Abstract
Modified barium gallo-germanate glass hosts are still worthy of attention in studying structure–property relationships. In this work, two different series of glass systems based on (60-x)GeO2-xTiO2-30BaO-10Ga2O3 and (60-x)GeO2-xB2O3-30BaO-10Ga2O3 (x = 10, 30, 50 mol%) were synthesized, and their properties were studied using spectroscopic techniques. X-ray diffraction (XRD) patterns revealed that all fabricated glasses were fully amorphous material. The absorption edge shifted toward the longer wavelengths with a gradual substitution of GeO2. The spectroscopic assignments of titanium ions were performed with excitation and emission spectra compared to the additional sample containing an extremely low content of TiO2 (0.005 mol%). On the basis of Raman and FT-IR investigations, it was found that increasing the TiO2 content caused a destructive effect on the GeO4 and GeO6 structural units. The Raman spectra of a sample containing a predominantly TiO2 (50 mol%) proved that the band was located near 650 cm−1, which corresponded to the stretching vibration of Ti-O in TiO6 unit. The deconvoluted IR results showed that the germanate glass network consisted of the coexistence of two BO3 and BO4 structural groups. Based on the experimental investigations, we concluded that the developed materials are a promising candidate for use as novel glass host matrices for doping rare-earth and/or transition metal ions.
1. Introduction
Glass, according to the definition, is a supercooled liquid and exhibits an amorphous nature. It should be noted that the common specific feature of all oxide glasses is the lack of long-range ordering. In the last few years, much research has been focused on explaining the relationship between the content of network formers and network modifiers and the stretching or bending vibrations occurring in the glasses. Special attention has been paid to the attractive infrared range characterizing the local structure of glassy systems using vibration analysis such as Raman and FTIR spectroscopy. There are extremely useful experimental techniques in investigating the structural properties of inorganic glasses [1,2,3]. Although there are well-documented scientific reports, the interpretation of the vibrations of glass host structural units occurring in oxide glasses is still a subject of ongoing scientific debate.
The above vibrational spectroscopies were successfully applied to examine the local structure of borate [4,5], phosphate [6,7], tellurite [8,9], silicate [10,11], and germanate [12,13,14,15,16] glass-host matrices. Studies in the last decade have shown that basic glass composition can be realized by adding several oxides and/or fluoride additives [17,18]. The main goal is to develop materials with excellent thermal, chemical, structural, and spectroscopic properties and various potential applications. Among those mentioned above, germanate glass is a promising starting material. First observed by Ivanov and Evstropiev [19], the coordination of Ge would continuously change from GeO4 to GeO6 with the addition of alkali oxides. Recently, the structural properties of germanate-based glasses have been examined in Na2O-GeO2-TeO2 [20], Li2O-GeO2-TeO2 [20], MnO-GeO2-PbO2 [21], TeO2-GeO2-PbO [22], Na2CO3-CaO-GeO2 [23], and Ga2O3-GeO2-BaO [24]. Among the reported germanate-based glasses, the barium gallo-germanate matrix has received the most attention due to its excellent properties, i.e., relatively large glass-forming region, high transmittance in a wide wavelength region, superior chemical durability, and thermal stability [25,26]. The structural properties of the BGG glass system modified by boron trioxide are less documented in the literature. Borate-based glass has excellent properties among the common glass-forming agents due to its high bond strength, coordination geometry, and maximum phonon energy (~1400 cm−1). The transformation of the coordination environment of boron, in terms of the equilibrium of the structural changes related to the conversion between BO3 and BO4, has been extensively studied [27]. Meanwhile, titanium oxide (TiO2) is one of the important constituents of luminescent glass, which acts as both network formers and network modifier [28]. Depending on the glass type, titanium can exhibit both trivalent and tetravalent valence states. The first attempts in this area were made by Cheng J. and Chen W. [29], who showed that stable titanate glasses could be obtained without adding glass network components (SiO2, B2O3). However, the formation of titanium ions in glasses has been reported, but most of the glass systems are partially crystallized. Up to now, fully amorphous barium gallo-germanate glasses containing higher TiO2 contents (10–50 mol%) were not obtained. It is well known that metal oxides such as B2O3 and TiO2 can be added to germanate-based glass to modify their optical and structural properties. The effect of the spectroscopic properties of properties for SrO-BaO-B2O2-SiO2 glass ceramics [30] with different TiO2/B2O3 ratios has been studied. Consequently, the fraction in the form of TiO4 and TiO6 increased when the fraction of BO3 decreased. For this reason, it is important to broaden our knowledge of the structural properties of the GeO2-BaO-Ga2O3 glass system modified by TiO2/B2O3.
In our previous work [31], Cr3+ ions were used in multicomponent germanate glasses as a spectroscopic probe. We observed that GeO2 and TiO2/B2O3 strongly influenced the profiles of luminescence bands and their relative intensity of trivalent chromium ions in the visible and infrared range. The calculated spectroscopic parameters from the Tanabe–Sugano diagram indicated that Cr3+ ions in the GeO2-BaO-Ga2O3 glassy phase occupied the intermediate (sample with B2O3) and strong (sample with TiO2) crystal field, respectively. It is assumed that adding glass formers such as TiO2 and B2O3 to the BGG network changes the local structure. We have extended this experimental approach to the analysis structural properties of undoped GeO2-TiO2-BaO-Ga2O3 and GeO-B2O3-BaO-Ga2O3 systems. Structural changes in the studied glasses were examined by XRD, FT-IR and Raman spectroscopy. Our study intends to present the role of titanium ions in germanate glass-host matrices based on excitation and emission spectra compared to the glass sample containing an extremely low content of TiO2. Another aspect worth exploring is a mathematical procedure of spectral decomposition as the possibility of determining the component bands resulting from matrix vibrations for the GeO2-B2O3-BaO-Ga2O3 glass system.
2. Materials and Methods
In the presented procedure, multicomponent germanate-based glass systems modified by TiO2/B2O3 were prepared using the conventional melt quench technique. The high purity chemicals such as (>99.99%) germanium oxide (GeO2), boron trioxide (B2O3), titanium oxide (TiO2), barium oxide (BaO), and gallium trioxide (Ga2O3) (Sigma Aldrich Chemical Co., St. Louis, MO, USA) were used to synthesize the glass samples. The glass samples were entitled as GT1 (GeO2:TiO2 = 5:1), GT2 (GeO2:TiO2 = 1:1), GT3 (GeO2:TiO2 = 1:5) and GB1 (GeO2:B2O3 = 5:1), GB2 (GeO2:B2O3 = 1:1), and GB3 (GeO2:B2O3 = 1:5), where the first letters refer of the GeO2, TiO2, and B2O3 metal oxides. The chemical compositions of the GeO2/TiO2 and GeO2/B2O3 molar ratios and nomenclature are listed in Table 1 and Table 2. The glass components were taken in appropriate proportions and mixed using agate-made mortar and pestle in a glove box in an inert atmosphere to constitute a 5 g batch. The homogenized mixtures were melted in a crucible (Łukasiewicz Research Network, Institute of Ceramics and Building Materials, Cracow, Poland) at a temperature of 1250 °C for 60 min in an electric furnace (CZYLOK Company, Jastrzębie-Zdrój, Poland). The molten glass sample was taken out of the electric furnace, cast on a porcelain plate, and cooled down to room temperature. At the end of the procedure, the glass samples were polished (semiautomatic grinding and polishing LaboPol-5 Struers, Denmark) in the desired shape. Finally, we fabricated glass samples of dimensions 15 mm × 15 mm and thickness ±3 mm for the following optical and structural measurements. Figure 1 and Figure 2 show images of the polished samples GT2 and GB2.

Table 1.
Nominal composition (mol%) and GeO2/TiO2 ratio of glass samples.

Table 2.
Nominal composition (mol%) and GeO2/B2O3 ratio of glass samples.
Figure 1.
Photographs of the obtained glass sample: 30GeO2-30TiO2-30BaO-10Ga2O3 (GT2).

Figure 2.
Photographs of the obtained glass sample: 30GeO2-30B2O3-30BaO-10Ga2O3 (GB2).
