNi high-entropy alloy via thermal-mechanical processing. J. Alloys Compd. 2022, 902, 163774. [Google Scholar] [CrossRef]
- Shahmir, H.; Mehranpour, M.S.; Derakhshandeh, A.; Nili-Ahmadabadi, M. Microstructure tailoring to enhance mechanical properties in CoCrFeNiMn high-entropy alloy by Ti addition and thermomechanical treatment. J. Mater. Charact. 2021, 182, 111513. [Google Scholar] [CrossRef]
- Fujieda, T.; Shiratori, H.; Kuwabara, K.; Hirota, M.; Kato, T.; Yamanaka, K.; Koizumi, Y.; Chiba, A.; Watanabe, S. CoCrFeNiTi-based high-entropy alloy with superior tensile strength and corrosion resistance achieved by a combination of additive manufacturing using selective electron beam melting and solution treatment. J. Mater. Lett. 2017, 189, 148–151. [Google Scholar] [CrossRef]
- Alijani, F.; Reihanian, M.; Gheisari, K. Study on phase formation in magnetic FeCoNiMnV high entropy alloy produced by mechanical alloying. J. Alloys Compd. 2019, 773, 623–630. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, Y. Simulations of irradiation resistance and mechanical properties under irradiation of high-entropy alloy NiCoCrFe. J. Mater. Today Commun. 2022, 33, 104308. [Google Scholar] [CrossRef]
- Chen, G.Z.; Fray, D.J.; Farthing, T.W. Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride. Nature 2000, 407, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.X.; Liu, G.T.; Yang, X. Isolating elemental phosphorus from sewage sludge ash by electrochemistry. J. Resour. Conserv. Recycl. 2022, 190, 106815. [Google Scholar] [CrossRef]
- Gao, M.Y.; Yang, C.; Zhang, Q.B.; Yu, Y.W.; Hua, Y.X.; Li, Y.; Dong, P. Electrochemical fabrication of porous Ni-Cu alloy nanosheets with high catalytic activity for hydrogen evolution. J. Electrochim. Acta 2016, 146, 44–51. [Google Scholar] [CrossRef]
- Wang, C.Y.; Lin, J.C.; Chang, Y.C.; Tseng, Y.T.; Ciou, Y.J.; Hwang, Y.R. Fabrication of Cu-Zn Alloy Micropillars by Potentiostatic Localized Electrochemical Deposition. J. Electrochem. Soc. 2019, 166, E252. [Google Scholar] [CrossRef]
- Sure, J.; Vishnu, D.S.M.; Schwandt, C. Single-step electrochemical synthesis of nano-crystalline CoCrFeNi high-entropy alloy powder. J. Electrochem. Commun. 2022, 143, 107392. [Google Scholar] [CrossRef]
- Sure, J.; Vishnu, D.S.M.; Schwandt, C. Electrochemical conversion of oxide spinels into high-entropy alloy. J. Alloys Compd. 2019, 776, 133–141. [Google Scholar] [CrossRef]
- Sure, J.; Vishnu, D.S.M.; Schwandt, C. Direct electrochemical synthesis of high-entropy alloys from metal oxides. J. Appl. Mater. 2017, 9, 111–121. [Google Scholar] [CrossRef]
- Okabe, T.H.; Nakamura, M.; Oishi, T.; Ono, K. Electrochemical deoxidation of titanium. J. Metall. Mater. Trans. B 1993, 24, 449–455. [Google Scholar] [CrossRef]