An XRD spectrum for prepared samples was recorded with an X’Pert Pro diffractometer with CuKα radiation with a λ = 1.54056 Å wavelength supplied by PANalytical (Almelo, The Netherlands) in the range 20–70°. The diffraction patterns were measured in step-scan mode with a step size of 0.050 and a time per step of 10 s. The UV-VIS spectrophotometer (Varian Cary 5000, Agilent Technology, Santa Clara, CA, USA) was used to measure the optical absorption spectra. The excitation and luminescence spectra of the glasses in a range of 260–650 nm were registered using a laser system that consisted of a PTI Quanta-Master 40 UV/VIS Steady State Spectrofluorometer (Photon Technology International, Birmingham, NJ, USA) coupled with a tunable pulsed optical parametric oscillator (OPO) pumped by the third harmonic of an Nd:YAG laser (Opotek Opolette 355 LD, OPOTEK, Carlsbad, CA, USA). The laser system was coupled with a Xe lamp (75 W). The resolution for the excitation and luminescence spectra was ±0.25 nm.
In the next step, the structural investigations for the obtained materials were evaluated. The bonding vibrations were determined via a Fourier Transform Infrared (FTIR) measurement in the region 1600-400 cm−1 (Nicolet™ iS™ 50, Thermo Fisher Scientific, Waltham, MA, USA) with a diamond attenuated total reflectance (ATR) module. The complementary structural characterization of the obtained glass samples was verified using Raman spectroscopy (Thermo Scientific, Waltham, MA, USA). The appropriate laser source with an excitation wavelength of 780 nm was used to obtain the Raman spectra. The laser was directly focused on the sample with an Olympus long-working-distance microscope objective (50×). The Raman and IR spectra were normalized and deconvoluted using Origin Pro 9.1 software. All the measurements were performed at room temperature.
3. Results
The aim of this work is to evaluate the effect of a boron trioxide (B2O3) and titanium dioxide (TiO2) substitution on the properties of barium gallo-germanate (BGG) glasses. Firstly, to emphasize the potential of titanium-rich glasses and boron-rich BGG glasses, X-ray diffraction (XRD) was used to verify the local structure of the studied glass systems. Absorption spectroscopy is a very useful technique for characterizing the optical properties within the range of 200–800 nm of fabricated glasses. The assignment of the titanium ions’ emission bands that were derived from the spectroscopic results allowed us to confirm the Ti3+ ions in barium gallo-germanate glass systems. Secondly, a thorough analysis of the structural properties was performed by means of FT-IR and Raman spectroscopy. In those pioneering works, the properties of barium gallo-germanate systems were studied for possible applications in low-loss fiber optics and optical components [32,33,34]. The morphology of barium germanate glasses was reported by Shelby [35]. It should be noted that the barium gallo-germanate system shows a broad glass-forming region [36]. The properties of these systems can also be modified by adding or substituting other components. The effect of various substitutions in the barium gallo-germanate glasses was studied by Jewell et al. [37]. As a result, gadolinium is a typical modifier ion because of its large field strengths. Next, aluminum acts as an intermediate with AlO4− substituting directly for Ga2O4− units. In the presence of GeO2, gallium atoms tend to reinforce the glass network as observed in BaO-Ga2O3-GeO2 glass compositions, where the corners bind GaO4 and GeO4 tetrahedra. Consequently, depending on the content of various components, their role can change from network modifier to network former. To the best of our knowledge, these phenomena were not yet examined for B2O3, one of the typical covalent network formers that meet Zachariasen’s rules for glass formation, and TiO2, whose role can change from glass network modifier to glass network former depending on its amount in the glass.
3.1. Glass Characterization
In order to examine the amorphous or crystalline state of the fabricated glasses, a phase analysis was conducted with the use of X-ray diffraction (XRD). Figure 3a,b present representative X-ray diffraction patterns measured of the barium gallo-germanate glasses with the varying content of TiO2 and B2O3 (from 10 to 50 mol%). According to the literature data [38], the barium gallo-germanate glass system can exhibit a tendency toward surface crystallization. However, it is well known that bulk crystallization can be made possible in various glass systems by phase separation followed by nucleation and crystal growth. It needs to be stressed that TiO2 was an effective crystal nucleating agent in various glass systems. Systematic studies clearly indicate that the crystallization of TiO2 in glass systems depends on several factors, e.g., ionic size, ionic chance, and the ability to be conditional glass former ions [39,40]. The main drawbacks of TiO2 content are related to decreasing the viscosity of the glasses at a high temperature process and importantly promoting glass liquid–liquid phase separation, which provides more phase interfaces. Mingshen et al. [41] exhibited the role of TiO2 (5–10 wt%) in the process of the phase separation, nucleation, and crystallization of CaO-MgO-Al2O3-SiO2-Na2O system glasses. Importantly, they showed that the number of the main crystalline phase increased with temperature and time. Our results indicated that this phenomenon was not observed in the fabricated glass system using the traditional high-temperature melt-quenching method. For this reason, for the structural analysis, we started using X-ray diffraction measurements.
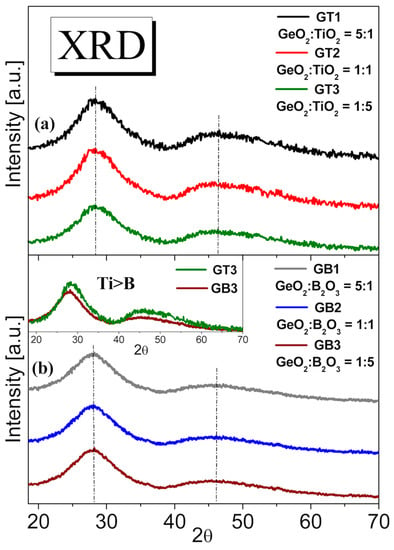
Figure 3.
XRD profiles of barium gallo-germanate glasses containing various molar ratios TiO2 (a) and B2O3 (b). Insets show the X-ray diffraction intensity for GT3 (GeO2:TiO2 = 1:5) and GB3 (GeO2:B2O3 = 1:5) glass samples.
Figure 3 shows the XRD patterns of the GT and GB precursor glasses. The titanate–germanate samples revealed only a broad diffuse scattering at different angles instead of narrow lines typical for crystalline materials, confirming a long-range structural disorder characteristic of the amorphous glassy network. The same behavior was observed in the case of a glass sample where GeO2 was partially substituted by B2O3 from Figure 3b. The broad low intense peak at the angles 20–30° confirmed the amorphous nature of the GB1, GB2, and GB3 samples. Additionally, the hump’s maximum did not shift, corroborating the absence of the evolution to a lower degree of the order of the local structure of the studied glasses with wide GeO2:TiO2 and GeO2:B2O3 molar ratios. Moreover, the GT3 glass sample containing 50 mol% TiO2 showed a higher peak intensity than the glass sample with predominantly boron trioxide content GB3. This phenomenon met the requirement that the higher the atomic number of an element (Ti > B), the higher the X-ray diffraction intensity shown in the inset of Figure 3.
Figure 4 illustrates the optical UV–visible absorption spectra for fabricated germanate glasses modified by titanium dioxide (Figure 4a) and boron trioxide (Figure 4b). It should be noted that the characteristic of both series of GeO2-TiO2-BaO-Ga2O3 and GeO2-B2O3-BaO-Ga2O3 glass matrices is the absorption edge which was shifting towards the longer wavelength with increasing TiO2 and B2O3 content. Following that, the UV cut-off wavelength, referred to as the intersection between the zero-base line and the extrapolation of the absorption edge, was estimated. The inset in Figure 4a,b presents the absorption edge for the GT1, GT2, GT3 and GB1, GB2, GB3 glasses. From absorption spectra, it is clear that with the increasing TiO2/B2O3 concentration, the absorption edges were shifted to longer wavelengths.
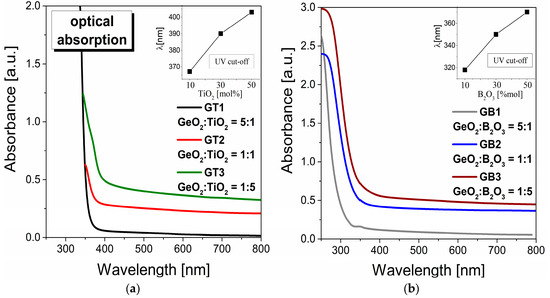
Figure 4.
UV–visible optical absorption spectra for glass systems modified by TiO2 (a) and B2O3 (b). Insets show the variation in UV cut-off as a function of TiO2 and B2O3 concentration.
According to the literature [42,43], titanium ions are also accepted to exist in glasses in various oxidation states. For this reason, we extended our experimental approach in optical research by designing and preparing a glass sample doped with an extremely low concentration of TiO2 (0.005 mol% TiO2). We registered the optical absorption spectrum for the spectroscopic characterization of titanium states in the glass sample (Figure 5a). Rao et al. [44] suggest that the intense and very broad absorption in the visible region was a result of superimposed bands from Ti3+ and Ti4+ ions. In the silica calcium aluminosilicate system, the band of about 310 nm corresponded to the Ti4+ ions [45]. However, this band was strongly masked by the absorption edge for titanium-doped germanate glass, which was significantly shifted toward longer wavelengths. Moreover, in the case of a fabricated sample doped with titanium ions, the inset of Figure 5a displays the high-resolution optical absorption spectrum in the 675–730 nm range. The low intense absorption band centered at 700 nm may be quite well interpreted and related to the Ti3+–Ti4+ ion pair interactions [45]. Based on it, it was stated that the presented experiment constitutes the next step toward measurements with different emission wavelengths
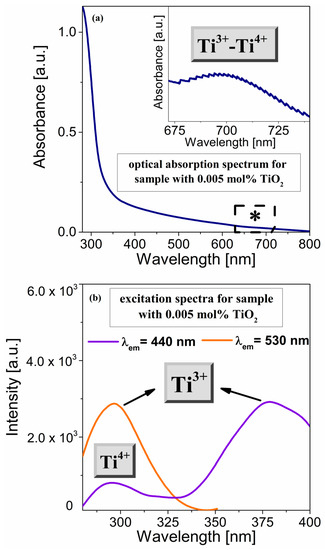
Figure 5.
Optical absorption spectrum (a) and excitation spectra (b) of the sample containing extremely low titanium dioxide content. The excitation spectra are monitored at 440 nm and 530 nm. The inset shows Ti3+–Ti4+ pair’s interaction in the range 675–730 nm (*) recorded with high resolution.
To analyze the emission spectra for the prepared glasses, it is necessary to know the excitation wavelengths of titanium ions. For this purpose, Figure 5 shows combined excitation spectra for the titanium-doped germanate glass sample (0.005 mol%). Upon excitation at 440 nm, the band at 270 nm might be identified as Ti ions in a tetravalent oxidation state [44,45]. On the other hand, our experimental investigations demonstrated the excitation band by monitoring the emission at 530 nm. The spectrum exhibited a relatively intense band at about 300 nm, which could be related to the Ti3+ ions [44, 45].
We performed the same procedure for the GT1, GT2, and GT3 glass samples (Figure 6). Obviously, changing the chemical glass composition leads to changes in the positions and intensities of the relevant excitation bands of the titanium species. Within the VIS range, the excitation spectra consisted of a characteristic band centered at 390 nm (Figure 6a). The relatively highest intensity of these excitation band glasses suggests a larger Ti3+ ions concentration in these samples. Notably, the band associated with titanium ions on the tetravalent valence state was very weakly detectable. Further analysis demonstrated notable changes in the excitation spectra (λem = 530 nm) dependent on the TiO2 content. To evaluate a full picture of the optical properties of the titanate–germanate glass system, we performed a luminescence experiment using the direct excitation wavelength between two excitation bands (λexc = 345 nm).
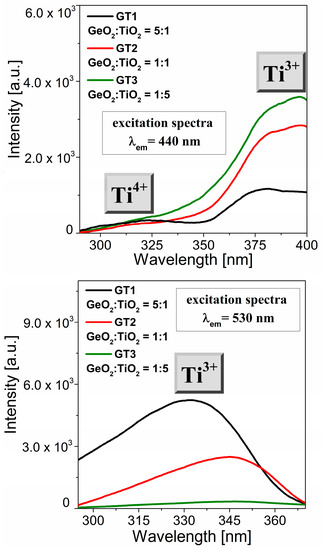
Figure 6.
Excitation spectra (λem = 440 nm and λem = 530 nm) for GT1, GT2, GT3 samples.
The emission properties of GeO2-TiO2-BaO-Ga2O3 glass systems with different GeO2:TiO2 molar ratios were investigated under the 345 nm excitation wavelength and recorded in the 400–650 nm range, as shown in Figure 7. The asterisk (*) refers to the extremely low titanium dioxide concentration of the glass samples. For the fabricated titanate–germanate glasses (GT1, GT2, GT3), the intensities of the recorded band at 560 nm increased with the increasing GeO2:TiO2 molar ratio. Interestingly, for the sample with the highest GeO2:TiO2 molar ratio (1:5), the concentration quenching of the emission of the Ti3+ ions was observed. Our research phenomenally demonstrated that the successive replacement of germanium dioxide by titanium dioxide shifts the redox equilibrium Ti3+–Ti4+ to obtain optically detectable amounts of Ti3+ ions. Hence, a sample with a 10 mol% concentration of TiO2 is preferable for achieving a high luminescence emission of Ti3+ ions. Broadband, low-intensity emission with a maximum of 440 nm is characteristic of the Ti4+ valence state [44,45].
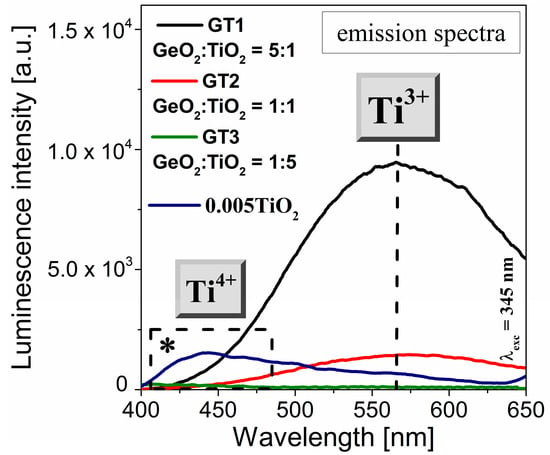
Figure 7.
Visible emission spectra (λexc = 345 nm) for GT1, GT2, and GT3 samples. The asterisk (*) refers to glass containing an extremely low titanium dioxide concentration.
The absorption, excitation, and luminescence results showed that titanium dioxide caused variations in the valence state of the titanium ions in the germanate glass network that may produce structural modifications and local-field variations in the structure. For this reason, this work discusses the IR and Raman spectroscopy of germanate-based glass systems modified by TiO2/B2O3.
3.2. Raman and FT-IR Spectroscopy of Barium Gallo-Germanate Glasses Containing TiO2/B2O3
Previous reports showed that many types of germanate glass had been studied using vibrational spectroscopy [46,47,48,49]. Initially, the precise determination of the network units characterized by multicomponent germanate matrices was a complicated task due to the character of composed network-forming or network-modifier oxides. The network of germanate glass is formed by tetrahedral GeO4 structural units, which share their corners, and the Ge atom is covalently bonded to four bridging oxygens. The thermodynamic instability of the GeO6 octahedral units produces a large concentration of nonbridging oxygen ions. This evolution clearly indicates the conversion of [GeO4] → [GeO6] structural units [50]. According to the literature [51], the vibrational spectrum of the germanate glasses is rather remarkable. The germanate matrix is characterized by the structural units’ dominant contributions in low- and high-frequency regions. The low-frequency region is mainly characterized by a peak around 560 cm−1 and is associated with the bending vibrations of Ge-O-Ge. The high-frequency region is reported to contain a band at approx. 915 cm−1, and low inflections at approx. 1000 cm−1 are attributed to the asymmetric vibrations of the Ge-O-(Ge) bridges. These bands occur in a typical infrared spectrum when the glassy germanium oxide is composed of germanium–oxygen tetrahedra with nonbridging oxygens. It was repeatedly demonstrated that changes in the local structure of the germanate network as the alkali concentration [52,53] increased resulted in a systematic shift in the band components associated with the vibrations of the GeO4 units. This is evidenced by the broken Ge-O- bridges at about 750 and 870 cm−1 for the Q2 and Q3 units. Comprehensive studies of germanate glasses have also provided strong evidence regarding the replacement of GeO4 by other units, including germanate–oxygen octahedra (GeO6). This strong modification is demonstrated by the appearance of a band at about 715 cm−1 in the midinfrared spectrum, evidently involving a change in the coordination number of the germanium ions from four (LK = 4) to six (LK = 6) [54,55]. According to the paper published by McKeaon and Marzbacher [56], when the GeO2 content decreases, the midfrequency envelope shifts to higher frequencies while the high-frequency features shift to lower frequencies. It was interpreted as a reduction in the average ring size, as well as an average lengthening of the T-O (where T is Ge or Ga) band.