- Li, W.; Jin, X.; Huang, F.; Chen, G.Z. Metal-to-Oxide molar volume ratio: The overlooked barrier to solid-state electroreduction and a “green” bypass through recyclable NH4HCO3. J. Angew. Chem. Int. Ed. Engl. 2010, 49, 3203–3206. [Google Scholar] [CrossRef]
- Jiang, K.; Hu, X.; Ma, M.; Wang, D.H.; Qiu, G.H.; Jin, X.B.; Chen, G.Z. “Perovskitization”-assisted electrochemical reduction of solid TiO2 in molten CaCl2. J. Angew. Chem. Int. Ed. 2006, 45, 428–432. [Google Scholar] [CrossRef]
- Schwandt, C.; Alexander, D.T.L.; Fray, D.J. The electro-deoxidation of porous titanium dioxide precursors in molten calcium chloride under cathodic potential control. J. Electrochim. Acta 2009, 54, 3819–3829. [Google Scholar] [CrossRef]
- Chen, G.Z.; Fray, D.J.; Farthing, T.W. Cathodic deoxygenation of the alpha case on titanium and alloys in molten calcium chloride. J. Metall. Mater. Trans. B 2001, 32, 1041–1052. [Google Scholar] [CrossRef]
- Jiang, L.; LU, Y.P.; Dong, Y.; Wang, T.M.; Cao, Z.Q.; Li, T.J. Annealing effects on the microstructure and properties of bulk high-entropy CoCrFeNiTi0.5 alloy casting ingot. J. Intermet. 2014, 44, 37–43. [Google Scholar] [CrossRef]
- Fujieda, T.; Chen, M.; Shiratori, H.; Kuwabara, K.; Yamanaka, K.; Koizumi, Y.; Chiba, A.; Watanabe, S. Mechanical and corrosion properties of CoCrFeNiTi-based high-entropy alloy additive manufactured using selective laser melting. J. Addit. Manuf. 2019, 25, 412–420. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Huang, Y.; Xie, L.; Xu, Q.; Zhao, T.X. Effect of Ti content on the microstructure and corrosion resistance of CoCrFeNiTix high entropy alloys prepared by laser cladding. J. Mater. 2020, 13, 2209. [Google Scholar] [CrossRef]
- Peng, Y.B.; Zhang, W.; Mei, X.L.; Wang, H.J.; Zhang, M.Y.; Wang, L.; Li, X.F.; Hu, Y. Microstructures and mechanical properties of FeCoCrNi-Mo High entropy alloys prepared by spark plasma sintering and vacuum hot-pressed sintering. J. Mater. Today. Commun. 2020, 23, 100893. [Google Scholar] [CrossRef]
- Shun, T.T.; Chang, L.Y.; Shiu, M.H. Microstructures and mechanical properties of multiprincipal component CoCrFeNiTix alloys. J. Mater. Sci. Eng. A 2012, 556, 170–174. [Google Scholar] [CrossRef]
- Wen, J.N.; Wu, R.J.; Mi, Y.H.; Zhang, N.; Lin, Z.H.; Li, Y.Q.; Yang, S.F. Effect of Ti as co-sputtering target on microstructure and mechanical properties of FeCoNi(CuAl)0.2 high-entropy alloy thin films. J. Mater. Chem. Phys. 2022, 269, 127214. [Google Scholar] [CrossRef]
- Xing, B.; Ding, Q.; Jin, B.; Zuo, X.; Zhang, N.; Yin, S. Corrosion Resistance and Passivation Behavior of CoCrFeNi-TiAl High-Entropy Alloy Coatings in Acidic Solutions. J. Therm. Spray. Technol. 2022, 31, 1637–1682. [Google Scholar] [CrossRef]
- Sahu, B.P.; Ray, M.; Mitra, R. Structure and properties of Ni1-xTixN thin films processed by reactive magnetron co-sputtering. J. Mater. Charact. 2020, 169, 110604. [Google Scholar] [CrossRef]