Figure 8 shows the measured FT-IR and Raman spectra in the wavelength range of 400–1600 cm−1 of barium gallo-germanate glasses with various GeO2:TiO2 molar ratios. The spectra exhibited two groups of bands (i) in the low-frequency region located from 400 to 600 cm−1 and (ii) in the high-frequency region from 700 to 900 cm−1. A single band dominates the first region of 400–600 cm−1 due to the GeO4 structural units, which share their corners, where the germanium atom bending is covalently bonded to four biding oxygens. The second high-frequency region, between 620–900 cm−1, is attributed to the GeO6 structural units, where the central atom is germanium and is surrounded by six oxygen atoms [57,58]. As expected, the molar ratio of TiO2 strongly affected these structural properties of glasses. As one can see from Figure 8, the intensities of the IR and Raman bands related to the GeO4 and GeO6 structural units underwent significant changes by incorporating the TiO2 content into the germanate glass host. With the introduction of TiO2 up to 30mol% in the GeO2-BaO-Ga2O3 glass network, the intensity band centered at about 450 cm−1 and 800 cm−1 was observed to significantly decrease with the shifting towards the lower frequency region. However, when the TiO2 concentration was greater than 30mol%, it was observed that the band due to the GeO6 structural units was shifted to higher frequencies. The explanation for these results lies in the dual role of titanium dioxide in the glass network. Titanium dioxide acted both as the network modifier and network former, which participated during the formation of the glass network in the form of TiO4 or existed in the gap outside the network in the form of TiO6 units [57,58,59,60]. In this work, the effect observed with the increased TiO2 content very well confirms that the doping titanium ions of the germanate matrix generated a strong destruction of germanate tetrahedra and octahedra units caused by the formation of more Ti-O structural units. We considered the Raman spectrum for the GeO2:TiO2 = 1:5 (GT3) glass sample when titanium dioxide acted as a network former. The main problem with this material is the interpretation of the local structure due to the overlapping bands. The most interesting observation concerning all registered spectra was the presence of a band in the frequency range of 600–700 cm−1. The mentioned band was very detectable with the increase in the amount of titanium ions introduced at the expense of germanium ions, which resulted in a systematic decrease in the amount of GeO4 tetrahedrons and GeO6 octahedrons. According to the data, this Raman peak is considered strong evidence of Ti-O stretching vibration connected with the TiO6 unit. Earlier studies of other glasses containing TiO2 exhibited a well-resolved band at about ~720 cm−1 identified due to the vibration of TiO4 structural units [61,62].
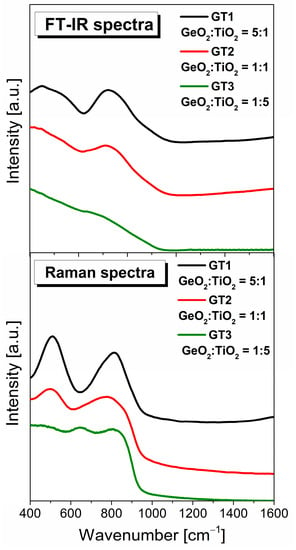
Figure 8.
Infrared and Raman spectra of the investigated samples with GeO2/TiO2 ratio (5:1, 1:1, and 1:5).
As a final point of our investigations, we characterized the structural properties of the barium gallo-germanate glass host containing B2O3 (Figure 9). As expected, independently, the chemical composition germanate glass structure resulted in the appearance of the spectra, revealing signals from the bending and stretching modes of the GeO4 and GeO6 structural units. In contrast to TiO2, the addition of boron oxide was found to be a weak scatterer in low-frequency and high-frequency ranges between 400–1600 cm−1. Adding B2O3 to glass causes progressive changes in the low and higher frequency range. These changes are accompanied by the decreasing of a strong band at 450 cm−1 (almost vanishing at 50 mol% B2O3 in the FT-IR spectrum) and the one at 800 cm−1 with increasing borate content and shifts to lower frequencies. The obtained results indicated that the Raman shift decreased from 1411 cm−1 (glass GeO2-rich composition) to 1340 cm−1 (glass B2O3-rich composition). A clear correspondence was observed between the bands in the Raman spectra and the measurements carried out of the infrared spectra for the obtained glasses. In general, in the boron-based glass host, boron is three-coordinated. Moreover, previous studies indicated the presence of trigonal and tetrahedral boron with different ratios and the partial conversion of BO3 into BO4 units [63,64].
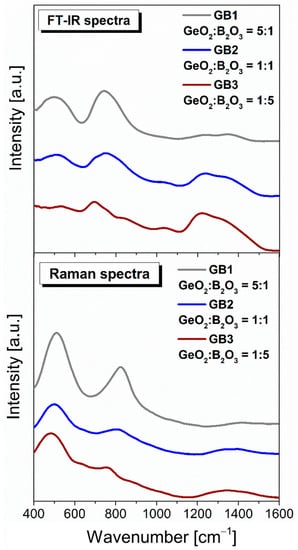
Figure 9.
Infrared and Raman spectra of investigated glass samples with GeO2/B2O3 ratio (5:1, 1:1, and 1:5).
To better visualize the obtained data, the deconvoluted components infrared bands were separated by a Gaussian deconvolution constructed for each sample GB1, GB2, and GB3 (see Figure 10). From the structural point of view, the most important observation was the change in the shift and the relative intensities of these two deconvoluted components positioned at 1230 cm−1 and 1360 cm−1 initiated as a function of the quantitative GeO2/B2O3 ratio. Hence, in assessing the effect of boron oxide on the barium gallo-germanate glass network, it is useful to analyze the location and mutual intensity of the appropriate deconvoluted bands, which have been previously described in detail in the scientific report of lead-borate glass systems [65].
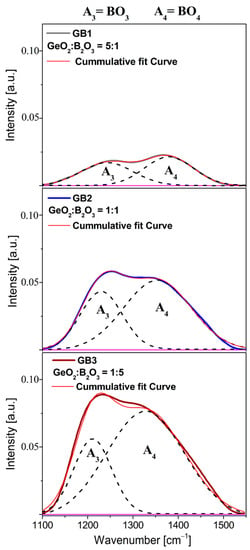
Figure 10.
Deconvoluted infrared bands of series glass samples (GB1, GB2, GB3) as a function of GeO2 and B2O3 concentration in spectra range 1100–1550 cm−1.
The peculiar structural properties of borate glasses come from the ability of boron to occur in three or four coordination. In general, the equilibrium of the structural conversion between the BO3 and BO4 units in the glass network depends on the chemical composition and the kind of modifiers [66,67]. Moreover, the relative fraction of the BO3 and BO4 groups proved to be a sensitive probe of the basic structural units of the network. Calculations were evaluated with the following formula presented in Figure 11, where A3 and A4 correspond the areas of the BO3 and BO4 groups. The relative integrated intensity A3/A4 of the GeO2-B2O3-BaO-Ga2O3 glass system upon adding oxides GeO2 and B2O3 in three different concentrations of 10%, 30%, and 50% each drastically reduced from 1.03 (glass sample GB1) to 0.38 (glass sample GB3), respectively. We can conclude that the incorporation of B2O3 changed the local structure of the barium gallo-germanate glasses, and the low polymeric states containing the BO3 units constantly transformed into the high polymerized network units that mainly consisted of BO4. Interestingly, the group Mogus-Milankovic et al. [68] noted results for the quaternary Li2O-B2O3-P2O5-Ge2O glass system. With the addition of lower GeO2 content, the dominant borate unit was BO4, whose charge was more delocalized and enhanced the ion transport. In contrast, the formation of neutral BO3 units at a higher GeO2 concentration could break the conduction pathways and reduce the mobility of the ions. According to the literature [69,70,71], the competition for charge compensation between highly charged cations is the fundamental reason for boron coordination. The field strength of different modifier cations such as Na+, Ba2+, and Ca2+ [72] affects the enhanced stabilization of tetraborate groups by involving higher field strength cations in NBO-rich glasses. A similar effect of Na+ in sodium borate glasses has been recently found for the highly charged state of lanthanide ions (which act as modifiers) [73], which boosts the formation of negatively charged tetrahedral boron.
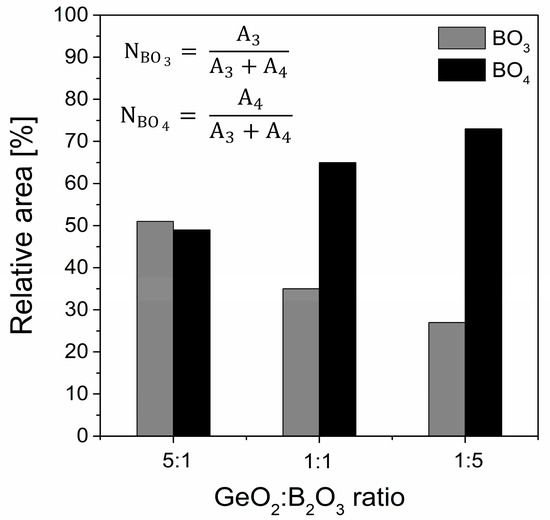
Figure 11.
Change with composition of the calculated total fraction of BO3 and BO4 units versus the GeO2/B2O3 molar ratio.
4. Discussion
The properties of precursor glasses were characterized using various experimental techniques to fulfill laser sources emitting midinfrared radiation and broadband optical amplifiers operating at near-IR range requirements. The obtained novel titanate–germanate and borogermanate glasses with a TiO2 and B2O3 content of up to 50 mol% were transparent and exhibited a fully amorphous nature. The luminescence studies of the GeO2-TiO2-BaO-Ga2O3 glasses perfectly confirmed the oxidation state of titanium ions in the function of the GeO2:TiO2 molar ratio. In the case of the GT series of glasses, the titanium acted as a glass network and a network modifier in the barium gallo-germanate network. It was proven that the incorporation of two various metal oxides in the barium-gallo germanate glass host influenced their structural GeO4 and GeO6 units. Indeed, this effort led to the better identification of the structural building blocks and the evolution of the Raman and FT-IR spectra of the BaO-Ga2O3-GeO2 glass system containing TiO2/B2O3 in the lower and higher frequency, respectively. It is well known that in glasses doped with lanthanide ions, the highest-energy phonons exercise the most influence in nonradiative relaxations because multiphonon decay occurs with the fewest number of phonons required to bridge the energy gap between two manifolds. On the other hand, the lower phonon energy of the glass host can reduce the probability of nonradiative relaxation and enable the higher quantum efficiency of photoluminescence and/or a higher luminescence lifetime of the excited state of lanthanide ions [74,75]. Moreover, we indicated [76] that the highest phonon energy for glass with GeO2:TiO2 equal to 1:1 decreased to 765 cm−1, which was smaller than that of the pure barium gallo-germanate glass reported (845 cm−1) [77]. For this reason, in the field of developing the glass structure system by embedding optically active ions to enhance their optical characteristics, titanium dioxide is a very useful component. Our systematic investigations demonstrated that barium gallo-germanate glasses containing TiO2 can be successfully used for near-IR laser applications at 1.06 µm through Nd3+ doping. The same glass systems doped with Er3+ ions are suitable for near-IR luminescence at 1.5 µm and could be useful for near-infrared broadband optical amplifiers. However, the optimal molar ratios of GeO2:TiO2 in these glass systems were completely different for Nd3+ [78] than Er3+ [79] ions. The further characterization of barium gallo-germanate glasses with TiO2 and their energy transfer processes between Yb3+ and Ln3+ ions (Ln = Pr, Er, Tm, Ho) [80] allowed us to demonstrate that the measured lifetimes decreased with an increasing TiO2 content, while changes in the energy transfer efficiency seemed to be nonlinear. To extend the optical applications of barium gallo-germanate glasses, the effect of B2O3 on structural modifications in the higher frequency range was observed, suggesting the important role of the molar ratio of GeO2:B2O3 in the formation of the glass host. In general, borate glasses are striking hosts because they are highly transparent, thermally stable, and show an appreciable solubility of lanthanide ions [81]. In the part of this work devoted to the results the Raman band near 1300 cm−1 was very well observed after B2O3 incorporation. This Raman band was related to the maximal phonon energy of the borate glass host, which caused emission quenching and the suppression of radiative emissions, especially in the near-IR spectral range. For that reason, barium gallo-germanate glasses containing relatively higher B2O3 concentrations are rather useless for near-IR luminescence applications, but they are interesting glass materials for the emission of visible light. These glass systems doped with Dy3+ ions emitted an intense greenish light, which was changed to yellowish light with an increasing GeO2:B2O3 molar ratio [82]. Interestingly, increasing B2O3 concentrations in barium gallo-germanate glasses did not negatively affect the reddish–orange luminescence and experimental lifetimes of Eu3+ ions despite their relatively high phonon energy [83]. This suggests evidently that barium gallo-germanate glass can be an excellent candidate for visible or near-IR luminescence depending on the glass modifiers (TiO2 or B2O3) and lanthanide doping.
5. Conclusions
Undoped germanate-based glasses modified by TiO2/B2O3 were studied experimentally using XRD, luminescence, FT-IR, and Raman spectroscopy. The results led to the following conclusions:
- Barium gallo-germanate glass hosts can accommodate 50 mol% TiO2 and B2O3, and the samples were still fully amorphous. Based on the absorption spectra measurements, the absorption edge was determined. It was proven that the intensities of the excitation and emissions and the position bands of Ti3+ and Ti4+ strongly depended on the chemical composition of the fabricated materials.
- Analysis of Raman and FT-IR spectra for the modified barium gallo-germanate glasses showed evidence of GeO4 and GeO6 structural units, independently of the GeO2/TiO2 and GeO2/B2O3 molar ratio. However, titanium dioxide strongly modified the structure of the glass between the 400 cm−1 and 1000 cm−1 frequency region, while boron trioxide modified the structure between the 1100 cm−1 and 1600 cm−1 frequency region. As the titanium dioxide increased, the bands were shifted to a lower frequency region. From the Raman spectra, we observed that the additional band located near 650 cm−1 confirmed the presence of the TiO6 unit. The dependence of the fractions of the BO3 and BO4 units on the kind of glass network formers was reduced from 1.03 to 0.38. Therefore, we confirmed such a hypothesis that there was a strict correlation between the local structure and optical properties of the barium gallo-germanate glass system in the function of two various network-former components (TiO2 and B2O3). The presented analysis confirmed that the developed materials are one of the most important classes of matrices for doping optically active ions for photonic applications.