| x | CoO | Cr2O3 | Fe2O3 | NiO | TiO2 | Total Mass |
|---|---|---|---|---|---|---|
| 0 | 3.68 | 3.73 | 3.92 | 3.68 | 0 | 15 |
| 0.5 | 3.25 | 3.29 | 3.47 | 3.25 | 1.73 | 15 |
| 1 | 2.91 | 2.95 | 3.11 | 2.91 | 3.11 | 15 |
| 1.5 | 2.64 | 2.68 | 2.82 | 2.64 | 4.23 | 15 |
| x | Co | Cr | Fe | Ni | Ti |
|---|---|---|---|---|---|
| 0 | 0.23 | 0.26 | 0.27 | 0.24 | 0 |
| 0.5 | 0.22 | 0.23 | 0.23 | 0.21 | 0.09 |
| 1.0 | 0.22 | 0.19 | 0.19 | 0.22 | 0.18 |
| 1.5 | 0.17 | 0.18 | 0.19 | 0.21 | 0.25 |
| Sample | Ti0 | Ti0.5 | Ti1.0 | Ti1.5 |
|---|---|---|---|---|
| Oxygen content/wt% | 0.35 | 0.51 | 0.78 | 0.93 |
| Carbon content/wt% | 1.03 | 1.15 | 1.23 | 1.21 |
| Sample | Solution | Ecorr (V) vs. SCE | Epit (V) vs. SCE | icorr (A/cm2) |
|---|---|---|---|---|
| Ti0 | 0.5 M H2SO4 | −0.425 | 0.921 | 4.26 × 10−5 |
| Ti0.5 | −0.276 | 0.968 | 1.39 × 10−5 | |
| Ti1.0 | −0.290 | 0.972 | 1.48 × 10−5 | |
| Ti1.5 | −0.300 | 0.977 | 1.64 × 10−5 |
| Sample | Solution | Ecorr (V) vs. SCE | Epit (V) vs. SCE | icorr (A/cm2) |
|---|---|---|---|---|
| Ti0 | 1 M KOH | −1.251 | 0.375 | 2.06 × 10−7 |
| Ti0.5 | −1.202 | −0.138 | 3.02 × 10−7 | |
| Ti1.0 | −1.235 | −0.083 | 1.09 × 10−7 | |
| Ti1.5 | −1.150 | −0.117 | 5.61 × 10−8 |
| Sample | Solution | Ecorr (V) vs. SCE | Epit (V) vs. SCE | icorr (A/cm2) |
|---|---|---|---|---|
| Ti0 | 3.5 wt% NaCl | −1.147 | −0.142 | 2.45 × 10−7 |
| Ti0.5 | −1.138 | −0.153 | 7.73 × 10−8 | |
| Ti1.0 | −1.224 | −0.190 | 1.07 × 10−7 | |
| Ti1.5 | −1.185 | −0.512 | 1.39 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Zhang, S.; Liang, J.; Hu, M.; Yang, Y. Effect of Ti on Characterization and Properties of CoCrFeNiTix High Entropy Alloy Prepared Via Electro-Deoxidization of the Metal Oxides and Vacuum Hot Pressing Sintering Process. Materials 2023, 16, 1547. https://doi.org/10.3390/ma16041547
Li H, Zhang S, Liang J, Hu M, Yang Y. Effect of Ti on Characterization and Properties of CoCrFeNiTix High Entropy Alloy Prepared Via Electro-Deoxidization of the Metal Oxides and Vacuum Hot Pressing Sintering Process. Materials. 2023; 16(4):1547. https://doi.org/10.3390/ma16041547
Chicago/Turabian StyleLi, Hui, Sheng Zhang, Jinglong Liang, Meilong Hu, and Yu Yang. 2023. "Effect of Ti on Characterization and Properties of CoCrFeNiTix High Entropy Alloy Prepared Via Electro-Deoxidization of the Metal Oxides and Vacuum Hot Pressing Sintering Process" Materials 16, no. 4: 1547. https://doi.org/10.3390/ma16041547
APA StyleLi, H., Zhang, S., Liang, J., Hu, M., & Yang, Y. (2023). Effect of Ti on Characterization and Properties of CoCrFeNiTix High Entropy Alloy Prepared Via Electro-Deoxidization of the Metal Oxides and Vacuum Hot Pressing Sintering Process. Materials, 16(4), 1547. https://doi.org/10.3390/ma16041547