Author Contributions
Conceptualization, J.P. and M.S.; methodology, K.K. and M.K.; formal analysis, K.K. and M.S.; investigation, K.K. and M.K.; writing—original draft preparation, K.K.; writing—review and editing, J.P., M.K., and W.A.P.; visualization, K.K.; project administration, W.A.P.; funding acquisition, W.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Centre (Poland), grant number 2018/31/B/ST8/00166.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Doweidar, H.; El-Egili, K.; Ramadan, R.; Khalil, E. Structural species in mixed-fluoride PbF2-CdF2-B2O3 borate glasses; FTIR investigation. Vib. Spectrosc. 2019, 102, 23–30. [Google Scholar] [CrossRef]
- Doweidar, H.; El-Damrawi, G.; Mansour, E.; Fetouh, R.E. Structural role of MgO and PbO in MgO-PbO-B2O3 glasses as revealed by FTIR; a new approach. J. Non-Cryst. Solids 2012, 358, 941–946. [Google Scholar] [CrossRef]
- Linda, D.; Dutreilh-Colas, M.; Hamani, D.; Thomas, P.; Mirgorodsky, A.; Duclere, J.R.; Masson, O.; Loukil, M.; Kabadou, A. New glasses within the Tl2O-Ag2)-TeO2 system: Thermal characteristics, Raman spectra and structural properties. Mater. Res. Bull. 2010, 24, 1816–1824. [Google Scholar] [CrossRef]
- Maniu, D.; Iliescu, T.; Ardelean, I.; Cinta-Pinzaru, S.; Tarcea, N.; Kiefer, W. Raman study on B2O3-CaO glasses. J. Mol. Struct. 2003, 651–653, 485–488. [Google Scholar] [CrossRef]
- Pascuta, P.; Pop, L.; Rada, S.; Bosca, M.; Culea, E. The local structure of bismuth borate glasses doped with europium ions evidenced by FT-IR spectroscopy. J. Mater. Sci. 2008, 19, 424–428. [Google Scholar] [CrossRef]
- Ivascu, C.; Timar Gabor, A.; Cozar, O.; Daraban, L.; Ardelean, I. FT-IR, Raman and thermoluminescence investigation of P2O5-BaO-Li2O glass system. J. Mol. Struct. 2011, 993, 249–253. [Google Scholar] [CrossRef]
- Lai, Y.M.; Liang, X.F.; Yang, S.Y.; Wang, J.X.; Cao, L.H.; Dai, B. Raman and FTIR spectra of iron phosphate glasses containing cerium. J. Mol. Struct. 2011, 992, 84–88. [Google Scholar] [CrossRef]
- Rong, Q.J.; Osaka, A.; Nanba, T.; Takada, J.; Miura, Y. Infrared and Raman spectra of binary tellurite glasses containing boron and indium oxides. J. Mater. Sci. 1992, 27, 3793–3798. [Google Scholar] [CrossRef]
- Lalla, E.A.; Sanz-Arranz, A.; Konstantinidis, M.; Freemantle, J.; Such, P.; Lozano-Gorrin, A.D.; Lavin, V.; Lopez-Reyes, G.; Rull-Perez, F.; Rodriguez-Mendoza, U.R. Raman-IR spectroscopic structural analysis of rare-earth (RE3+) doped fluorotellurite glasses at different laser wavelengths. Vib. Spectrosc. 2020, 106, 103020. [Google Scholar] [CrossRef]
- Silva, A.M.B.; Queiroz, C.M.; Agathopoulos, S.; Correia, R.N.; Fernandes, M.H.V.; Oliveira, J.M. Structure of SiO2-MgO-Na2O glasses by FTIR, Raman and 29Si MAS NMR. J. Mol. Struct. 2011, 986, 16–21. [Google Scholar] [CrossRef]
- Eniu, D.; Gruian, C.; Vanea, E.; Patcas, L.; Simon, V. FTIR and EPR spectroscopic investigation of calcium-silicate glasses with iron and dysprosium. J. Mol. Struct. 2015, 1084, 23–27. [Google Scholar] [CrossRef]
- Baia, L.; Iliescu, T.; Simon, S.; Kiefer, W. Raman and IR spectroscopic studies of manganese doped GeO2-Bi2O3 glasses. J. Mol. Struct. 2001, 599, 9–13. [Google Scholar] [CrossRef]
- Sigaev, V.N.; Gregora, I.; Pernice, P.; Champagnon, B.; Smelyanskaya, E.N.; Aronne, A.; Sarkisov, P.D. Structure of lead germanate glasses by Raman spectroscopy. J. Non. Cryst. Solids 2001, 279, 136–144. [Google Scholar] [CrossRef]
- Pascuta, P.; Pop, L.; Rada, S.; Bosca, M.; Culea, E. The local structure of bismuth germanate glasses and glass ceramics doped with europium ions evidenced by FT-IR spectroscopy. Vib. Spectrosc. 2008, 48, 281–283. [Google Scholar] [CrossRef]
- Koroleva, O.N.; Shtenberg, M.V.; Ivanova, T.N. The structure of potassium germanate glasses as revealed by Raman and IR spectroscopy. J. Non-Cryst. Solids 2019, 510, 143–150. [Google Scholar] [CrossRef]
- Hongisto, M.; Danto, S.; Ghena, M.; Iancu, D.; Ighigeanu, D.; Mihai, L.; Jubera, V.; Petit, L. Response of Various Yb3+-Doped Oxide Glasses to Different Radiation Treatments. Materials 2022, 15, 3162. [Google Scholar] [CrossRef]
- Agathopoulos, S.; Tulyaganov, D.U.; Ventura, J.M.G.; Kannan, S.; Saranti, A.; Karakassides, M.A.; Ferreira, J.M.F. Structural analysis and devitrification of glasses based on the CaO-MgO-SiO2 system with B2O3, Na2O, CaF2, and P2O5 additives. J. Non-Cryst. Solids 2006, 352, 322–328. [Google Scholar] [CrossRef]
- Rada, M.; Rus, L.; Rada, S.; Culea, E.; Rusu, T. The network modifier and former role of the bismuth ions in the bismuth-lead-germanate glasses. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 132, 533–537. [Google Scholar] [CrossRef]
- Ivanov, A.O.; Evstropiev, K.S. Structure of simple germanate glass. Dokl. Akad. Nauk. SSSR 1962, 145, 797. [Google Scholar]
- Tagiara, N.S.; Chatzipanagis, K.I.; Bradtmuller, H.; Rodrigues, A.C.M.; Moncke, D.; Kamitos, E.I. Network former mixing effects in alkali germanotellurite glasses: A vibrational spectroscopic study. J. Alloys Compd. 2021, 882, 160782. [Google Scholar] [CrossRef]
- Rada, S.; Erhan, R.V.; Bodnarchuk, V.; Barbu-Tudoran, L.; Culea, E. SANS, RAMAN and SEM studies of lead-germanate glasses doped with the manganese oxide. J. Alloys Compd. 2021, 882, 160721. [Google Scholar] [CrossRef]
- Mattos, G.R.S.; Bordon, C.D.S.; Kassab, L.R.P.; Issa, S.A.; ALMisned, G.; Tekin, H.O. Towards obtaining the optimum physical, optical, and nuclear radiation attenuation behaviours of tellurite-germanate glasses through Eu2O3 reinforcement: Glass synthesis, experimental and theoretical characterization study. Ceram. Int. 2023, 49, 986–994. [Google Scholar] [CrossRef]
- Pipes, R.S.; Shelby, J.E. Formation and properties of soda lime germanate glasses. J. Non-Cryst. Solids 2021, 553, 120506. [Google Scholar] [CrossRef]
- Falci, R.F.; Guerineau, T.; Delarosbil, J.L.; Messaddeq, Y. Spectroscopic properties of gallium-rich germane gallate glasses doped with Tm3+. J. Lumin. 2022, 249, 119014. [Google Scholar] [CrossRef]
- Jianga, X.P.; Yang, Z.M.; Liu, T.; Xu, S.H. Energy transfer between Yb3+ and Er3+ in barium gallogermanate glass. J. Appl. Phys. 2009, 105, 103113. [Google Scholar] [CrossRef]
- Pisarska, J.; Sołtys, M.; Górny, A.; Kochanowicz, M.; Zmojda, J.; Dorosz, J.; Dorosz, D.; Sitarz, M.; Pisarski, W.A. Rare earth-doped barium gallo-germanate glasses and their near-infrared luminescence properties. Spectrochim. Acta A 2018, 201, 362–366. [Google Scholar] [CrossRef]
- Edwards, T.; Endo, T.; Walton, J.H.; Sen, S. Observation of the transition state for pressure-induced BO3 → BO4 conversion in glass. Mater. Sci. 2014, 345, 1261201. [Google Scholar] [CrossRef]
- Farouk, M. Effect of TiO2 on the structural, thermal, and optical properties of BaO-Li2O-diborate glasses. J. Non-Cryst. Solids 2014, 402, 74–78. [Google Scholar] [CrossRef]
- Cheng, J.; Chen, W. Formation, and structure of titanate glasses. J. Non-Cryst. Solids 1986, 80, 135–140. [Google Scholar]
- Zhang, B.; He, F.; Cao, X.; Wei, M.; Zheng, C.; Xie, J. The effect of TiO2 and B2O3 on sintering behavior and crystallization behavior of SrO-BaO-B2O3-SiO2 glass-ceramics. Ceram. Int. 2022, 48, 7013–7023. [Google Scholar] [CrossRef]
- Kowalska, K.; Kuwik, M.; Polak, J.; Pisarska, J.; Pisarski, W.A. EPR and optical spectroscopy of Cr3+ ions in barium gallo-germanate glasses containing B2O3/TiO2. J. Lumin. 2022, 48, 118775. [Google Scholar] [CrossRef]
- Calzavara, F.; Allix, M.; Dussauze, M.; Jubera, V.; Nalin, M.; Cardinal, T.; Fargin, E. Glass forming regions, structure and properties of lanthanum barium germanate and gallate glasses. J. Non Cryst. Solids 2021, 571, 121064. [Google Scholar] [CrossRef]
- Leśniak, M.; Mach, G.; Starzyk, B.; Sadowska, K.; Ragiń, T.; Zmojda, J.; Kochanowicz, M.; Kuwik, M.; Miluski, P.; Jimenez, G.L.; et al. The Effect of Fluorides (BaF2, MgF2, AlF3) on Structural and Luminescence Properties of Er3+-Doped Gallo-Germanate Glass. Materials 2022, 15, 5230. [Google Scholar] [CrossRef] [PubMed]
- Skopak, T.; Calzavara, F.; Ledemi, Y.; Celarie, F.; Allix, M.; Veron, E.; Dussauze, M.; Cardinal, T.; Fargin, E.; Messaddeq, Y. Properties, structure and crystallization study of germane-gallate glasses in the Ga2O3-GeO2-BaO-K2O system. J. Non Cryst. Solids 2019, 514, 98–107. [Google Scholar] [CrossRef]
- Shelby, J. Properties and Morphology of Barium Germanate Glasses. J. Am. Ceram. Soc. 1984, 67, 557–560. [Google Scholar] [CrossRef]
- Bayya, S.S.; Chin, G.D.; Sanghera, J.S.; Aggarwal, I.D. Germanate glass as a window for high energy laser systems. Opt. Express 2006, 14, 11687–11693. [Google Scholar] [CrossRef]
- Jewell, J.M.; Higby, P.L.; Aggarwal, I.D. Properties of BaO-R2O3-Ga2O3-GeO2 (R=Y, Al, La, and Gd) Glasses. J. Am. Ceram. Soc. 1994, 77, 698–700. [Google Scholar] [CrossRef]
- Bayya, S.S.; Harbison, B.B.; Sanghera, J.S.; Aggarwal, I.D. BaO-Ga2O3-GeO2 glasses with enhanced properties. J. Non-Cryst. Solids 1997, 212, 198–207. [Google Scholar] [CrossRef]
- ElBatal, F.H.; Marzouk, M.N.; ElBatal, H.A. Optical and crystallization studies of titanium dioxide doped sodium and potassium silicate glasses. J. Mol. Struct. 2016, 1121, 54–59. [Google Scholar] [CrossRef]
- Raghavaiah, B.V.; Laxmikanth, C.; Veeraiah, N. Spectroscopic studies of titanium ions in PbO-Sb2O3-As2O3 glass system. Opt. Commun. 2004, 235, 341–349. [Google Scholar] [CrossRef]
- Mingshen, M.; Wen, N.; Yali, W.; Zhongjie, W.; Fengmei, L. The effect of TiO2 on phase separation and crystallization of glass-ceramic in CaO-MgO-Al2O3-SiO2-Na2O system. J. Non. Cryst. Solids 2008, 354, 5395–5401. [Google Scholar] [CrossRef]
- Drabik, J.; Cichy, B.; Marciniak, L. New Type on Nanocrystalline Luminescent Thermometers Based on Ti3+/Ti4+ and Ti4+/Ln3+ (Ln = Nd3+, Eu3+, Dy3+) Luminescence Intensity Ratio. J. Phys. Chem C 2018, 122, 14928–14936. [Google Scholar] [CrossRef]
- Colak, S.C. Role of titanium ions on the optical and thermal properties of zinc borate glass doped with TiO2. Phys. Chem. Glasses Part B 2017, 58, 41–48. [Google Scholar] [CrossRef]
- Rao, R.B.; Rao, D.K.; Veeraiah, N. The role of titanium ions on structural, dielectric, and optical properties of Li2O-MgO-B2O3 glass system. Mater. Chem. Phys. 2004, 87, 357–369. [Google Scholar] [CrossRef]
- Andrade, L.H.; Lima, S.M.; Novatski, A.; Neto, A.M.; Bento, A.C.; Baesso, L.M.; Gandra, F.C.G.; Guyot, Y.; Boulon, G. Spectroscopic assignments of Ti3+ and Ti4+ in titanium-doped OH- free low-silica calcium aluminosilicate glass and role of structural defects on the observed long lifetime and high fluorescence of Ti3+ ions. Phys. Rev. B 2008, 78, 224202. [Google Scholar] [CrossRef]
- Biswas, K.; Sontakke, A.D.; Annapurna, K. Effect of TiO2 on thermal, structural and third-order nonlinear optical properties of Ca-La-B-O glass system. J. Alloys Compd. 2010, 489, 493–498. [Google Scholar] [CrossRef]
- Pernice, P.; Aronne, A.; Catauro, M. Glass transition temperature and devitrification study of barium germanate glasses. J. Non-Cryst. Solids 1997, 210, 23–31. [Google Scholar] [CrossRef]
- Rojas, S.S.; De Souza, J.E.; Andreeta, M.R.B.; Hernandes, A.C. Influence of ceria addition on thermal properties and local structure of bismuth germanate glasses. J. Non-Cryst. Solids 2010, 356, 2942–2946. [Google Scholar] [CrossRef]
- Szal, R.; Zmojda, J.; Kochanowicz, M.; Miluski, P.; Dorosz, J.; Lesniak, M.; Jeleń, P.; Starzyk, B.; Sitarz, M.; Kuwik, M.; et al. Spectroscopic properties of antimony modified germanate glass doped with Eu3+ ions. Ceram. Int. 2019, 45, 24811–24817. [Google Scholar] [CrossRef]
- Sahar, M.R.; Wahab, A.; Hussein, M.A.; Hussin, R. Structural characteristic of Na2O-P2O5-GeO2 glass systems. J. Non-Cryst. Solids 2007, 353, 1134–1140. [Google Scholar] [CrossRef]
- Zmojda, J.; Kochanowicz, M.; Miluski, P.; Lesniak, M.; Sitarz, M.; Pisarski, W.A.; Pisarska, J.; Dorosz, D. Effect of GeO2 content on structural and spectroscopic properties of antimony glasses doped with Sm3+ ions. J. Mol. Struct. 2016, 1126, 207–212. [Google Scholar] [CrossRef]
- Di Martino, D.; Santos, L.F.; Marques, A.C.; Almeida, R.M. Vibrational spectra and structure of alkali germanate glasses. J. Non-Cryst. Solids 2001, 293–295, 394–401. [Google Scholar] [CrossRef]
- Alvarado-Rivera, J.; Rodriguez-Carvajal, D.A.; Acosta-Enrniquez, M.d.C.; Manzanares-Martinez, M.B.; Alvarez, E.; Lozada-Morales, R.; Diaz, G.C.; de Leon, A.; Zayas, M.E. Effect of CeO2 on the Glass Structure of Sodium Germanate Glasses. J. Am. Ceram. Soc. 2014, 97, 3493–3500. [Google Scholar] [CrossRef]
- Rachkovskaya, G.E.; Zakharevich, G.B. IR spectra of tellurium germanate glasses and their structure. J. Appl. Spectrosc. 2007, 74, 86–89. [Google Scholar] [CrossRef]
- Henderson, G.S.; Fleet, M.E. The structure of glasses along the Na2O-GeO2 join. J. Non-Cryst. Solids 1991, 134, 259–269. [Google Scholar] [CrossRef]
- McKeown, D.A.; Merzbacher, C.I. Raman spectroscopic studies of BaO-Ga2O3-GeO2 glasses. J. Non-Cryst. Solids 1995, 183, 61–72. [Google Scholar] [CrossRef]
- Curtis, B.; Hynek, D.; Kaizer, S.; Feller, S.; Martin, S.W. Composition dependence of the short range order structures in 0.2Na2O+0.9[xBO3/2+(1-x)GeO2] mixed glass formers. J. Non-Cryst. Solids 2018, 500, 61–69. [Google Scholar] [CrossRef]
- Kukharenko, S.A.; Shilo, A.E.; Itsenko, P.P.; Kutsai, A.N. The effect of titanium dioxide on the structure of silicate multicomponent glasses, J. Superhard Mater. 2010, 32, 396–405. [Google Scholar] [CrossRef]
- Klyuev, V.P. Dependences of the dilatometric properties of glasses on their structure: II. Silicate, phosphate, fluorine-containing, and titanium-containing glasses. Glass Phys. Chem. 2006, 32, 196–205. [Google Scholar] [CrossRef]
- El-Ahafi, N.A.; Morsi, M.M. Optical absorption and infrared studies of some silicate glasses containing titanium. J. Mater. Sci. 1997, 32, 5185–5189. [Google Scholar] [CrossRef]
- Elsaghier, H.M.; Azooz, M.A.; Zidan, N.A.; Abbas, W.; Okasha, A.; Marzouk, S.Y. The influence of Er3+ ions on the spectroscopic and lasing characteristics of alkaline earth titanium borate glasses for photonic applications. Opt. Mater. 2022, 131, 112624. [Google Scholar] [CrossRef]
- Cernosek, Z.; Chladkova, M.; Holubova, J. The influence of TiO2 on the structure and properties of sodium-zinc phosphate glasses. J. Non-Cryst. Solids 2020, 531, 119866. [Google Scholar] [CrossRef]
- Subhadra, M.; Kistaiah, P. Infrared and Raman spectroscopic studies of alkali bismuth borate glasses: Evidence of mixed alkali effect. Vib. Spectrosc. 2012, 62, 23–27. [Google Scholar] [CrossRef]
- Fajans, K.; Barber, S.W. Properties and Structures of Vitreous and Crystalline Boron Oxide. J. Am. Chem. Soc. 1952, 74, 2761–2768. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Pisarska, J.; Ryba-Romanowski, W. Structural role of rare earth ions in lead borate glasses evidenced by infrared spectroscopy: BO3→BO4 conversion. J. Mol. Struct. 2005, 744–747, 515–520. [Google Scholar] [CrossRef]
- Huang, S.; Wang, W.; Jiang, H.; Zhao, H.; Ma, Y. Network Structure and Properties of Lithium Aluminosilicate Glass. Materials 2022, 15, 4555. [Google Scholar] [CrossRef]
- Culea, E.; Ristoiu, T.; Bratu, I. Magnetic and structural behavior of some borate glasses containing holmium ions. Mater. Sci. Eng. 1999, 57, 259–261. [Google Scholar] [CrossRef]
- Mogus-Milankovic, A.; Sklepic, K.; Mosner, P.; Koudelka, L.; Kalendra, P. Lithium-Ion Mobility in Quaternary Boro-Germano-Phosphate Glasses. J. Phys. Chem. B 2016, 120, 3978–3987. [Google Scholar] [CrossRef]
- Cormier, L.; Ghaleb, D.; Delaye, J.M.; Calas, G. Competition for charge compensation in borosilicate glasses: Wide-angle X-ray scattering and molecular dynamics calculations. Phys. Rev. B 2000, 61, 14495. [Google Scholar] [CrossRef]
- Costa-silva, D.L.; Bartolome, J.F.; Silva, A.C.; Mello-Castanho, S. Structural and thermal influence of niobia in aluminoborosilicate glasses. Ceram. Int. 2022, 48, 18433–18440. [Google Scholar] [CrossRef]
- Yin, H.; Gao, Y.; Guo, H.; Wang, C.; Yang, C. Effect of B2O3 Content and Microstructure on Verdet Constant of Tb2O3-Doped GBSB Magneto-Optical Glass. J. Phys. Chem. C 2018, 122, 16894–16900. [Google Scholar] [CrossRef]
- Wu, J.; Stebbins, J.F. Cation Field Strength Effects on Boron Coordination in Binary Borate Glasses. J. Am. Ceram. Soc. 2014, 97, 2794–2801. [Google Scholar] [CrossRef]
- Fabian, M.; Gergely, F.; Osan, J.; Cendak, T.; Kesari, S.; Rao, R. Structural investigation of borosilicate glasses containing lanthanide ions. Sci. Rep. 2020, 10, 7835. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Xu, L.; Hu, L.; Zhang, J. Structural Origin and Laser Performance of Thulium-Doped Germanate Glasses. J. Phys. Chem. A 2011, 115, 14163–14167. [Google Scholar] [CrossRef]
- Ebendorff-Heidepriem, H.; Wang, P. Chapter 3—Oxide Glass and Optical Fiber Fabrication. Mid-Infrared Fiber Photonics 2022, Chapter 3, 111–176. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Kowalska, K.; Kuwik, M.; Polak, J.; Pietrasik, E.; Goryczka, T.; Pisarska, J. Novel Multicomponent Titanate-Germanate Glasses: Synthesis, Structure, Properties, Transition Metal, and Rare Earth Doping. Materials 2020, 13, 4422. [Google Scholar] [CrossRef]
- Wen, X.; Tang, G.; Yang, Q.; Chen, X.; Qian, Q.; Zhang, Q.; Yang, Z. Highly Tm3+ doped germanate glass and its single mode fiber for 2.0 μm laser. Sci Rep. 2016, 6, 20344. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Kowalska, K.; Kuwik, K.; Pisarska, J.; Dorosz, D.; Żmojda, J.; Kochanowicz, M.; Dorosz, D. Nd3+ doped titanate-germanate glasses for near-IR laser applications. Opt. Mater. Express 2022, 7, 2912–2926. [Google Scholar] [CrossRef]
- Kowalska, K.; Kuwik, M.; Pisarska, J.; Leśniak, M.; Dorosz, D.; Kochanowicz, M.; Żmojda, J.; Dorosz, A.; Pisarski, W.A. Influence of TiO2 concentration on near-infrared luminescence of Er3+ ions in barium gallo-germanate glasses. J. Mater. Res. Technol. 2022, 21, 4761–4772. [Google Scholar] [CrossRef]
- Kowalska, K.; Kuwik, M.; Pisarska, J.; Pisarski, W.A. Near-IR Luminescence of Rare-Earth Ions (Er3+, Pr3+, Ho3+, Tm3+) in Titanate–Germanate Glasses under Excitation of Yb3+. Materials 2022, 15, 3660. [Google Scholar] [CrossRef]
- Kaur, S.; Mohan, S.; Singh, D.P. Spectroscopic properties and lasing potentialities of Sm3+ doped multi-component borate glasses. Opt. Commun. 2021, 482, 126523. [Google Scholar] [CrossRef]
- Kuwik, M.; Górny, A.; Pisarski, W.A.; Pisarska, J. Influence of glass formers and glass modifiers on spectral properties and CIE coordinated of Dy3+ ions in lead-free borate glasses. Spectrochim. Acta A 2022, 268, 120693. [Google Scholar] [CrossRef]
- Kowalska, K.; Kuwik, M.; Polak, J.; Pisarska, J.; Pisarski, W.A. Transition Metals (Cr3+) and Lanthanides (Eu3+) in Inorganic Glasses with Extremely Different Glass-Formers B2O3 and GeO2. Materials 2021, 14, 7156. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).